Abstract
The development of osteogenic biomaterials relies on updates in research methodologies. Establishing reasonable modes is the basis for obtaining reliable experimental conclusions. With the advancement in bone immunology, osteoimmunomodulatory properties have become one of the crucial indexes for evaluating osteogenic biomaterials. Summarizing the current models of bone immunomodulation is beneficial for optimizing experimental protocols and promoting the clinical application of osteogenic biomaterials. In this review, we introduced the crosstalk between the skeletal system and the immune system, in particular, the roles of different immune cells in the process of bone regeneration. Moreover, the mechanisms of osteogenic biomaterials in regulating the osteoimmune microenvironment were analyzed, followed by summarizing the benefits and limitations of current osteoimmunomodulation models in evaluating osteogenic biomaterials. Finally, we discussed the expected future directions of the applications of osteoimmunomodulation models.
1. Introduction
Bone defects resulting from trauma, tumors, and congenital developmental diseases have a profound impact on the function of the patient’s skeletal system and quality of life []. Although autogenous bone represents the gold standard for bone defect repair, its clinical application is constrained by several factors []. These include donor site morbidity, limited graft quantity, post-graft resorption, prolonged healing time, risk of infection, and other complications []. The implantation of osteogenic biomaterials continues to be one of the common treatments []. Developing new biomaterials with efficient osteogenic efficacy depends on effective preclinical testing. Thus, the construction of a reasonable evaluation model is of significant importance for the development of osteogenic materials and the reduction in failure rates in clinical applications. In vivo, implanted osteogenic biomaterials, as foreign objects, can initiate immune responses and form a new microenvironment, which decides the fate of bone healing []. The term “osteoimmunology” was first proposed in 2000, marking the beginning of a new era in the study of the intricate relationships between the skeletal system and the immune system at the cellular and biomolecular levels [,]. This research field has led to significant advancements in evaluating osteogenic biomaterials []. After implanting osteogenic biomaterials, immune cells will be activated immediately, which crosstalk with bone-related cells to decide the implant fate. On the other hand, biomaterials modulate the phenotype and function of immune cells by releasing biophysical, biochemical, and biological cues. To investigate these complex interaction mechanisms, we further analyzed the establishment and applications of current osteoimmunomodulation models in evaluating osteogenic biomaterials. Finally, continuing challenges and future directions of the model development were discussed.
2. Crosstalk Between Bone Cells and Immune Cells in the Process of Bone Regeneration
Bone regeneration is a dynamic, complex, and orderly biological process that usually undergoes three overlapping phases: inflammation, bone formation, and remodeling []. In these processes, osteoblasts (OBs) continuously receive biological signals from the surrounding environment. The modern theory of “osteoimmunology” indicates that bone regeneration results from the crosstalk of skeletal and immune systems (Figure 1A) [,]. Immune cells, as the main players in the inflammatory response, are involved in establishing the immune microenvironment for bone regeneration []. On the other hand, bone cells, especially bone marrow mesenchymal stem cells (BMSCs), can regulate immune cell polarization and behavior [].
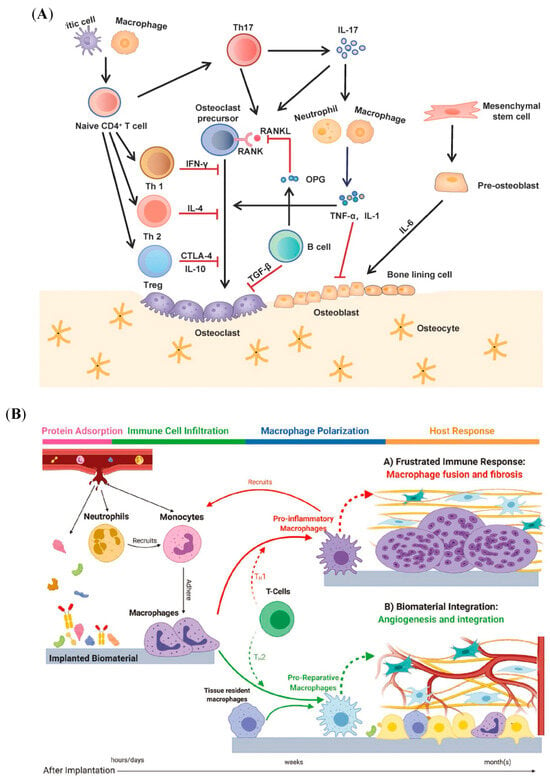
Figure 1.
Schematic illustration of the crosstalk between bone and immune cells in biomaterial-induced bone regeneration. (A) The communication between bone-related cells and immune cells during bone formation ( means promotion;
means promotion;  means inhibition). Reprinted from Ref. []. (B) The local immune–bone–biomaterial microenvironment. Reprinted with permission from Ref. []. Copyright 2020, John Wiley and Sons.
means inhibition). Reprinted from Ref. []. (B) The local immune–bone–biomaterial microenvironment. Reprinted with permission from Ref. []. Copyright 2020, John Wiley and Sons.
Osteogenic biomaterials implanted as foreign bodies can stimulate a series of host responses, including protein adsorption, immune cell infiltration, macrophage polarization, and host response, as shown in Figure 1B []. Initial protein adsorption affects immune cell recruitment and adhesion, followed by immune cell polarization [,,]. Immune cells with different phenotypes influence the bone development microenvironment by releasing receptor activators of nuclear factor κ-B ligand (RANKL), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor α (TNF-α), which participate in deciding the fate of bone formation []. In turn, bone cells recruit immune cells by secreting chemokines, such as macrophage chemoattractant protein-1 (MCP-1) and stromal cell-derived factor 1 (SDF-1). This close relationship between the skeletal system and the immune system claims that changes in the physiology and pathology of one system can impact the other.
2.1. The Roles of Neutrophils in Bone Regeneration
Neutrophils are the most abundant type of leukocyte derived from hematopoietic stem cells in the bone marrow, constituting about 50–70% of the white blood cells, particularly during the foreign body response (FBR) [,]. After implanting, osteogenic biomaterials are recognized as foreign and activate the immune systems []. Neutrophils are among the first immune cells to be recruited in the implant site in response to danger-associated molecular patterns (DAMPs) released by damaged tissues, such as interleukin-8 (IL-8), leukotriene B4 (LTB4), and reactive oxygen species (ROS) []. These recruited neutrophils will also recruit further immune cells by secreting IL-8, macrophage inflammatory protein-1 (MIP-1), MCP-1, and matrix metalloproteinase-9 (MMP-9) []. At this initial stage, neutrophils help to clear debris and pathogens from the injury site, which is the process of engulfing and digesting foreign particles or damaged cells produced by implantation surgery. Besides discussing the spatiotemporal dynamics of neutrophils, advanced studies focused on the phenotypes in bone regeneration []. Zvi G Fridlender et al. first suggested that neutrophils could be polarized to “N1” or “N2” phenotypes, like M1 and M2 in macrophages, by establishing a flank tumor model in mice []. And then, neutrophils are classified into inflammatory N1 and regenerative N2 phenotypes []. A recent study indicated that neutrophils played an essential role in the initial stage of bone regeneration []. Furthermore, in the mice model, N2-polarized neutrophils recruited BMSCs by secreting stromal cell-derived factor-1α (SDF-1α), followed by increased migration of BMSCs by activating the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/β-catenin pathway [].
Extracellular vesicles (EVs) contain rich biological information, which can be released by various cells, including neutrophils. Le Wang et al. pretreated BMSCs with neutrophil-EVs to make neutrophil-EVs-integrated BMSC sheets (BS@PMN-EVs) in vitro, followed by implanting the BS@PMN-EVs in the cranial defect model of rats []. The results of this study indicated that neutrophil-EVs promoted BMSC proliferation and osteogenic differentiation; further, BS@PMN-EVs enhanced the new bone maturation in vivo (BV/TV revealed new bone formation at ∼39.5%, 5.8%, and 23.8% upon the implantation of BS@PMN-EVs, the blank, and BS@PBS) []. In addition, neutrophils are involved in regulating bone regeneration by interacting with other cell types. They contributed to reprogramming macrophages and T cells to M2 and Treg phenotypes, respectively, which benefit osteogenic differentiation [,]. In addition, the study by TDK Herath et al. suggested that neutrophils promote angiogenesis and osteogenesis, two critical and interrelated processes in bone formation []. In particular, they established a novel triple cell co-culture model, consisting of neutrophils, human umbilical vein endothelial cells (HUVECs), and human osteoblasts (HOBs) []. Further results showed that neutrophils promoted the expression of angiogenic markers in HUVECs, such as vascular endothelial growth factor A (VEGF-A), CD34, and fibroblast growth factor-2 (FGF-2), and it increased the levels of alkaline phosphatase (ALP), osteocalcin (OCN), type I collagen (COL-1), and osteopontin (OPN) in HOBs []. Taken together, neutrophils perform multifaceted roles in regulating bone regeneration by clearing debris, modulating the inflammatory response, and influencing the activity of bone-related tissue cells.
2.2. The Roles of Dendritic Cells (DCs) in Bone Regeneration
The DCs are acknowledged as professional antigen-presenting cells (APCs) while sharing certain common features with osteoclasts (OCs) []. Both of them originate from hematopoietic tissue and display phagocytic capabilities. In steady conditions, there were rarely DCs in the bone tissue and no absence of bone in DC-deficient mice, which suggested that DCs do not exert a major role in normal bone []. However, DCs can act as precursors to OCs under inflammation, which are involved in the bone damage process []. Rivollier et al. first proposed that immature DCs can transdifferentiate into functional OCs []. The DC-derived OCs are further directly involved in the development of inflammatory bone disease []. This process may contribute to the sensitivity of DCs to RANKL. Although RANKL was first described as an important regulator of interactions between T cells and DCs, studies have demonstrated that RANKL can also influence the behavior of DCs []. Further, RANKL can protect mature DCs and prolong their survival, followed by promoting the production of pro-inflammatory cytokines [,]. In addition to its role in immunomodulation, DCs were transdifferentiated into mature resorbing OCs by treatment with RANKL in an inflammation model []. Thereby, DCs are a critical pool of OCs under inflammatory conditions to directly regulate bone homeostasis. A single-cell sequencing (scRNA-seq) analysis of the patient’s program showed that more CD14+ DCs existed in nonunions compared to the native bone []. These DCs were proposed to differentiate into OC-like cells, which may induce bone healing failure due to the increase in bone resorption []. The above studies indicated that the roles of DCs in the bone system depend on the specific conditions. Furthermore, the mechanisms of DCs in bone regeneration should be analyzed in the optimal model based on the purpose of the studies.
2.3. The Roles of Lymphocytes in Bone Regeneration
The two primary types of lymphocytes are T cells and B cells. Both originate from hematopoietic stem cells in the bone marrow and play crucial roles in bone formation []. In the process of bone regeneration, T cell and B cell infiltration presented two waves, during days 3–7 and after day 14, respectively [].
2.3.1. T Cells
The T cells regulate bone regeneration by secreting cytokines and interacting with other cells. Based on the expression of surface markers and the cytokines they produce, T cells are mainly classified into helper T cells (Th, CD4+ T cells) and cytotoxic T cells (CD8+ T cells) []. CD4+ T cells can be polarized to Th1 and Th17 subsets, followed by secreting the inflammatory cytokines, such as interferon-γ (IFN-γ), IL-17, and TNF-α, to form a pro-inflammatory microenvironment and induce heavy macrophage infiltration []. On the other hand, the subsets of Th2 and Treg reduce tissue inflammation by secreting interleukin-4 (IL-4), IL-10, and transforming growth factor-β (TGF-β) []. To explore the effects of T cells on bone regeneration of titanium (Ti) biomaterial implantation, Derek Avery et al. established the model of CD4 and/or CD8 knockout mice, and T cells were extracted from the treated mice (WT, Cd4−/−, or Cd8−/−), respectively. These different groups of T cells were directly co-cultured with macrophages to investigate the impact of CD4+ and CD8+ T cells on macrophage polarization in vitro []. Further, the authors made the macrophage-T cell-MSC co-culture model by culturing adipose-derived MSCs in transwell or conditioned media (CM) of macrophage-T cell co-culture []. The in vitro results were finally confirmed by implanting Ti implants in WT, Cd4−/−, and Cd8−/− mice []. The results of this study demonstrated that the deficiency of either CD4+ or CD8+ T cells increased the proportion of pro-inflammatory macrophages, diminished MSC recruitment, and reduced new bone formation at the implantation site []. Moreover, compared to CD8+ T cell deficiency, CD4+ T cell deficiency exacerbated these effects [].
As mentioned above, CD4+ T cells were further divided into Th1, Th2, Treg, and Th17 subsets. Current evidence suggests that Th1 cells primarily produce IFN-γ and Th2 cells secrete IL-4 and interleukin-13 (IL-13), thereby participating in regulating the behaviors of OCs and OBs [,]. However, the detailed mechanisms remain to be explored. In addition, Treg cells produce anti-inflammatory cytokines such as IL-10 and TGF-β, which can suppress bone resorption by inhibiting the activation of osteoclast precursors and promoting osteoprotegerin (OPG) expression, a decoy receptor for RANKL []. In the last few years, Th17 cells have attracted wide attention. They mainly promote bone resorption by secreting pro-inflammatory cytokines, such as interleukin-17 (IL-17), interleukin-22 (IL-22), and interleukin-26 (IL-26) [,]. In particular, IL-17 stimulates the production of RANKL by OBs, thereby promoting osteoclast differentiation, followed by bone resorption []. Moreover, CD8+ T cells can inhibit osteoclastogenesis and bone resorption by releasing IFN-γ, similar to the effects mediated by Th1 cells []. However, the precise mechanisms by which CD8+ T cells regulate bone cells are still being elucidated. A recent study explored the role of MSCs-based therapy in bone defect healing by Cytometry by Time Of Flight (CyTOF), which revealed the cellular and immunomodulatory profiles []. The results showed that MSCs increased the T cell population to about 24%, but the control group varied from 1% to 40% []. Further, CD4+ T cells consisted of about 68% of all T cells in the MSCs group, whereas CD8+ T cells were about 10% []. However, double-negative T cells (about 86%) formed a major population in the control group []. Therefore, the modulation of T cell subtypes by designing osteogenic biomaterials, which in turn promotes osteogenesis, deserves further investigation.
2.3.2. B Cells
B cells are mainly involved in the process of adaptive immune response []. Similar to T cells, the differentiation and maturation of B cells are also closely related to osteogenesis. The RANKL/RANK/OPG axis contributes to recruiting B cells and the crosstalk between B cells and OBs []. More than two decades ago, William C. Dougall et al. established the RANK-deficient mice (RANK−/−) []. Following this model, RANK−/− mice exhibited obvious osteopetrosis as a consequence of the impeded differentiation of OCs []. At the same time, a deficiency of B cells was observed within the spleen []. However, RANK is not necessary for the development and function of macrophages and DCs []. In the same year, Young-Yun Kong et al. found that RANKL was a new regulator in the early differentiation of B cells and an essential factor in osteoclast differentiation by establishing the RANKL-deficient mice (RANKL−/−) []. In addition to being regulated by RANKL, B cells can also produce RANKL to maintain their development in normal conditions instead of keeping bone homeostasis []. To further examine the relationship between B cells and bone-related cells, some studies have concentrated on the impact of B cells in pathological conditions. In the ovariectomy (OVX) mouse model, precursors of B (pre-B) cells were selectively increased in bone marrow, and osteoclastogenesis was enhanced, which may be attributed to estrogen deficiency [,]. Moreover, a study indicated that RANKL overexpression in pre-B cells induced by estrogen deficiency may be a significant contributor to the acceleration of osteoclastogenesis []. Another study further demonstrated that this accelerated osteoclastogenesis resulted from the accumulation of osteoclast precursors, followed by abnormal bone resorption []. The authors then suggested that estrogen-deficiency-induced pre-B cells could differentiate into OCs by using in vitro co-culture models []. Interestingly, mature B cells were found to inhibit osteoclastogenesis and shorten the life span of OCs by secreting the apoptotic cytokine TGF-β []. The study of B Klausen et al. also showed that a temporary and moderate B cell deficiency could aggregate the periodontal bone loss in a rat model of periodontitis [], suggesting that B cells may exert osteoprotective effects in some pathological conditions. The communication between B cells and bone cells depends on the developmental stage of the B cells and the surrounding conditions; thus, it is essential to establish rational models to explore their potential mechanisms.
2.4. The Roles of Macrophages in Bone Regeneration
Among these immune cells, macrophages are regarded as playing a central role in osteogenesis due to their high plasticity and pivotal role in regulating the osteogenic microenvironment []. Macrophages have a spectrum of activation states, ranging from pro-inflammatory M1 to anti-inflammatory M2. In the early phase of bone damage, macrophages polarize into M1 phenotype through “classical activated”, which clears cell debris through phagocytosis and secreting pro-inflammatory cytokines, such as IL-1β, IFN-γ, and TNF-α []. This clearance function was a critical process in creating a conducive environment for subsequent bone formation to maintain skeletal metabolism. In addition, M1 can recruit stem cells and initiate the bone healing process by secreting cytokines such as TNF-α, oncostatin M (OSM), bone morphogenetic protein 2 (BMP-2), and bone morphogenetic protein 6 (BMP-6) [,]. However, the prolonged presence of M1 is demonstrated to be associated with the failure of bone regeneration []. During the subsequent bone regeneration phase, macrophages transform into an “alternatively activated” M2 phenotype, which supports bone repair via secreting osteogenesis-related proteins BMP-2, VEGF, and TGF-β1 []. At the same time, an array of anti-inflammatory cytokines, including IL-4, IL-10, and IL-13, are also released to establish a microenvironment supportive of bone healing []. As mentioned above, both M1 and M2 participate in bone formation, and appropriate switching of M1/M2 is crucial for achieving success in bone regeneration.
Native bone is a highly vascularized tissue. Adequate blood supply is involved in the regeneration of bone tissue, to ensure the enough oxygen and nutrients are delivered to activate OBs []. Thus, the vascularized osteogenesis generated by osteogenic biomaterials plays a crucial role in remodeling the bone defect []. In addition to directly regulating bone-related cells, macrophages can regulate bone formation by promoting angiogenesis, serving as a bridge []. A topological scaffold was functionalized with M2 macrophage-derived exosome (M2-exosome) and measured for inflammatory regulation property and osteogenic potential in both in vitro and in vivo experiments []. This study indicated that, based on the scaffold biomaterial, M2-exosome played the coupling effect of angiogenesis, osteoclastogenesis, and osteogenesis by stimulating type H vessel formation []. In addition to directly promoting osteogenesis by regulating macrophage polarization, macrophage-induced angiogenesis can be used to guide osteogenic biomaterial design.
2.5. The Roles of BMSCs in Regulating Immune Cells
The BMSCs are directly involved in bone regeneration due to their multidirectional differentiation potential, and they have been used as seed cells to treat bone defects [,]. In addition, they can create specific microenvironments by releasing various cytokines, chemokines, and exosomes to communicate with other cells, including immune cells []. Dandan Chen et al. demonstrated that BMSCs exert immunosuppressive effects by regulating T cell survival and differentiation using a transwell model in vitro []. BMSCs and LPS-stimulated CD4+ T cells were seeded in the upper and lower parts of the transwell dishes, respectively. The results showed that BMSCs suppressed T cell proliferation and reduced Th1/Th2 and Th17/Treg ratios by secreting cytokines and decreasing programmed cell death-1 (PD-1) expression in T cells [].
In addition to the major paracrine pathways, immune cells can interact with BMSCs through direct cell–cell contact []. When the combination of BMSCs and T cells was directly cocultured, PD-ligand 1 (PD-L1) shRNA-BMSCs might activate CD4+ T cells. The vivo mice model further confirmed that PD-L1 inhibition attenuated BMSCs-induced imbalance of Th1/Th2 and Th17/Treg. These results suggested that the BMSCs interact with T cells via the PD-1/PD-L1 pathway []. The immune and bone systems were closely linked with each other; however, the detailed mechanism between them remains to be further explored. To investigate bone formation mechanisms and develop bone regeneration biomaterials, it is necessary to establish rational in vitro and in vivo research models.
3. Mechanisms of Osteogenic Biomaterials in Regulating Osteo-Immune Microenvironment
In contrast to the previous focus on the relationships between osteogenic biomaterials and OBs, the profound influence of osteogenic biomaterials in the immune response-modulated osteogenesis attracts wide attention. Based on the tight relationship between bone and immune system, the development of osteogenic biomaterials evolved from only focusing on mechanical-physical-chemical principles and directly promoting osteoblastic lineage cells to regulating bone-immune microenvironment, which is called “osteoimmunomodulation” []. Macrophages are extensively researched due to their high plasticity and critical role in regulating the osteoimmune microenvironment. The relevant studies are listed in Table 1.
The macrophages sense and respond to the endogenous and exogenous stimuli in the rounding microenvironment. As mentioned above, the M1 and M2 phenotypes participate in different stages of bone formation. Thus, understanding the potential mechanisms of osteogenic biomaterials regulating macrophage polarization is crucial, as it could guide the development of the next biomaterials.
3.1. Biophysical Cues
Living cells in the body are constantly exposed to mechanical stimuli originating from the surrounding microenvironment, which alter cellular fates and regulate their functions []. Macrophages, as a type of multi-functional cells, can sense the implanted biomaterials and their surrounding microenvironment []. They further transduce these mechanical cues into biological signals, such as surface topography, roughness, hydrophily, porosity, stiffness, and bioelectric signal [,]. These transduction ways involve regulating mechanical sensors, rearranging cytoskeleton-related structures, and activating associated signaling pathways (Figure 2A).
Macrophages adhere to the substrates and sense their topography by mechanical sensors, such as integrins and mechanosensitive ion channel proteins []. Integrins are transmembrane receptors on the cell surfaces, which assist cell–cell and/or cell-extracellular matrix (ECM) interaction []. Lin Lv et al.’s study showed that biomaterial surface hydrophilicity regulated macrophage polarization and osteogenesis through selective expression of integrin β1 or β2, which influenced macrophage behavior []. The hydrophilic surface activated the PI3K/Akt pathway to drive M2 polarization by interacting with integrin β1, but the hydrophobic surface resulted in M1 polarization by nuclear factor κ-B (NF-κB) pathway activation, which is associated with integrin β2 []. Other study results also suggested that the physical properties of biomaterials modulated macrophage polarization by expressing different integrins, which resulted in the cascade of intracellular pathways, such as PI3K/Akt-NF-κB pathway, c-Jun N-terminal Kinase (JNK) pathway, and RhoA/Rho-associated kinase (ROCK)-related pathway [,]. Based on transcriptomic analysis, the Yizhou Zhu et al.’s study revealed that a TiO2 honeycomb-like structure also upregulated the RhoA/ROCK signaling pathway to induce M2 macrophage polarization, followed by promoting osteogenic differentiation of MSCs in vitro and facilitating bone-to-implant osteointegration in vivo (Figure 2B) []. In addition, the cytoplasmic domains of activated integrin link to actin filaments, by which they transfer extracellular information and rearrange the cell shape []. Several cytoskeleton-related proteins and structures participate in this process, such as F-actin, talin, vinculin, and podosomes [,]. M1 and M2 macrophages exhibit dramatically different cell shapes, which are mainly controlled by the cytoskeleton []. By employing a micropatterning approach to directly control the cytoskeleton structure, the elongation of macrophages induced polarization toward an M2 phenotype []. These results implied that actin polymerization, actin/myosin contractility, and ROCK and myosin light chain kinase (MLCK) activities play a crucial role in controlling macrophage polarization by cell shape []. Consistently, to precisely design biomaterial porosity, the other investigation cultured human macrophages in poly(ε-caprolactone) (PCL) scaffold with different pore sizes; the results showed that cell elongation tended to increase M2 phenotype polarization by smaller pore size []. Further, Vijaykumar S. Meli et al. study showed that substrate stiffness and cytoskeletal polymerization regulated macrophage behaviors by Yes-associated protein (YAP) nuclear localization []. These studies suggested that the cytoskeleton dynamics are closely related to macrophage polarization []. In addition, proteins in the body adsorb to biomaterial surfaces immediately after implantation, reestablishing an ECM microenvironment []. Macrophages can sense the mechanical properties of the ECM through cytoskeleton rearrangement, by which they are polarized toward different phenotypes [,]. In addition, mechanosensitive ion channel proteins, such as Piezo1, are also involved in regulating macrophage polarization []. Piezo1 plays an important role in sensing the different stiffness of biomaterials and then participating in both inflammatory and healing pathways by mediating calcium ion (Ca2+) influx []. Above all, the effects of physical cues on macrophages should be considered during the design and development of osteogenic biomaterials.
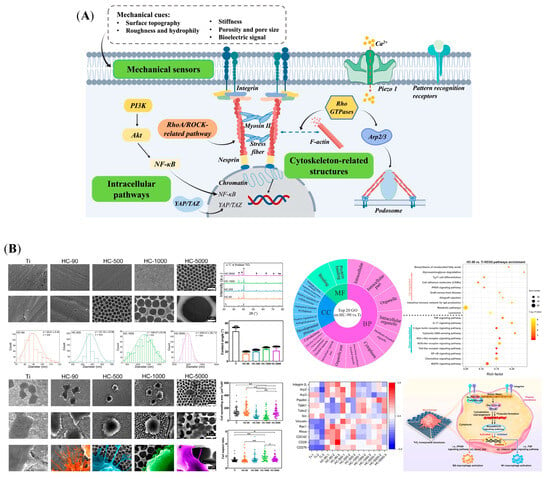
Figure 2.
Macrophages transform biophysical cues of biomaterials into biological signals, followed by regulating bone regeneration. (A) Potential mechanotransduction pathways for regulating macrophage behavior: mechanical sensors, cytoskeleton-related structures, and intracellular pathways. (B) Surface characterizations of TiO2 honeycomb-like nanostructure; the morphological changes in macrophages induced by it, and a mechanistic analysis of macrophage polarization. (*: p < 0.05; **: p < 0.01). Reprinted from Ref. [].
3.2. Biochemical Cues
Chemical modification methods are widely used to optimize the properties of osteogenic biomaterials. Several chemical cues regulate macrophage polarization, such as ion release, functional groups, and pH, as shown in Figure 3. These factors play crucial roles in modulating the osteoimmune microenvironment by orchestrating inflammatory responses and regulating tissue repair.
Metallic elements are integral to cellular composition and regulate biological behaviors through signaling pathways. Immune cells are sensitive to metal ions released from biomaterials. Ca2+ is a key component of bone tissue and plays an immunomodulatory role [,]. Controlled Ca2+ release from biphasic calcium phosphate (BCP) can activate the calcium-sensing receptor (CaSR) in macrophages, thereby enhancing the expression of M2 macrophage markers via the Wnt/β-catenin pathway []. M2-polarized macrophages facilitate the differentiation of MSCs into OBs []. Other divalent cations, like magnesium ion (Mg2+), zinc ion (Zn2+), or copper ion (Cu2+), was delivered by the alginate-based hydrogel (Alg), and their underlying mechanisms in promoting bone formation were explored []. As shown in Figure 3A, the results of this study demonstrated that the divalent cations triggered prostaglandin E2 (PGE2) production from macrophages, which can activate the PGE2 receptor4 (EP4) at the sensory nerve to tune down sympathetic tones via the cAMP-response element binding protein (CREB) signaling in the ventromedial hypothalamus (VMH), resulting in the increase in osteogenesis in the periosteum, and decreasing osteoclastogenesis []. Zn element loaded onto the TiO2 nanotubes’ (TNTs) surface (Zn-incorporated TNT) increased the expressions of M2 markers in macrophages []. The conditioned medium of macrophages in the Zn-incorporated TNT can enhance the osteogenic differentiation of OBs []. Similarly, R. Zhang et al. reported that micro/nanostructured TiO2/ZnO coating produced an immune microenvironment, which promoted osteogenesis []. Furthermore, the microarray was used to investigate the gene expression profile of macrophages on Zn-coated sulfonated polyetheretherketone (SPEEK) []. The results showed that the M2 markers (CD206 and CD163) and osteogenic genes (BMP-2, VEGF, and TGF-β) were increased in the Zn-coated SPEEK group []. In contrast, C-C Chemokine Receptor Type 7 (CCR7), inducible nitric oxide synthase (iNOS), and M1 markers were decreased []. The Kyoto Encyclopedia of Genes and Genomes (KEGG) was employed to analyze the pathways []. Pro-inflammatory and M1-phenotype-related pathways, TNF and NF-κB pathways, were downregulated, and the pathway regulating M2 polarization and bone formation, Janus kinase (Jak)-signal transducer and activator of transcription (STAT), and VEGF pathways were upregulated []. Incorporating more than one element on the biomaterial surface enables the synergistic effect of the elements to be utilized. For example, compared with Ti-Ca and Ti–strontium (Sr), Ti-Ca-Sr was superior in modulating M2 polarization, which increased the osteogenic differentiation of BMSCs in co-culture [].
The functional groups of biomaterial surfaces play crucial roles in directing immune cell behavior by regulating protein adsorption from serum or reprogramming cell phenotypes. R. M. Visalakshan et al. modified acrylic acid (AC), 2-methyl-2-oxazoline (MEOX), allylamine (AA), and 1,7-octadiene (OD) in the substrate surface by employing plasma polymerization (Figure 3B) []. The results showed that protein absorption was influenced by different chemical functionalities, which possessed a range of wettability []. Digitally, hydrophilic surface-induced protein absorption increased the release of anti-inflammatory cytokines []. In turn, the hydrophobic surface increased the production of pro-inflammatory cytokines []. Other studies revealed that modified biomaterials with different functional groups can reprogram macrophage phenotypes, further influencing the tissue microenvironment [].
The human body usually maintains its pH at a narrow range of 7.35–7.45 []. The implanted biomaterials, especially biodegradable bone material, could change the microenvironmental pH around the materials by degrading or releasing ions []. Some polymers, like polylactide acid (PLA), polyglycolide acid (PGA), polylactide-co-glycolide acid (PLGA), and PCL, generate several acidic products, subsequently decreasing the surrounding pH [,,,]. In contrast, bio-ceramic/glass materials can increase the local pH by releasing alkaline ions []. Several studies revealed that relatively high-pH environments are more favorable for the synthesis of osteogenesis proteins, such as collagen and ALP [,]. In turn, osteoclastic activity was inhibited []. In addition, immune cells can alter their phenotypes and cytokine patterns in response to the environmental pH. Hong Wu et al. reported that various environmental pH values induced changes in macrophage morphology and OB mineralization [], as shown in Figure 3C,D. Specifically, an acidic condition with a pH of 6.6 promotes M2 polarization, evidenced by increasing M2 markers arginase-1 (Arg-1) and CD206 []. The alkaline condition with a pH of 8.2 increased the expression of CD11c, TNF-α, IL-1β, and IL-6, indicating an enhancement of M1 polarization []. Their results further showed that culturing macrophages in pH 8.2 media increased ATP activity, collagen synthesis, in vitro mineralization, and the expression of ALP, Col-I, and OCN in OBs []. Thus, the microenvironmental pH might be an important switch to control macrophage polarization.
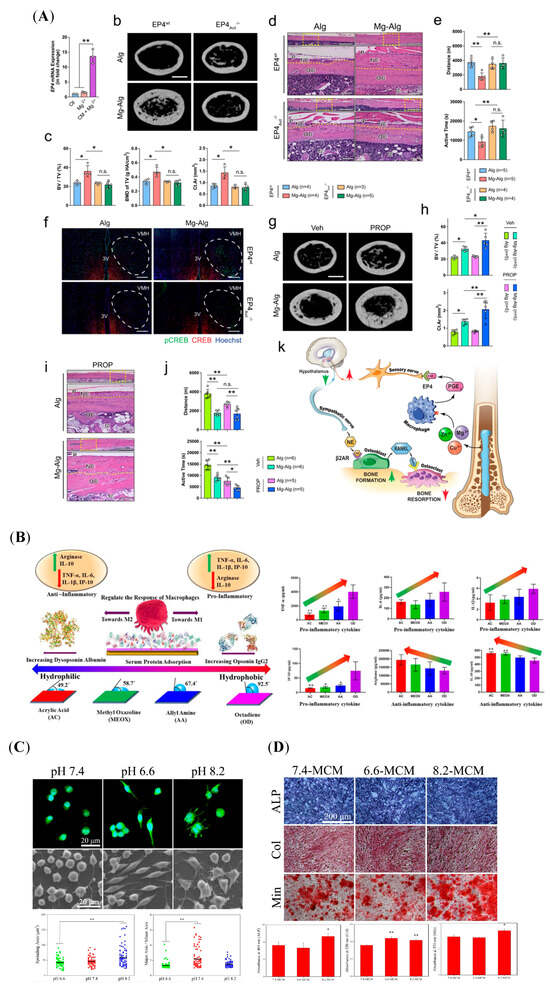
Figure 3.
Biomaterials act on macrophages with biochemical signals, followed by regulating bone regeneration. (A) The divalent cations increased bone formation and decreased bone resorption by regulating the immune–neural axis. (n.s.: not significant, *: p < 0.05, **: p < 0.01). Reprinted from Ref. []. (B) The influence of modified-surface wettability in serum protein adsorption and further immune responses in macrophages. (*: p < 0.05, **: p < 0.01). Reprinted with permission from Ref. []. Copyright 2019, American Chemical Society. (C) The macrophage morphology in response to different pH environments was observed via laser confocal microscopy and scanning electron microscopy (SEM). (Green: FITC-Phalloidin, Blue: 4′-6-diamidino-2-phenylindole (DAPI), **: p < 0.01, compared to the pH 7.4 group). Reprinted with permission from Ref. []. Copyright 2019, American Chemical Society. (D) ALP activity, collagen synthesis, and in vitro mineralization of the OBs cultured by various macrophage-CM for 5, 10, or 14 days, respectively. (*: p <0.05, **: p < 0.01, compared to the 7.4-MCM group). Reprinted with permission from Ref. []. Copyright 2019, American Chemical Society.
3.3. Biological Cues
Several cytokines and chemokines, such as IL-4, IL-10, BMP-2, and VEGF, regulate the osteoimmune environment. Besides being produced by immune cells responding to biomaterials, they can be released directly from osteogenic biomaterials to act on tissue cells. A study showed that BMP-2 activated macrophages by the pSmad1/5/8 pathway, and the CM harvested from BMP-2-treated macrophages accelerated the osteogenesis []. Further, the gelatin sponge incorporated with BMP-2 significantly increased macrophage infiltration and decreased the expression of M1 markers, such as IL-1β, IL-6, and iNOS, simultaneously []. In addition, Wang et al. modified the Ti surface with poly(dopamine) (pDA)-assisted immobilization of IL-4 (Figure 4A) []. The sandblasted and acid-etched (SLA)-pDA-IL4 surfaces retained IL-4 bioactivity and increased the M2/M1 proportion, which is anticipated to accelerate further bone integration [].
Bone regeneration is a dynamic and orderly biological process that includes inflammatory reactions, angiogenesis, and osteogenesis []. Some cytokines modulate this temporal and spatial pattern to coordinate bone remodeling. Kara et al. designed scaffolds to promote bone regeneration by sequentially switching macrophage polarization []. The scaffolds induced M1 polarization by shortly releasing IFN-γ, which plays a role in the early stage of healing [,]. Subsequently, they facilitated M2 polarization by releasing IL-4 to promote vascularization []. This study suggested that sequential cytokine release may promote angiogenesis by altering M1 and M2 polarization. In addition, a composite scaffold of 3D-printed titanium alloy with BMP-2 and IL-4 could polarize macrophages to the M2 phenotype, resulting in an osteogenic immune microenvironment []. Simultaneously, it promoted the differentiation of hBMSCs to OBs []. Moreover, BMP-2 exerts osteogenic effects in a dose-dependent manner []. High-dose BMP-2 inhibited M1 macrophage infiltration by inducing IL-1Ra secretion from MSCs []. Based on the in vitro and in vivo results, the bioactive scaffold accelerated bone regeneration by delivering multifunctional cytokines to elicit synergistic reactions.
Rapid cell adhesion is beneficial for avoiding the encapsulation of non-adhesive proteins after implanting the biomaterials []. As a natural polymer, COL-I favors cell initial adhesion by promoting focal adhesion formation []. Decorating COL-I on Ti substrates to develop the nanoporous network surfaces (T-ADC) could timely convert macrophages from pro-inflammatory M1 to pro-healing M2 phenotype, creating favorable osteoimmune microenvironments to enhance angio-/osteogenesis (Figure 4B) []. Further RNA sequence analysis showed that T-ADC promoted macrophage adhesion, the extension of lamellipodia and filopodia, and M2 polarization by synergistically activating RhoA/ROCK, PI3K-AKT, and classical MAPK signaling pathways []. Subsequently, it was proven that T-ADC prominently facilitated the effects of angiogenesis and osteogenesis by culturing cells with the specimen-conditioned medium of macrophages (SCMM), OBs (SCMO), or endothelial cells (SCME) []. In vivo experiments consistently revealed that T-ADC generated abundant new bone mass and ameliorative osseointegration [].
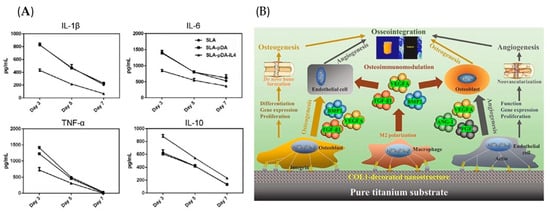
Figure 4.
Biomaterials act on macrophages with biological signals, followed by regulating bone regeneration. (A) IL-4 loading on the biomaterial surface reduced the release of pro-inflammatory factors and increased anti-inflammatory cytokines from macrophages. Reprinted with permission from Ref. []. Copyright 2019, John Wiley and Sons. (B) The COL-I decorated nanoporous network on Ti surfaces could significantly regulate early inflammatory reaction and subsequent angio-/osteogenesis processes, resulting in favorable osseointegration. Reprinted with permission from Ref. []. Copyright 2022, Elsevier.

Table 1.
Osteogenic biomaterials were involved in osteoimmunomodulation via biophysical, biochemical and biological cues.
Table 1.
Osteogenic biomaterials were involved in osteoimmunomodulation via biophysical, biochemical and biological cues.
| Engineering Parameters | Properties | Models | Effects | References | |
|---|---|---|---|---|---|
| Biophysical cues | surface topography | Surface grain size in nano-scale (~100 nm) to micron (~500 nm) range | In vitro: macrophages cultured on the sample surfaces In vivo: bilateral muscle pouches in mice | Nano/micro-topographies of hydroxyapatite (HA) resulted in differential integrin expression in macrophages, subsequently affecting cellular behaviors. The nano-topography could reduce tissue inflammation and promote M2 polarization. | [] |
| Cell micropatterning with 20 μm and 50 μm wide lines. | In vitro: macrophages cultured on the sample surfaces. | Macrophage polarization was regulated by reshaping the actin cytoskeleton. The elongation of macrophages could lead to M2 phenotype marker expression and reduce inflammatory cytokine secretion. | [] | ||
| Honeycomb-like TiO2 structures | In vitro: macrophage-CM In vivo: rat tibia implantation model | Honeycomb-like TiO2 structures facilitated macrophage filopodia formation and upregulated the Rho family of guanosine triphosphatases (RhoA, Rac1, and CDC42), which reinforced the polarization of macrophages through the activation of the RhoA/Rho–associated protein kinase signaling pathway. | [] | ||
| Nano-topography | In vitro: macrophage-CM In vivo: New Zealand white rabbits femoral implantation model | HA nano-particles-Ti formed an osteoimmune microenvironment to promote osteo-/angiogenesis via TGF-β, OPG/RANKL, and VEGF signaling pathways. | [] | ||
| Roughness | 100 nm < Ra < 500 nm | In vitro: macrophage-CM | Following Ra values of Ti surfaces increased, macrophages tended to M1 polarization, followed by the promoting osteoblast differentiation. | [] | |
| 500 nm < Ra < 2 μm | In vitro: macrophages cultured on the sample surfaces | Only a narrow range of roughness (Ra = 0.51–1.36 μm) in Ti surfaces tended to polarize macrophages toward the M2 phenotype. | [,] | ||
| In vitro: macrophage-CM/exosomes In vivo: mouse thigh muscle implantation model | Compared to micron-scale BCP (BCP2, ∼3.07 μm), BCP with submicron-scale structure (BCP1, ∼0.66 μm) facilitated M2 macrophage polarization. BCP1 ceramic markedly elevated miR-142a-5p levels in macrophage-derived exosomes, activating the PTEN/AKT signaling pathway, and consequently guiding the differentiation of MSCs towards osteoblast lineage. | [] | |||
| Ra > 2 μm | In vitro: macrophages cultured on the sample surfaces. | Following Ra values increased, pro-inflammatory cytokines (TNF-α, IL-6) release increased, and anti-inflammatory cytokines (IL-4, IL-10) release decreased. | [] | ||
| Hydrophily | hydrophilic | In vitro: macrophage-CM | The hydrophilic micro-rough Ti surfaces switched M1 macrophages to M2 phenotype, and enhanced osteogenesis by reducing inflammation. | [,] | |
| In vitro: macrophage-MSCs transwell co-culture; macrophage-CM; macrophage-T cell direct co-culture In vivo: mouse femoral implantation model | The rough-hydrophilic Ti surface could influence macrophage response to modulate the adaptive immune system, which ultimately controls stem cell recruitment and tissue regeneration. | [] | |||
| In vitro: macrophages cultured on the sample surfaces. In vivo: mouse femoral implantation model and Csf1r-iCre+; Wlsfl/fl mice | The Ti surface characteristics of roughness and hydrophilicity regulated macrophage polarization by Wnt signaling, and influenced macrophage to recruit other cells, which is critical to osseous healing. | [] | |||
| super-hydrophilic | In vitro: macrophages cultured on the sample surfaces | The super-hydrophilic nanotubular surface preferentially activated macrophages toward an anti-inflammatory M2 phenotype under standard conditions, and attenuated M1 responses under LPS stimulation, followed by regulating the microenvironment to accelerate inflammation resolution, facilitate tissue repair, and ultimately promote osseointegration. | [] | ||
| Porosity and pore size | Nanoporous anodic alumina with 20–200 nm sized pores | In vitro: macrophage-CM | Nanoporous structures regulated macrophage differentiation by changing cellular shape and activating the autophagy pathways. The osteoimmune environment formed by the 50 nm nanoporous structure was beneficial to the osteogenic differentiation of BMSCs. | [] | |
| Nanoporous alumina membranes with 20–200 nm sized pores | In vitro: macrophages cultured on sample surfaces In vivo: subcutaneous model in mouse | The 200 nm pores induced a stronger inflammatory response as compared to the alumina with 20 nm pores, which increased macrophage activation in vitro and promoted cell recruitment to generate pro-inflammatory cytokines in vivo. | [] | ||
| Fibrous scaffolds with box-shaped pores | In vitro: macrophages cultured on the scaffolds | The scaffolds facilitate primary human macrophage elongation accompanied by differentiation towards the M2 type, which was most pronounced for the 40 μm pore size. | [] | ||
| Scaffold generated from microgel with 40 μm, 70 μm, and 130 μm sizes | In vitro: macrophages were encapsulated in microporous annealed particle scaffolds (MAPS) | The activation levels of M1/M2 macrophages were correlated with changes in morphology, cell motility and nucleus shape regulated by the scaffolds. | [] | ||
| Stiffness | Polyacrylamide hydrogels | In vitro: macrophages cultured on the sample surfaces In vivo: subcutaneous model in mouse | Piezo1 is a mechanosensor of stiffness in macrophages, and its activity modulates polarization responses. | [] | |
| Polyacrylamide gels (PA gels) | In vitro: macrophages cultured on the sample surfaces | Gel stiffness regulated the macrophage behavior by Rho-A kinase (ROCK) and podosome-related pathways, including cell polarization, function and migration. | [] | ||
| Bioelectric signal | Piezoelectric hydrogel | In vitro: macrophage-CM In vivo: rat large-sized cranial injury model | Cs/Gel/PDA-modified HA/PDAmodified BaTiO3 (CG/PHA/PBT) piezoelectric hydrogels activated the PI3K/Akt signaling axis to promote macrophage M2 polarization, followed by accelerating angiogenesis and bone regeneration. | [,] | |
| Ti6Al4V scaffold coated with BaTiO3 (BT/Ti (poled)) | In vitro: cyclic loading on macrophage-scaffold composites was applied to form piezoelectric condition; macrophage-CM In vivo: ultrasound stimulation of piezoelectric scaffolds in a subcutaneous rat model; sheep cervical corpectomy model | BT/Ti (poled) facilitated macrophage M2 polarization by inhibiting MAPK/JNK signaling pathway and activating oxidative phosphorylation and ATP synthesis, followed by promoting bone regeneration. | [] | ||
| β-PVDF film under ultrasound treatment and the release of a localized charge | In vitro: ultrasound was applied as stimulation to cells cultured in the piezoelectric β-PVDF film | Ultrasound-stimulated piezoelectric β-PVDF film enhanced M1 polarization and inhibited M2 polarization via voltage-gated channels and Ca2+-CAMK2A-NF-κB axis to regulate Ca2+ influx. | [] | ||
| Biochemical cues | Inorganic ions | Ca2+ | In vitro: macrophage-CM In vivo: gastrocnemius muscle defect models in mouse lower limbs | BCP-released Ca2+ targeted the Wnt/β-catenin signaling pathway and activated Arg1 and IL-10 transcription through the CaSR in macrophages. | [] |
| Ca2+ + Sr2+ | In vitro: macrophages cultured on the sample surfaces | Ca and Sr elements modified the nanoscale topographical Ti surfaces upregulated M2 macrophage phenotype expression. | [,] | ||
| Zn2+ | In vitro: macrophage-CM | Zn released from the Zn-incorporated TiO2 nanotube (TNT) biomaterials enhanced gene and protein expression of M2 markers, and M1 markers were inhibited. The macrophage-CM of Zn-TNT group strengthened OB proliferation, adhesion and osteogenic differentiation. | [] | ||
| Mg2+, Zn2+, Cu2+ | In vitro: macrophage-CM In vivo: tunnel defect models in femur; macrophage-depleted mouse model | The divalent cation released from Mg–Alg, Cu–Alg, or Zn–Alg alginate can regulate the calcitonin gene-related polypeptide-α+ nerve fibers by enhancing PGE2 secretion from macrophages, followed by downregulating sympathetic activity and promoting new bone formation. | [] | ||
| Organic functional groups | AC, MEOX, AA), and OD | In vitro: macrophages cultured on the sample surfaces | These organic functional groups regulated the protein adsorption patterns in human serum by forming hydrophilic or hydrophobic surfaces, leading to distinct macrophage polarization. | [] | |
| pH | pH6.2–8.6 | In vitro: macrophage-CM | The acidic environment (pH 6.6) tended to polarize macrophages to M2 phenotype, while alkaline environment (pH 8.2) led to M1 polarization. | [] | |
| PGA scaffold degradation | In vitro: macrophages cultured on the medium with PGA degradation products. In vivo: subcutaneous model in mouse | The fast degradation of porous scaffolds triggered M1 macrophages into the implantation site, whilst the slow degradation of PGA fibers promoted the polarization of macrophages into M2 pro-healing phenotypes. | [] | ||
| Biological cues | Cytokines | IL-10 | In vivo: subcutaneous model in rats | IL-10 loaded in the hexamethylenediisocyanate-crosslinked dermal sheep collagen (HDSC) disks downmodulates the foreign body reaction (FBR), impairing the progression of the FBR. | [] |
| IL-4 | In vitro: macrophages cultured on the sample surfaces | The SLA-pDA-IL4 surfaces described here are able to activate adherent macrophages into M2 phenotype and reduce the release of pro-inflammatory cytokines. | [] | ||
| Proteins | BMP-2 | In vitro: macrophages- MSCs transwell model In vivo: femoral defect model and subcutaneous model in mice | BMP-2/CPC induced bone regeneration by regulating macrophage-MSC interaction. | [] | |
| COL-I | In vitro: macrophage-CM In vivo: Tibia implant model in rats | COL-I decorated nanoporous network on titanium implant surface inhibited inflammation and osteoclastic-related gene expression in macrophages by activating RhoA/ROCK, PI3K-AKT, and classical MAPK signaling pathways, followed by facilitating angiogenesis and osteogenesis. | [] | ||
| spatiotemporal immunomodulation | Phenolic ligand (tannic acid, TA) + indometacin (IND) | In vitro: macrophage-CM In vivo: sample was inserted in the femur of mice | In the normal biological environment, the coating was relatively stable, while TA and IND motifs could be triggered in the inflammatory environment to downregulate pro-inflammatory cytokines and upregulate anti-inflammatory cytokines and osteogenic-related factors. | [] | |
| calcium-strontium-zinc-phosphate (CSZP) coating + IL-4 | In vitro: BMMSCs and macrophages directly co-cultured In vivo: rat subcutaneous implant model; rat femoral defect repair model | The macrophages were recruited and polarized to M1 by CSZP coating, followed by M2 polarization by adding IL-4. | [] | ||
| IFN-γ + IL-4 | In vitro: macrophages cultured on the sample surfaces In vivo: subcutaneous model in mice | The modified decellularized bone scaffolds shortly released IFN-γ to promote the M1 phenotype, followed by a more sustained release of IL-4 to promote the M2 phenotype. | [] |
4. Current Osteoimmunomodulation Models in Evaluating Osteogenic Biomaterials
Osteogenic biomaterial implantation rebuilt a new osteoimmune microenvironment, which mainly involved various bone lineage cells, vascular cells, and immune cells. To enhance a better preclinical evaluation of osteogenic materials, it is necessary to explore further the interaction between biomaterials and surrounding cells from the perspective of osteoimmunomodulation. However, the experimental results usually depend on the model selected []. Analyzing the properties of each model can inform the selection of an appropriate model to evaluate the new osteogenic biomaterials. As shown in Table 2, the characteristics of different evaluation models were compared, such as physiological relevance, cost-efficiency, measurement methods, complexity, and translational potential.

Table 2.
Comparison of different assessment models.
4.1. Two-Dimensional (2D) Models
The two-dimensional model cultures cells on a flat, two-dimensional surface, such as a culture plate, dish, slide, or transwell, forming a cell layer. These models are widely employed to investigate the cellular crosstalk, signaling pathways, and molecular mechanisms that link the skeletal and immune systems [,,]. In addition, various disease conditions can be mimicked by altering the medium composition. For example, adding high-concentration glucose is used to simulate a diabetic environment, and LPS is added to induce inflammation []. While 2D models are cost-effective and facilitate straightforward analysis, they are unable to replicate the three-dimensional osteoimmune microenvironment, thereby limiting their capacity to fully capture complex interactions such as matrix-mediated signaling or spatial relationships within bone tissue []. Despite these limitations, 2D models remain a valuable tool for high-throughput screening of biomaterials, drugs, or signaling modulators with the objective of modulating osteoimmune responses.
4.1.1. CM Models
One cell type (donor cell) is first cultured with osteogenic biomaterials in the media to induce biological responses, followed by mixing the media (CM) with the osteogenic medium in a certain proportion, such as 1:1, 1:2, or 1:3 [,,,]. The CM comprises the components of cytokines, chemokines, and exosomes, which perform various functions and are the essential link between immune and bone systems []. The CM model has been widely used in exploring the osteoimmunomodulation characteristics of osteogenic biomaterials, largely due to its simple procedure, convenient storage, straightforward analysis, and no special instrument. Collected CM was employed to culture bone-related cells (acceptor cells), with the objective of evaluating the role of biomaterials-induced immune cells, most notably macrophages, in osteogenesis. In vivo, macrophage polarization adjusts to surrounding stimuli cues, followed by rebuilding the microenvironment by releasing various cytokines and regulating OBs differentiation []. Under in vitro models, polarized macrophages release cytokines into the culture media. Thus, the components of CM can represent the polarization condition and be used to explore the interactions between macrophages and bone-related cells. For example, to verify the role of macrophage-CM in OBs, a study investigated the cellular behaviors of BMSCs after culturing in different CMs generated by unpolarized macrophages (M0) or polarized macrophages (M1 or M2) []. Based on their results, the M0-CM had a remarkable effect on cell osteogenic differentiation, the M2-CM also facilitated BMSCs osteogenesis, whereas the M1-CM supported the adipogenic differentiation of BMSCs []. These findings were consistent with the in vivo results, which means that macrophage-CM contained sufficient components to regulate BMSC differentiation.
The CM is easily accessible, and one or several components can be isolated from culture media, followed by investigating their potential roles in regulating bone formation. For example, as shown in Figure 5, to determine the effects of macrophage exosomes on osteoblast differentiation, exosomes produced by plasma immersion ion implantation (PIII)+IL4-stimulated macrophages were isolated from the supernatants, which can be immediately used in BMSCs culture or stored at −80 °C []. The results revealed that PIII + IL4-exosomes significantly increase the expression of osteoblastic differentiation markers [].
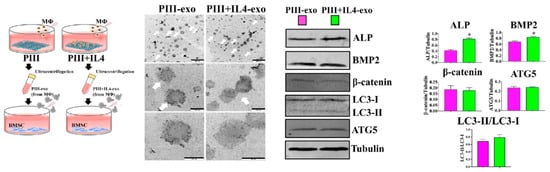
Figure 5.
Macrophage-CM works as a medium to transmit biological information between macrophage and bone-related cells. Exosomes (white arrow) were isolated from the media of culturing PIII/PIII + IL4-stimulated macrophages, followed by treating the BMSC (*: p <0.05). Reprinted with permission from Ref. []. Copyright 2023, American Chemical Society.
It should be noted that the compositions of the media differ between individual cells, such as glucose concentration, amino acid content, and inorganic ion species []. In addition to reacting to the desired cell-secreted factors from donor cells, the acceptor cells can be interfered with by the medium components. These factors have the potential to impact the outcome; however, they can easily be overlooked. In addition, as we mentioned above, there is no standardized ratio of CM (the more frequently used ratios: 1:1, 1:2, or 1:3), which is a crucial factor influencing the cytokine concentration in the CM. The concentration of cytokines secreted by macrophages mediated different effects on the behavior of bone cells []. For example, TNF-α, a pro-inflammatory cytokine, showed a concentration-dependent effect on regulating bone homeostasis []. Low-level TNF-α stimulates mesenchymal precursor cells to differentiate into OBs, whereas high-level TNF-α is an inhibitor of OB differentiation and an activator of osteoclastogenesis []. In practical research, the concentration of CM can be determined by cytotoxicity assays. Different ratios of CM (1:100 to 1) were applied in culturing cells, and 1:1 was chosen for the next experiment based on the results of the MTT assay []. Thus, it is better to further optimize the ratio of CM through experiments to establish a standardized model. In addition, the CM model focuses more on the unidirectional effect of macrophages on bone cells by accumulating secreted cytokines in the medium, resulting in ignoring the crosstalk between macrophages and bone cells.
4.1.2. Indirect Co-Culture Models
The indirect co-culture model is characterized by the absence of intercellular physical contact, with cells instead engaging in interactions via paracrine pathways. The transwell insert system has become a standard co-culture system in studies involving two distinct but interacting cell types []. To fulfill the requirements of the studies, there are also some models established with three or four cell types [,,].
The transwell system utilizes a semipermeable membrane to separate the cells while enabling the bidirectional exchange of secreted molecules within each well. It consists of an insert with a permeable membrane at its base, placed within a culture dish or well plate to create two chambers: an upper chamber for cell culture and a lower chamber for media or other cell types. The membrane, composed of materials such as polycarbonate or polyester, is available in a 0.4 µm to 8.0 µm range of pore sizes to facilitate selective permeation of substances [,], mimicking natural interactions such as nutrient transport or immune cell migration. Due to the dose- and time-dependent effects of inflammatory cytokines on bone regeneration, the transwell system is more suitable for exploring the dynamic impact of osteoimmunomodulation, which can reflect the real-time crosstalk among cells []. Fan Zhang et al. designed an ultrasound-controlled hydrogel in situ with self-assembled ultrashort peptide nanofibers (UPN@hydrogel), which can temporally release ultrashort peptide (Ser-Glu-Ser-Ser-Glu, SESSE) to regulate M2-type macrophage polarization, followed by the change in surrounding environment []. Based on the released property of this structure, the transwell system was used to further explore the BMSC differentiation, which was influenced by the dynamic immune microenvironment []. The transwell is suitable for evaluating the temporally variable osteogenic biomaterials.
The transwell system enables compartmentalized cell culture while allowing communication between chambers, making them versatile for modeling in vivo conditions. They are widely employed to investigate cell migration and to construct the barrier models [,,,]. A study investigated the effects of BCP on BMSC homing by regulating macrophages, in which the transwell system played an essential role []. Specifically, macrophages were seeded on the BCP surfaces, followed by collecting the supernatants and cells, which were analyzed with cytokine array and real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), respectively []. The results showed that BCP promoted the expression of C-C motif chemokine ligand 2 (CCL2)/MCP-1 and C-C motif chemokine ligand 3 (CCL3)/MIP-1α in macrophages, which were proved to accelerate BMSC migration []. The macrophages were further seeded in the bottom of transwell dishes and co-cultured with BMSCs that had been seeded in the inserts []. In this system, BMSC migration can be influenced by the cytokines and chemokines secreted by macrophages, and the results suggested that the CCL3/C-C chemokine receptor type 1 (CCR1), CCL2/C-C chemokine receptor type 2 (CCR2) axis may exert a predominant chemotactic effect for BMSC recruitment []. Another study functionalized the Ti surface with PTL@Sr coating to enhance osteogenesis and osteoimmunomodulation []. In this study, the macrophages were first cultured on the sample surfaces to harvest the CM; the BMSCs were then cultured in macrophage-CM in the transwell system to evaluate the cell migration []. Compared with the CM model, the transwell co-culture model allows real-time crosstalk between macrophages, bone cells, and osteogenic biomaterials (Figure 6) []. It is more beneficial for exploring the dynamic effect of osteoimmunomodulation. In addition, it can be employed to explore the cell migration and the recruitment of immune cells to bone cells. However, some factors other than experimental variables, like membrane properties and the mixed media, may interfere with the results.
Figure 6.
Schematic illustration of the application of the transwell model in cell co-culture. A model of BMSC or OB co-culture with treated macrophages. Chitosan/Agarose/NanoHA bone scaffold-treated macrophages promoted BMSC osteogenic differentiation. Reprinted from Ref. [].
4.1.3. Direct Co-Culture Models
In addition to paracrine pathways, there are complex interrelationships between immune and bone cells, which communicate via cell-to-cell contact. The direct co-culture model means that different cell types are cultured with/in/on the same biomaterial, which is convenient for dynamic communication between cells. Thus, this model can better mimic the in vivo environment by allowing direct contact of cell–cell and/or cell–biomaterials []. For example, a study intended to investigate how 3D-printed submicron patterns influence bone regeneration by modulating interactions between OBs and macrophages (Figure 7) []. M. Nouri-Goushki et al. established a direct co-culture model to mimic in vivo conditions, allowing the real-time study of cellular interactions. Briefly, they seeded OBs and M1 macrophages in the samples as 1:2 and cultured them with a mixed media of DMEM and α-MEM as 1:1 []. The key findings in this study showed that inflammation initially inhibited osteoblast differentiation; however, the patterned surfaces enhanced osteogenic markers like runt-related transcription factor 2 (RUNX2) over time, promoting bone formation []. To further reveal the effects of macrophage phenotypic switching on osteogenesis, after co-culturing M1 macrophages and MC3T3-E1 cells, exogenous IL-4 was added at different time points to polarize the M1 macrophage towards M2 []. The final results indicated that a transient inflammatory phase is crucial for promoting osteogenesis [].
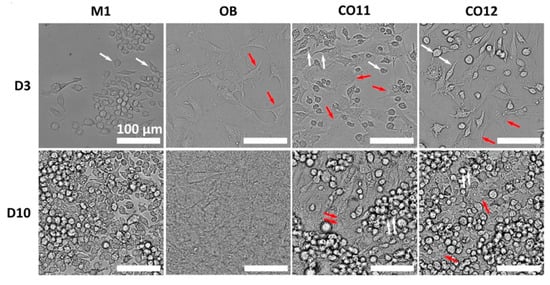
Figure 7.
M1 macrophage and OBs direct co-culture model. Spatial distribution of OBs and M1 macrophages after culturing for 3 and 10 days. Reprinted from Ref. []. (White arrow: macrophages, red arrow: OBs).
Compared with CM and indirect co-culture models, the direct co-culture model involves both cell-to-cell/biomaterials contact and soluble factor interactions. The effects of these three models on pro-osteogenesis were evaluated in a study []. The results suggested that the capacity of macrophages to improve osteogenic differentiation and mineralization was as follows: direct co-culture model > indirect co-culture model > CM []. Therefore, a more appropriate experimental model should be chosen according to the purpose of the experiment.
The direct co-culture model also presents several challenges and limitations. One significant disadvantage is the complexity of this system, which can make it difficult to distinguish the specific contributions and mechanisms of direct cell–cell contact from those of soluble factors. Additionally, a direct co-culture system is susceptible to the ratios and densities of the different cell types. It is, therefore, essential to optimize the parameters through adequate pre-experiments to avoid dominance by one cell type. Moreover, the dynamic and interconnected signaling processes place a greater demand on testing tools. In some cases, it was necessary to isolate the cells prior to conducting the assay. In addition, similarly to another co-culture model, it should be noted that the culture media is also an important factor in influencing the results [].
4.2. Three-Dimensional Models
Traditional 2D culture models are widely used in studies due to their simplicity and convenience. However, in these models, the cells are mostly in a flattened monolayer growth pattern, which limits the accumulation of ECM and the multidirectional cell-to-cell interactions []. To evaluate the osteogenic biomaterials in conditions closer to the in vivo environment, 3D models are increasingly being used in studies []. In the dynamic osteogenesis process, the secretion and mineralization of ECM play crucial roles, which can be influenced by the interactions between immune and bone cells. The two most commonly used 3D models for osteoimmunomodulation of osteogenic biomaterials are described below.
4.2.1. Three-Dimensional Scaffold Models
To restore and replace damaged bone tissue, the concept of bone tissue engineering (BTE) was introduced, and its design is undergoing continuous optimization []. The BTE typically involves three main components: cells, scaffolds, and bioactive molecules. Scaffold, as the framework, provides mechanical support and 3D structure for cells to adhere, proliferate, and differentiate. The ideal scaffold is usually designed to mimic the physical and chemical properties of natural bone, including porosity, mechanical strength, and biodegradability []. Recently, osteoimmunomodulation has also been known as an important indicator for evaluating biomaterials. Min He et al. designed a tannic acid–indometacin (TA-IND) nanoparticle-loaded PCL scaffold, which can spatiotemporally manipulate the osteoimmune microenvironment by scaffold biodegradation and releasing drugs []. In this study, the LPS-treated macrophages (M1) were seeded in the nanofibrous scaffold, which was converted to an anti-inflammatory M2 type by the releasing drugs from scaffolds []. However, this early process was slow and controlled by the “shielding effect” of the substrate to avoid over-inhibiting the activation of M1 macrophage, which is beneficial to tissue regeneration []. At the later stage, the collapse of the scaffolds exposed a greater quantity of TA-IND, effectively relieving the biodegradation-induced chronic inflammation []. This 3D model was able to control drug delivery and enabled the facilitation of macrophage polarization []. In addition, the scaffold model can provide a 3D space for direct co-culture of multiple cells, thus better simulating the cell–cell contact. The scaffolds synthesized by COL-I were used in a study, and macrophages and OBs were encapsulated in the 3D scaffolds or cultured on the 2D surface []. The results of culturing OBs indicated that the scaffold was beneficial in forming the mineralized matrix and upregulated the NF-κB expression, compared to the results of 2D culturing []. The macrophages and OBs were further co-encapsulated in the scaffolds to explore their interactions []. This 3D collagen matrix provided a closer physical environment, which may account for the difference in results from the 2D model []. Similarly, other studies also showed the different behaviors of macrophages and OBs in the 3D model and 2D model [,].
Despite significant advancements, 3D scaffolds still have some limitations. It is difficult to completely control the material biodegradation, which can be influenced by its inherent components, design, and the surrounding complex microenvironment. Uneven degradation rates may cause mechanical instability and disrupt tissue regeneration. At the same time, some degradation byproducts can disrupt the microenvironmental homeostasis, such as influencing the surrounding pH value, inducing inflammatory response, or cytotoxicity. Therefore, further development of scaffold models is required to more accurately simulate in vivo physiological conditions.
4.2.2. Microfluidic Platforms
With the development of research technologies and methodologies, the microfluidic system has been used in the field of bone research (Figure 8A) []. To establish the interactional models of bone and immune systems, various microfluidic devices have been designed, such as conditional culture by CM, co-culture of immune and bone cells [], purpose-dependent structures, and multi-organ on one chip.
Different from the microenvironment provided by the traditional model, the microfluid platform is a more dynamic and tunable system, characterized by the bionic mechanical environment. The OB behaviors, such as proliferation, differentiation, and mineralization, are sensitive to mechanical stimuli from the surrounding environment [,]. The results of culturing the MSCs and adipose-derived stem cells under the same mechanical stimulation in a microfluidic device show that MSCs were more sensitive and had a higher potential in osteogenic differentiation []. As shown in Figure 8B, a multi-shear microfluidic system was developed to measure Ca2+ concentration dynamics of osteoblast cytosolic, which can provide four-level shear flows []. After treatment with the different shear flows, the OBs cultured in separate chambers showed different Ca2+ concentrations in the cytosolic []. These results suggested that the in vitro models without the mechanical environment may influence the study outcomes []. Another study was further consistent with these results. A 3D osteocyte network was reconstructed by assembling murine early osteocytes (MLO-A5) in the BCP microbeads []. After culturing for three weeks, the expression trend of Sost gene in MLO-A5 cells cultured in microfluidic chambers exhibited consistency with that observed in vivo, which is a crucial osteocyte-specific marker for the mechanotransduction function []. However, cells cultured in a 2D model did not exhibit a similar trend []. Similarly, these mechanical stimuli can modulate immune cell functions. For example, leukocytes tended to migrate toward areas of higher shear stress, which is a crucial process in immune response []. Thus, the shear stress of dynamic fluid flow should be optimized to construct an osteoimmune microfluidic model with a closer physiological environment.
The construction of a microfluidic platform of the osteoimmune model offers a novel approach to assessing osteogenic biomaterials. To evaluate the biological properties of medical grade Ti disks for bone repair, Sarah-Sophia D. Carter et al. fabricated two different microfluidic channel designs, Ti-polydimethylsiloxane (PDMS)-chip and Ti-glass-chip []. Based on this study, considering the inertness of the materials, the robustness of the fabrication processes, the accessibility of the equipment, and the integration with standard biochemical assays, the Ti-glass-chip was better suited for longer-term cell experiments []. The schematic illustration of Ti-glass-chip is shown in Figure 8C []. In addition, the microfluidic system can mimic intricate physiological and pathological conditions and evaluate the whole-body level through the real-time observation of communication between different unions. It could link the engineered tissues, such as bone, immune, and vasculature, via vascular flow containing circulating monocytes []. This allowed for maintaining dynamic interactions among tissues and the surrounding environment [], including the osteoimmune microenvironment. Thus, the establishment of a microfluidic platform is a reasonable methodology to mimic the immune environment of bone tissue to evaluate biomaterials.
It is convenient to investigate the dynamic interactions of cell–cell, cell–biomaterials, and cell–microenvironment in the microfluidic platform because it allows the co-culture of multiple cell types and biomaterials. In addition, microfluidic devices can integrate multiple experimental conditions on a single platform, enabling high-throughput screening and real-time monitoring of various cellular responses [,,]. However, there are still some limitations of microfluidic platforms in evaluating osteogenic biomaterials. The adaptation of standard biological assays and imaging techniques to microfluidic systems is also a challenging process, requiring specialized optimization. Moreover, operating these devices demands technical expertise, and issues such as clogging or leakage can impact reproducibility. These factors, combined with cost and accessibility concerns, make microfluidic platforms less universally applicable despite their precision and innovative potential.
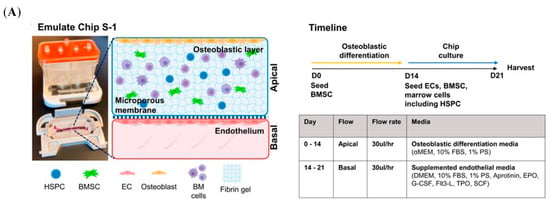
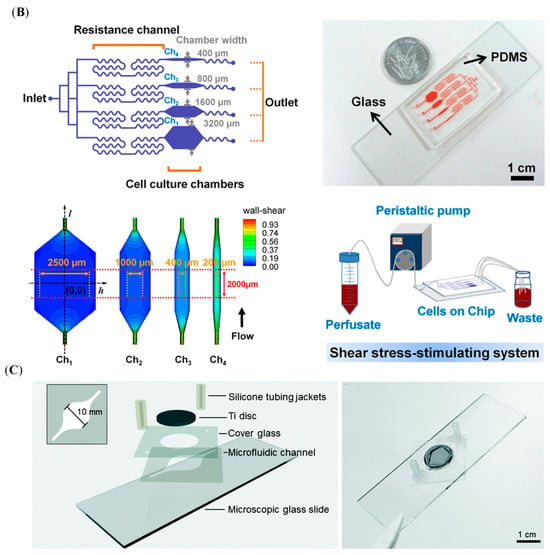
Figure 8.
Schematic illustration of the microfluidic models. (A) BMME-on-chip with osteoblastic, endothelium layer and whole bone marrow cells, as well as the culture timeline. Reprinted from Ref. []. (B) A schematic illustration of the microfluidic shear device design and shear stress-stimulating system. Reprinted with permission from Ref. []. Copyright 2011, Elsevier. (C) The different layers of the Ti-glass-chip and a top-view photograph of the completed device. Reprinted from Ref. [].
4.2.3. Bone Organoids
Bone organoid is a 3D in vitro cell culture system that provides a more comprehensive view of the interactions and spatial patterns between multiple cells, and cells and matrices []. It closely mimics the structural and functional microenvironment of native bone tissue, involving a series of highly coordinated biological events, such as cellular self-organization, osteogenic differentiation, ECM secretion, and mineralized collagen formation [,]. This cascade of developmental processes relies on complex intrinsic regulatory mechanisms, which cannot be accomplished by traditional 2D or 3D models. Mengru Zhu et al. developed a dynamic DNA/Gelatin methacryloyl (GelMA) hydrogel (CGDE) to recapitulate key biochemical and mechanical features of the bone ECM, providing a supportive microenvironment for bone organoid formation []. This dual-network hydrogel facilitated cellular migration, enhanced cellular self-organization, and downregulated innate immune responses, promoting woven bone organoid (WBO) formation via intramembranous ossification []. In addition to simulating the physiology of bone tissue, it is useful for exploring disease processes. A trabecular bone organoid model was developed to reproduce the bone remodeling cycle and its spatiotemporal profiles, which can be used to address issues such as osteoporosis and reduced bone density []. Thus, it depends on the bone organoid status and properties to apply them in mimicking bone diseases, promoting bone regeneration, and screening biomaterials [,]. Based on the highly bionic nature of bone organoids, they will be the next generation of in vitro models for evaluating osteogenic biomaterials and have great potential for clinical translation. However, to further acquire reliable safety, it should be considered in enhancing organoid matrix biocompatibility, precisely controlling cellular behavior, and standardizing fabrication techniques [].
4.3. In Vivo Models
In vitro experiments play a pivotal role in evaluating the direct cell response to osteogenic biomaterials and exploring the potential mechanisms. The animal models represent a crucial intermediate step between in vitro studies and clinical trials, offering comprehensive assessments, such as biocompatibility, mechanical stability, and safety []. They provide a realistic and complex in vivo environment that reflects the interactions between the immune system and bone remodeling processes []. These models make it possible to observe how biomaterials influence immune responses, such as immune cell activation and cytokine collapse, and how these, in turn, impact bone formation []. In addition, animal models can simulate bone defects in different physiologic microenvironments of the human body. To evaluate the bone regeneration biomaterials applied in the alveolar bone, an animal alveolar defect model is beneficial to reflect the distribution the forces from chewing, which may disturb the bone regeneration process []. For loss of tooth-supporting tissues due to periodontitis, inhibiting the colonization of periodontal pathogens is an effective strategy to promote the recovery of periodontal hard tissues []. Modeling periodontal inflammation can better respond to the interaction of biomaterials with oral microorganisms and their role in regulating immune responses and osteogenesis in the oral complex environment []. In addition, animal models can more accurately reflect the degradation of biomaterials because it is difficult to completely simulate complex tissue microenvironments in vitro []. Compared to cellular experiments in vitro, animal models also enable long-term observation of potential chronic immune reactions, material degradation, or inflammation, which could interfere with bone regeneration and provide evidence on biocompatibility and safety before clinical trials.
Depending on the purpose of the experiment, different animal models are established in the studies. In addition to assessing the osteogenic capacity, the osteoimmunomodulation model also focused on the osteoimmune microenvironment and explored the intrinsic connection between the bone and immune systems. The air pouch model is commonly employed to evaluate the biocompatibility and immunomodulatory effects of biomaterials []. Compared to implanting in hard tissue, biomaterial samples are easily inserted into the subcutaneous tissue at the animal back [,,,]. This model is commonly constructed in mice or rats without the need for large animals. In addition, the exudates and peri-implant tissue can be collected for a comprehensive analysis, such as inflammatory cytokines, infiltration of immune cells, and fibrous encapsulation []. A study established a mouse air pouch model and injected carrageenan solution into the pouch []. The cells were harvested from the pouch and showed that a significant cell infiltration occurred in response to carrageenan, and the cell types changed over time []. In addition, a subcutaneous air pocket was developed on the back of mice by injecting the sterile air, followed by insertion into the scaffold materials []. Four days later, the skin tissues were harvested and evaluated []. As shown in Figure 9A, the thinnest fibrous layer was observed in the skin sections of modified samples []. The immunofluorescence staining further confirmed that this fibrous layer had more Arg-1 expression and less iNOS expression []. The bone integration model was also established in this study. A rat femur defect was created first, and the cylindrical implant was then inserted parallel to the long axis of the femur []. To observe the new bone formation, the Micro-CT was employed to scan the femur samples containing the implants, and the images and data were quantitatively analyzed by the software (Figure 9B) []. Besides the femur defect model [], depending on the composition and size of the osteogenic material and the implantation site in the human body, other models were constructed in the studies, such as the tibia defect model [], alveolar bone defect model (Figure 9C) [], and calvarial bone defect model []. Analyzing the data from the air pouch model and bone integration model together facilitates a more comprehensive analysis of osteogenic mechanisms in biomaterials. Taken together, animal models offer crucial insights into how biomaterials interact with both bone and immune systems, guiding their development for clinical applications. However, it is essential to acknowledge the financial, ethical, and individual variability inherent to animal models as a research tool.
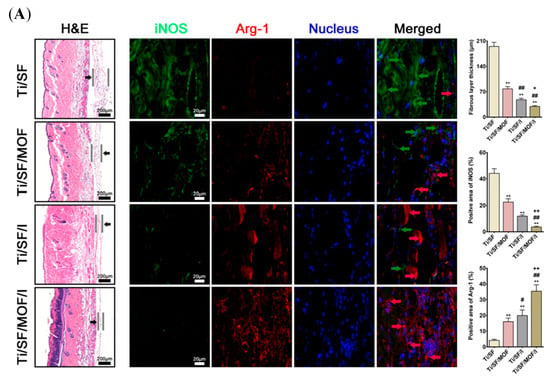
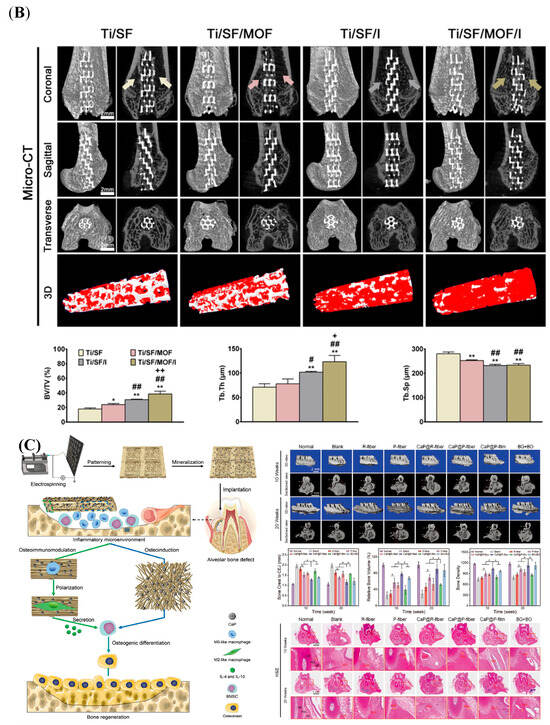
Figure 9.
The application of animal models in osteoimmunomodulation evaluation. (A) In vivo evaluation of the skin in air pouch model. (# and + represent p < 0.05 when compared with Ti/SF, Ti/SF/MOF, and Ti/SF/I, respectively; **, ##, and ++ represent p < 0.01). Reprinted from Ref. []. (B) Micro-CT analysis of osteoporotic osseointegration. (*, # and + represent p < 0.05 when compared with Ti/SF, Ti/SF/MOF, and Ti/SF/I, respectively; **, ## and ++ represent p < 0.01). Reprinted from Ref. []. (C) Schematic illustration of hierarchical-structured mineralized nanofiber (HMF) scaffold for enhancing alveolar bone repair (Left). In vivo bone regeneration assessments, including micro-CT evaluation and H&E staining (Right) (* p < 0.05). Reprinted with permission from Ref. []. Copyright 2021, John Wiley and Sons.
5. Future Outlook
Based on the above theoretical and practical foundations, we recommend that the osteoimmunomodulation evaluation of osteogenic biomaterials can be used to complement the ISO 10993 guideline []. Incorporating this characteristic into guidelines for the evaluation of implants can help prevent implant failure due to osteoimmune microenvironment plays a central regulatory role in the bone regeneration process. Immune cells (e.g., macrophages and T cells) directly regulate osteogenic differentiation of MSCs and osteoclast activity by secreting pro-inflammatory or anti-inflammatory factors, and the early immune response after biomaterial implantation significantly affects subsequent osseointegration results. Traditional evaluation systems focus only on the mechanical properties and in vitro osteogenic capacity of biomaterials, but ignore the decisive role of immune response on bone regeneration, which may lead to biomaterial failure in vivo due to excessive inflammation or immune rejection. By complementing osteoimmune indicators such as macrophage polarization, cytokine dynamics, and immune cell infiltration and phenotyping, it can accurately predict the long-term performance of biomaterials. In particular, the development of microfluidic chips and bone organoid technologies will be beneficial in guiding the design of osteogenic biomaterials with immunomodulation functions, followed by solving the clinical challenges such as osteoporosis and infected bone defects. This integrated strategy will promote the paradigm shift in biomaterials from “passive osteogenesis” to “active immune-osteogenic co-regulation” and significantly increase the success rate of clinical translation.
6. Conclusions
The bone and the immune systems are closely linked in the tissue origin and functioning. There is growing evidence that the success of osteogenic biomaterials depends on the osteoimmune microenvironment in which they facilitate. In the early stage of biomaterial implantation, bone damage triggers the acute inflammatory response. The immune cells play an anabolic role in eliminating tissue debris and dead cells while recruiting bone precursor cells through releasing cytokines. In the later phase of bone integration, long-term chronic inflammation produces a catabolic effect that induces failure of bone formation. The osteogenic biomaterials communicate with the surrounding microenvironment, including immune and bone-related cells, via biophysical cues, biochemical cues, and biological cues, resulting in the immune cell conversion from the pro-inflammatory phenotype to the pro-restorative phenotype, followed by the reconstruction of the osteoimmune microenvironment. Understanding the complex spatiotemporal interactions among osteogenic biomaterials, immune cells, and bone-related cells can help guide the biomaterial design and drive advances in BTE.
The development and application of osteogenic biomaterials require rational experimental models for evaluation. In this review, we introduced the current osteoimmunomodulation models for evaluating osteogenic biomaterials and discussed their properties. The CM is the most common experimental approach because it is easy to operate and does not require a special instrument. However, it overlooks the dynamic interactions between cell-to-cell and cell-to-biomaterials. To address this limitation, indirect and direct co-culture models provide a communication platform for cells and biomaterials, enabling their real-time interaction. Besides these traditional approaches, to better mimic the spatial and temporal variability of the osteogenic process in vivo, 3D scaffolds, microfluidic systems, and bone organoids were used to evaluate osteogenic biomaterials. In particular, microfluidic platforms and bone organoids, which can combine bone and the immune system in one system, are used to evaluate osteogenic biomaterials from the organoid level. Although animal experiments remain indispensable in the current evaluation of the osteoimmunomodulatory effects of osteogenic biomaterials, it is promising to build a bone-immuno-biomaterials assay platform with the advancement of microfluidic and single-cell technologies, which can realize the integration of cell culture-measure-analysis and partially replace animal models. Taken together, this paper is beneficial in further standardizing experimental protocols and facilitating the optimization of osteogenic biomaterials by reviewing the current applications of osteoimmunomodulation models in evaluating osteogenic biomaterials.
Author Contributions
Conceptualization, Y.W.; writing—original draft preparation, Y.W. and Y.H.; writing—review and editing, Y.W. and Y.Z.; visualization, Y.W., Z.Z. and G.L.; supervision, W.F.; project administration, W.F. and G.M.; funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Natural Science Foundation of China (No. 62271104, No. 62171077, and No. 61871068).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript
| AA | Allylamine |
| AC | Acrylic acid |
| Akt | Protein Kinase B |
| Alg | Alginate-based hydrogel |
| ALP | Alkaline phosphatase |
| APCs | Antigen-presenting cells; |
| Arg-1 | Arginase-1 |
| BCP | Biphasic calcium phosphate |
| BMP-2 | Bone morphogenetic protein 2 |
| BMP-6 | Bone morphogenetic protein 6 |
| BMSCs | Bone marrow mesenchymal stem cells |
| BTE | Bone tissue engineering |
| CaSR | Calcium-sensing receptor |
| CCL2 | C-C motif chemokine ligand 2 |
| CCL3 | C-C motif chemokine ligand 3 |
| CCR1 | C-C chemokine receptor type 1 |
| CCR2 | C-C chemokine receptor type 2 |
| CCR7 | C-C Chemokine Receptor Type 7 |
| CM | Conditioned media |
| COL-I | Type I collagen |
| CREB | cAMP-response element binding protein |
| CyTOF | Cytometry by Time of Flight |
| Cu2+ | Copper ion |
| DAMPs | Danger-associated molecular patterns |
| DAPI | 4′-6-diamidino-2-phenylindole |
| DCs | Dendritic cells |
| ECM | Extracellular matrix |
| EP4 | PGE2 receptor4 |
| EVs | Vesicles |
| FBR | Foreign body response |
| FGF-2 | Fibroblast growth factor-2 |
| HOBs | Human osteoblasts |
| HUVECs | Human umbilical vein endothelial cells |
| MEOX | 2-methyl-2-oxazoline |
| Mg2+ | Magnesium ion |
| MLCK | Myosin light chain kinase |
| MMP-9 | Matrix metalloproteinase-9 |
| MIP-1 | Macrophage inflammatory protein-1 |
| iNOS | Inducible nitric oxide synthase |
| IFN-γ | Interferon-γ |
| IL-1β | Interleukin-1β |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-13 | Interleukin-13 |
| IL-17 | Interleukin-17 |
| IL-22 | Interleukin-22 |
| IL-26 | Interleukin-26 |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LTB4 | Leukotriene B4 |
| MCP-1 | Macrophage chemoattractant protein-1 |
| OBs | Osteoblasts |
| OCN | Osteocalcin |
| OCs | Osteoclasts |
| OD | 1,7-octadiene |
| OPN | Osteopontin |
| OPG | Osteoprotegerin |
| OSM | Oncostatin M |
| OVX | Ovariectomy |
| PCL | Poly(ε-caprolactone) |
| PDMS | Polydimethylsiloxane |
| PD-1 | Programmed cell death-1 |
| pDA | Poly(dopamine) |
| PD-L1 | PD-ligand 1 |
| PGA | Polyglycolide acid |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| PIII | Plasma immersion ion implantation |
| PLA | Polylactide acid |
| PLGA | Polylactide-co-glycolide acid |
| pre-B | Precursors of B |
| qRT-PCR | Real-time quantitative reverse transcriptase polymerase chain reaction |
| RANKL | Receptor activator of nuclear factor κ-B ligand |
| ROS | Reactive oxygen species |
| RUNX2 | Runt-related transcription factor 2 |
| SCME | Specimen-conditioned medium of endothelial cells |
| SCMM | Specimen-conditioned medium of macrophages |
| SCMO | Specimen-conditioned medium of osteoblasts |
| SDF-1 | Stromal cell-derived factor 1 |
| SDF-1α | Stromal cell-derived factor-1α |
| SEM | Scanning electron microscopy |
| SLA | Sandblasted and acid-etched |
| SPEEK | Sulfonated polyetheretherketone |
| Sr | Strontium |
| STAT | Signal transducer and activator of transcription |
| TGF-β | Transforming growth factor-β |
| Th cells | Helper T cells |
| Ti | Titanium |
| TNF-α | Tumor necrosis factor α |
| TNTs | TiO2 nanotubes |
| T-ADC | Decorating COL-I on Ti substrates to develop the nanoporous network surfaces |
| 2D | Two-dimensional |
| VEGF-A | Vascular endothelial growth factor A |
| VMH | Ventromedial hypothalamus |
| YAP | Yes-associated protein |
| Zn2+ | Zinc ion |
References
- Zhou, L.; Quan, R.; Yang, J.; Xu, H. Healing of bone defects by induced pluripotent stem cell-derived bone marrow mesenchymal stem cells seeded on hydroxyapatite-zirconia. Ann. Transl. Med. 2021, 9, 1723. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Res. 2021, 9, 12. [Google Scholar] [CrossRef]
- Amani, H.; Alipour, M.; Shahriari, E.; Taboas, J.M. Immunomodulatory Biomaterials: Tailoring Surface Properties to Mitigate Foreign Body Reaction and Enhance Tissue Regeneration. Adv. Healthc. Mater. 2024, 13, e2401253. [Google Scholar] [CrossRef]
- Arron, J.R.; Choi, Y. Bone versus immune system nature. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Negrescu, A.M.; Cimpean, A. The State of the Art and Prospects for Osteoimmunomodulatory Biomaterials. Materials 2021, 14, 1357. [Google Scholar] [CrossRef]
- Mestres, G.; Carter, S.D.; Hailer, N.P.; Diez-Escudero, A. A practical guide for evaluating the osteoimmunomodulatory properties of biomaterials. Acta Biomater. 2021, 130, 115–137. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef]
- Miron, R.J.; Bohner, M.; Zhang, Y.; Bosshardt, D.D. Osteoinduction and osteoimmunology: Emerging concepts. Periodontology 2000 2024, 94, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mi, B.B.; Lin, Z.; Hu, Y.Q.; Yu, L.; Zha, K.K.; Panayi, A.C.; Yu, T.; Chen, L.; Liu, Z.P.; et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: From mechanism to therapeutic opportunity. Mil. Med. Res. 2022, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Peng, T.; Zhang, Z.; Guo, S.; Su, Y.; Zhang, K.; Wang, J.; Liu, C. Mesenchymal stem cells overexpressing XIST induce macrophage M2 polarization and improve neural stem cell homeostatic microenvironment, alleviating spinal cord injury. J. Tissue Eng. 2024, 15, 20417314231219280. [Google Scholar] [CrossRef] [PubMed]
- Davenport Huyer, L.; Pascual-Gil, S.; Wang, Y.; Mandla, S.; Yee, B.; Radisic, M. Advanced Strategies for Modulation of the Material–Macrophage Interface. Adv. Funct. Mater. 2020, 30, 1909331. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, Z.; Zhang, J.; Ni, Y.; Ouyang, S.; Qi, H.; Yu, Y.; Miron, R.J.; Tang, H.; Zhang, Y. Fn-HMGB1 Adsorption Behavior Initiates Early Immune Recognition and Subsequent Osteoinduction of Biomaterials. Adv. Healthc. Mater. 2024, 13, e2301808. [Google Scholar] [CrossRef]
- Visalakshan, R.M.; MacGregor, M.N.; Sasidharan, S.; Ghazaryan, A.; Mierczynska-Vasilev, A.M.; Morsbach, S.; Mailander, V.; Landfester, K.; Hayball, J.D.; Vasilev, K. Biomaterial Surface Hydrophobicity-Mediated Serum Protein Adsorption and Immune Responses. ACS Appl. Mater. Interfaces 2019, 11, 27615–27623. [Google Scholar] [CrossRef]
- Hachemi, Y.; Perrin, S.; Ethel, M.; Julien, A.; Vettese, J.; Geisler, B.; Göritz, C.; Colnot, C. Multimodal analyses of immune cells during bone repair identify macrophages as a therapeutic target in musculoskeletal trauma. Bone Res. 2024, 12, 56. [Google Scholar] [CrossRef]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tabrizi, Z.; Bhatt, N.N.; Franciosa, S.A.; Bracko, O. A Brief Overview of Neutrophils in Neurological Diseases. Biomolecules 2023, 13, 743. [Google Scholar] [CrossRef]
- Zhao, T.; Chu, Z.; Ma, J.; Ouyang, L. Immunomodulation Effect of Biomaterials on Bone Formation. J. Funct. Biomater. 2022, 13, 103. [Google Scholar] [CrossRef]
- Morandini, L.; Avery, D.; Angeles, B.; Winston, P.; Martin, R.K.; Donahue, H.J.; Olivares-Navarrete, R. Reduction of neutrophil extracellular traps accelerates inflammatory resolution and increases bone formation on titanium implants. Acta Biomater. 2023, 166, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Lin, D.; Li, Y.; Wang, L.; Xie, J.; Dai, T.; Liu, F.; Tang, M.; Tian, L.; Yuan, Y.; et al. N2-Polarized Neutrophils Guide Bone Mesenchymal Stem Cell Recruitment and Initiate Bone Regeneration: A Missing Piece of the Bone Regeneration Puzzle. Adv. Sci. 2021, 8, e2100584. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Lu, F.; Verleg, S.; Groven, R.V.M.; Poeze, M.; van Griensven, M.; Blokhuis, T.J. Is there a role for N1-N2 neutrophil phenotypes in bone regeneration? A systematic review. Bone 2024, 181, 117021. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Gao, Y.; Dai, T.; Yu, J.; Liu, Y.; Bao, H.; She, J.; Hou, Y.; Kong, L.; et al. Extracellular Vesicles Derived from Neutrophils Accelerate Bone Regeneration by Promoting Osteogenic Differentiation of BMSCs. ACS Biomater. Sci. Eng. 2024, 10, 3868–3882. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Wang, Y.; Brown, Z.J.; Xia, Y.; Huang, Z.; Shen, C.; Hu, Z.; Beane, J.; Ansa-Addo, E.A.; et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J. Hepatol. 2021, 75, 1271–1283. [Google Scholar] [CrossRef]
- Herath, T.D.K.; Larbi, A.; Teoh, S.H.; Kirkpatrick, C.J.; Goh, B.T. Neutrophil-mediated enhancement of angiogenesis and osteogenesis in a novel triple cell co-culture model with endothelial cells and osteoblasts. J. Tissue Eng. Regen. Med. 2018, 12, e1221–e1236. [Google Scholar] [CrossRef]
- Steinman, R.M.; Nussenzweig, M.C. Dendritic cells: Features and functions. Immunol. Rev. 1980, 53, 127–147. [Google Scholar] [CrossRef]
- McKenna, H.J.; Stocking, K.L.; Miller, R.E.; Brasel, K.; De Smedt, T.; Maraskovsky, E.; Maliszewski, C.R.; Lynch, D.H.; Smith, J.; Pulendran, B.; et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000, 95, 3489–3497. [Google Scholar] [CrossRef]
- Liu, Y.C.G.; Teng, A.Y. Potential contribution of immature myeloid CD11c(+)dendritic cells-derived osteoclast precursor to inflammation-induced bone loss in the TRAF6-null chimeras in-vivo. J. Dent. Sci. 2023, 18, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Rivollier, A.; Mazzorana, M.; Tebib, J.; Piperno, M.; Aitsiselmi, T.; Rabourdin-Combe, C.; Jurdic, P.; Servet-Delprat, C. Immature dendritic cell transdifferentiation into osteoclasts: A novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 2004, 104, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Oliva, D.; Barrena-Blázquez, S.; Jiménez-Álvarez, L.; Fraile-Martinez, O.; García-Montero, C.; López-González, L.; Torres-Carranza, D.; García-Puente, L.M.; Carranza, S.T.; Álvarez-Mon, M.; et al. The RANK-RANKL-OPG System: A Multifaceted Regulator of Homeostasis, Immunity, and Cancer. Medicina 2023, 59, 1752. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Choi, Y. Regulation of T cell-associated tissues and T cell activation by RANKL-RANK-OPG. J. Bone Miner. Metab. 2021, 39, 54–63. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Chi, M.; Zhang, M.; Shan, H.; Zhang, Q.H.; Zhang, J.; Shi, J.; Zhang, J.Z.; Wu, R.M.; et al. Osteoprotegerin mediate RANK/RANKL signaling inhibition eases asthma inflammatory reaction by affecting the survival and function of dendritic cells. Allergol. Immunopathol. 2019, 47, 179–184. [Google Scholar] [CrossRef]
- Beurel, E.; Jope, R.S. Differential regulation of STAT family members by glycogen synthase kinase-3. J. Biol. Chem. 2008, 283, 21934–21944. [Google Scholar] [CrossRef]
- Avin, K.G.; Dominguez, J.M., 2nd; Chen, N.X.; Hato, T.; Myslinski, J.J.; Gao, H.; Liu, Y.; McKinley, T.O.; Brown, K.M.; Moe, S.M.; et al. Single-cell RNAseq provides insight into altered immune cell populations in human fracture nonunions. J. Orthop. Res. 2023, 41, 1060–1069. [Google Scholar] [CrossRef]
- Narisawa, M.; Kubo, S.; Okada, Y.; Yamagata, K.; Nakayamada, S.; Sakata, K.; Yamaoka, K.; Tanaka, Y. Human dendritic cell-derived osteoclasts with high bone resorption capacity and T cell stimulation ability. Bone 2021, 142, 115616. [Google Scholar] [CrossRef]
- Sun, S.; Wijanarko, K.; Liani, O.; Strumila, K.; Ng, E.S.; Elefanty, A.G.; Stanley, E.G. Lymphoid cell development from fetal hematopoietic progenitors and human pluripotent stem cells. Immunol. Rev. 2023, 315, 154–170. [Google Scholar] [CrossRef]
- Könnecke, I.; Serra, A.; El Khassawna, T.; Schlundt, C.; Schell, H.; Hauser, A.; Ellinghaus, A.; Volk, H.D.; Radbruch, A.; Duda, G.N.; et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 2014, 64, 155–165. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- de Franca, M.N.F.; Rodrigues, L.S.; Barreto, A.S.; da Cruz, G.S.; Aragão-Santos, J.C.; da Silva, A.M.; de Jesus, A.R.; Palatnik-de-Sousa, C.B.; de Almeida, R.P.; Corrêa, C.B. CD4(+) Th1 and Th17 responses and multifunctional CD8 T lymphocytes associated with cure or disease worsening in human visceral leishmaniasis. Front. Immunol. 2024, 15, 1277557. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, M.P.d.; Vieira, J.H. Entrance to the multifaceted world of CD4+ T cell subsets. Explor. Immunol. 2024, 4, 152–168. [Google Scholar] [CrossRef]
- Avery, D.; Morandini, L.; Sheakley, L.; Grabiec, M.; Olivares-Navarrete, R. CD4(+) and CD8(+) T cells reduce inflammation and promote bone healing in response to titanium implants. Acta Biomater. 2024, 179, 385–397. [Google Scholar] [CrossRef]
- Gao, Y.; Grassi, F.; Ryan, M.R.; Terauchi, M.; Page, K.; Yang, X.; Weitzmann, M.N.; Pacifici, R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J. Clin. Investig. 2007, 117, 122–132. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef]
- Alvarez, C.; Suliman, S.; Almarhoumi, R.; Vega, M.E.; Rojas, C.; Monasterio, G.; Galindo, M.; Vernal, R.; Kantarci, A. Regulatory T cell phenotype and anti-osteoclastogenic function in experimental periodontitis. Sci. Rep. 2020, 10, 19018. [Google Scholar] [CrossRef]
- Ramezanpour, M.; Moraitis, S.; Smith, J.L.; Wormald, P.J.; Vreugde, S. Th17 Cytokines Disrupt the Airway Mucosal Barrier in Chronic Rhinosinusitis. Mediat. Inflamm. 2016, 2016, 9798206. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, H.R.; Kim, B.M.; Cho, M.L.; Lee, S.H. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis. Am. J. Pathol. 2015, 185, 3011–3024. [Google Scholar] [CrossRef]
- Tau, G.Z.; Cowan, S.N.; Weisburg, J.; Braunstein, N.S.; Rothman, P.B. Regulation of IFN-gamma signaling is essential for the cytotoxic activity of CD8(+) T cells. J. Immunol. 2001, 167, 5574–5582. [Google Scholar] [CrossRef]
- Huang, E.E.; Zhang, N.; Ganio, E.A.; Shen, H.; Li, X.; Ueno, M.; Utsunomiya, T.; Maruyama, M.; Gao, Q.; Su, N.; et al. Differential dynamics of bone graft transplantation and mesenchymal stem cell therapy during bone defect healing in a murine critical size defect. J. Orthop. Transl. 2022, 36, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lan, C.; Benlagha, K.; Camara, N.O.S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; Forsman, H.; et al. The interaction of innate immune and adaptive immune system. MedComm 2024, 5, e714. [Google Scholar] [CrossRef] [PubMed]
- Dougall, W.C.; Glaccum, M.; Charrier, K.; Rohrbach, K.; Brasel, K.; De Smedt, T.; Daro, E.; Smith, J.; Tometsko, M.E.; Maliszewski, C.R.; et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999, 13, 2412–2424. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef]
- Manilay, J.O.; Zouali, M. Tight relationships between B lymphocytes and the skeletal system. Trends Mol. Med. 2014, 20, 405–412. [Google Scholar] [CrossRef]
- Masuzawa, T.; Miyaura, C.; Onoe, Y.; Kusano, K.; Ohta, H.; Nozawa, S.; Suda, T. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J. Clin. Investig. 1994, 94, 1090–1097. [Google Scholar] [CrossRef]
- Most, W.; van der Wee-Pals, L.; Ederveen, A.; Papapoulos, S.; Löwik, C. Ovariectomy and orchidectomy induce a transient increase in the osteoclastogenic potential of bone marrow cells in the mouse. Bone 1997, 20, 27–30. [Google Scholar] [CrossRef]
- Kanematsu, M.; Sato, T.; Takai, H.; Watanabe, K.; Ikeda, K.; Yamada, Y. Prostaglandin E2 induces expression of receptor activator of nuclear factor-kappa B ligand/osteoprotegrin ligand on pre-B cells: Implications for accelerated osteoclastogenesis in estrogen deficiency. J. Bone Miner. Res. 2000, 15, 1321–1329. [Google Scholar] [CrossRef]
- Sato, T.; Shibata, T.; Ikeda, K.; Watanabe, K. Generation of bone-resorbing osteoclasts from B220+ cells: Its role in accelerated osteoclastogenesis due to estrogen deficiency. J. Bone Miner. Res. 2001, 16, 2215–2221. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Cenci, S.; Haug, J.; Brown, C.; DiPersio, J.; Pacifici, R. B lymphocytes inhibit human osteoclastogenesis by secretion of TGFbeta. J. Cell Biochem. 2000, 78, 318–324. [Google Scholar] [CrossRef]
- Klausen, B.; Hougen, H.P.; Fiehn, N.E. Increased periodontal bone loss in temporarily B lymphocyte-deficient rats. J. Periodontal Res. 1989, 24, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Nathan, K.; Lu, L.Y.; Lin, T.; Pajarinen, J.; Jämsen, E.; Huang, J.F.; Romero-Lopez, M.; Maruyama, M.; Kohno, Y.; Yao, Z.; et al. Precise immunomodulation of the M1 to M2 macrophage transition enhances mesenchymal. Bone Jt. Res. 2019, 8, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Valles, G.; Bensiamar, F.; Maestro-Paramio, L.; Garcia-Rey, E.; Vilaboa, N.; Saldana, L. Influence of inflammatory conditions provided by macrophages on osteogenic ability of mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 57. [Google Scholar] [CrossRef]
- Gou, M.; Wang, H.; Xie, H.; Song, H. Macrophages in guided bone regeneration: Potential roles and future directions. Front. Immunol. 2024, 15, 1396759. [Google Scholar] [CrossRef]
- Liang, B.; Wang, H.; Wu, D.; Wang, Z. Macrophage M1/M2 polarization dynamically adapts to changes in microenvironment and modulates alveolar bone remodeling after dental implantation. J. Leukoc. Biol. 2021, 110, 433–447. [Google Scholar] [CrossRef]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef]
- Lee, E.J.; Jain, M.; Alimperti, S. Bone Microvasculature: Stimulus for Tissue Function and Regeneration. Tissue Eng. Part B Rev. 2021, 27, 313–329. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, Y.; Sun, Z.; Wu, X.; Li, J.; Jiang, S.; Wang, Y.; Wang, J.; Du, Y.; Zhang, S. Hypoxia-triggered exosome-mimetics accelerate vascularized osteogenesis. Mater. Today 2024, 73, 16–29. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, H.; Fan, Y.; Li, J.; Guo, Z.; Xu, Q.; Liu, Y.; Gao, K.; Ait Lahcine, N.; Zhang, J.; et al. Macrophages regulate angiogenesis-osteogenesis coupling induced by mechanical loading through the Piezo1 pathway. J. Bone Miner. Res. 2024, 6, zjae198. [Google Scholar] [CrossRef]
- Jin, S.; Wen, J.; Zhang, Y.; Mou, P.; Luo, Z.; Cai, Y.; Chen, A.; Fu, X.; Meng, W.; Zhou, Z.; et al. M2 macrophage-derived exosome-functionalized topological scaffolds regulate the foreign body response and the coupling of angio/osteoclasto/osteogenesis. Acta Biomater. 2024, 177, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, W.; Zhuang, N.; Wen, S.; Huang, S.; Lu, W.; Zhou, Y.; Liao, G.; Zhang, B.; Liu, C. Experimental study on bone defect repair by BMSCs combined with a light-sensitive material: g-C3N4/rGO. J. Biomater. Sci. Polym. Ed. 2020, 32, 248–265. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Liu, J.; Li, Z.; Xie, J.; Luo, Y.; Wang, M. Exploring the mechanism of bone marrow mesenchymal stromal cell exosomes in respiratory syncytial virus infection based on miRNA sequencing. Sci. Rep. 2025, 15, 13797. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, P.; Liu, L.; Wang, F.; Xing, H.; Sun, L.; Jiang, Z. Bone marrow-derived mesenchymal stem cells promote cell proliferation of multiple myeloma through inhibiting T cell immune responses via PD-1/PD-L1 pathway. Cell Cycle 2018, 17, 858–867. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Deng, Y.Y.; Lai, W.; Zheng, S.Y.; Bian, H.N.; Liu, Z.A.; Huang, Z.F.; Sun, C.W.; Li, H.H.; Luo, H.M.; et al. Effect of bone marrow mesenchymal stem cells on the polarization of macrophages. Mol. Med. Rep. 2018, 17, 4449–4459. [Google Scholar] [CrossRef]
- Ouyang, L.; Cao, J.; Dai, Q.; Qiu, D. New insight of immuno-engineering in osteoimmunomodulation for bone regeneration. Regen. Ther. 2021, 18, 24–29. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Wang, T. How the mechanical microenvironment of stem cell growth affects their differentiation: A review. Stem Cell Res. Ther. 2022, 13, 415. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Clark, N.M.; Olivares-Navarrete, R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials 2018, 182, 202–215. [Google Scholar] [CrossRef]
- Meli, V.S.; Atcha, H.; Veerasubramanian, P.K.; Nagalla, R.R.; Luu, T.U.; Chen, E.Y.; Guerrero-Juarez, C.F.; Yamaga, K.; Pandori, W.; Hsieh, J.Y.; et al. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci. Adv. 2020, 6, eabb8471. [Google Scholar] [CrossRef]
- Zhou, H.; Xue, Y.; Dong, L.; Wang, C. Biomaterial-based physical regulation of macrophage behaviour. J. Mater. Chem. B 2021, 9, 3608–3621. [Google Scholar] [CrossRef]
- Wilson, H.M. Modulation of macrophages by biophysical cues in health and beyond. Discov. Immunol. 2023, 2, kyad013. [Google Scholar] [CrossRef] [PubMed]
- Meizlish, M.L.; Kimura, Y.; Pope, S.D.; Matta, R.; Kim, C.; Philip, N.; Meyaard, L.; Gonzalez, A.; Medzhitov, R. Macrophages sense ECM mechanics and growth factor availability through cytoskeletal remodeling to regulate their tissue repair program. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lv, L.; Xie, Y.; Li, K.; Hu, T.; Lu, X.; Cao, Y.; Zheng, X. Unveiling the Mechanism of Surface Hydrophilicity-Modulated Macrophage Polarization. Adv. Healthc. Mater. 2018, 7, e1800675. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, F.; Tang, Y.; Wang, J.; Chen, X.; Li, X.; Zhang, X. Regulation of macrophage polarization and functional status by modulating hydroxyapatite ceramic micro/nano-topography. Mater. Des. 2022, 213, 110302. [Google Scholar] [CrossRef]
- Shao, J.; Li, J.; Weng, L.; Cheng, K.; Weng, W.; Sun, Q.; Wu, M.; Lin, J. Remote Activation of M2 Macrophage Polarization via Magneto-Mechanical Stimulation To Promote Osteointegration. ACS Biomater. Sci. Eng. 2023, 9, 2483–2494. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, H.; Liu, X.; Wu, J.; Yang, C.; Wong, T.M.; Kwan, K.Y.H.; Cheung, K.M.C.; Wu, S.; Yeung, K.W.K. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci. Adv. 2021, 7, eabf6654. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Choi, C.K.; Horwitz, A.R. Integrins in cell migration—The actin connection. J. Cell Sci. 2009, 122, 199–206. [Google Scholar] [CrossRef]
- Soe, Z.Y.; Park, E.J.; Shimaoka, M. Integrin Regulation in Immunological and Cancerous Cells and Exosomes. Int. J. Mol. Sci. 2021, 22, 2193. [Google Scholar] [CrossRef]
- Chen, Z.; Bachhuka, A.; Wei, F.; Wang, X.; Liu, G.; Vasilev, K.; Xiao, Y. Nanotopography-based strategy for the precise manipulation of osteoimmunomodulation in bone regeneration. Nanoscale 2017, 9, 18129–18152. [Google Scholar] [CrossRef]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and functions. ChemTexts 2022, 8, 12. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [PubMed]
- Tylek, T.; Blum, C.; Hrynevich, A.; Schlegelmilch, K.; Schilling, T.; Dalton, P.D.; Groll, J. Precisely defined fiber scaffolds with 40 mum porosity induce elongation driven M2-like polarization of human macrophages. Biofabrication 2020, 12, 025007. [Google Scholar] [CrossRef] [PubMed]
- Kizhakkedathu, J.N.; Conway, E.M. Biomaterial and cellular implants: Foreign surfaces where immunity and coagulation meet. Blood 2022, 139, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Meizlish, M.A.-O.; Kimura, Y.A.-O.X.; Pope, S.A.-O.; Matta, R.; Kim, C.A.-O.; Philip, N.A.-O.; Meyaard, L.A.-O.; Gonzalez, A.; Medzhitov, R.A.-O. Mechanosensing regulates tissue repair program in macrophages. Sci. Adv. 2024, 10, eadk6906. [Google Scholar] [CrossRef]
- Atcha, H.; Jairaman, A.; Holt, J.R.; Meli, V.S.; Nagalla, R.R.; Veerasubramanian, P.K.; Brumm, K.T.; Lim, H.E.; Othy, S.; Cahalan, M.D.; et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021, 12, 3256. [Google Scholar] [CrossRef]
- Lee, M.N.; Hwang, H.S.; Oh, S.H.; Roshanzadeh, A.; Kim, J.W.; Song, J.H.; Kim, E.S.; Koh, J.T. Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef]
- Bose, T.; Cieślar-Pobuda, A.; Wiechec, E. Role of ion channels in regulating Ca2+ homeostasis during the interplay between immune and cancer cells. Cell Death Dis. 2015, 6, e1648. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Q.; Yin, C.; Jia, X.; Zhao, Z.; Zhang, X.; Yuan, G.; Hu, H.; Zhao, Q. Sustained calcium ion release from bioceramics promotes CaSR-mediated M2 macrophage polarization for osteoinduction. J. Leukoc. Biol. 2021, 110, 485–496. [Google Scholar] [CrossRef]
- Qiao, W.; Pan, D.; Zheng, Y.; Wu, S.; Liu, X.; Chen, Z.; Wan, M.; Feng, S.; Cheung, K.M.C.; Yeung, K.W.K.; et al. Divalent metal cations stimulate skeleton interoception for new bone formation in mouse injury models. Nat. Commun. 2022, 13, 535. [Google Scholar] [CrossRef]
- Chen, B.; You, Y.; Ma, A.; Song, Y.; Jiao, J.; Song, L.; Shi, E.; Zhong, X.; Li, Y.; Li, C. Zn-Incorporated TiO(2) Nanotube Surface Improves Osteogenesis Ability Through Influencing Immunomodulatory Function of Macrophages. Int. J. Nanomed. 2020, 15, 2095–2118. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.; Xiong, Z.; Huang, Q.; Yang, X.; Yan, H.; Ma, J.; Feng, Q.; Shen, Z. The immunomodulatory effects of Zn-incorporated micro/nanostructured coating in inducing osteogenesis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. Zinc-Modified Sulfonated Polyetheretherketone Surface with Immunomodulatory Function for Guiding Cell Fate and Bone Regeneration. Adv. Sci. 2018, 5, 1800749. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Cao, H.; Wang, J.; Tang, K.; Li, B.; Zhao, Y.; Cheng, M.; Qin, H.; Liu, X.; Zhang, X. Immunomodulatory Effects of Calcium and Strontium Co-Doped Titanium Oxides on Osteogenesis. Front. Immunol. 2017, 8, 1196. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Kundu, S. Protein (BSA) adsorption on hydrophilic and hydrophobic surfaces. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Billing, F.A.-O.; Walter, B.; Fink, S.; Arefaine, E.; Pickarski, L.; Maier, S.; Kretz, R.; Jakobi, M.; Feuerer, N.; Schneiderhan-Marra, N.; et al. Altered Proinflammatory Responses to Polyelectrolyte Multilayer Coatings Are Associated with Differences in Protein Adsorption and Wettability. ACS Appl. Mater. Interfaces 2021, 13, 55534–55549. [Google Scholar] [CrossRef]
- Bygd, H.C.; Bratlie, K.M. Investigating the Synergistic Effects of Combined Modified Alginates on Macrophage Phenotype. Polymers 2016, 8, 422. [Google Scholar] [CrossRef]
- Clancy, J.; McVicar, A. Short-term regulation of acid-base homeostasis of body fluids. Br. J. Nurs. 2007, 16, 1016–1021. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, B.; Xi, Z.; Zhao, L.; Cen, L.; Yang, Y. A comparable study of polyglycolic acid’s degradation on macrophages’ activation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110574. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Huang, W.; Day, D.E.; Kittiratanapiboon, K.; Rahaman, M.N. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci. Mater. Med. 2006, 17, 583–596. [Google Scholar] [CrossRef]
- Wang, C.X.; Ma, T.; Wang, M.Y.; Guo, H.Z.; Ge, X.Y.; Zhang, Y.; Lin, Y. Facile distribution of an alkaline microenvironment improves human bone marrow mesenchymal stem cell osteogenesis on a titanium surface through the ITG/FAK/ALP pathway. Int. J. Implant. Dent. 2021, 7, 56. [Google Scholar] [CrossRef]
- Kohn, D.H.; Sarmadi, M.; Helman, J.I.; Krebsbach, P.H. Effects of pH on human bone marrow stromal cells in vitro: Implications for tissue engineering of bone. J. Biomed. Mater. Res. 2002, 60, 292–299. [Google Scholar] [CrossRef]
- Tian, H.; Gu, C.; Li, W.; Tong, T.; Wang, Y.; Yang, Y.; Wang, H.; Dai, Z.; Chen, P.; Wang, F.; et al. Neutralization of Intracellular pH Homeostasis to Inhibit Osteoclasts Based on a Spatiotemporally Selective Delivery System. Nano Lett. 2023, 23, 4101–4110. [Google Scholar] [CrossRef]
- Wu, H.; Yin, Y.; Hu, X.; Peng, C.; Liu, Y.; Li, Q.; Huang, W.; Huang, Q. Effects of Environmental pH on Macrophage Polarization and Osteoimmunomodulation. ACS Biomater. Sci. Eng. 2019, 5, 5548–5557. [Google Scholar] [CrossRef]
- Wei, F.; Zhou, Y.; Wang, J.; Liu, C.; Xiao, Y. The Immunomodulatory Role of BMP-2 on Macrophages to Accelerate Osteogenesis. Tissue Eng. Part A 2018, 24, 584–594. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Miron, R.J.; Zhang, Y. Modulating macrophage polarization on titanium implant surface by poly(dopamine)-assisted immobilization of IL4. Clin. Implant. Dent. Relat. Res. 2019, 21, 977–986. [Google Scholar] [CrossRef]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef]
- Kuninaka, Y.; Ishida, Y.; Ishigami, A.; Nosaka, M.; Matsuki, J.; Yasuda, H.; Kofuna, A.; Kimura, A.; Furukawa, F.; Kondo, T. Macrophage polarity and wound age determination. Sci. Rep. 2022, 12, 20327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, Z.; Liu, X.; Yang, C.; Gao, R.; Liu, W.; Ou-Yang, W.; Dong, A.; Zhang, C.; Huang, P.; et al. Titanium alloy composited with dual-cytokine releasing polysaccharide hydrogel to enhance osseointegration via osteogenic and macrophage polarization signaling pathways. Regen. Biomater. 2022, 9, rbac003. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.D.; Jiang, W.M.; Yang, H.L.; Shi, J.H. Exploratory meta-analysis on dose-related efficacy and complications of rhBMP-2 in anterior cervical discectomy and fusion: 1,539,021 cases from 2003 to 2017 studies. J. Orthop. Transl. 2020, 24, 166–174. [Google Scholar] [CrossRef]
- Jiang, F.; Qi, X.; Wu, X.; Lin, S.; Shi, J.; Zhang, W.; Jiang, X. Regulating macrophage-MSC interaction to optimize BMP-2-induced osteogenesis in the local microenvironment. Bioact. Mater. 2023, 25, 307–318. [Google Scholar] [CrossRef]
- Mitchell, J.; Lo, K.W. The Use of Small-Molecule Compounds for Cell Adhesion and Migration in Regenerative Medicine. Biomedicines 2023, 11, 2507. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, L.; Zhang, Y.; Yao, R.; Sun, Y.; Hang, R.; Chen, X.; Wang, H.; Yao, X.; Xiao, Y.; et al. Type I collagen decorated nanoporous network on titanium implant surface promotes osseointegration through mediating immunomodulation, angiogenesis, and osteogenesis. Biomaterials 2022, 288, 121684. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Du, Z.; Weng, Z.; Yao, W.; Zhang, X.; Huang, X.; Yao, X.; Crawford, R.; Hang, R.; et al. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018, 76, 344–358. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Elkhooly, T.A.; Liu, Y.; Wu, H.; Feng, Q.; Liu, L.; Fang, Y.; Zhu, W.; Hu, T. Effects of titanium surface roughness on the mediation of osteogenesis via modulating the immune response of macrophages. Biomed. Mater. 2018, 13, 045013. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Jansen, J.A.; Yang, F.; van den Beucken, J. Titanium surfaces characteristics modulate macrophage polarization. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 143–151. [Google Scholar] [CrossRef]
- Lu, J.; Webster, T.J. Reduced immune cell responses on nano and submicron rough titanium. Acta Biomater. 2015, 16, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, X.; Xiao, Y.; Zhu, X.; Chen, X.; Zhang, X. Calcium phosphate ceramic-induced osteoimmunomodulation: Submicron-surface-treated macrophage-derived exosomes driving osteogenesis. Mater. Des. 2024, 241, 112903. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.J.; Lv, Z.Y.; Liu, K.; Meng, C.X.; Zou, B.; Li, K.Y.; Liu, F.Z.; Zhang, B. The observed difference of macrophage phenotype on different surface roughness of mineralized collagen. Regen. Biomater. 2020, 7, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Hamlet, S.M.; Lee, R.S.B.; Moon, H.J.; Alfarsi, M.A.; Ivanovski, S. Hydrophilic titanium surface-induced macrophage modulation promotes pro-osteogenic signalling. Clin. Oral. Implants Res. 2019, 30, 1085–1096. [Google Scholar] [CrossRef]
- Dai, X.; Wei, Y.; Zhang, X.; Meng, S.; Mo, X.; Liu, X.; Deng, X.; Zhang, L.; Deng, X. Attenuating Immune Response of Macrophage by Enhancing Hydrophilicity of Ti Surface. J. Nanomater. 2015, 2015, 712810. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Chaubal, M.; Hotchkiss, K.M.; Olivares-Navarrete, R. Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials 2020, 243, 119920. [Google Scholar] [CrossRef]
- Gao, S.; Lu, R.; Wang, X.; Chou, J.; Wang, N.; Huai, X.; Wang, C.; Zhao, Y.; Chen, S. Immune response of macrophages on super-hydrophilic TiO(2) nanotube arrays. J. Biomater. Appl. 2020, 34, 1239–1253. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, S.; Han, S.; Crawford, R.; Lu, S.; Wei, F.; Chang, J.; Wu, C.; Xiao, Y. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale 2017, 9, 706–718. [Google Scholar] [CrossRef]
- Pujari, S.; Hoess, A.; Shen, J.; Thormann, A.; Heilmann, A.; Tang, L.; Karlsson-Ott, M. Effects of nanoporous alumina on inflammatory cell response. J. Biomed. Mater. Res. A 2014, 102, 3773–3780. [Google Scholar] [CrossRef]
- Liu, Y.; Suarez-Arnedo, A.; Riley, L.; Miley, T.; Xia, J.; Segura, T. Spatial confinement modulates macrophage response in microporous annealed particle (MAP) scaffolds. Adv. Healthc. Mater. 2023, 12, e2300823. [Google Scholar] [CrossRef]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Shen, L.; Liu, H.F.; Zou, X.H.; Zhao, J.; Huang, Y.; Zhu, Y.F.; Li, Z.Y.; Xu, C.; Luo, L.H.; et al. The marriage of immunomodulatory, angiogenic, and osteogenic capabilities in a piezoelectric hydrogel tissue engineering scaffold for military medicine. Mil. Med. Res. 2023, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, Y.; Zhu, Y.; Wu, P.; Deng, Z.; Dong, Q.; Wu, M.; Cai, L. Bioinspired Piezoelectric Periosteum to Augment Bone Regeneration via Synergistic Immunomodulation and Osteogenesis. ACS Appl. Mater. Interfaces 2023, 15, 12273–12293. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dong, H.; Tang, Z.; Chen, Y.; Liu, Y.; Wang, M.; Wei, X.; Wang, N.; Bao, S.; Yu, D.; et al. Electrical stimulation of piezoelectric BaTiO3 coated Ti6Al4V scaffolds promotes anti-inflammatory polarization of macrophages and bone repair via MAPK/JNK inhibition and OXPHOS activation. Biomaterials 2023, 293, 121990. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, F.; Ma, B.; Duan, J.; Yuan, W.; Sang, Y.; Han, L.; Wang, S.; Liu, H. Wireless Localized Electrical Stimulation Generated by an Ultrasound-Driven Piezoelectric Discharge Regulates Proinflammatory Macrophage Polarization. Adv. Sci. 2021, 8, 2100962. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, Y.J.; Jang, J.H.; Park, J.W. Modulating macrophage polarization with divalent cations in nanostructured titanium implant surfaces. Nanotechnology 2016, 27, 085101. [Google Scholar] [CrossRef]
- Liu, X.; Sui, Q.; Wang, W.; Liu, H. Biological activity of tantalum oxide coating doped with calcium and strontium by micro-arc oxidation under osteoimmunomodulation. Surf. Interfaces 2024, 50, 104500. [Google Scholar] [CrossRef]
- van Putten, S.M.; Wübben, M.; Hennink, W.E.; van Luyn, M.J.; Harmsen, M.C. The downmodulation of the foreign body reaction by cytomegalovirus encoded interleukin-10. Biomaterials 2009, 30, 730–735. [Google Scholar] [CrossRef]
- He, M.; Yang, B.; Huo, F.; Xie, L.; Yang, M.; Tian, W. A novel coating with universal adhesion and inflammation-responsive drug release functions to manipulate the osteoimmunomodulation of implants. J. Mater. Chem. B 2021, 9, 5272–5283. [Google Scholar] [CrossRef]
- Zhao, D.W.; Zuo, K.Q.; Wang, K.; Sun, Z.Y.; Lu, Y.P.; Cheng, L.; Xiao, G.Y.; Liu, C. Interleukin-4 assisted calcium-strontium-zinc-phosphate coating induces controllable macrophage polarization and promotes osseointegration on titanium implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111512. [Google Scholar] [CrossRef]
- Luo, M.L.; Jiao, Y.; Gong, W.P.; Li, Y.; Niu, L.N.; Tay, F.R.; Chen, J.H. Macrophages enhance mesenchymal stem cell osteogenesis via down-regulation of reactive oxygen species. J. Dent. 2020, 94, 103297. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kishen, A. Dysfunctional crosstalk between macrophages and fibroblasts under LPS-infected and hyperglycemic environment in diabetic wounds. Sci. Rep. 2025, 15, 17233. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Wang, R.; Boulbes, D.R.; Zhang, H.; Watowich, S.S.; Xia, L.; Ye, X.; Bhattacharya, R.; Ellis, L.M. Macrophage conditioned medium promotes colorectal cancer stem cell phenotype via the hedgehog signaling pathway. PLoS ONE 2018, 13, e0190070. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, Y.; Zhao, R.; Li, X.; Jia, W. A novel hierarchical biofunctionalized 3D-printed porous Ti6Al4V scaffold with enhanced osteoporotic osseointegration through osteoimmunomodulation. J. Nanobiotechnol. 2022, 20, 68. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, D.; Ni, Z.; Ou, T.; Wang, Y.; Zhang, H.; Mao, L.; Lin, K.; Xu, S.; Liu, J. Calcium silicate bioactive ceramics induce osteogenesis through oncostatin M. Bioact. Mater. 2021, 6, 810–822. [Google Scholar] [CrossRef]
- Cai, L.; Lv, Y.; Yan, Q.; Guo, W. Cytokines: The links between bone and the immune system. Injury 2024, 55, 111203. [Google Scholar] [CrossRef]
- Bi, Z.; Cai, Y.; Shi, X.; Chen, J.; Li, D.; Zhang, P.; Liu, J. Macrophage-mediated immunomodulation in biomaterial-assisted bone repair: Molecular insights and therapeutic prospects. Chem. Eng. J. 2024, 488, 150631. [Google Scholar] [CrossRef]
- He, X.T.; Li, X.; Yin, Y.; Wu, R.X.; Xu, X.Y.; Chen, F.M. The effects of conditioned media generated by polarized macrophages on the cellular behaviours of bone marrow mesenchymal stem cells. J. Cell Mol. Med. 2018, 22, 1302–1315. [Google Scholar] [CrossRef]
- Wei, F.; Mu, Y.; Tan, R.P.; Wise, S.G.; Bilek, M.M.; Zhou, Y.; Xiao, Y. Osteo-Immunomodulatory Role of Interleukin-4-Immobilized Plasma Immersion Ion Implantation Membranes for Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 2590–2601. [Google Scholar] [CrossRef]
- Biggers, J.D.; Summers, M.C. Choosing a culture medium: Making informed choices. Fertil. Steril. 2008, 90, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Zhang, W.; Zhu, A.; Backman, L.J.; Chen, J. Regulation of osteogenic differentiation by the pro-inflammatory cytokines IL-1β and TNF-α: Current conclusions and controversies. Hum. Cell 2022, 35, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Osta, B.; Benedetti, G.; Miossec, P. Classical and Paradoxical Effects of TNF-α on Bone Homeostasis. Front. Immunol. 2014, 5, 48. [Google Scholar] [CrossRef]
- Jin, Q.H.; Kim, H.K.; Na, J.Y.; Jin, C.; Seon, J.K. Anti-inflammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci. Rep. 2022, 12, 4754. [Google Scholar] [CrossRef]
- Vis, M.A.M.; Ito, K.; Hofmann, S. Impact of Culture Medium on Cellular Interactions in in vitro Co-culture Systems. Front. Bioeng. Biotechnol. 2020, 8, 911. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, S.; Zou, R.; Gao, X.; Ruan, J.; Weir, M.D.; Reynolds, M.A.; Qin, W.; Chang, X.; Fu, H.; et al. Calcium phosphate cement scaffold with stem cell co-culture and prevascularization for dental and craniofacial bone tissue engineering. Dent. Mater. 2019, 35, 1031–1041. [Google Scholar] [CrossRef]
- Churm, R.; Dunseath, G.J.; Prior, S.L.; Thomas, R.L.; Banerjee, S.; Owens, D.R. Development and characterization of an in vitro system of the human retina using cultured cell lines. Clin. Exp. Ophthalmol. 2019, 47, 1055–1062. [Google Scholar] [CrossRef]
- Malik, J.R.; Fletcher, C.V.; Podany, A.T.; Dyavar, S.R.; Scarsi, K.K.; Pais, G.M.; Scheetz, M.H.; Avedissian, S.N. A novel 4-cell in-vitro blood-brain barrier model and its characterization by confocal microscopy and TEER measurement. J. Neurosci. Methods 2023, 392, 109867. [Google Scholar] [CrossRef]
- Wang, M.; Chen, F.; Wang, J.; Chen, X.; Liang, J.; Yang, X.; Zhu, X.; Fan, Y.; Zhang, X. Calcium phosphate altered the cytokine secretion of macrophages and influenced the homing of mesenchymal stem cells. J. Mater. Chem. B 2018, 6, 4765–4774. [Google Scholar] [CrossRef]
- Mosaad, E.; Chambers, K.; Futrega, K.; Clements, J.; Doran, M.R. Using high throughput microtissue culture to study the difference in prostate cancer cell behavior and drug response in 2D and 3D co-cultures. BMC Cancer 2018, 18, 592. [Google Scholar] [CrossRef]
- Zhang, F.; Lv, M.; Wang, S.; Li, M.; Wang, Y.; Hu, C.; Hu, W.; Wang, X.; Wang, X.; Liu, Z.; et al. Ultrasound-triggered biomimetic ultrashort peptide nanofiber hydrogels promote bone regeneration by modulating macrophage and the osteogenic immune microenvironment. Bioact. Mater. 2024, 31, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, Q.; Gu, B.; Yin, C.; Shen, K.; Tang, H.; Xia, H.; Zhang, X.; Zhao, Y.; Yang, X.; et al. Minimally invasive implantation and decreased inflammation reduce osteoinduction of biomaterial. Theranostics 2020, 10, 3533–3545. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Van Delen, M.; De Laere, M.; Sterkens, A.; Costas Romero, C.; Berneman, Z.; Cools, N. Transmigration across a Steady-State Blood-Brain Barrie Induces Activation of Circulating Dendritic Cells Partly Mediated by Actin Cytoskeletal Reorganization. Membranes 2021, 11, 700. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Chen, X.; Chen, J.; Gautam, S.C.; Xu, Y.; Chopp, M. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology 2002, 7, 113–117. [Google Scholar] [CrossRef]
- Lou, T.; Chen, K.; Luo, Q.; Liu, C.; Yuan, Y.; Fan, C. Periosteum-inspired in situ CaP generated nanocomposite hydrogels with strong bone adhesion and superior stretchability for accelerated distraction osteogenesis. Biomater. Res. 2022, 26, 91. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Liu, Z.; Ma, S.; Sun, Y.; Wu, X.; Zhang, X.; Gao, P. Application of a Strontium-Loaded, Phase-Transited Lysozyme Coating to a Titanium Surface to Enhance Osteogenesis and Osteoimmunomodulation. Med. Sci. Monit. 2019, 25, 2658–2671. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Koziol, M.; Przekora, A. The Chitosan/Agarose/NanoHA Bone Scaffold-Induced M2 Macrophage Polarization and Its Effect on Osteogenic Differentiation In Vitro. Int. J. Mol. Sci. 2021, 22, 1109. [Google Scholar] [CrossRef]
- Nouri-Goushki, M.; Eijkel, B.I.M.; Minneboo, M.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Osteoimmunomodulatory potential of 3D printed submicron patterns assessed in a direct co-culture model. Biomater. Adv. 2022, 139, 212993. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Zhang, R.; Pajarinen, J.; Lin, T.H.; Goodman, S.B.; Yao, Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res. Ther. 2016, 7, 15. [Google Scholar] [CrossRef]
- Zauchner, D.; Müller, M.Z.; Horrer, M.; Bissig, L.; Zhao, F.; Fisch, P.; Lee, S.S.; Zenobi-Wong, M.; Müller, R.; Qin, X.H. Synthetic biodegradable microporous hydrogels for in vitro 3D culture of functional human bone cell networks. Nat. Commun. 2024, 15, 5027. [Google Scholar] [CrossRef]
- Casajuana Ester, M.; Day, R.M. Production and Utility of Extracellular Vesicles with 3D Culture Methods. Pharmaceutics 2023, 15, 663. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cui, J.; Lin, K.; Xie, J.; Wang, X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, Q.; Feng, Y.; Gao, X.; He, C.; Li, J.; Zhao, W.; Tian, W.; Zhao, C. Spatiotemporal Management of the Osteoimmunomodulation of Fibrous Scaffolds by Loading a Novel Amphiphilic Nanomedicine. ACS Appl. Mater. Interfaces 2022, 14, 13991–14003. [Google Scholar] [CrossRef]
- Nyangoga, H.; Aguado, E.; Goyenvalle, E.; Baslé, M.F.; Chappard, D. A non-steroidal anti-inflammatory drug (ketoprofen) does not delay beta-TCP bone graft healing. Acta Biomater. 2010, 6, 3310–3317. [Google Scholar] [CrossRef]
- Jacho, D.; Babaniamansour, P.; Osorio, R.; Toledano, M.; Rabino, A.; Garcia-Mata, R.; Yildirim-Ayan, E. Deciphering the Cell-Specific Effect of Osteoblast-Macrophage Crosstalk in Periodontitis. Tissue Eng. Part A 2023, 29, 579–593. [Google Scholar] [CrossRef]
- Adams, S.; Wuescher, L.M.; Worth, R.; Yildirim-Ayan, E. Mechano-Immunomodulation: Mechanoresponsive Changes in Macrophage Activity and Polarization. Ann. Biomed. Eng. 2019, 47, 2213–2231. [Google Scholar] [CrossRef]
- Jacho, D.; Rabino, A.; Garcia-Mata, R.; Yildirim-Ayan, E. Mechanoresponsive regulation of fibroblast-to-myofibroblast transition in three-dimensional tissue analogues: Mechanical strain amplitude dependency of fibrosis. Sci. Rep. 2022, 12, 16832. [Google Scholar] [CrossRef]
- Sharipol, A.; Lesch, M.L.; Soto, C.A.; Frisch, B.J. Bone Marrow Microenvironment-On-Chip for Culture of Functional Hematopoietic Stem Cells. Front. Bioeng. Biotechnol. 2022, 10, 855777. [Google Scholar] [CrossRef]
- Middleton, K.; Al-Dujaili, S.; Mei, X.; Günther, A.; You, L. Microfluidic co-culture platform for investigating osteocyte-osteoclast signalling during fluid shear stress mechanostimulation. J. Biomech. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50, e12338. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, S.; Matyas, J.R.; Rancourt, D.E.; Duncan, N.A. The effect of mechanical stimulation on mineralization in differentiating osteoblasts in collagen-I scaffolds. Tissue Eng. Part A 2014, 20, 3142–3153. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Sim, W.Y.; Min, B.H.; Yang, S.S.; Khademhosseini, A.; Kaplan, D.L. Chip-based comparison of the osteogenesis of human bone marrow- and adipose tissue-derived mesenchymal stem cells under mechanical stimulation. PLoS ONE 2012, 7, e46689. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Pan, L.; van Noort, D.; Meng, G.; Wu, X.; Sun, H.; Xu, J.; Lee, I. A multishear microfluidic device for quantitative analysis of calcium dynamics in osteoblasts. Biochem. Biophys. Res. Commun. 2011, 408, 350–355. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, W.; Sun, Q.; Hao, Y.; Zilberberg, J.; Lee, W.Y. Microbeads-Guided Reconstruction of 3D Osteocyte Network during Microfluidic Perfusion Culture. J. Mater. Chem. B 2015, 3, 3625–3633. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Lo, K.-Y.; Wang, Y.-H.; Sun, Y.-S. Use microfluidics to study cell migration in response to fluid shear stress gradients. Microchem. J. 2024, 206, 111612. [Google Scholar] [CrossRef]
- Carter, S.D.; Barbe, L.; Tenje, M.; Mestres, G. Exploring microfluidics as a tool to evaluate the biological properties of a titanium alloy under dynamic conditions. Biomater. Sci. 2020, 8, 6309–6321. [Google Scholar] [CrossRef]
- Smith, R.J., Jr.; Nasiri, B.; Kann, J.; Yergeau, D.; Bard, J.E.; Swartz, D.D.; Andreadis, S.T. Endothelialization of arterial vascular grafts by circulating monocytes. Nat. Commun. 2020, 11, 1622. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Teles, D.; Yeager, K.; Tavakol, D.N.; Zhao, Y.; Chramiec, A.; Tagore, S.; Summers, M.; Stylianos, S.; Tamargo, M.; et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat. Biomed. Eng. 2022, 6, 351–371. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fernández-Garibay, X.; Castaño, A.G.; De Chiara, F.; Hernández-Albors, A.; Balaguer-Trias, J.; Ramón-Azcón, J. Muscle-on-a-chip with an on-site multiplexed biosensing system for in situ monitoring of secreted IL-6 and TNF-α. Lab. Chip 2019, 19, 2568–2580. [Google Scholar] [CrossRef]
- Barata, D.; van Blitterswijk, C.; Habibovic, P. High-throughput screening approaches and combinatorial development of biomaterials using microfluidics. Acta Biomater. 2016, 34, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Atif, A.R.; Aramesh, M.; Carter, S.S.; Tenje, M.; Mestres, G. Universal Biomaterial-on-Chip: A versatile platform for evaluating cellular responses on diverse biomaterial substrates. J. Mater. Sci. Mater. Med. 2024, 35, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Du, Y.; Hou, Z.; Zhang, Y.; Cui, W.; Chen, W. A Review of Advanced Biomaterials and Cells for the Production of Bone Organoid. Small Sci. 2023, 3, 2300027. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhou, D.; Li, G.; Liu, J.; Chen, X.; Su, J. Engineering bone/cartilage organoids: Strategy, progress, and application. Bone Res. 2024, 12, 66. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, H.; Zhou, Q.; Sheng, S.; Gao, Q.; Geng, Z.; Chen, X.; Lai, Y.; Jing, Y.; Xu, K.; et al. Dynamic GelMA/DNA Dual-Network Hydrogels Promote Woven Bone Organoid Formation and Enhance Bone Regeneration. Adv. Mater. 2025, e2501254. [Google Scholar] [CrossRef]
- Park, Y.; Cheong, E.; Kwak, J.G.; Carpenter, R.; Shim, J.H.; Lee, J. Trabecular bone organoid model for studying the regulation of localized bone remodeling. Sci. Adv. 2021, 7, eabd6495. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Z.; Yang, B.; Huang, H. The Application of Organoids in the Research of Skeletal Diseases: Current Status and Prospects. Stud. Health Technol. Inform. 2023, 308, 597–604. [Google Scholar] [CrossRef]
- Xie, C.; Liang, R.; Ye, J.; Peng, Z.; Sun, H.; Zhu, Q.; Shen, X.; Hong, Y.; Wu, H.; Sun, W.; et al. High-efficient engineering of osteo-callus organoids for rapid bone regeneration within one month. Biomaterials 2022, 288, 121741. [Google Scholar] [CrossRef]
- Domínguez-Oliva, A.; Hernández-Ávalos, I.; Martínez-Burnes, J.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Mota-Rojas, D. The Importance of Animal Models in Biomedical Research: Current Insights and Applications. Animals 2023, 13, 1223. [Google Scholar] [CrossRef]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef]
- Guarch-Perez, C.; Riool, M.; Zaat, S.A. Current osteomyelitis mouse models, a systematic review. Eur. Cell Mater. 2021, 42, 334–374. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Nakayama, T. Recent Clinical Treatment and Basic Research on the Alveolar Bone. Biomedicines 2023, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Daghrery, A.; Dal-Fabbro, R.; dos Reis-Prado, A.H.; de Souza Araújo, I.J.; Fischer, N.G.; Rosa, V.; Silikas, N.; Aparicio, C.; Watts, D.C.; Bottino, M.C. Guidance on the assessment of the functionality of biomaterials for periodontal tissue regeneration: Methodologies and testing procedures. Dent. Mater. 2025, 41, 306–318. [Google Scholar] [CrossRef]
- Su, G.-L.; Peng, Y.-J.; Ruan, H.-Z.; Cheng, J.; Deng, T.; Zhang, Y.-F. Regulating periodontal disease with smart stimuli-responsive systems: Antimicrobial activity, immunomodulation, periodontium regeneration. Mater. Today Bio 2025, 32, 101863. [Google Scholar] [CrossRef]
- Woitschach, F.; Kloss, M.; Kischkel, S.; Macháček, T.; Reinholdt, C.; Senz, V.; Schlodder, K.; Löbermann, M.; Grabow, N.; Reisinger, E.C.; et al. Utilization of a highly adaptable murine air pouch model for minimally invasive testing of the inflammatory potential of biomaterials. Front. Bioeng. Biotechnol. 2024, 12, 1367366. [Google Scholar] [CrossRef]
- Duarte, D.B.; Vasko, M.R.; Fehrenbacher, J.C. Models of inflammation: Carrageenan air pouch. Curr. Protoc. Pharmacol. 2012, 56, 5.6.1–5.6.8. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Wan, Y.; Zhang, Y.; Wang, Z.; Klausen, L.H.; Huang, P.; Dong, M.; Han, X.; Cui, B.; et al. A hierarchically ordered compacted coil scaffold for tissue regeneration. NPG Asia Mater. 2020, 12, 55. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, X.; Yuan, Z.; Shen, M.; Song, Y.; Liu, H.; Deng, J.; Zhong, X.; Zhang, X. Establishing an osteoimmunomodulatory coating loaded with aspirin on the surface of titanium primed with phase-transited lysozyme. Int. J. Nanomed. 2019, 14, 977–991. [Google Scholar] [CrossRef]
- Croes, M.; Boot, W.; Kruyt, M.C.; Weinans, H.; Pouran, B.; van der Helm, Y.J.M.; Gawlitta, D.; Vogely, H.C.; Alblas, J.; Dhert, W.J.A.; et al. Inflammation-Induced Osteogenesis in a Rabbit Tibia Model. Tissue Eng. Part C Methods 2017, 23, 673–685. [Google Scholar] [CrossRef]
- He, Y.; Tian, M.; Li, X.; Hou, J.; Chen, S.; Yang, G.; Liu, X.; Zhou, S. A Hierarchical-Structured Mineralized Nanofiber Scaffold with Osteoimmunomodulatory and Osteoinductive Functions for Enhanced Alveolar Bone Regeneration. Adv. Healthc. Mater. 2022, 11, e2102236. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Liu, D.; Bai, J.; Haugen, H.J.; Li, B.; Yan, H. Early osteoimmunomodulation by mucin hydrogels augments the healing and revascularization of rat critical-size calvarial bone defects. Bioact. Mater. 2023, 25, 176–188. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-1:2018; Biological evaluation of medical devices. International Organization for Standardization: Geneva, Switzerland, 2018.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).