Strength–Ductility Synergy in Biodegradable Mg-Rare Earth Alloy Processed via Multi-Directional Forging
Abstract
1. Introduction
2. Materials and Methods
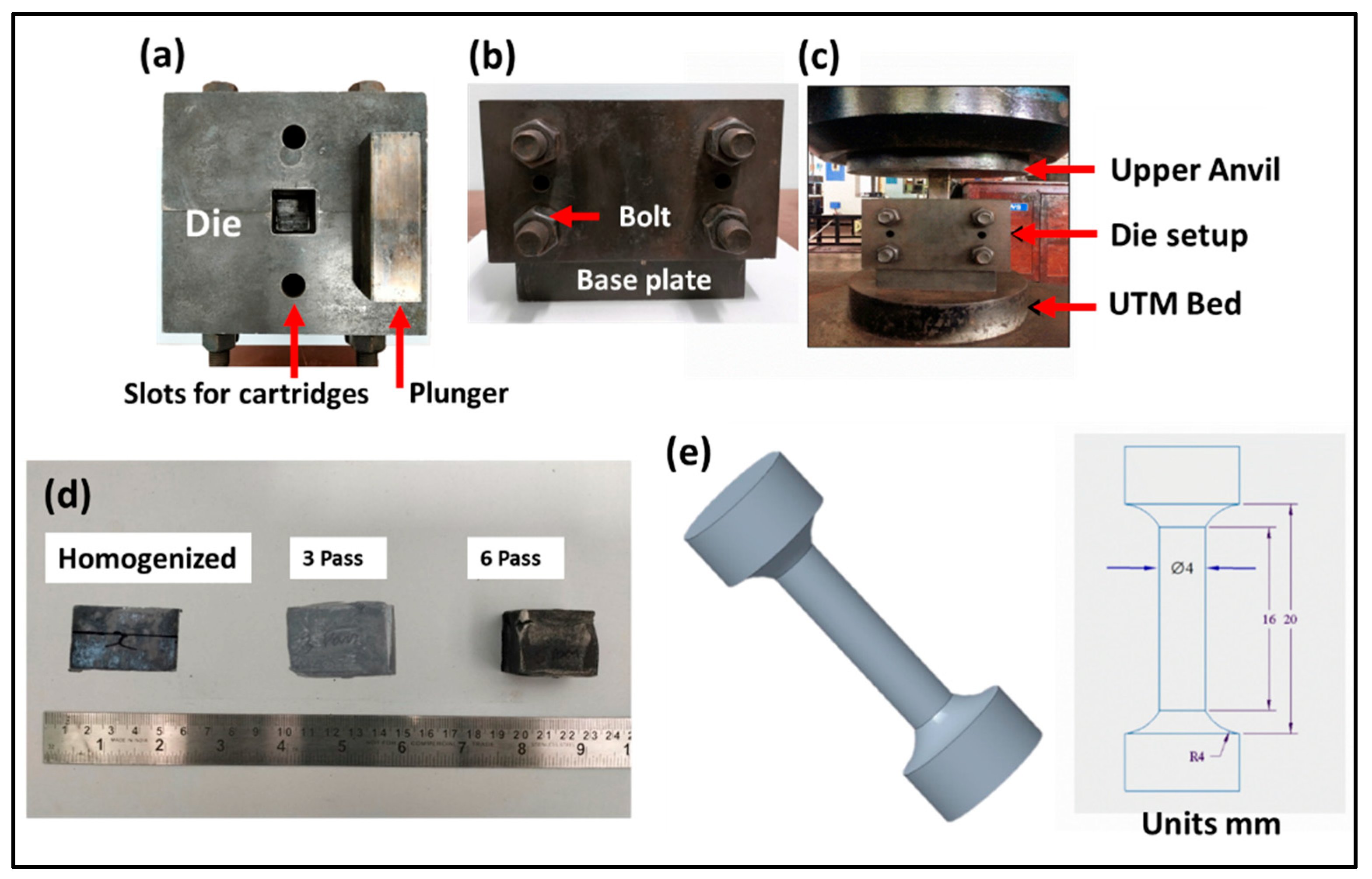
2.1. Multi-Directional Forging
2.2. Microstructural Characterization
2.3. Mechanical Properties
2.4. Electrochemical Corrosion Testing
3. Results and Discussion
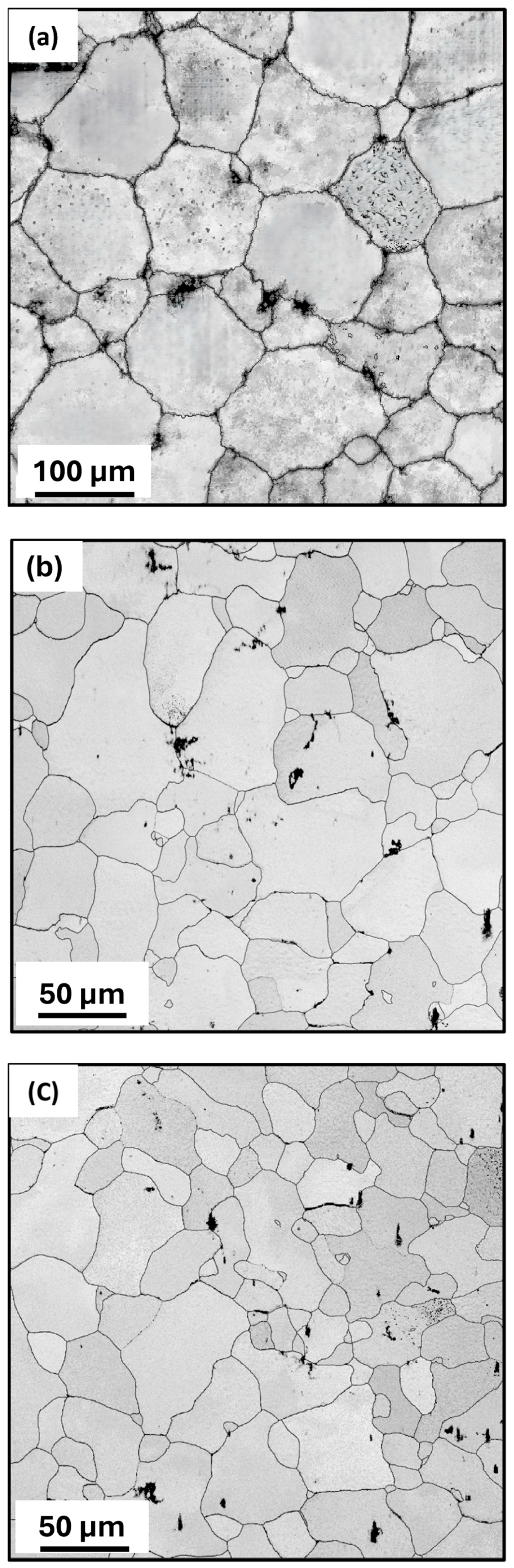
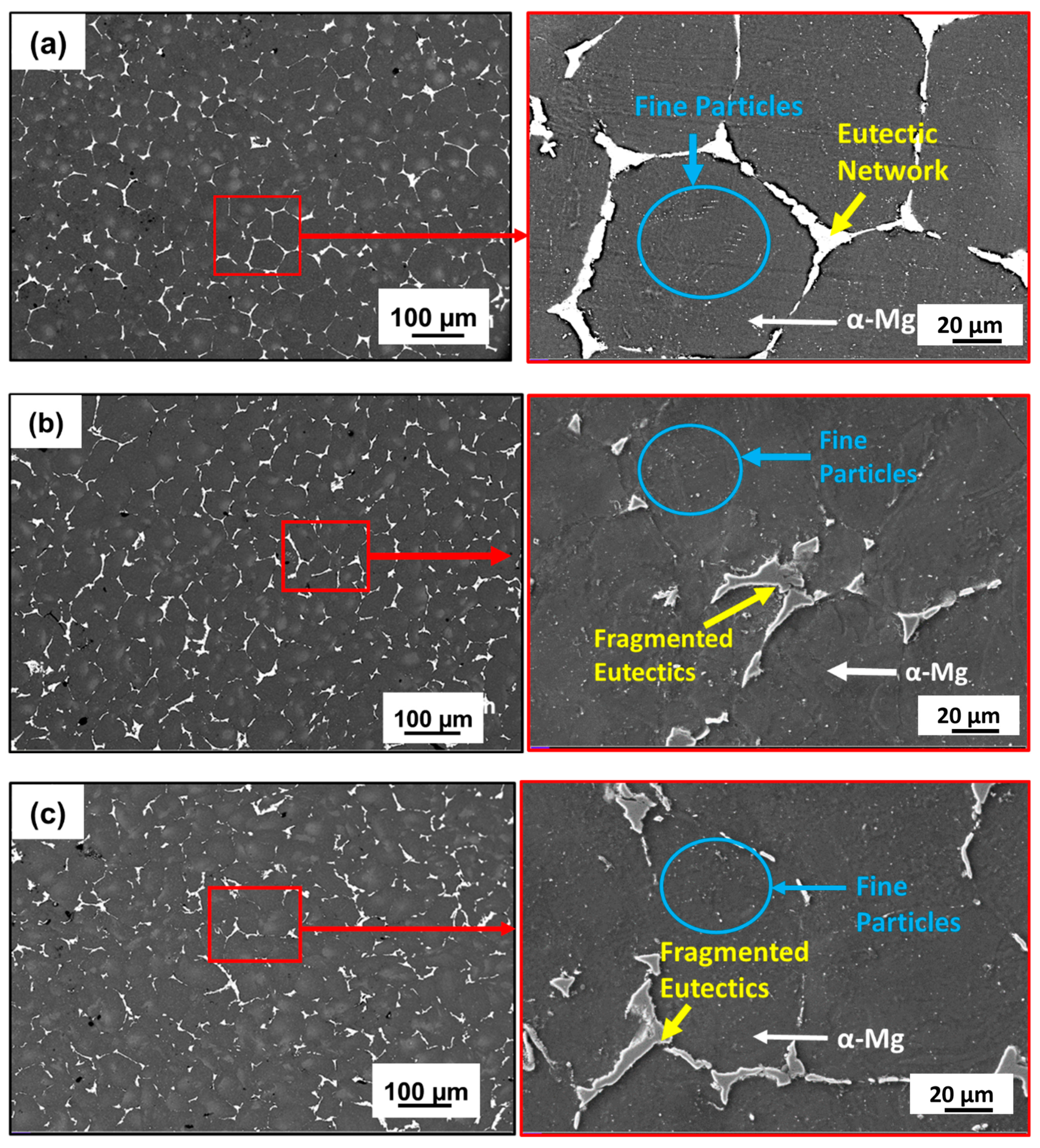
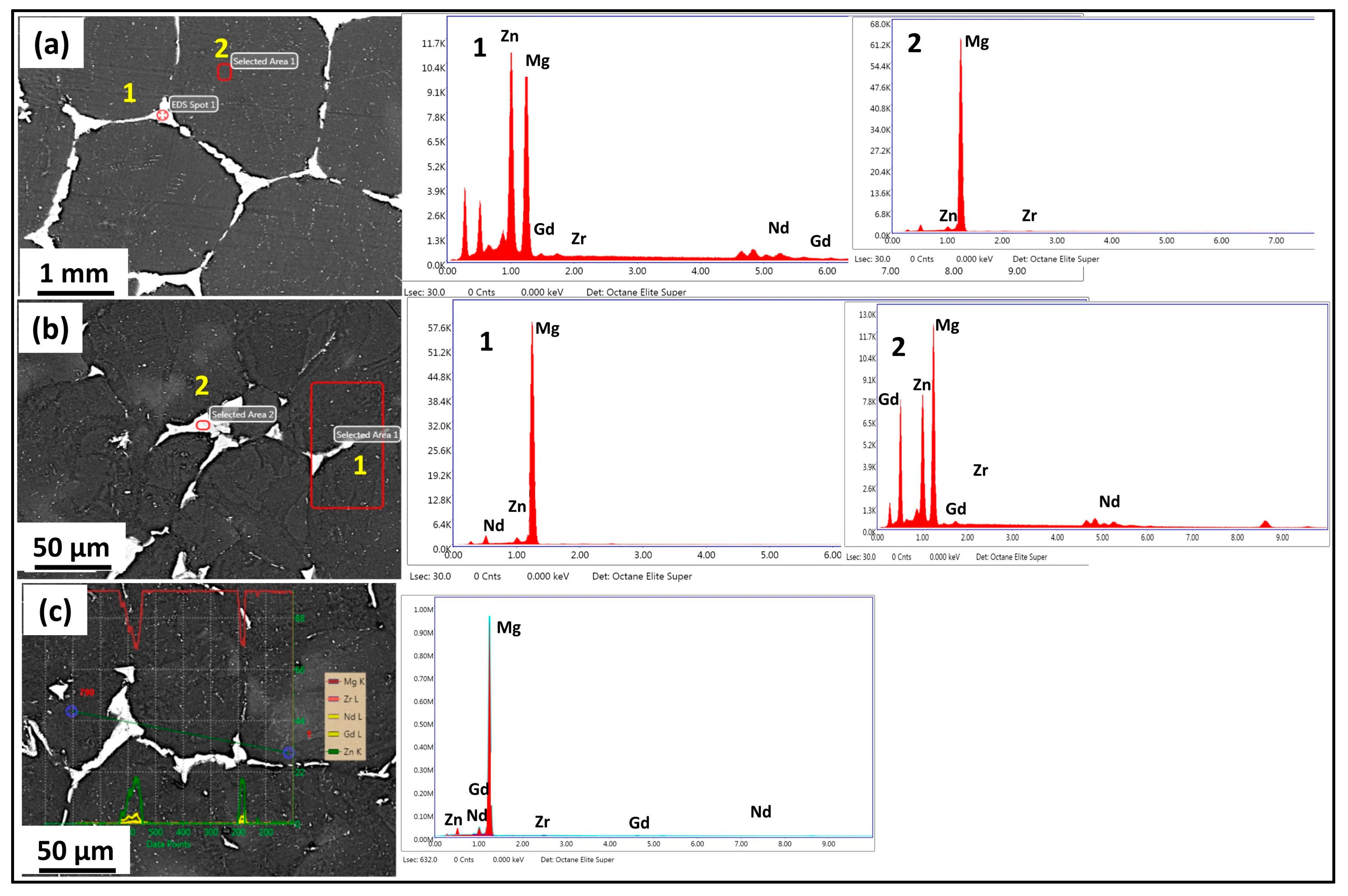
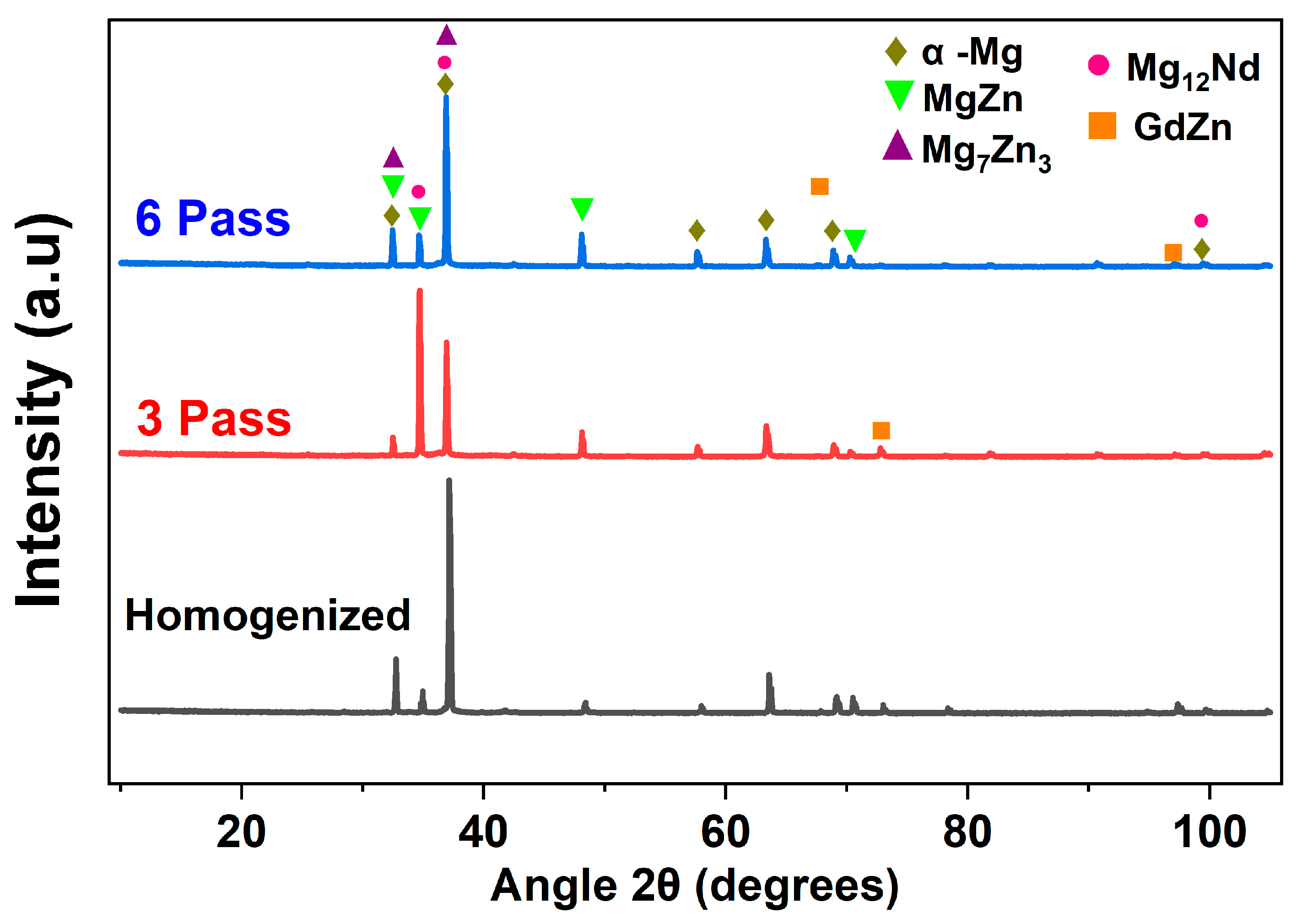
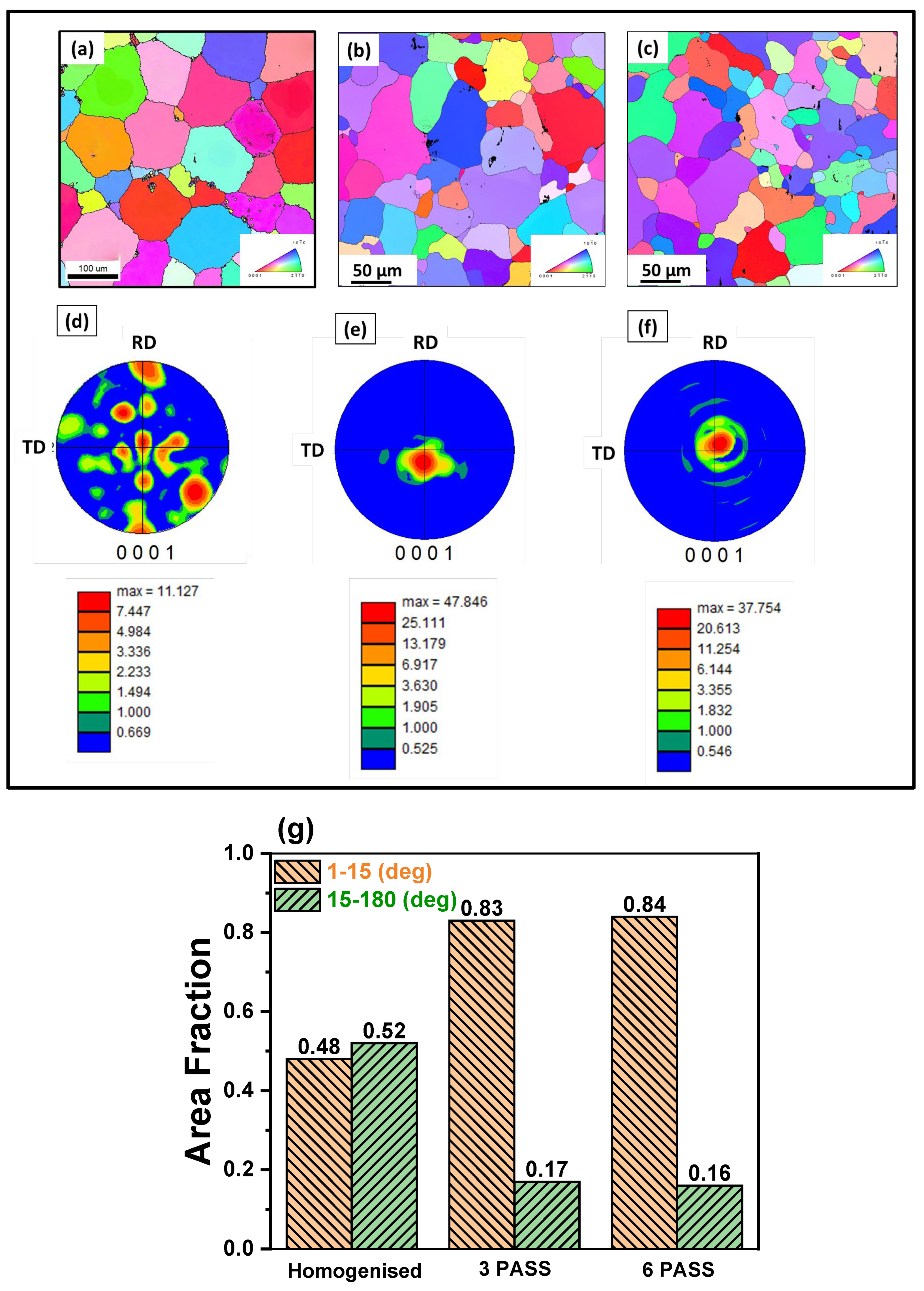
3.1. Microstructure and Phase Evolution of the MDF Samples
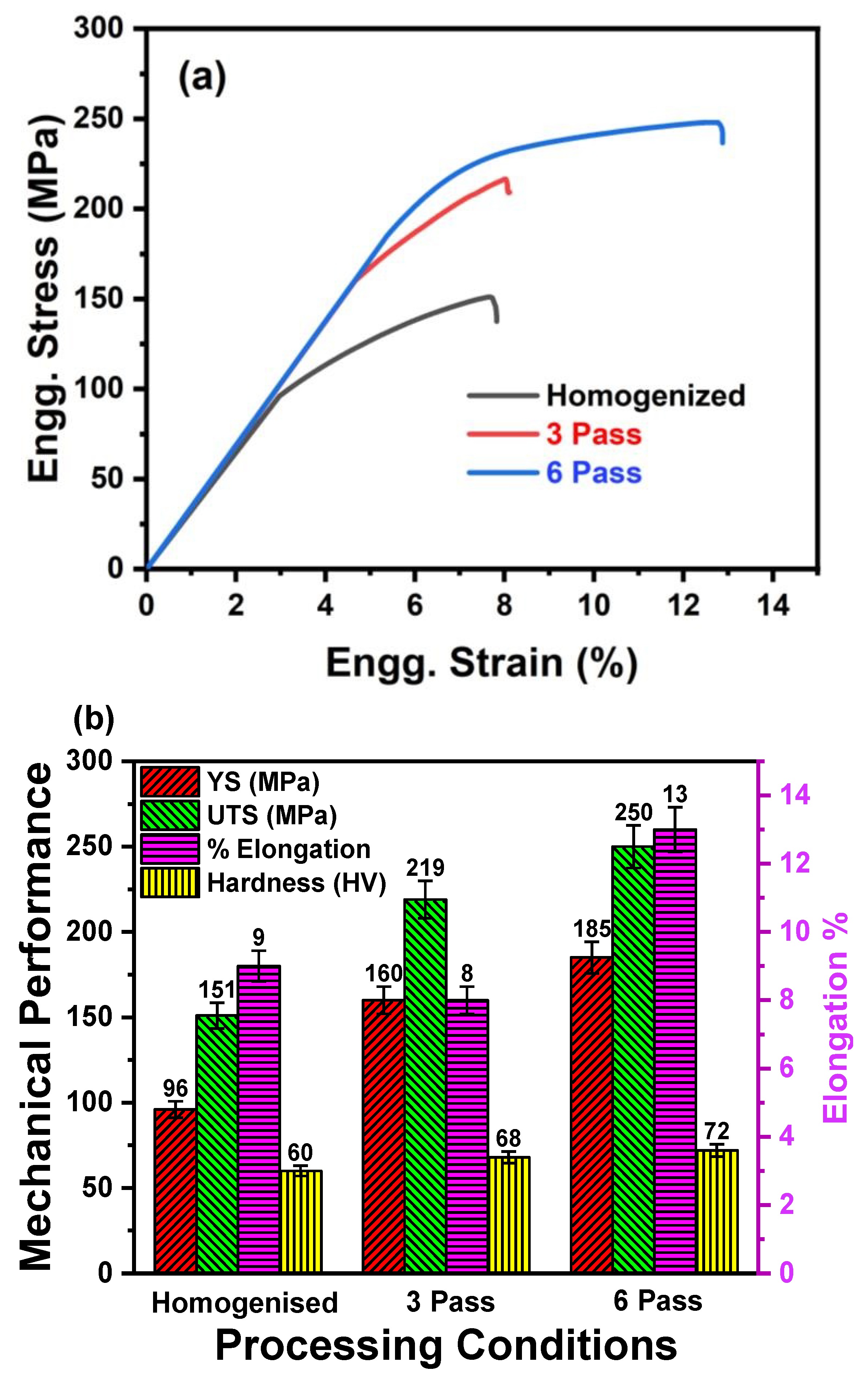
3.2. Mechanical Response
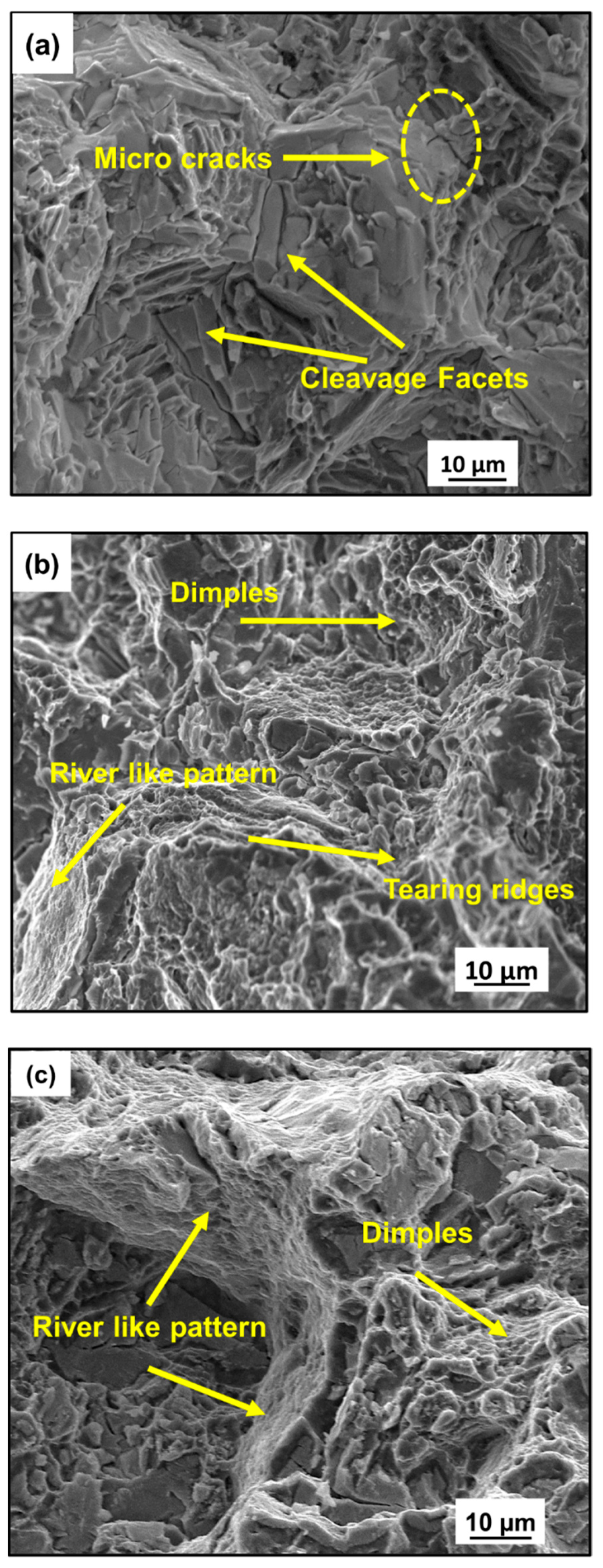
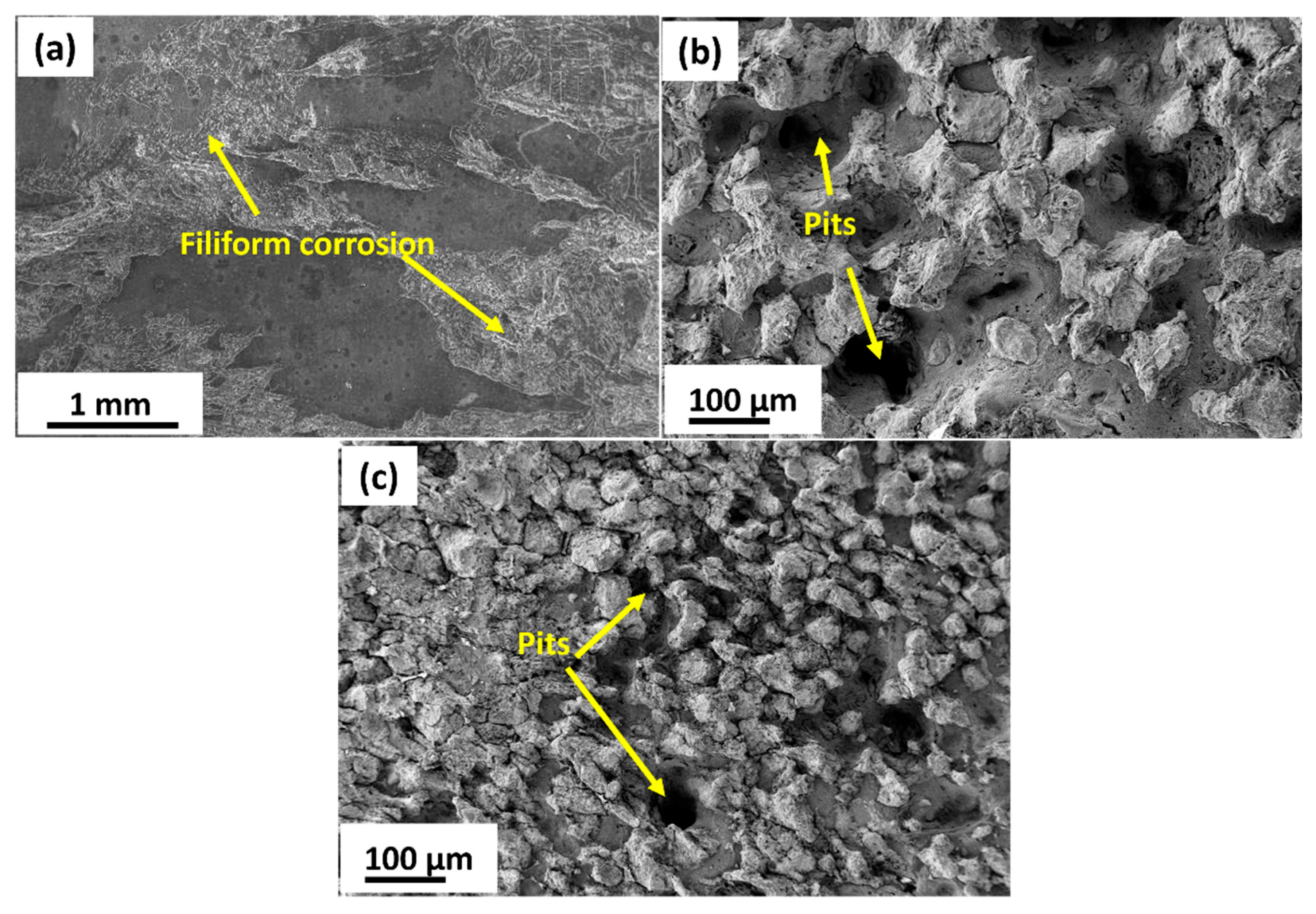
3.2.1. Tensile Behavior and Fracture Analysis
3.2.2. Microhardness Evolution
3.3. Electrochemical Corrosion Behavior
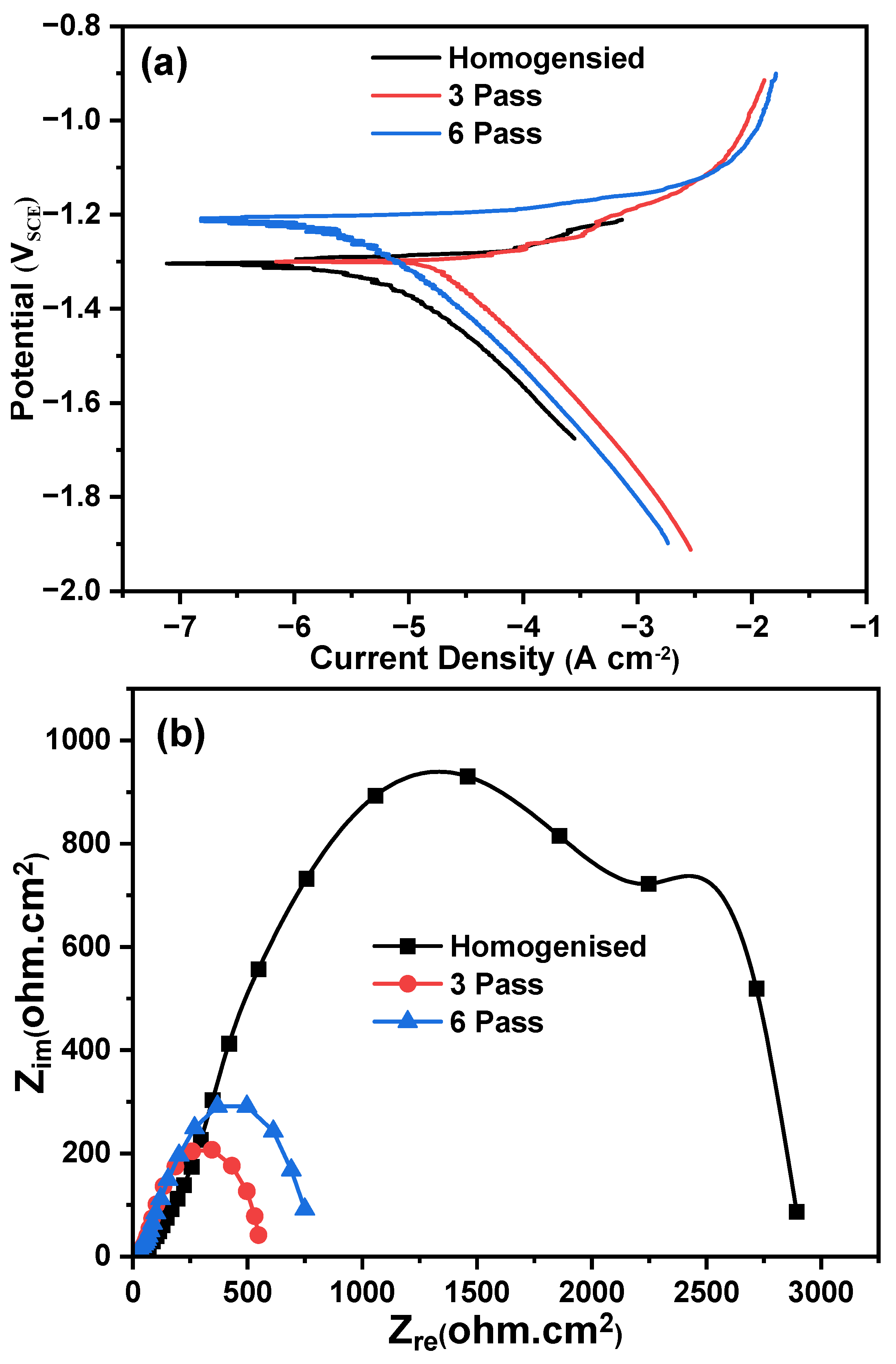
3.3.1. Potentiodynamic Polarization (PDP) Studies
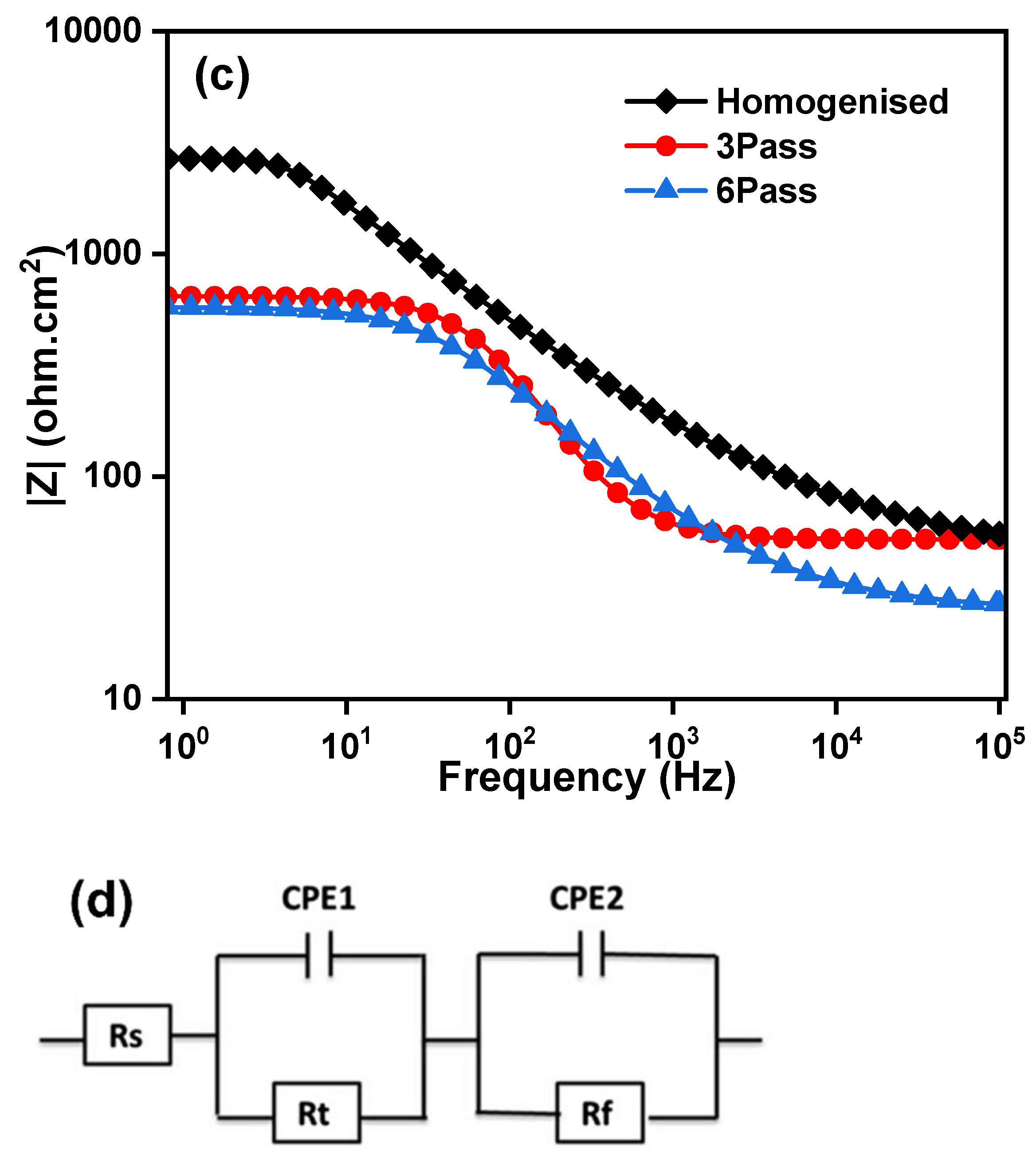
3.3.2. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, L.; Zhao, S.; Li, Y.; Xu, J.; Yan, W.; Guo, B.; Xu, J.; Jiang, L.; Zhang, Y.; Wei, H.; et al. Engineered MgO nanoparticles for cartilage-bone synergistic therapy. Sci. Adv. 2024, 10, eadk6084. [Google Scholar] [CrossRef]
- Zan, R.; Shen, S.; Huang, Y.; Yu, H.; Liu, Y.; Yang, S. Smart Materials in Medicine Research hotspots and trends of biodegradable magnesium and its alloys. Smart Mater. Med. 2023, 4, 468–479. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.B.; Molnar, B.; et al. Biodegradable magnesium fixation screw for barrier membranes used in guided bone regeneration. Bioact. Mater. 2022, 14, 15–30. [Google Scholar] [CrossRef]
- Wei, H.; Wee, D.; Toong, Y.; Chen, J.; Ng, K.; Ow, V.; Lu, S.; Poh, L.; En, P.; Wong, H.; et al. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef]
- Kumar, K.; Gill, R.S.; Batra, U. Challenges and opportunities for biodegradable magnesium alloy implants. Mater. Technol. 2018, 33, 153–172. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Mehdizade, M.; Eivani, A.R.; Esmaielzadeh, O.; Tabatabaei, F. Fabrication of osteogenesis induced WE43 Mg-Hydroxyapatite composites with low biodegradability and increased biocompatibility for orthopedic implant applications. J. Mater. Res. Technol. 2023, 25, 4277–4298. [Google Scholar] [CrossRef]
- Gu, X.N.; Li, S.S.; Li, X.M.; Fan, Y.B. Magnesium based degradable biomaterials: A review. Front. Mater. Sci. 2014, 8, 200–218. [Google Scholar] [CrossRef]
- Cao, F.; Song, G.L.; Atrens, A. Corrosion and passivation of magnesium alloys. Corros. Sci. 2016, 111, 835–845. [Google Scholar] [CrossRef]
- Atrens, A.; Shi, Z.; Mehreen, S.U.; Johnston, S.; Song, G.; Chen, X.; Pan, F. Review of Mg alloy corrosion rates. J. Magnes. Alloys 2020, 8, 989–998. [Google Scholar] [CrossRef]
- Zhao, M.C.; Zhao, Y.C.; Yin, D.F.; Wang, S.; Shangguan, Y.M.; Liu, C.; Tan, L.L.; Shuai, C.J.; Yang, K.; Atrens, A. Biodegradation Behavior of Coated As-Extruded Mg–Sr Alloy in Simulated Body Fluid. Acta Metall. Sin. Engl. Lett. 2019, 32, 1195–1206. [Google Scholar] [CrossRef]
- Razzaghi, M.; Kasiri-Asgarani, M.; Bakhsheshi-Rad, H.R.; Ghayour, H. In Vitro Degradation, Antibacterial Activity and Cytotoxicity of Mg-3Zn-xAg Nanocomposites Synthesized by Mechanical Alloying for Implant Applications. J. Mater. Eng. Perform. 2019, 28, 1441–1455. [Google Scholar] [CrossRef]
- Gungor, A.; Incesu, A. Effects of alloying elements and thermomechanical process on the mechanical and corrosion properties of biodegradable Mg alloys. J. Magnes. Alloys 2021, 9, 241–253. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Yoshida, Y.; Okazaki, M.; Shimazu, A.; Kubo, T.; Akagawa, Y.; Uchida, T. Action of FGMgCO3Ap-collagen composite in promoting bone formation. Biomaterials 2003, 24, 4913–4920. [Google Scholar] [CrossRef]
- Rokkala, U.; Jana, A.; Bontha, S.; Ramesh, M.R.; Balla, V.K. Comparative investigation of coating and friction stir processing on mg-Zn-Dy alloy for improving antibacterial, bioactive and corrosion behaviour. Surf. Coat. Technol. 2021, 425, 127708. [Google Scholar] [CrossRef]
- Khan, M.F.; Rokkala, U. Development of high strength and corrosion resistance Mg-Zn-Dy/HA-Ag composite for temporary implant applications. Mater. Lett. 2023, 347, 134604. [Google Scholar] [CrossRef]
- Prithivirajan, S.; Narendranath, S.; Desai, V. Analysing the combined effect of crystallographic orientation and grain refinement on mechanical properties and corrosion behaviour of ECAPed ZE41 Mg alloy. J. Magnes. Alloys 2020, 8, 1128–1143. [Google Scholar] [CrossRef]
- Bahmani, A.; Arthanari, S.; Shin, K.S. Achieving a high corrosion resistant and high strength magnesium alloy using multi directional forging. J. Alloys Compd. 2021, 856, 158077. [Google Scholar] [CrossRef]
- Kumar, A.; MD, F.K.; Panigrahi, S.K.; Chaudhari, G.P. Microstructural evolution and corrosion behaviour of friction stir-processed QE22 magnesium alloy. Corros. Rev. 2021, 39, 351–360. [Google Scholar] [CrossRef]
- Sun, L.; Li, F.; Zhang, J.Y.; Niu, W.T.; Cao, M.Z. Mechanism of work hardening and softening behavior of AZ31 magnesium alloy sheets with hard plate accumulative roll bonding. J. Magnes. Alloys 2024, 13, 3430–3449. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Wang, Q.; Attarilar, S. A comprehensive review of magnesium-based alloys and composites processed by cyclic extrusion compression and the related techniques. Prog. Mater. Sci. 2023, 131, 101016. [Google Scholar] [CrossRef]
- Miura, H.; Yang, X.; Sakai, T. Evolution of Ultra-Fine Grains in AZ31 and AZ61 Mg Alloys during Multi Directional Forging and Their Properties Temperature/K (b) Single pass. Mater. Trans. 2008, 49, 1015–1020. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, L.; Zheng, M.; Wei, Q.; Shan, D.; Guo, B. Achievement of high strength and good ductility in the large-size AZ80 Mg alloy using a designed multi-directional forging process and aging treatment. J. Mater. Process. Technol. 2023, 311, 117828. [Google Scholar] [CrossRef]
- Anne, G.; Sampath, R.; Kumar, G. Development, Characterization, Mechanical and Corrosion Behaviour Investigation of Multi-direction Forged Mg–Zn Alloy. In Magnesium Technology 2019; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Ramesh, S.; Anne, G.; Nayaka, H.S.; Sahu, S.; Ramesh, M.R. Influence of Multidirectional Forging on Microstructural, Mechanical, and Corrosion Behavior of Mg-Zn Alloy. J. Mater. Eng. Perform. 2019, 28, 2053–2062. [Google Scholar] [CrossRef]
- Cao, F.; Deng, K.; Nie, K.; Kang, J.; Niu, H. Microstructure and corrosion properties of Mg-4Zn-2Gd-0.5Ca alloy influenced by multidirectional forging. J. Alloys Compd. 2019, 770, 1208–1220. [Google Scholar] [CrossRef]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drücker, H.; Vogt, C.; Hort, N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef]
- Kottuparambil, R.R.; Bontha, S.; Rangarasaiah, R.M.; Arya, S.B.; Jana, A.; Das, M.; Balla, V.K.; Amrithalingam, S.; Prabhu, T.R. Effect of zinc and rare-earth element addition on mechanical, corrosion, and biological properties of magnesium. J. Mater. Res. 2018, 33, 3466–3478. [Google Scholar] [CrossRef]
- Myrissa, A.; Braeuer, S.; Martinelli, E.; Willumeit-Römer, R.; Goessler, W.; Weinberg, A.M. Gadolinium accumulation in organs of Sprague–Dawley® rats after implantation of a biodegradable magnesium-gadolinium alloy. Acta Biomater. 2017, 48, 521–529. [Google Scholar] [CrossRef]
- Zhao, M.-C.; Liu, M.; Song, G.-L.; Atrens, A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41. Corros. Sci. 2008, 50, 3168–3178. [Google Scholar] [CrossRef]
- Li, J.; Han, Q.; Han, X.; Yi, X. Strength—Ductility synergy in Mg-Gd-Y-Zr alloys via texture engineering in bi-directional forging. J. Magnes. Alloys 2024, 12, 4709–4721. [Google Scholar] [CrossRef]
- Wang, R.; Yan, F.; Sun, J.; Xing, W.; Li, S. Microstructural Evolution and Mechanical Properties of Extruded AZ80 Magnesium Alloy during Room Temperature Multidirectional Forging Based on Twin Deformation Mode. Materials 2024, 17, 5055. [Google Scholar] [CrossRef]
- Mohammed, S.M.A.K.; Nisar, A.; John, D.; Sukumaran, A.K.; Fu, Y.; Paul, T.; Hernandez, A.; Seal, S.; Agarwal, A. Boron nitride nanotubes induced strengthening in aluminum 7075 composite via cryomilling and spark plasma sintering. Adv. Compos. Hybrid Mater. 2025, 8, 155. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Z.; Wang, Z.; Zhao, S.; Wang, K.; Li, D.; Wang, X. Microstructural optimization and strengthening mechanisms of in-situ TiB2/Al–Cu composite after multidirectional forging for six passes. Int. J. Miner. Metall. Mater. 2025, 32, 1703–1718. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, Z.; Zhou, L. Microstructure evolution and mechanical properties of Mg-Gd-Nd-Zn-Zr alloy processed by equal channel angular pressing. Mater. Sci. Eng. A 2015, 647, 184–190. [Google Scholar] [CrossRef]
- Khan, F.; Panigrahi, S.K. Achieving excellent superplasticity in an ultrafine-grained QE22 alloy at both high strain rate and low-temperature regimes. J. Alloys Compd. 2018, 747, 71–82. [Google Scholar] [CrossRef]
- Rokkala, U.; Bontha, S.; Ramesh, M.R.; Balla, V.K.; Srinivasan, A.; Kailas, S.V. Tailoring surface characteristics of bioabsorbable Mg-Zn-Dy alloy using friction stir processing for improved wettability and degradation behavior. J. Mater. Res. Technol. 2021, 12, 1530–1542. [Google Scholar] [CrossRef]
- MD, F.K.; Karthik, G.M.; Panigrahi, S.K.; Janaki Ram, G.D. Friction stir processing of QE22 magnesium alloy to achieve ultrafine-grained microstructure with enhanced room temperature ductility and texture weakening. Mater. Charact. 2019, 147, 365–378. [Google Scholar] [CrossRef]
- Xin, R.; Zheng, X.; Liu, Z.; Liu, D.; Qiu, R.; Li, Z.; Liu, Q. Microstructure and texture evolution of an Mg–Gd–Y–Nd–Zr alloy during friction stir processing. J. Alloys Compd. 2016, 659, 51–59. [Google Scholar] [CrossRef]
- Rokkala, U.; Bontha, S. Influence of friction stir processing on microstructure, mechanical properties and corrosion behaviour of Mg-Zn-Dy alloy. J. Mater. Sci. 2023, 58, 2893–2914. [Google Scholar] [CrossRef]
- Dong, B.; Che, X.; Zhang, Z.; Yu, J.; Meng, M. Microstructure evolution and microhardness of Mg-13Gd-4Y-2Zn-0.5Zr alloy via pre-solution and multi-directional forging (MDF) process. J. Alloys Compd. 2021, 853, 157066. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Li, S.; Yu, J. Strengthening mechanism based on dislocation-twin interaction under room temperature multi-directional forging of AZ80 Mg alloy. J. Mater. Res. Technol. 2024, 29, 3656–3672. [Google Scholar] [CrossRef]
- Wei, Q.; Yuan, L.; Ma, X.; Zheng, M.; Shan, D.; Guo, B. Strengthening of low-cost rare earth magnesium alloy Mg-7Gd-2Y–1Zn-0.5Zr through multi-directional forging. Mater. Sci. Eng. A 2022, 831, 142144. [Google Scholar] [CrossRef]
- Wei, Q.; Yuan, L.; Shan, D.; Guo, B. Study on the microstructure and mechanical properties of ZK60 magnesium alloy with submicron twins and precipitates obtained by room temperature multi-directional forging. J. Mater. Sci. 2023, 58, 13236–13250. [Google Scholar] [CrossRef]
- Khaleghi, A.A.; Salevati, M.A.; Sabbaghian, M.; Fekete-Horváth, K.; Drozdenko, D.; Máthis, K.; Akbaripanah, F. Comparing the microstructural and mechanical improvements of AZ80/SiC nanocomposite using DECLE and MDF processes. Mater. Sci. Eng. A 2024, 892, 146020. [Google Scholar] [CrossRef]
- Li, J.; He, Z.; Fu, P.; Wu, Y.; Peng, L.; Ding, W. Heat treatment and mechanical properties of a high-strength cast Mg-Gd-Zn alloy. Mater. Sci. Eng. A 2016, 651, 745–752. [Google Scholar] [CrossRef]
- Mohammed, S.M.A.K.; Chen, D.L.; Liu, Z.Y.; Ni, D.R.; Wang, Q.Z.; Xiao, B.L.; Ma, Z.Y. Deformation behavior and strengthening mechanisms in a CNT-reinforced bimodal-grained aluminum matrix nanocomposite. Mater. Sci. Eng. A 2021, 817, 141370. [Google Scholar] [CrossRef]
- Praveen, T.R.; Shivananda Nayaka, H.; Swaroop, S.; Gopi, K.R. Strength enhancement of magnesium alloy through equal channel angular pressing and laser shock peening. Appl. Surf. Sci. 2020, 512, 145755. [Google Scholar] [CrossRef]
- Song, G. The Effect of Texture on the Corrosion Behavior of AZ31 Mg Alloy. JOM 2012, 64, 671–679. [Google Scholar] [CrossRef]
- Xin, R.; Luo, Y.; Zuo, A.; Gao, J.; Liu, Q. Texture effect on corrosion behavior of AZ31 Mg alloy in simulated physiological environment. Mater. Lett. 2012, 72, 1–4. [Google Scholar] [CrossRef]
- Maric, M.; Muránsky, O.; Karatchevtseva, I.; Ungár, T.; Hester, J.; Studer, A.; Scales, N.; Ribárik, G.; Primig, S.; Hill, M.R. The effect of cold-rolling on the microstructure and corrosion behaviour of 316L alloy in FLiNaK molten salt. Corros. Sci. 2018, 142, 133–144. [Google Scholar] [CrossRef]
- Cui, Q.; Yi, D.; Wang, H.; Zhang, J.; Xu, J.; Wang, B. Effects of grain size and secondary phase on corrosion behavior and electrochemical performance of Mg-3Al-5Pb-1Ga-Y sacrificial anode. J. Rare Earths 2019, 37, 1341–1350. [Google Scholar] [CrossRef]
- Bahmani, A.; Lotfpour, M.; Taghizadeh, M.; Kim, W. Corrosion behavior of severely plastically deformed Mg and Mg alloys. J. Magnes. Alloys 2022, 10, 2607–2648. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Li, J. Microstructure, mechanical properties, corrosion behavior and film formation mechanism of Mg-Zn-Mn-xNd in Kokubo’s solution. J. Alloys Compd. 2018, 730, 458–470. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Li, Y.; Luo, D.-M.; Ding, Y.; Hodgson, P. Microstructures, mechanical and corrosion properties and biocompatibility of as extruded Mg–Mn–Zn–Nd alloys for biomedical applications. Mater. Sci. Eng. C 2015, 49, 93–100. [Google Scholar] [CrossRef]
- Jana, A.; Balla, V.K.; Das, M. In-vitro corrosion and biocompatibility properties of heat treated Mg-4Y-2.25Nd-0.5Zr alloy. Mater. Chem. Phys. 2023, 304, 127873. [Google Scholar] [CrossRef]
- Patel, V.; Li, W.; Andersson, J.; Li, N. Enhancing grain refinement and corrosion behavior in AZ31B magnesium alloy via stationary shoulder friction stir processing. J. Mater. Res. Technol. 2022, 17, 3150–3156. [Google Scholar] [CrossRef]
| Elements | Zn | Nd | Gd | Zr | Mg |
|---|---|---|---|---|---|
| Composition (wt. %) | 3.07 | 1.70 | 1.40 | 0.95 | Balance |
| Sample Condition | Icorr (mA/cm2) | Ecorr (V) | βa | βc | CR (mm/yr) |
|---|---|---|---|---|---|
| Homogenized | 0.0051 | −1.2799 | 0.0071 | −0.1987 | 0.1165 |
| 3 Pass | 0.0199 | −1.2967 | 0.0285 | −0.2557 | 0.4560 |
| 6 Pass | 0.0109 | −1.1999 | 0.0044 | −0.2606 | 0.2499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, F.K.; Rokkala, U.; Mohammed, S.M.A.K.; Altammar, H.; Moinuddin, S.Q.; Mohammed, R. Strength–Ductility Synergy in Biodegradable Mg-Rare Earth Alloy Processed via Multi-Directional Forging. J. Funct. Biomater. 2025, 16, 391. https://doi.org/10.3390/jfb16100391
Mohammad FK, Rokkala U, Mohammed SMAK, Altammar H, Moinuddin SQ, Mohammed R. Strength–Ductility Synergy in Biodegradable Mg-Rare Earth Alloy Processed via Multi-Directional Forging. Journal of Functional Biomaterials. 2025; 16(10):391. https://doi.org/10.3390/jfb16100391
Chicago/Turabian StyleMohammad, Faseeulla Khan, Uzwalkiran Rokkala, Sohail M. A. K. Mohammed, Hussain Altammar, Syed Quadir Moinuddin, and Raffi Mohammed. 2025. "Strength–Ductility Synergy in Biodegradable Mg-Rare Earth Alloy Processed via Multi-Directional Forging" Journal of Functional Biomaterials 16, no. 10: 391. https://doi.org/10.3390/jfb16100391
APA StyleMohammad, F. K., Rokkala, U., Mohammed, S. M. A. K., Altammar, H., Moinuddin, S. Q., & Mohammed, R. (2025). Strength–Ductility Synergy in Biodegradable Mg-Rare Earth Alloy Processed via Multi-Directional Forging. Journal of Functional Biomaterials, 16(10), 391. https://doi.org/10.3390/jfb16100391










