Fracture Resistance of CAD/CAM-Fabricated Zirconia and Lithium Disilicate Crowns with Different Margin Designs: Implications for Digital Dentistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Sample Selection and Storage
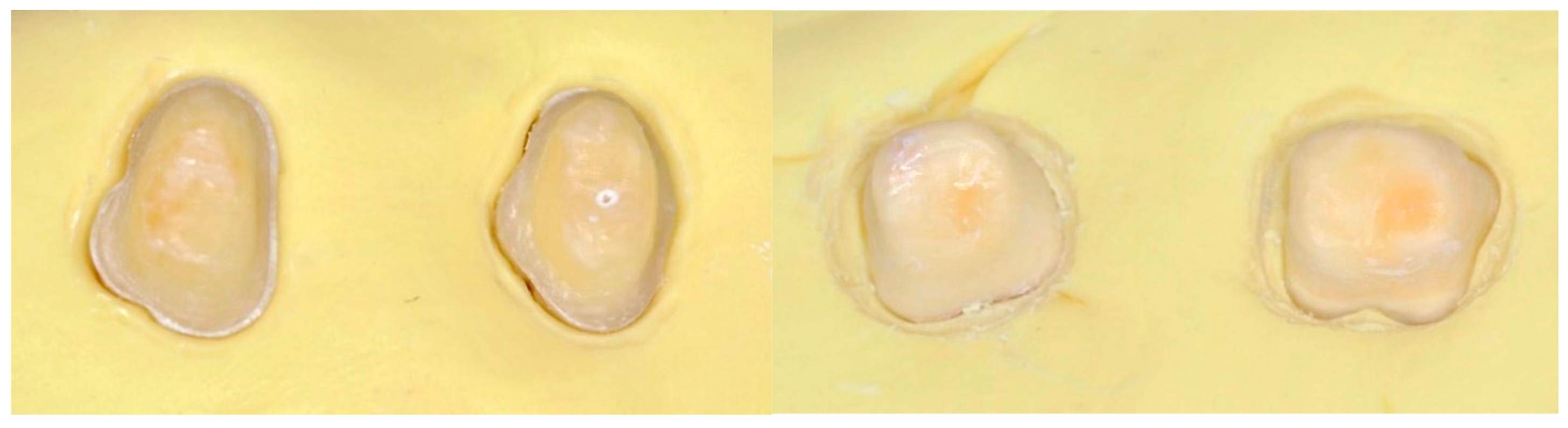
2.3. Tooth Preparation Protocol
2.4. Crown Fabrication Workflow
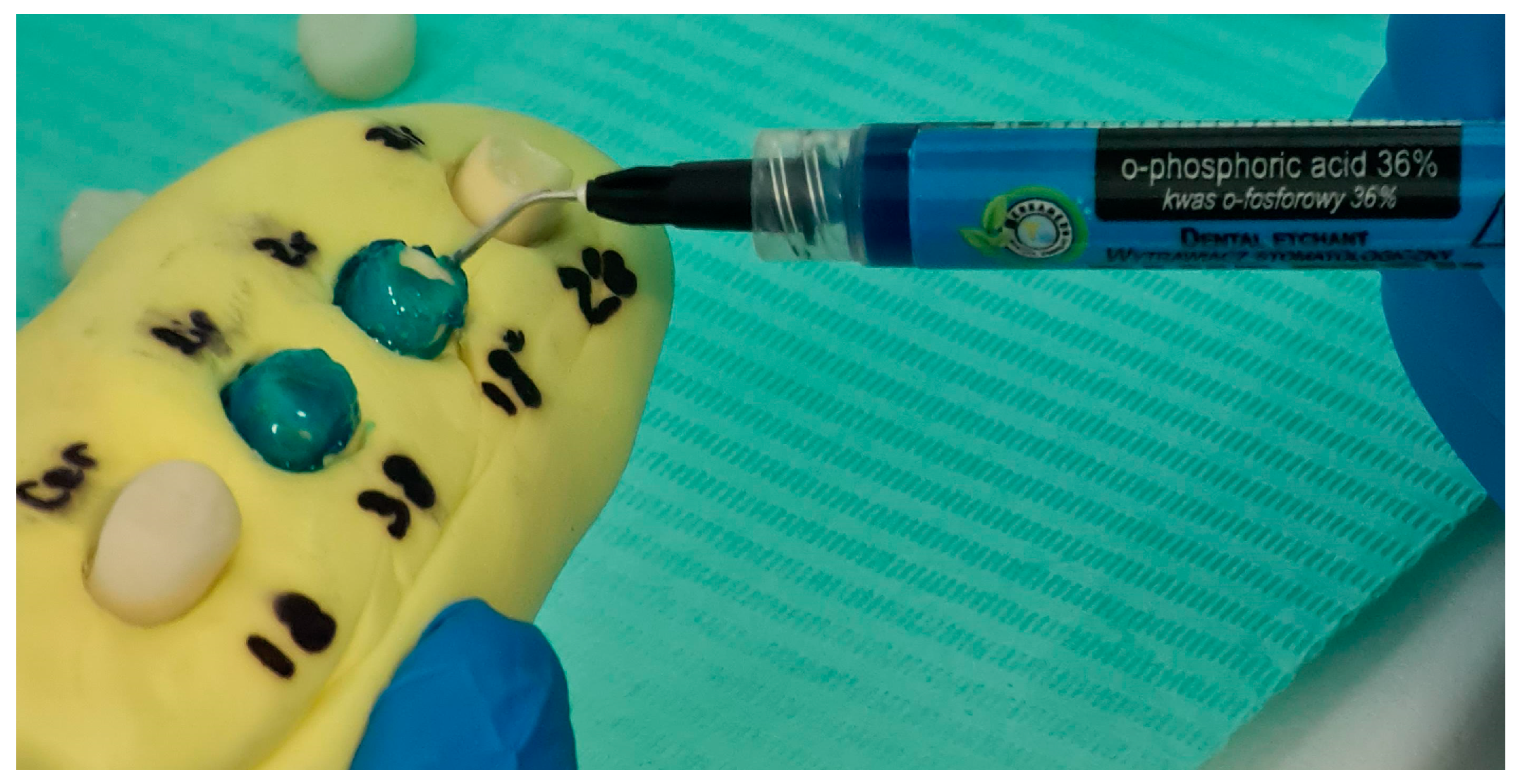
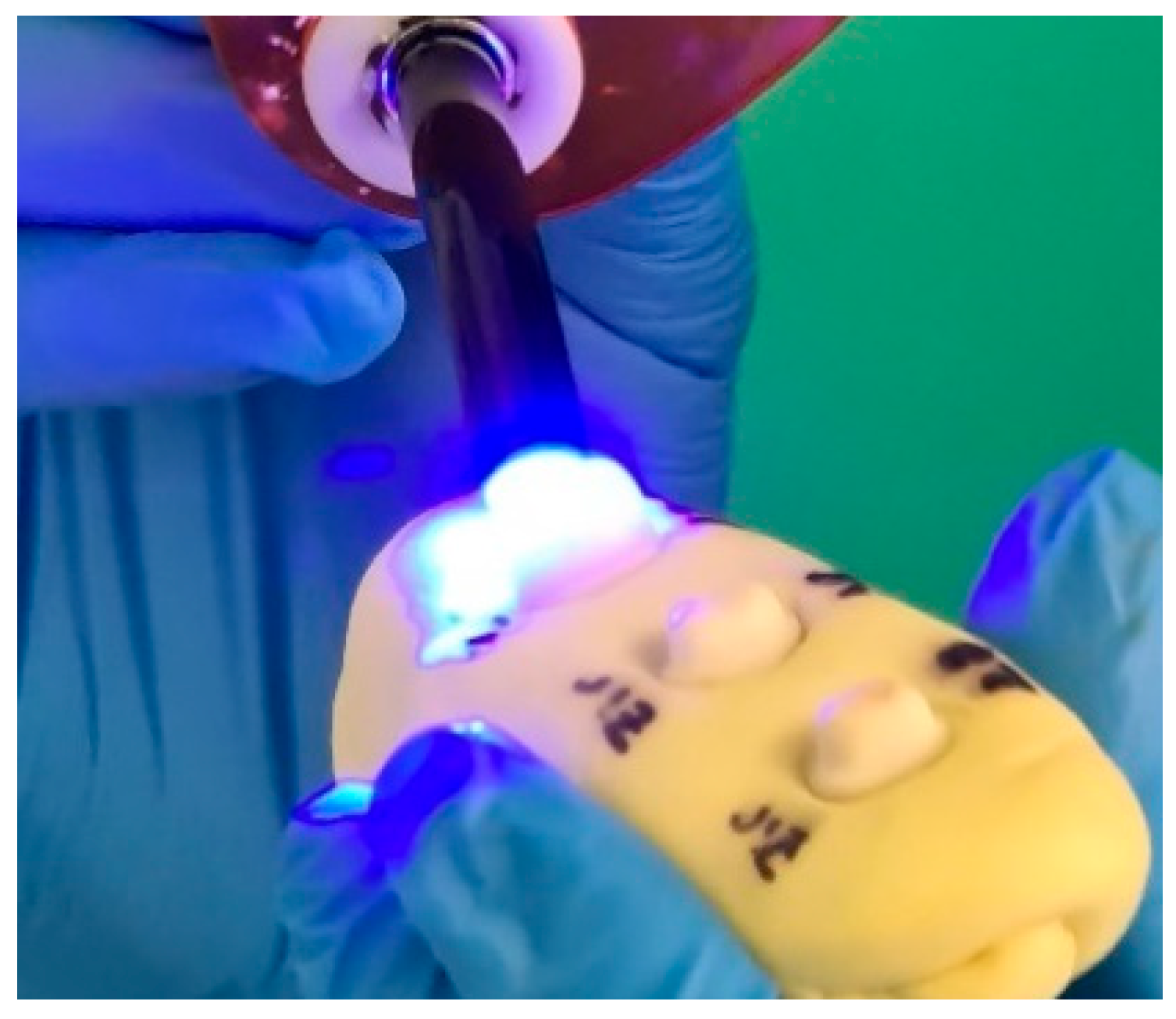
2.5. Surface Conditioning and Cementation
2.6. Fracture Resistance Testing
2.7. Statistical Analysis
3. Results
3.1. Fracture Resistance and Failure Mode
3.2. Influence of Margin Design and Material Type
3.3. Statistical Significance and Interactions Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD/CAM | Computer-Aided Design/Computer-Aided Manufacturing |
| FEA | Finite Element Analysis |
| LD | Lithium Disilicate |
| PFM | Porcelain-Fused-to-Metal |
| SD | Standard Deviation |
| SEM | Scanning Electron Microscopy |
| Zr | Zirconia |
| n.s. | Not Significant |
References
- Comlekoglu, M.; Dundar, M.; Ozcan, M.; Gungor, M.A.; Gokce, B.; Artunc, C. Influence of Cervical Finish Line Type on the Marginal Adaptation of Zirconia Ceramic Crowns. Oper. Dent. 2009, 34, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Hategan, S.I.; Belea, A.L.; Tanase, A.D.; Gavrilovici, A.M.; Petrescu, E.L.; Marsavina, L.; Sinescu, C.; Negrutiu, M.L.; Manole, M.C. The Evaluation of the Prosthetic Preparations Finish Lines for Provisional Crowns with the Impact on Periodontal Health. Rom. J. Oral Rehabil. 2024, 16, 453–459. [Google Scholar] [CrossRef]
- Lee, E.A.; Cambra, V.; Bergler, M. Staged Esthetic Crown Lengthening: Classification and Guidelines for Periodontal-Restorative Therapy. J. Esthet. Restor. Dent. 2024, 36, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Waerhaug, J. Healing of the Dento-Epithelial Junction Following Subgingival Plaque Control. II: As Observed on Extracted Teeth. J. Periodontol. 1978, 49, 119–134. [Google Scholar] [CrossRef]
- Miura, S.; Kasahara, S.; Yamauchi, S.; Egusa, H. Effect of Finish Line Design on Stress Distribution in Bilayer and Monolithic Zirconia Crowns: A Three-Dimensional Finite Element Analysis Study. Eur. J. Oral Sci. 2018, 126, 159–165. [Google Scholar] [CrossRef]
- Pan, C.Y.; Lan, T.H.; Lan, T.H.; Liu, P.H. Comparison of Different Cervical Finish Lines of All-Ceramic Crowns on Primary Molars in Finite Element Analysis. Materials 2020, 13, 1094. [Google Scholar] [CrossRef]
- Skalskyi, V.; Makeev, V.; Stankevych, O.; Pavlychko, R. Features of Fracture of Prosthetic Tooth-Endocrown Constructions by Means of Acoustic Emission Analysis. Dent. Mater. 2018, 34, e46–e55. [Google Scholar] [CrossRef]
- Agustin-Panadero, R.; Martin-de Llano, J.J.; Fons-Font, A.; Roman-Rodriguez, J.L.; Sola-Ruiz, M.F. Histological Study of Human Periodontal Tissue Following Biologically Oriented Preparation Technique (BOPT). J. Clin. Exp. Dent. 2020, 12, e597–e602. [Google Scholar] [CrossRef]
- Kihara, H.; Hatakeyama, W.; Komine, F.; Takata, Y.; Kanazawa, M. Accuracy and Practicality of Intraoral Scanner in Dentistry: A Literature Review. J. Prosthodont. Res. 2020, 64, 109–113. [Google Scholar] [CrossRef]
- Fadeev, R.A.; Kuznetsov, A.V. Key Stages of Dentistry and Prosthetic Dentistry Evolution: From Ancient Times to the Early 20th Century. Acta Univ. Dent. Chir. Maxillofac. 2024, 2, 151–159. [Google Scholar]
- Johnson, W.W. The History of Prosthetic Dentistry. J. Prosthet. Dent. 1959, 9, 841–846. [Google Scholar] [CrossRef]
- Neves, A.; Friedel, R.; Callapez, M.E.; Ferreira, M.J. Safeguarding Our Dentistry Heritage: A Study of the History and Conservation of Nineteenth–Twentieth Century Dentures. Herit. Sci. 2023, 11, 142. [Google Scholar] [CrossRef]
- Stephens, C. A Brief History of the Development and Use of Vulcanised Rubber in Dentistry. Br. Dent. J. 2023, 234, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, E.L.; Martu, I.; Sinescu, C.; Romînu, M.; Stoia, A.E.; Onisei, D.; Negrutiu, M.L.; Tanase, A.D.; Pop, D.M. Factors Involved in the Chromatic Failures of Non-Metallic Fixed Dental Prostheses. Rom. J. Oral Rehabil. 2024, 16, 549–556. [Google Scholar] [CrossRef]
- Madfa, A.A.; Al-Sanabani, F.A.; Al-Qudami, N.H.; Al-Sanabani, J.S.; Amran, A.G. Use of Zirconia in Dentistry: An Overview. Open Biomater. J. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Goodacre, C.J.; Campagni, W.V.; Aquilino, S.A. Tooth Preparations for Complete Crowns: An Art Form Based on Scientific Principles. J. Prosthet. Dent. 2001, 85, 363–376. [Google Scholar] [CrossRef]
- ISO 6872: 2015; Dentistry—Ceramic Materials. International Organization for Standardization: Geneva, Switzerland, 2015.
- Vichi, A.; Zhao, Z.; Paolone, G.; Scotti, N.; Mutahar, M.; Goracci, C.; Louca, C. Factory Crystallized Silicates for Monolithic Metal-Free Restorations: A Flexural Strength and Translucency Comparison Test. Materials 2022, 15, 7834. [Google Scholar] [CrossRef]
- Abduo, J.; Elseyoufi, M. Accuracy of Intraoral Scanners: A Systematic Review of Influencing Factors. Eur. J. Prosthodont. Restor. Dent. 2018, 26, 101–121. [Google Scholar]
- Mangano, F.; Gandolfi, A.; Luongo, G.; Logozzo, S. Intraoral Scanners in Dentistry: A Review of the Current Literature. BMC Oral Health 2017, 17, 149. [Google Scholar] [CrossRef]
- Sorrentino, R.; Ruggiero, G.; Leone, R.; Cagidiaco, E.F.; Mauro, M.I.D.; Ferrari, M.; Zarone, F. Trueness and Precision of an Intraoral Scanner on Abutments with Subgingival Vertical Margins: An In Vitro Study. J. Dent. 2024, 144, 104943. [Google Scholar] [CrossRef]
- Son, K.; Lee, K.-B. Effect of Finish Line Locations of Tooth Preparation on the Accuracy of Intraoral Scanners. Int. J. Comput. Dent. 2021, 24, 17–24. [Google Scholar]
- Ferrari Cagidiaco, E.; Goracci, C.; Marconi, A.; Vichi, A.; Grandini, S. Analysis of the Reproducibility of Subgingival Vertical Margins Using Intraoral Optical Scanning (IOS): A Randomized Controlled Pilot Trial. J. Clin. Med. 2021, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Rădulescu, V.; Boariu, M.; Rusu, D.; Boldeanu, C.; Christodorescu, R.; Roman, A.; Surlin, P.; Didilescu, A.C.; Vela, O.; Kardaras, G.; et al. Is the Diagnosis of Generalized Stage IV (Severe) Periodontitis Compatible with the Survival of Extended Stabilizing Prosthetic Restorations? A Medium-Term Retrospective Study. Diagnostics 2022, 12, 3053. [Google Scholar] [CrossRef] [PubMed]
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia Based Dental Ceramics: Structure, Mechanical Properties, Biocompatibility and Applications. Dalton Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef]
- Torabi, K.; Vojdani, M.; Giti, R.; Taghva, M.; Pardis, S. The Effect of Various Veneering Techniques on the Marginal Fit of Zirconia Copings. J. Adv. Prosthodont. 2015, 7, 233–239. [Google Scholar] [CrossRef]
- Grech, J.; Antunes, E. Zirconia in Dental Prosthetics: A Literature Review. J. Mater. Res. Technol. 2019, 8, 4956–4964. [Google Scholar] [CrossRef]
- Hajaj, T.; Lile, I.E.; Veja, I.; Titihazan, F.; Rominu, M.; Negruțiu, M.L.; Sinescu, C.; Novac, A.C.; Talpoș Niculescu, S.; Zaharia, C. Influence of Pontic Length on the Structural Integrity of Zirconia Fixed Partial Dentures (FPDs). J. Funct. Biomater. 2025, 16, 116. [Google Scholar] [CrossRef]
- Magne, P.; Belser, U. Biomimetic Restorative Dentistry, 2nd ed.; Quintessence Publishing: Berlin, Germany, 2021. [Google Scholar]
- Sinescu, C.; Bradu, A.; Duma, V.-F.; Topala, F.; Negrutiu, M.; Podoleanu, A.G. Effects of Temperature Variations during Sintering of Metal Ceramic Tooth Prostheses Investigated Non-Destructively with Optical Coherence Tomography. Appl. Sci. 2017, 7, 552. [Google Scholar] [CrossRef]
- Serban, C.; Lungeanu, D.; Bota, S.D.; Cotca, C.C.; Negrutiu, M.L.; Duma, V.F.; Sinescu, C.; Craciunescu, E.L. Emerging Technologies for Dentin Caries Detection-A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 674. [Google Scholar] [CrossRef]
- Gavrilovici, A.M.; Anitas, E.M.; Chirigiu, L.; Bica, I.; Negruțiu, M.L. Magnetodielectric Effects in Magnetorheological Elastomers Based on Polymer Fabric, Silicone Rubber, and Magnetorheological Suspension. Adv. Polym. Technol. 2019, 2019, 1983547. [Google Scholar] [CrossRef]
- Fouad, M.; Allam, E. Failures in Fixed Dental Prostheses: A Clinical Survey on Causes and Longevity. J. Dent. Sci. Res. Rev. 2024, 6, 1–4. [Google Scholar] [CrossRef]
- Almilhatti, H.J.; Neppelenbroek, K.H.; Vergani, C.E.; Machado, A.L.; Pavarina, A.C.; Giampaolo, E.T. Adhesive bonding of resin composite to various titanium surfaces using different metal conditioners and a surface modification system. J. Appl. Oral Sci. 2013, 21, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Avram, L.T.; Galațanu, S.V.; Opriș, C.; Pop, C.; Jivănescu, A. Effect of Different Etching Times with Hydrofluoric Acid on the Bond Strength of CAD/CAM Ceramic Material. Materials 2022, 15, 7071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olariu, I.; Marian, D.; Veja, I.; Flueras, R.; Popovici, R.A.; Pitic, D.E.; Stana, H.A.; Vaida, L.L.; Lile, I.E. Exploring Dentists’ Preferences in Selecting Adhesive Systems: A Survey Analysis. Appl. Sci. 2024, 14, 10119. [Google Scholar] [CrossRef]







| Material | Fracture Mode | Number of Samples | Percentage (%) |
|---|---|---|---|
| Zirconia | Cohesive | 7 | 70% |
| Zirconia | Catastrophic | 3 | 30% |
| Zirconia | Adhesive | 0 | 0% |
| Lithium Disilicate | Cohesive | 4 | 40% |
| Lithium Disilicate | Catastrophic | 6 | 60% |
| Lithium Disilicate | Adhesive | 0 | 0% |
| Restoration Type + Margin Design | Mean (N) | SD (N) | 95% CI (N) |
|---|---|---|---|
| Lithium Disilicate–Tangential | 1862 | 251.20 | [1715, 2009] |
| Lithium Disilicate–Chamfer | 2494 | 460.50 | [2195, 2793] |
| Zirconia–Tangential | 2425 | 240.50 | [2286, 2564] |
| Zirconia–Chamfer | 2658 | 541.31 | [2329, 2987] |
| Source of Variation | F-Value | p-Value | Partial η2 | Significance |
|---|---|---|---|---|
| Material (Zr vs. LD) | 26.47 | <0.001 | 0.49 | Yes |
| Margin Design (Chamfer vs. Tangential) | 3.21 | 0.08 | 0.07 | No |
| Interaction (Material × Margin) | 9.76 | <0.01 | 0.17 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajaj, T.; Marian, D.; Zaharia, C.; Niculescu, S.T.; Negru, R.M.; Titihazan, F.; Rominu, M.; Sinescu, C.; Novac, A.C.; Dobrota, G.; et al. Fracture Resistance of CAD/CAM-Fabricated Zirconia and Lithium Disilicate Crowns with Different Margin Designs: Implications for Digital Dentistry. J. Funct. Biomater. 2025, 16, 205. https://doi.org/10.3390/jfb16060205
Hajaj T, Marian D, Zaharia C, Niculescu ST, Negru RM, Titihazan F, Rominu M, Sinescu C, Novac AC, Dobrota G, et al. Fracture Resistance of CAD/CAM-Fabricated Zirconia and Lithium Disilicate Crowns with Different Margin Designs: Implications for Digital Dentistry. Journal of Functional Biomaterials. 2025; 16(6):205. https://doi.org/10.3390/jfb16060205
Chicago/Turabian StyleHajaj, Tareq, Diana Marian, Cristian Zaharia, Serban Talpos Niculescu, Radu Marcel Negru, Florina Titihazan, Mihai Rominu, Cosmin Sinescu, Andreea Codruta Novac, Gabriel Dobrota, and et al. 2025. "Fracture Resistance of CAD/CAM-Fabricated Zirconia and Lithium Disilicate Crowns with Different Margin Designs: Implications for Digital Dentistry" Journal of Functional Biomaterials 16, no. 6: 205. https://doi.org/10.3390/jfb16060205
APA StyleHajaj, T., Marian, D., Zaharia, C., Niculescu, S. T., Negru, R. M., Titihazan, F., Rominu, M., Sinescu, C., Novac, A. C., Dobrota, G., & Veja, I. (2025). Fracture Resistance of CAD/CAM-Fabricated Zirconia and Lithium Disilicate Crowns with Different Margin Designs: Implications for Digital Dentistry. Journal of Functional Biomaterials, 16(6), 205. https://doi.org/10.3390/jfb16060205












