Comparison of Anatomical Maxillary Sinus Implant and Polydioxanone Sheets in Treatment of Orbital Floor Blowout Fractures: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
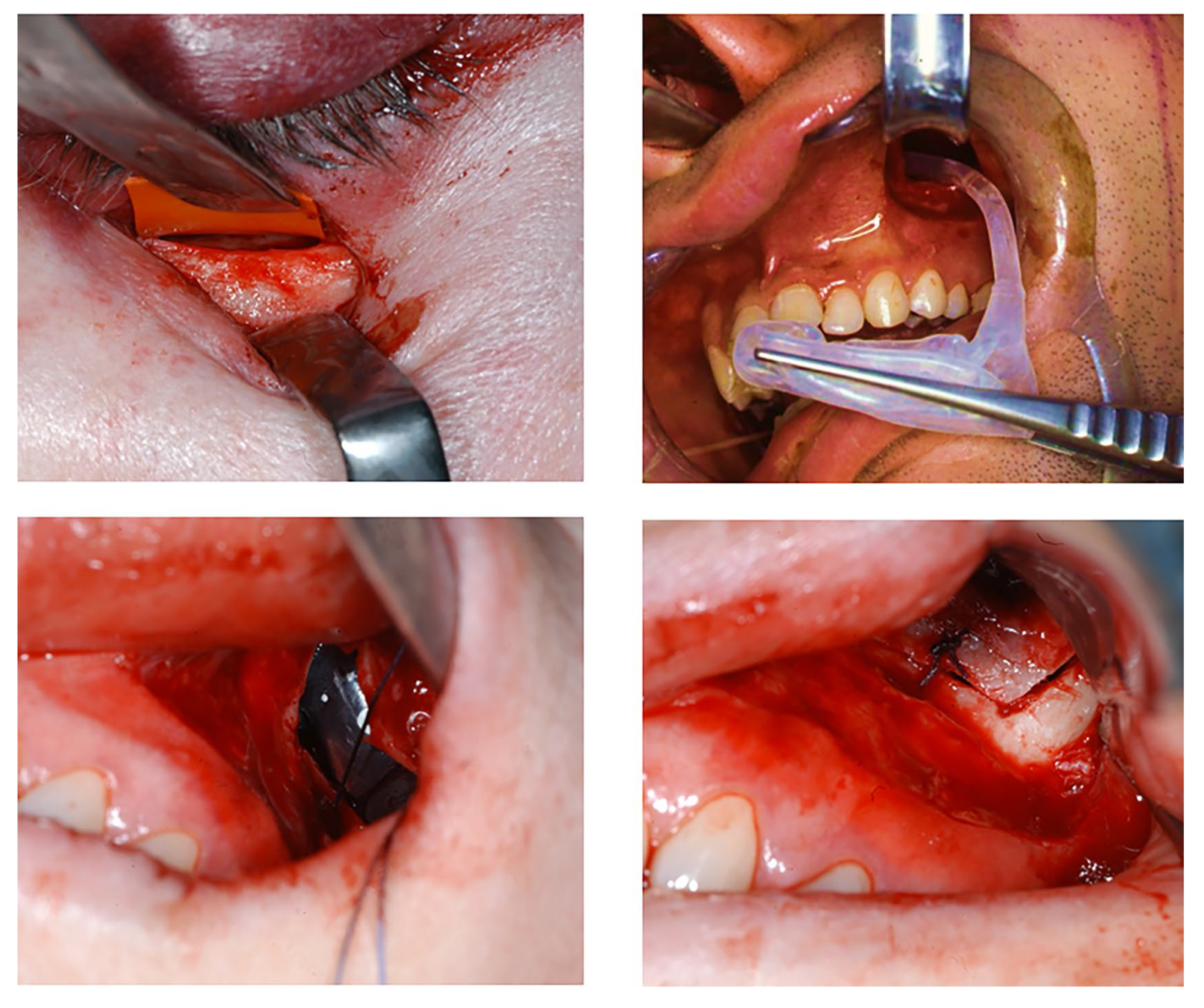
2.3. Procedures
2.4. Clinical Data
2.5. Geometric Measurements
2.6. Statistic Analysis
3. Results
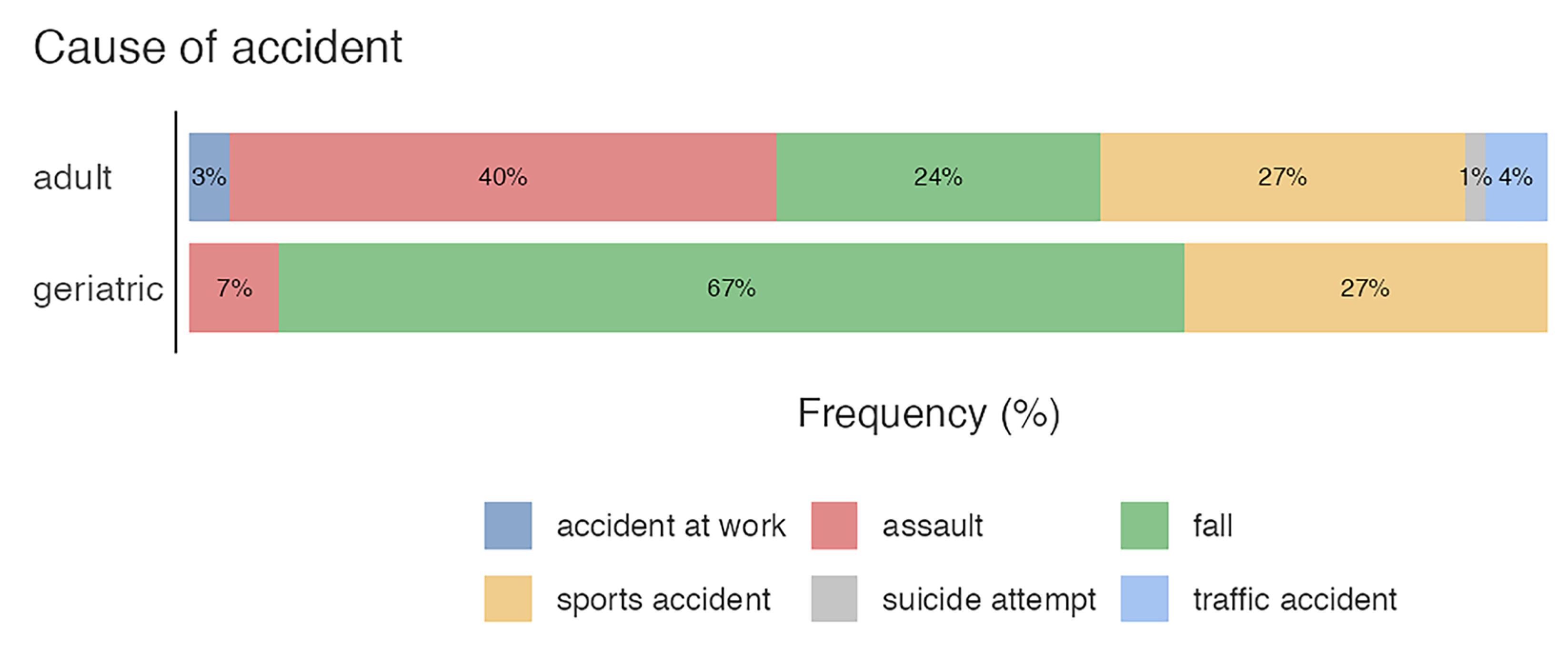
3.1. Age, Sex, and Cause of Accident
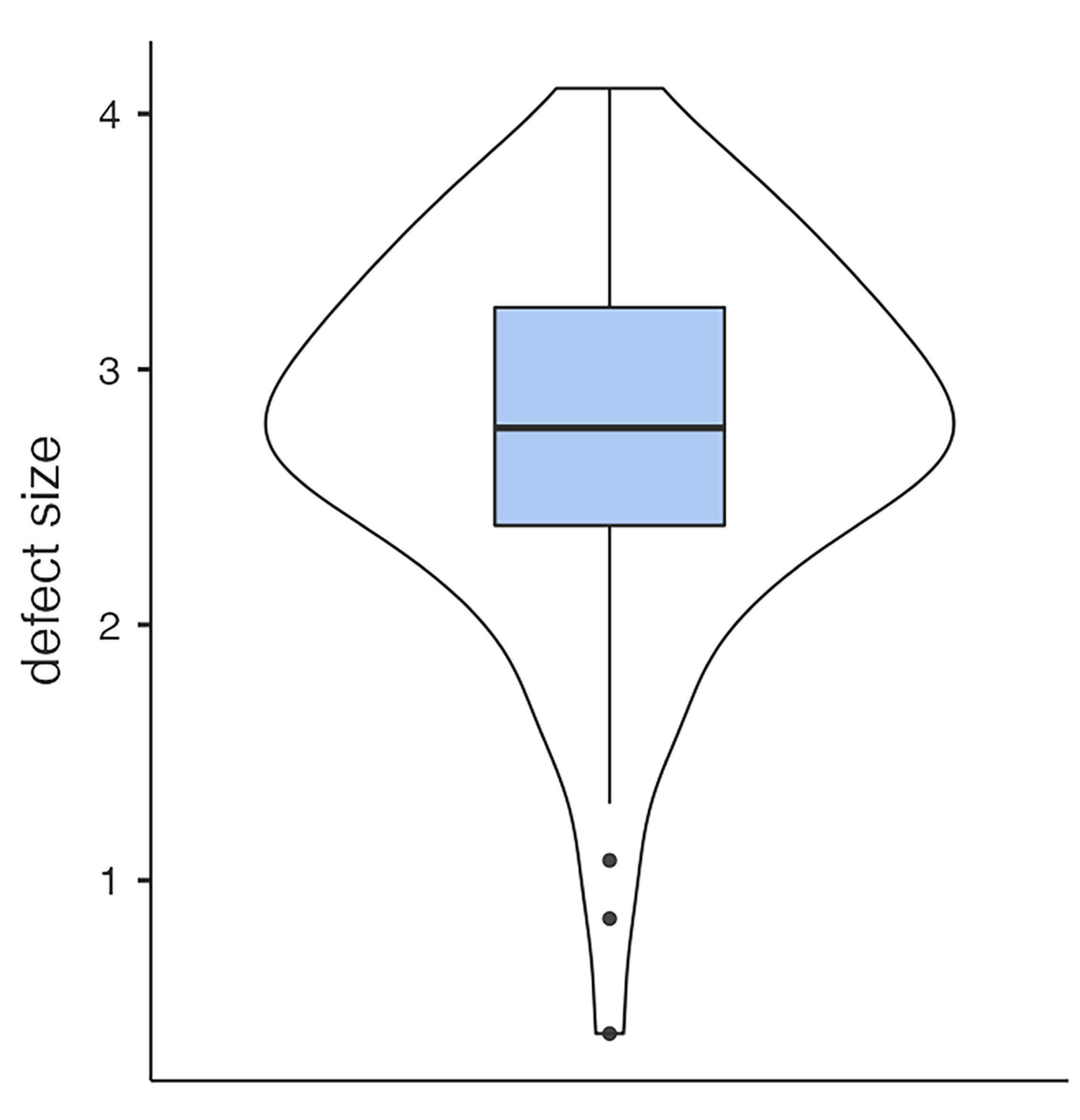
3.2. Defect Size, Volume of Hernia, Initial Diplopia, Enophthalmos, and Infraorbital Nerve Impairment
3.3. Treatment Methods
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shumway, C.L.; Motlagh, M.; Wade, M. Anatomy, Head and Neck, Orbit Bones; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hwang, K.; You, S.H.; Sohn, I.A. Analysis of orbital bone fractures: A 12-year study of 391 patients. J. Craniofac. Surg. 2009, 20, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.M.; Gehlot, N.; Kv, A.; Prasad, P.; Mehta, P.; Paul, T.R.; Dupare, A.; Cvns, C.S.; Rahman, S. Ophthalmic Complications in Maxillofacial Trauma: A Prospective Study. Cureus 2022, 14, e27608. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Artymowicz, A.; Muscente, J.; Shinder, R.; Mostafavi, D. Do Not Fall for This; Diagnostic Challenges in Orbital Floor Fractures with Extraocular Muscle Entrapment. Cureus 2023, 15, e35268. [Google Scholar] [CrossRef] [PubMed]
- Jaquiéry, C.; Aeppli, C.; Cornelius, P.; Palmowsky, A.; Kunz, C.; Hammer, B. Reconstruction of orbital wall defects: Critical review of 72 patients. Int. J. Oral. Maxillofac. Surg. 2007, 36, 193–199. [Google Scholar] [CrossRef]
- Winegar, B.A.; Gutierrez, J.E. Imaging of Orbital Trauma and Emergent Non-traumatic Conditions. Neuroimaging Clin. N. Am. 2015, 25, 439–456. [Google Scholar] [CrossRef]
- Borumandi, F.; Gaggl, A.; Hachleitner, J. Anatomic maxillary sinus balloon for orbital floor repair: A preliminary volumetric study. Orbit 2014, 33, 318. [Google Scholar] [CrossRef]
- Seifert, L.B.; Mainka, T.; Herrera-Vizcaino, C.; Verboket, R.; Sader, R. Orbital floor fractures: Epidemiology and outcomes of 1594 reconstructions. Eur. J. Trauma Emerg. Surg. 2022, 48, 1427–1436. [Google Scholar] [CrossRef]
- Gosau, M.; Schöneich, M.; Draenert, F.G.; Ettl, T.; Driemel, O.; Reichert, T.E. Retrospective analysis of orbital floor fractures—Complications, outcome, and review of literature. Clin. Oral Investig. 2011, 15, 305–313. [Google Scholar] [CrossRef]
- Bourry, M.; Hardouin, J.B.; Fauvel, F.; Corre, P.; Lebranchu, P.; Bertin, H. Clinical evaluation of the efficacy of materials used for primary reconstruction of orbital floor defects: Meta-analysis. Head Neck 2021, 43, 679–690. [Google Scholar] [CrossRef]
- Baumann, A.; Burggasser, G.; Gauss, N.; Ewers, R. Orbital floor reconstruction with an alloplastic resorbable polydioxanone sheet. Int. J. Oral Maxillofac. Surg. 2002, 31, 367–373. [Google Scholar] [CrossRef]
- Beck-Broichsitter, B.E.; Acar, C.; Kandzia, C.; Jochens, A.; Wiltfang, J.; Becker, S.T. Reconstruction of the orbital floor with polydioxanone: A long-term clinical survey of up to 12 years. Br. J. Oral Maxillofac. Surg. 2015, 53, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Crozet, A.; Lebranchu, P.; Vabre, B.; Paillé, C.; Bourry, M.; Corre, P.; Bertin, H. Management of orbital floor fractures in France: Results of a national online survey. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101389. [Google Scholar] [CrossRef] [PubMed]
- Taxis, J.; Ungerboeck, L.; Motel, C.; Eckert, A.W.; Platz Batista da Silva, N.; Nieberle, F.; Ludwig, N.; Meier, J.K.; Ettl, T.; Reichert, T.E.; et al. Thin PDS Foils Represent an Equally Favorable Restorative Material for Orbital Floor Fractures Compared to Titanium Meshes. Tomography 2023, 9, 1515–1525. [Google Scholar] [CrossRef]
- Winnand, P.; Ooms, M.; Ayoub, N.; Schick, D.; Paulßen von Beck, F.; Hölzle, F.; Mücke, T.; Modabber, A. The impact of polydioxanone (PDS) foil thickness on reconstruction of the orbital geometry after isolated orbital floor fractures. Eur. J. Trauma Emerg. Surg. 2024, 50, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.K.; Kang, D.H.; Oh, S.A.; Gu, J.H. Orbital Floor Restoration Using the Transnasal Balloon Technique for Inferior Orbital Wall Fracture. Ann. Plast. Surg. 2015, 75, 522–525. [Google Scholar] [CrossRef]
- Park, I.H.; Lee, H.M.; Yanagi, K. Endoscopic transantral and transnasal repair of orbital floor fracture with the ballooning technique, and classification and characterization of orbital floor fractures. Am. J. Rhinol. Allergy 2015, 29, 445–448. [Google Scholar] [CrossRef]
- Lee, S.M.; Leem, D.H. Effective Reduction of Orbital Floor Fracture with Customized Balloon Using Contrast Agent And Micro Saw. J. Craniofac. Surg. 2021, 32, 1540–1544. [Google Scholar] [CrossRef]
- Jo, E.; Kim, J.; Yang, H. Inferior Blow-Out Fracture Reduction Using Two Urinary Balloon Catheters. Arch. Craniofac. Surg. 2015, 16, 114. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, D.H.; Jeon, H.B.; Kim, H. Orbital wall restoration with primary bone fragments in complex orbital fractures. Arch. Craniofac. Surg. 2023, 24, 52–58. [Google Scholar] [CrossRef]
- Ku, J.K.; Leem, D.H. Intraoral Approach for Zygomaticomaxillary Complex and Orbital Floor Fracture With a Customized Ballooning Technique. J. Craniofac. Surg. 2024, 35, e213–e215. [Google Scholar] [CrossRef]
- Konstantinov, D.I.; Erashov, M.A.; Denisova, O.A.; Polunin, M.M.; Soldatsky, Y.L.; Polyakov, A.A.; Gorbunova, E.D.; Kononov, L.B.; Edgem, S.R. Optimized interdisciplinary approach in the treatment of maxillary sinus upper wall fractures in children. Vestn. Otorinolaringol. 2024, 89, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Tedaldi, M.; Vetrano, S.; Zotos, G.; Buonaccorsi, S.; Marini Balestra, F.M.; Cerulli, G.; Piccolino, P. Management of Orbital Medial Wall Fracture with Endonasal Balloon. J. Craniofac. Surg. 2023, 34, 1076–1077. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, K.; Maeda, Y.; Kataoka, N.; Kagokawad, H.; Nagaminea, M.; Otad, I.; Fujita, T. Algorithm for pediatric orbital blowout fractures: A 20-year retrospective cohort study. Braz. J. Otorhinolaryngol. 2023, 89, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, C.; Hachleitner, J.; Thaller-Antlanger, H. Experience with evacuable maxillary sinus endothesis for orbital and maxillary trauma. Dtsch. Z. Mund- Kiefer- Gesichts-Chir. 1989, 13, 252–255. [Google Scholar]
- Steinmassl, O.; Laimer, J.; Offermanns, V.; Wildauer, M.; Steinmassl, P.-A.; Grams, A.E.; Kofler, F.; Rasse, M.; Bruckmoser, E. Clinical Outcome Following Surgical Repair of Small Versus Large Orbital Floor Fractures Using Polyglactin 910/Polydioxanone (Ethisorb®). Materials 2020, 13, 206. [Google Scholar] [CrossRef]
- Hassan, B.; Hricz, N.; Er, S.; Yoon, J.; Resnick, E.; Liang, F.; Yang, R.; Manson, P.N.; Grant, M.P. Development and validation of a risk calculator for postoperative diplopia following orbital fracture repair in adults. Sci. Rep. 2024, 14, 3654. [Google Scholar] [CrossRef]
- Garcia, B.G.; Ferrer, A.D. Surgical indications of orbital fractures depending on the size of the fault area determined by computed tomography: A systematic review. Rev. Española Cirugía Oral Maxilofac. 2016, 38, 42–48. [Google Scholar] [CrossRef][Green Version]
- Safi, A.F.; Richter, M.T.; Rothamel, D.; Nickenig, H.J.; Scheer, M.; Zöller, J.; Kreppel, M. Influence of the volume of soft tissue herniation on clinical symptoms of patients with orbital floor fractures. J. Cranio-Maxillofac. Surg. 2016, 44, 1929–1934. [Google Scholar] [CrossRef]
- Scolozzi, P.; Bachelet, J.T.; Courvoisier, D.S. Are Inferior Rectus Muscle Displacement and the Fracture’s Size Associated with Surgical Repair Decisions and Clinical Outcomes in Patients with Pure Blowout Orbital Fracture? J. Oral Maxillofac. Surg. 2020, 78, 2280.e1–2280.e10. [Google Scholar] [CrossRef]
- Ploder, O.; Klug, C.; Voracek, M.; Burggasser, G.; Czerny, C. Evaluation of computer-based area and volume measurement from coronal computed tomography scans in isolated blowout fractures of the orbital floor. J. Oral. Maxillofac. Surg. 2002, 60, 1267–1272; discussion 1273–1274. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Agostini, F.; Marotta, N.; Santilli, G.; Boffano, P.; Scaturro, D.; Letizia Mauro, G.; Ammendolia, A.; de Sire, A. Oral health-related quality of life in elderly: An umbrella review of systematic reviews from a multidisciplinary rehabilitation point-of-view. Clin. Ter. 2024, 175, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Janto, M.; Iurcov, R.; Daina, C.M.; Neculoiu, D.C.; Venter, A.C.; Badau, D.; Cotovanu, A.; Negrau, M.; Suteu, C.L.; Sabau, M.; et al. Oral Health among Elderly, Impact on Life Quality, Access of Elderly Patients to Oral Health Services and Methods to Improve Oral Health: A Narrative Review. J. Pers. Med. 2022, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Soejima, K.; Shimoda, K.; Kashimura, T.; Yamaki, T.; Kono, T.; Sakurai, H.; Nakazawa, H. Endoscopic transmaxillary repair of orbital floor fractures: A minimally invasive treatment. J. Plast. Surg. Hand Surg. 2013, 47, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Kashimura, T.; Soejima, K.; Kikuchi, Y.; Nakazawa, H. Stability of Orbital Floor Fracture Fixation After Endoscope-Assisted Balloon Placement. J. Craniofac. Surg. 2017, 28, e669–e672. [Google Scholar] [CrossRef]
- Kang, D.H. Orbital wall restoring surgery with primary orbital wall fragments in blowout fracture. Arch. Craniofac. Surg. 2019, 20, 347–353. [Google Scholar] [CrossRef]
- Ramesh, S.; Hubschman, S.; Goldberg, R. Resorbable Implants for Orbital Fractures: A Systematic Review. Ann. Plast. Surg. 2018, 81, 372–379. [Google Scholar] [CrossRef]
- Gunarajah, D.R.; Samman, N. Biomaterials for repair of orbital floor blowout fractures: A systematic review. J. Oral. Maxillofac. Surg. 2013, 71, 550–570. [Google Scholar] [CrossRef]
- Touil, H.; Mabrouk, H.; Msellmi, F.; Bouzaiene, M. Reconstruction of orbital floor fractures with Polypropylen mesh. Tunis. Med. 2020, 98, 49–54. [Google Scholar]
- Düzgün, S.; Kayahan Sirkeci, B. Comparison of post-operative outcomes of graft materials used in reconstruction of blow-out fractures. Ulus. Travma Acil Cerrahi Derg. 2020, 26, 538–544. [Google Scholar] [CrossRef]
- Abukhder, M.; Onions, E.; Flaherty, E.; Tarassoli, S.; Hassan, M.R.; Whelan, R. A systematic literature review and narrative synthesis on the use of autologous cartilage in the repair of orbital fractures. Ann. Med. Surg. 2024, 86, 968–974. [Google Scholar] [CrossRef]
- Kyriakidou, E.; O’Connor, N.; Larkin, E.B. An orbital floor fracture may not always be the cause of new-onset diplopia: Literature review of the aetiology and types of diplopia. Oral Surg. 2016, 9, 170–176. [Google Scholar] [CrossRef]
- Bartoli, D.; Fadda, M.T.; Battisti, A.; Cassoni, A.; Pagnoni, M.; Riccardi, E.; Sanzi, M.; Valentini, V. Retrospective analysis of 301 patients with orbital floor fracture. J. Craniomaxillofac. Surg. 2015, 43, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Colangeli, W.; Ferragina, F.; Kallaverja, E.; Celano, C.; Cristofaro, M.G. Orbital fractures treated in a university hospital of southern Italy: Epidemiology, outcomes and prognostic factors resulting from 538 retrospectively analyzed cases. Oral Maxillofac. Surg. 2024, 28, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Dhabaria, H.; Kolari, V.; Sequeira, J.; Shah, A. Evaluation of Infraorbital Nerve Recovery and its Effect on Quality of Life following Open Reduction and Internal Fixation of Zygomaticomaxillary Complex Fractures—An Evaluative Study. Ann. Maxillofac. Surg. 2022, 12, 128–132. [Google Scholar] [CrossRef]
- Baradaran, A.; El-Hawary, H.; Efanov, J.I.; Xu, L. Peripheral Nerve Healing: So Near and Yet So Far. Semin. Plast. Surg. 2021, 35, 204–210. [Google Scholar] [CrossRef]
- Lam, T.C.; Leung, Y.Y. Innovations in Peripheral Nerve Regeneration. Bioengineering 2024, 11, 444. [Google Scholar] [CrossRef]
- Holtmann, H.; Eren, H.; Sander, K.; Kübler, N.R.; Handschel, J. Orbital floor fractures—Short and intermediate-term complications depending on treatment procedures. Head Face Med. 2016, 12, 1. [Google Scholar] [CrossRef]
- Hughes, D.; McQuillan, J.; Holmes, S. Quantitative analysis of diplopia following orbital fracture repair. Br. J. Oral Maxillofac. Surg. 2023, 61, 202–208. [Google Scholar] [CrossRef]
- Damgaard, O.E.; Larsen, C.G.; Felding, U.A.; Toft, P.B.; von Buchwald, C. Surgical Timing of the Orbital “Blowout” Fracture: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2016, 155, 387–390. [Google Scholar] [CrossRef]
- Kholaki, O.; Hammer, D.A.; Schlieve, T. Management of Orbital Fractures. Atlas Oral Maxillofac. Surg. Clin. North. Am. 2019, 27, 157–165. [Google Scholar] [CrossRef]
- Timkovic, J.; Stransky, J.; Handlos, P.; Janosek, J.; Tomaskova, H.; Stembirek, J. Detecting Binocular Diplopia in Orbital Floor Blowout Fractures: Superiority of the Orthoptic Approach. Medicina 2021, 57, 989. [Google Scholar] [CrossRef] [PubMed]
- Cena, P.; Raco, I.; Roccia, F.; Federica, S.; Dediol, E.; Kos, B.; Bottini, G.B.; Goetzinger, M.; Samieirad, S.; Gorla, L.F.d.O.; et al. An 11-year multicentric surgical experience on pediatric orbital floor trapdoor fracture: A World Oral Maxillofacial Trauma (WORMAT) project. J. Stomatol. Oral Maxillofac. Surg. 2025, 126, 102033. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Koh, H.K.; Lee, H.; Shin, H.J. Deep-Learning Method for the Diagnosis and Classification of Orbital Blowout Fracture Based on Computed Tomography. J. Oral Maxillofac. Surg. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Santilli, G.; Mangone, M.; Agostini, F.; Paoloni, M.; Bernetti, A.; Diko, A.; Tognolo, L.; Coraci, D.; Vigevano, F.; Vetrano, M.; et al. Evaluation of Rehabilitation Outcomes in Patients with Chronic Neurological Health Conditions Using a Machine Learning Approach. J. Funct. Morphol. Kinesiol. 2024, 9, 176. [Google Scholar] [CrossRef]




| Sex | Counts | % of Total | Cumulative% |
|---|---|---|---|
| female | 23 | 28.0 % | 28.0% |
| male | 59 | 72.0 % | 100.0% |
| Cause of Accident | Counts | % of Total | Cumulative% |
|---|---|---|---|
| accident at work | 2 | 2.4% | 2.4% |
| assault | 28 | 34.1% | 36.6% |
| fall | 26 | 31.7% | 68.3% |
| sports accident | 22 | 26.8% | 95.1% |
| suicide attempt | 1 | 1.2% | 96.3% |
| traffic accident | 3 | 3.7% | 100.0% |
| Predictor | Estimate | 95% Confidence Interval | SE | Z | p | Odds Ratio | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Intercept | −8.24739 | −12.9971 | −3.4977 | 2.4234 | −3.403 | <0.001 | 2.62 × 10−4 |
| Implant or PDS: | |||||||
| PDS–Implant | 2.0076 | 0.5134 | 3.5019 | 0.7624 | 2.633 | 0.008 | 7.45 |
| Age | 0.00658 | −0.027 | 0.0402 | 0.0171 | 0.384 | 0.701 | 1.01 |
| Defect size | 1.3175 | −0.092 | 2.727 | 0.7191 | 1.832 | 0.067 | 3.73 |
| Hernia volume | 0.82226 | 0.1121 | 1.5324 | 0.3623 | 2.269 | 0.023 | 2.28 |
| Predictor | Estimate | 95% Confidence Interval | SE | Z | p | Odds Ratio | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Intercept | −3.13834 | −6.1402 | −0.1364 | 1.5316 | −2.049 | 0.04 | 0.0434 |
| Implant or PDS: | |||||||
| PDS–Implant | −0.2914 | −1.5195 | 0.9367 | 0.6266 | −0.465 | 0.642 | 0.7472 |
| Age | 0.00484 | −0.0236 | 0.0333 | 0.0145 | 0.333 | 0.739 | 1.0049 |
| Defect size | 0.21674 | −0.9002 | 1.3337 | 0.5699 | 0.38 | 0.704 | 1.242 |
| Hernia volume | 0.6342 | −5.04 × 10−4 | 1.2689 | 0.3238 | 1.958 | 0.05 | 1.8855 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walch, B.; Gaggl, A.; Bottini, G.B.; Hachleitner, J.; Huber, F.; Römhild, H.; Geroldinger, M.; Götzinger, M. Comparison of Anatomical Maxillary Sinus Implant and Polydioxanone Sheets in Treatment of Orbital Floor Blowout Fractures: A Retrospective Cohort Study. J. Funct. Biomater. 2025, 16, 204. https://doi.org/10.3390/jfb16060204
Walch B, Gaggl A, Bottini GB, Hachleitner J, Huber F, Römhild H, Geroldinger M, Götzinger M. Comparison of Anatomical Maxillary Sinus Implant and Polydioxanone Sheets in Treatment of Orbital Floor Blowout Fractures: A Retrospective Cohort Study. Journal of Functional Biomaterials. 2025; 16(6):204. https://doi.org/10.3390/jfb16060204
Chicago/Turabian StyleWalch, Benjamin, Alexander Gaggl, Gian Battista Bottini, Johannes Hachleitner, Florian Huber, Hannes Römhild, Martin Geroldinger, and Maximilian Götzinger. 2025. "Comparison of Anatomical Maxillary Sinus Implant and Polydioxanone Sheets in Treatment of Orbital Floor Blowout Fractures: A Retrospective Cohort Study" Journal of Functional Biomaterials 16, no. 6: 204. https://doi.org/10.3390/jfb16060204
APA StyleWalch, B., Gaggl, A., Bottini, G. B., Hachleitner, J., Huber, F., Römhild, H., Geroldinger, M., & Götzinger, M. (2025). Comparison of Anatomical Maxillary Sinus Implant and Polydioxanone Sheets in Treatment of Orbital Floor Blowout Fractures: A Retrospective Cohort Study. Journal of Functional Biomaterials, 16(6), 204. https://doi.org/10.3390/jfb16060204






