Abstract
Background: Marine ecosystems, covering 70% of Earth’s surface, hold immense biodiversity and potential for biomaterials. Cuttlefish bone (CB) and marine resources have gained attention as eco-friendly biomaterials. Objectives: We aim to comprehensively study biomedical applications of CB-derived materials. By evaluating both in vivo and in vitro investigations, the review seeks to uncover the diverse potential of CB in the biomedical field. Methods: A comprehensive search of electronic databases yielded 51 articles from 2408 studies. These studies encompassed in vivo animal studies and in vitro investigations. Results: In vivo studies employed for bone repair, dorsal subcutaneous defects, thermal wound healing, muscle injections, and avian blood testing. In vitro studies focused on HAp synthesis, scaffold development, dental material enhancement, and antimicrobial properties. Risk of bias assessments revealed varying degrees of methodological quality in both animal and in vitro studies, underscoring the need for standardised reporting and rigorous study design in future research. Conclusions: This review fills a gap in the literature by providing a comprehensive overview of the applications of CB-derived materials in the biomedical field. Additionally, it offers valuable insights for researchers, clinicians, and policymakers interested in sustainable and effective biomaterials for diverse medical purposes, advancing the fields of regenerative medicine and dentistry.
1. Introduction
The diversity of marine life is enormous, with an estimated 200,000 species already known and countless yet-undiscovered wonders occupying more than 70% of our planet’s surface [1]. This aqueous expanse lies a treasure trove of materials, particularly calcium carbonate polymorphs that are intertwined with various marine structures. Marine-derived ceramic materials offer several benefits, including non-toxicity and higher availability, sparking interest among specialists in various biomedical applications [2]. These marine-derived biomaterials possess distinctive attributes, including low electrical and thermal conductivity, minimal toxicity, exceptional biocompatibility, biodegradability, and remarkable resistance to compression. Their surface chemistry and unique bone-bonding properties position them as ideal candidates for applications in bone substitution engineering and medicine [2]. Marine sources’ distinctive shape and bioactive components enable their usage in tissue engineering and drug delivery systems, allowing biomimetics and intelligent biomaterials tailored for personalised medicine [3].
Utilising marine byproducts for biomaterials reduces reliance on synthetic alternatives, lowers costs, and mitigates environmental harm, promoting ecosystem health. This approach also prevents environmental contamination by reducing biomass waste accumulation [4]. Additionally, nanoparticles like silver, titanium, and gold derived from marine sources have various applications in pharmaceuticals, environmental management, cosmetics, and drug delivery [5]. Moreover, the synthesis of these materials often aligns with green synthesis approaches, which prioritise environmentally friendly and sustainable methods [6]. By adopting green synthesis techniques, the development of marine-derived biomaterials can be both innovative and ecologically responsible.
Recent advances in the development of green and marine biomaterials underscore the significant scientific interest in utilising marine-derived matrices and mineral powders for biomedical applications. For instance, Gandolfi et al. [7] developed innovative green hydrogels composed of sodium mannuronate/guluronate, gelatin, and biointeractive calcium silicates/dicalcium phosphate dihydrate specifically designed for the regeneration of oral bone defects through their bioactive properties. Similarly, Yan et al. [8] created an injectable alginate/hydroxyapatite gel scaffold combined with gelatin microspheres, demonstrating its effectiveness for bone tissue engineering applications. This composite material leverages the osteoconductive properties of hydroxyapatite and the biocompatibility of alginate to enhance osteoblast activity and in turn bone tissue regeneration. These examples illustrate the growing trend and promising future of marine-derived biomaterials in advancing regenerative medicine and tissue engineering.
Delving into the marine sources, we encounter a rich mosaic of organisms like coral, mussels, oyster seashells, crab shells, and sponges [9,10,11,12,13,14,15]. Among these, corals, marine invertebrates adorned with calcium carbonate (CaCO3) skeletons have earned notable attention since the 1970s. Their commercial production of HAp for bone graft materials, underpinned by porous structures and intricate pore interconnections, showcases excellent mechanical bonding and biocompatibility, signifying promise in bone substitution engineering and medicine [16]. The conversion of coral to coralline HAp still keeps its unique microstructure. This biomaterial has osteoconductivity, bioactivity, and biocompatibility, allowing for direct bone-to-biomaterial coupling and gradual host-bone replacement following implantation [17]. Nevertheless, this technology’s substantial drawback lies in its impact on coral reefs, vital bastions for marine life and biodiversity conservation [18].
Cuttlefish bone (Sepia officinalis), alternatively known as cuttlebone (CB), has emerged as a compelling source of bioceramics characterised by chemistry and crystallography akin to corals [19]. A comprehensive exploration of marine habitats has unveiled CB’s vast potential, particularly in biomedical applications. CB’s significance lies in its composition of natural aragonite, a crystallised form of CaCO3 readily convertible to HAp using simple methods [20].
This marine resource boasts global accessibility, affordability, interconnected pore structures, and adaptable sizing properties, rendering it well suited for supporting diverse physiological processes. Furthermore, cuttlefish bone (CB) exhibits exceptional machinability, facilitating rapid customisation to meet specific requirements. It demonstrates robust in vitro bioactivity and boasts high biocompatibility, as corroborated by osteoblast tests. Additionally, AB-type carbonated hydroxyapatite, which closely mirrors the composition of human bones, can be obtained from CB after specific treatments [21]. However, its application in bone tissue engineering faces limitations due to CB’s inherent brittleness and low strength. These challenges can be mitigated by incorporating varying concentrations of polymers into the scaffold [22].
CB’s captivating structure further piques scientific curiosity. Comprising a fragile ventral (interior) shell and a robust dorsal shield, CB features an interconnected chamber-like architecture with high porosity, contributing to the cuttlefish’s buoyancy at various depths during underwater sojourns [15,23]. These microchambers within CB exhibit size variations linked to their specific locations within the structure. Horizontal striations adorn the arches, giving them a parallel, wave-like appearance. These arches account for CB’s high porosity (approximately 93 percent porosity), encompassing pores ranging from 200 to 600 µm, which regulate CB’s water content [24].
CB, a naturally occurring substance, showcases multifaceted attributes, including high porosity, remarkable flexural stiffness, and compressive strength, exemplifying nature’s prowess in designing optimised cellular structures [25]. Its intricately organised interior shell, composed of aragonite polymorph fused with a small amount (3 to 4.5 wt%) of organic matter, primarily β-chitin and protein, enhances CB’s utility in templating inorganic growth [26]. The crux of regenerative medicine lies in restoring healthy biological processes, spanning from cellular to tissue-level regeneration, culminating in repairing or replacing damaged or diseased cells, tissues, and organs. Notably, HAp, alpha-tricalcium phosphate (α-TCP), and beta-tricalcium phosphate (β-TCP) play pivotal roles in bone grafting for bone tissue regeneration and repair. Crafting 3D HAp-based scaffolds was a premier technique for producing bone graft materials [27]. The versatility of CB-derived HAp is a beacon in regenerative medicine, enabling the creation of composite materials that mimic the natural properties of bone. These composite materials have yielded superior bone growth, repair, and regeneration [1].
The superior cellular structure, biological nature, and mechanical behaviour of CB, along with its CaCO3 (aragonite) composition, make it highly suitable for HAp and scaffold synthesis. Unlike nacre and coral, which collapse after hydrothermal transformation due to high temperature and pressure, CB maintains its stability [28]. Additionally, the presence of chitin, which is biocompatible, biodegradable, hemostatic, and antimicrobial, enhances its suitability for biomedical applications. The essential properties of cuttlefish-based biocomposites, such as high porosity, optimal modulus of elasticity, biocompatibility, strength, and non-toxicity, further contribute to its advantages over other marine-based bio-ceramics like corals, sea sponges, sea urchins, crab shells, and oyster shells, particularly in bone tissue engineering and repairing bone defects with its excellent mechanical strength [15,29]. Table 1 summarises the key differences and similarities between CB and other marine biomaterials.

Table 1.
Summarising the key differences and similarities between cuttlefish bone and other marine sources.
Remarkably, despite the promise of CB in biomedical fields, a systematic review to comprehensively explore its applications in these disciplines remains conspicuously absent. This systematic review embarks on a journey to illuminate the landscape of in vitro and in vivo studies investigating the biological applications of CB in tissue engineering, regenerative medicine, and dentistry. By shedding light on this uncharted territory, this paper aims to comprehensively explore the published literature about the potential biological application of cuttlefish bone-derived materials, expanding the knowledge about these promising biomaterials and opening new horizons for future medical and dental research endeavours.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered with the Open Science Framework Database (https://osf.io) on 15 January 2023 and possesses a registration DOI of https://doi.org/10.17605/OSF.IO/2VA8W.
2.2. Research Question and Search Strategy
The research question guiding this review is “Is the use of cuttlefish bone biomaterials effective in biomedical applications?”. To compile relevant studies, an extensive search strategy was implemented across three prominent electronic databases: Scopus, PubMed, and Web of Science. The search query employed a comprehensive array of keywords with interchangeable usage, alternate spellings, synonymous expressions, and MeSH terms. The search strategy was integrated with the use of Boolean operators (i.e., “OR” and “AND”). The keywords, which closely aligned with the objective of our study, included the following: (“cuttlebone” OR “cuttlebones” OR “sepia officinalis” OR “cuttlebone powder” OR “cuttlebone hydroxyapatite” OR “cuttlebone hydroxyapatites” OR “calcium carbonate” OR “aragonite” OR “cuttlebone aragonite” OR “CaCO3”) AND (“tooth bioengineering” OR “bone tissue engineering” OR “hard tissue treatment” OR “bone graft” OR “scaffold” OR” scaffolds” OR “bone scaffold” OR “bone tissue engineering scaffolds” OR “natural biomaterial” OR “marine biomaterial” OR “marine biomaterials” OR “bio-ceramic materials” OR “bioceramic materials” OR “bio-ceramic scaffolds” OR “bioceramic scaffolds” OR “biomedical application” OR “biomedical uses” OR “regenerative medicine” OR “regenerative dentistry”).
This process commenced on 16 January 2023 and was updated on 17 March 2024, encompassing studies published from 2000 to 2024. While research on this topic dates back to the 1970s, significant advancements in HAp scaffold preparation began with Rocha, Lemos [37]. Therefore, this date range captures the most crucial period for HAp scaffold synthesis using CB.
2.3. Data Processing
After conducting a database search to extrapolate the findings, two reviewers, R.A.A. and H.M.A., independently assessed the studies based on selection criteria that fit the purpose of the review, The selected studies were downloaded in the EndNote software (Version 21 (Bld 17096), Clarivate, London, UK). Four rounds of screening and filtering were performed to evaluate the selection process. First, duplicates were removed. Second, the search results were appraised for relevance based on their titles. Third, unrelated articles were removed after screening the abstracts. Finally, the full texts were read and analysed.
2.4. Inclusion and Exclusion Criteria
Predefined inclusion and exclusion criteria guided the final selection of studies for in-depth examination. As for the type of publication, only research studies were included. All conference papers, abstracts, pilot studies, reviews, communications, letters to the editor, and editorials were excluded. Studies published in languages other than English were also excluded to ensure relevance and consistency. As for the study relevance, only studies (in vivo and in vitro) related to biomedical applications of CB were included. Studies dealing with the fabrication or synthesis of a wide variety of materials, material compositions, and applications other than biological ones were excluded. Considering the materials of interest, studies on tricalcium phosphate, eggshells, chitosan, bioglass, marine sources other than CB, and industrial applications of CB were also excluded. The eligibility criteria were then applied, excluding studies that focused solely on physical, mechanical, and chemical properties without any medical application, as well as studies that dealt only with morphology and tribological behaviour. Two independent reviewers (R.A.A. and H.M.A.) comprehensively assessed the full text of the selected studies based on strict eligibility criteria. Discrepancies in study inclusion or exclusion were resolved through consensus among the reviewers. The inter-reviewer agreement was evaluated using Cohen’s kappa coefficient [38].
A comprehensive depiction of the study search and selection process is presented in Figure 1, following the PRISMA guidelines. The two reviewers verified the accuracy and alignment of the extracted information for the selected studies. The data were extracted from each of the selected studies and then organised, summarised, and tabulated, including details such as the author(s), year of publication, the aim of the study, methodology (preparation of CB, characterisation tests of the materials, and preparation of the animal), findings, and study limitations.
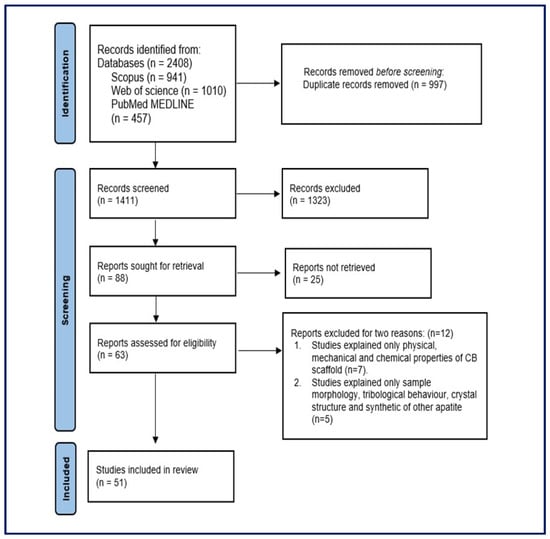
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of the included studies.
2.5. Risk of Bias (ROB) Assessment
The assessment of the ROB entailed a systematic categorisation of the final dataset based on study design, resulting in two principal groups: in vivo (animal studies) and in vitro investigations. These assessments were independently conducted by two reviewers, R.A.A. and H.M.A.
For animal studies, SYRCLE’s ROB tool, which is rooted in the Cochrane ROB tool, was adapted to account for bias unique to animal intervention studies [39]. This tool encompassed multiple domains to evaluate various facets of bias, including selection bias (with components like sequence generation, baseline characteristics, and allocation concealment), performance bias (encompassing random housing and blinding procedures), detection bias (evaluating randomisation and blinding in outcome assessment), attrition bias (assessing incomplete outcome data), reporting bias (scrutinising selective outcome reporting), and other potential sources of bias. Signal questions were integral to this evaluation, with responses categorised as “Yes” indicating a low risk of bias, “No” signifying a high risk of bias, and “Unclear” indicating insufficient information to accurately assess bias risk.
Conversely, in vitro studies underwent evaluation using the QUIN (Quality Assessment Tool for In Vitro Studies) ROB tool. This standardised tool for assessing bias in in vitro studies incorporated 12 domains to evaluate study quality [40]. These domains encompassed clarity of aims/objectives, detailed sample size calculation, elucidation of sampling techniques, descriptions of the comparison group, operator specifications, randomisation, outcome measurement methods, details on outcome assessors, blinding, statistical analysis, and presentation of results. Scoring entailed assigning 2, 1, or 0 based on whether a criterion was well specified, inadequately specified, or not applicable, respectively. The final score for each study was calculated using a specific formula:
Final score = (Total score × 100)/(2 × Number of applicable criteria)
The calculated scores were interpreted as follows: a score of >70% = low ROB, 50–70% = medium ROB, and <50% = high ROB. These comprehensive assessments aimed to enhance the credibility and validity of this systematic review, ensuring a robust foundation for data interpretation and synthesis.
3. Results
3.1. Study Selection
The comprehensive search query resulted in a total of 2408 research publications across three prominent databases: 941 from Scopus, 457 from PubMed, and 1010 from Web of Science. After eliminating duplicate studies, 1411 studies remained and were subjected to further scrutiny. Of the initial studies, 1323 were excluded based on irrelevant titles, leaving 88 studies for more detailed examination. After a thorough review of abstracts, 25 non-relevant studies were excluded from consideration. The remaining 63 studies underwent a comprehensive evaluation by reviewing their full texts based on predefined eligibility criteria. A total of 12 studies were excluded for two main reasons as shown in Figure 1. Of those, seven studies focused only on the physical, mechanical, and chemical properties of the CB scaffold without mentioning any medical application. The other five studies addressed only sample morphology, tribological behaviour, crystal structure, and the synthetic nature of apatites. Following the application of these eligibility criteria to all studies, the final dataset for this systematic review encompassed 51 studies. The inter-reviewer agreement for inclusion and exclusion studies based on Cohen’s kappa coefficient demonstrated substantial agreement (kappa = 0.74).
3.2. Literature Taxonomy
To facilitate future research and address various associated issues and scientific gaps, a taxonomy was developed based on the utilisation of CB in biomedical applications as shown in Figure 2. This taxonomy classified studies into in vivo and in vitro categories.
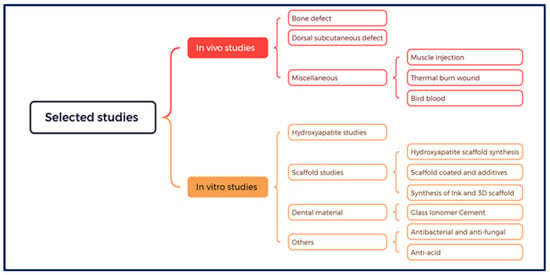
Figure 2.
Taxonomy of the included studies.
3.2.1. In Vivo Category
In vivo studies involving animal experiments, encompassing rabbits, rats, or birds, accounted for 16 studies (16/51). These studies were further categorised into three main groups. The first group of studies focused on animal bone structure, defect, or fracture (11 studies) at diverse anatomical sites including the calvaria, tibia, and sinus. The second group included studies that addressed dorsal subcutaneous defects (two studies). The miscellaneous group (third one) was further subdivided into three subgroups that examined thermal wound healing, muscle injection for tested substances, and bird blood testing (one study each). Detailed characteristics of these in vivo studies are presented in Table 2.

Table 2.
Studies on CB applications in animal-experiment-based bone regeneration and biomaterial assessment.
3.2.2. In Vitro Category
This category was subdivided into four main groups: the HAp group, scaffold group, dental materials group, and others.
HAp Group
The studies in this group (7/51 studies) focused on synthesising and characterising HAp as a potential biomaterial for bone tissue engineering (BTE). These studies explored the properties and suitability of HAp for biomedical applications. The study characteristics of this group are presented in Table 3.

Table 3.
Studies on HAp synthesis from CB (HAp group) and its characterisation using various methods.
Scaffold Group
The main research subject in this group of studies (21/51 studies) was the synthesis, characterisation, and biomedical application of scaffolds. Accordingly, this group of studies was further subdivided into three subgroups. The first subgroup included five studies that mainly dealt with the pure HAp scaffold synthesis, revealing its importance for tissue engineering or direct clinical use. The second subgroup had eight studies that dealt with scaffold coating, which improves the materials, and mechanical properties. The synthesis of ink and 3D scaffolds was the main area of interest in the third subgroup (eight studies). Characteristics of studies belonging to this group are presented in Table 4.

Table 4.
Preparation methods, characterisation, and key findings of CB-derived scaffolds (scaffold group) for BTE.
Dental Material Group
Three out of fifty-one studies were assigned to this group, which mostly focused on glass ionomer cement (GIC). These studies specifically addressed the incorporation of CB-derived HAp powder into GIC, assessing the potential enhancement in its physical properties. Table 5 shows the characteristics of this study group.

Table 5.
Effects of CB-derived HAp on glass ionomer cement (GIC group) mechanical properties.
Others
This group included four out of fifty-one studies. Three studies explored the antibacterial and antifungal effects, discussing natural sources of safe antimicrobial agents. One study focused on synthesising antiacid substances from CB raw material. The study characteristics are explained in Table 6.

Table 6.
Preparation, material synthesis, characterisation, and applications of CB-derived materials in scientific studies (other).
3.3. Risk of Bias Assessment
The assessment of ROB for in vivo studies was conducted using SYRCLE’s ROB tool as seen in Figure 3. The results revealed a low ROB in the reporting bias domain, attributed to the comprehensive reporting of primary and secondary outcomes and well-clarified methods and results sections in all studies. However, for other domains, such as allocation concealment, random housing, blinding, and random outcome assessment, more than 50% of the studies displayed an unclear ROB, mainly due to insufficient data and less likely due to insufficient details on housing conditions and the timing of outcome assessment. The assessment of ROB for in vitro studies was performed using the QUIN ROB tool as seen in Figure 4. Among the 35 studies assessed, 31 studies exhibited a low ROB, with final scores exceeding 70%. Four studies were classified as having a medium ROB with final scores exceeding 60%. These findings indicate that most studies provided clear aims, objectives, comparison groups, detailed methodologies, outcome assessments, statistical analyses, and results. However, some domains did not apply to these studies. These comprehensive assessments collectively contribute to the credibility and reliability of this systematic review, establishing a robust foundation for the interpretation and synthesis of data.
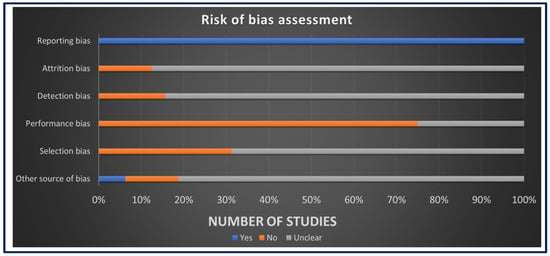
Figure 3.
The risk of bias assessment using SYRCLE’s tool.
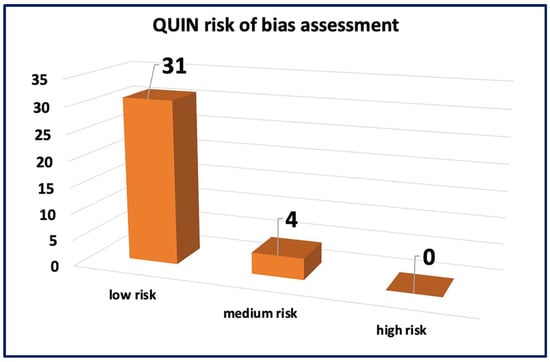
Figure 4.
The risk of bias assessment using the QUIN tool.
4. Discussion
CB has attracted a tremendous deal of interest from scientists who are working to understand its special characteristics and consider its potential applications. Numerous studies have delved into CB’s chemical and mechanical structures, revealing versatile attributes that can be harnessed for biological and industrial endeavours [25,28,87,88,89,90]. CB stands as an economical, abundantly available, and remarkably bioactive natural material, poised for transformation into HAp and bone-mimetic scaffolds. In the fields of tissue engineering and regenerative medicine, the utility of CB scaffolds has been increasingly investigated [91]. In pursuit of the ideal bone substitute—one that avoids immune responses while remaining bioactive, osteoconductive, osteoinductive, biodegradable, sterilisable, accessible, and cost-effective—CB emerges as a promising alternative to autogenous bone grafts [92]. To shed light on this prospect, our discussion segment will emphasise in vivo and in vitro studies that have examined the application of CB in biomedical and regenerative contexts.
4.1. Application of CB in an In Vivo Setting
The conversion of CB-derived materials into augmenting biomaterials for bone defects or as potential autogenous bone substitutes has garnered substantial research attention. It has been conclusively established that CB material holds promise as a bone graft material, BTE scaffold, bone filler, and regenerating bone. CB-based derivatives, which were mostly synthesised based on hydrothermal techniques, have been distinguished by their excellence in serving as bone substitutes either solely or in combination with other biological materials [43,44].
It has been reported that the most optimal osteogenic differentiation has been observed with CB-derived scaffolds in aerogel form, which fosters cell adhesion, proliferation, alkaline phosphatase (ALP) activity, targeted gene expression, and mineralisation. Surface roughness modifications have been shown to enhance cell adhesion, osteoblast proliferation, and differentiation [42]. Biocompatibility assessments of CB typically involve scrutinising its potential cytotoxicity impact on MG-63 cells (osteoblast-like human osteosarcoma cells) [52], as well as evaluating the adhesion and growth of human marrow mesenchymal stem cells (hMSCs) on the scaffold [54]. The extraordinary topographical structure of these substances enables the synthesis of novel periosteum substitutes that demonstrate remarkable osteogenic and angiogenic properties [41]
The porous nature of CB, with its capacity for blood circulation and support for bone cell adhesion, proliferation, and nutrient solution microcirculation, has been lauded for its ability to boost neovascularisation and bone tissue formation [45,52,54]. Moreover, the high surface area for bone–material interaction in CB-based HAp augments the biomechanical environment, creating favourable conditions [46]. CB exhibits remarkable biocompatibility, osteoconductivity, and bioactivity, with no discernible rejection, infection, or cytotoxic effects on bone healing. Also, it reduces free radicals in soft tissues and enhances bone healing without triggering oxidative stress [45,53].
Histologically, CB-based materials have accelerated callus formation and increased osteoblast production, whether used as bone graft materials or as subcutaneous implants, thus hastening the bone repair process. [47]. The initiation of bone healing involves fibrous tissue formation followed by vascularisation and osteochondral union. CB-based materials have demonstrated exceptional effectiveness in promoting bone union, achieving 100% bone union in radiographic assessments [53]. Moreover, the encapsulation of the grafting material has led to enhanced inflammatory cell infiltration, which further enhances osteoconduction [18].
CB-based HAp graft materials have shown osteoconductivity and biocompatibility through observations of inflammatory reactions around the implanted material, the proliferation of fibroblasts and connective tissue, and the eventual encapsulation of the implant [51]. Additionally, cell infiltration and proliferation have shown a non-toxic effect of CB material when implanted in bone or muscle, comparable to that of synthetic bone grafts [49,50]. CB has also demonstrated substantial influence on healing skin burns and wounds, enhancing re-epithelialisation, granulation tissue formation, hemostasis, and inflammation inhibition, attributed to chitin polysaccharide action [55]. Of particular interest is the sulphated chitosan (SP-LMWSC) extracted from CB, which has exhibited both in vitro anticoagulant and antiviral properties, indicating its potential utility in various medical applications [48]. CB exhibits a multitude of exceptional properties, including biocompatibility, osteoconductivity, cell adhesion promotion, non-toxicity, osteoblast proliferation, and differentiation. Collectively, these findings robustly advocate for the potential of CB in BTE and augmentation. However, the results should be interpreted cautiously, considering the outcomes of the ROB assessments.
4.2. In Vitro Category
4.2.1. Biomedical Application of HAp
HAp [Ca10(PO4)6(OH)2] has long been explored as a potential implant material due to its structural resemblance to the mineral component of bone and teeth. The investigation into HAp ceramics derived from natural materials has yielded innovative biomedical applications. HAp’s porous morphology is conducive to vascularisation, bone cell invasion, and angiogenesis. It exhibits rapid absorption by living cells, surpassing stoichiometric HAp, and accelerating bone regeneration [93,94,95].
Given the easy transformation of CB’s aragonite into HAp, it has been used as a biomaterial in BTE and other biomedical applications. Key features, such as the interconnected channelled structure, which facilitates cell growth, nutrient passage, and waste elimination, enhance its biological excellence [32,61]. The biological activity of nanocrystalline HAp derived from CB has been attributed to the presence of different ions such as the strontium ion (Sr2+) and magnesium ion (Mg2+) [57,60].
To enhance the mechanical strength of HAp, researchers have introduced biological macromolecules like silk fibroin, forming injectable hydrogels. This approach has improved cell proliferation rates and cell-to-cell communication among osteoclasts and osteoblasts. Additionally, CB-based HAp nanocomposites have demonstrated antibacterial activity against microorganisms such as S. aureus [59]. It is worth noting that adding carbon and silver nanoparticles has also been practised to significantly increase the mechanical and biological properties, enhance the proliferation rate of MG63 cells, and provide higher antibacterial action against microorganisms (e.g., E. coli and S. aureus) [58]. The hydrogel derived from CB-based HAp and its non-toxic features has shown exceptional qualities for use in medical substances [56].
4.2.2. Biomedical Application of Scaffold
Scaffolds represent highly specialised biomaterials suitable for tissue engineering and therapeutic use [37]. When analysing marine-derived biomaterials, focusing on bioactivity and biointeractivity properties is crucial. In bone tissue engineering, osteoinductive scaffolds promote bone growth and integration. An ideal pore size for scaffolds in BTE is over 100 micrometres, supporting cell infiltration, vascularisation, nutrient transport, cell adhesion, and growth. These materials possess porous matrices with interconnected pores that facilitate nutrient and oxygen transfer and support cell adhesion, proliferation, and differentiation. Mechanical properties such as stiffness, strength, and toughness are vital for tissue replacement [96]. Additionally, it is crucial to synchronise the scaffold’s degradation rate with the rate of new tissue formation. This ensures the scaffold provides temporary support, is gradually replaced by natural bone, and enhances angiogenesis and osteogenesis for bone tissue engineering [62,70,71].
Inspired by the clinical need for simple custom shapes, biomimetic scaffolds have been successfully employed as clinical implants [21,37,79]. Other compositions, such as porous biphasic calcium phosphate (BCP) scaffolds, have shown promise for osteointegration and osteoinduction [20]. CB has emerged as a favourable substrate for cell development and the growth of hMSCs. Its lamellar matrix allows for better cellular penetration than the dorsal shield and it can serve as both a bone replacement and a barrier against fibroblast infiltration near bone defects [78]. To enhance mechanical properties and maintain microstructure, scaffolds are coated with biodegradable polymers [97].
Polymers such as polycaprolactone (PCL) and polyvinyl alcohol, poly DL-lactide, a polyester amide (PEA), polyester urea, polylactic acid, and sol-gel improve the mechanical (e.g., hardness, compressive strength, and elastic modulus) and biological (e.g., cell adhesion, proliferation, and differentiation) features of the HAp scaffold for BTE [21,37,79]. On the other hand, unprocessed CB with collagen has been used to synthesise CB-HAp-collagen scaffolds, which can also be beneficial for BTE as it is bioabsorbable and has low immunogenicity [73]. HAp with whitlockite and Mg2+, used as porous Mg-substituted calcium phosphate scaffolds, has shown improvements in mechanical and biological properties and can thus be used as a candidate in BTE [67].
Scholars have attempted to create three-dimensional (3D) scaffolds similar to cancellous bone structures for multifunctional purposes. For instance, researchers designed 3D cellulose-based scaffolds [70,72] and regenerating cellulose RC/CB scaffolds [68] with simulated body fluid (SBF) coatings to enhance cell adhesion, promote osteoconduction in BTE, and increase calcium phosphate deposition. This was facilitated by the ability of β-chitin to bind proteins and trace elements that are crucial for mineralisation. Moreover, sophisticated 3D scaffolds with layer-by-layer deposition that were designed with BioCAD software with crosslinking steps were mechanically efficient and had good energy absorption and low weight, and, as a result, could be used in a wide range of medical applications [66]. Nano-BCP 3D printing powder derived from CB was mixed with glass–ceramic powder and different pore geometries were created with sufficient porosity. This type of scaffold showed quick adherence with hMSCs, which enter the pores and colonise the porous structure [65].
To fully control and create sufficient scaffold porosity, powder 3D printing was confirmed to be an appropriate approach for BTE with good reproducibility. Such scaffolds have been prepared from nano-BCP powder derived from CB mixed with glass–ceramic powder and different pore geometries. [64]. Moreover, paste-like 3D printing inks [63] are made from an extracellular matrix based on CB and from biomineral composite scaffolds of five materials (eggshell, pearl, turtle shell, degelatinated deer antler, and CB) with appropriate porosity [62]. These scaffolds have good biocompatibility and can encourage bone regrowth and stimulate biomineralisation. In a nutshell, the superior properties of the 3D-CB scaffold motivate researchers to apply it as a promising technology in biomedical and regenerative medicine.
4.2.3. Incorporation of CB-HAp into Dental Materials
Glass ionomer cement (GIC) is one of the restorative materials widely used in dentistry. While its advantages include easy bonding to dental tissue, biocompatibility, ease of handling, and fluoride release, its mechanical properties are relatively low [98]. Consequently, the incorporation of CB-HAp into GIC has improved its mechanical properties. Noteworthy, the incorporation of CB-microparticles was preferable, as it is easier to be mixed with resin [99], compared to nanoparticles, which have rough surfaces (cauliflower-like morphology), extending the GIC’s setting time [100].
Bilić-Prcić et al. [82] showed that compressive strength, flexural strength (FS), and diametral tensile strength of chemically set Fuji IX groups were not improved when manually mixed with micro-HAp (<180 µm) at varied wt% concentrations (2, 5, and 10 wt%). However, the mechanical properties of light-cured Fuji II groups showed improvement, particularly with 10 wt% HAp, which yielded the best results in terms of FS. Additionally, the surface roughness and microhardness were not improved by adding HAp-CB to commercial GICs (Fuji IX and Fuji II). This was not the case for the Fuji II, which had been mixed with a 10 wt% HAp group, and that was attributed to the size of the particles and air inclusion due to manual mixing. However, adding HAp-CB significantly improved the fluoride release property of GIC [81].
Ivanišević, Rajić et al. [80] incorporated a mixture of titanium dioxide (TiO2) nanoparticles and CB-HAp microparticles into conventional GIC. TiO2 at the nanoscale has antibacterial properties, good chemical stability, and high biocompatibility and potentially reinforces GIC. Although the CB-HAp microparticles enhanced GIC fluoride release, there were no improvements in compressive strength, breaking strength, or compressive modulus due to an inadequate powder-to-liquid ratio that left many particles unreacted [80].
4.2.4. Incorporation of CB-HAp into Antimicrobial, Antifungal, and Antiacid Agents
Antibacterial activity is a crucial feature in preventing implant failure. HAp may induce rapid bacterial biofilm growth due to bioactive calcium and phosphate that provide nutrients for bacterial colony growth [101]. However, nano-HAp derived from CB has exhibited potent antibacterial activity against Gram-positive Bacillus subtilis, surpassing its effectiveness against Gram-negative E. coli [84,86]. The mechanism involves the penetration of nanoparticles into the bacterial cell membranes, increasing their osmotic potential and leading to irreversible cellular damage. Because CaCO3 and polysaccharides in CB’s chitosan can cause bacterial death by releasing cellular contents, powdered CB has shown effective antibacterial and antifungal properties against Klebsiella oxytoca and Aspergillus flavus, respectively. However, in as study, no antibacterial action was reported against Staphylococcus aureus as it could not dissolve the S. aureus peptidoglycan cell wall [83].
Only one study presented the CB as a natural antiacid tablet, overcoming the side effects of synthetic chemical drugs. The results demonstrated that CB outperformed commercially available antacid tablets such as CaCO3 and Al-Mg tablets. Excellent antacid properties were reported by using calcined CB, which is characterised by high purity, the absence of organic components, and appropriate physicochemical qualities [85]. CB can be considered a promising material for synthesising affordable, antibacterial, anti-fungal, and antiacid agents.
This systematic literature review has included studies that dealt with different medical applications, whether in vitro or in vivo. However, it has potential limitations as it has included only three databases with no grey literature conducted and is limited to English studies. Additionally, no meta-analysis was performed due to the lack of consistent numerical values from the included studies.
4.3. Limitations and Challenges of Clinical Translation of CB
The current research on CB biomaterials primarily relies on preclinical studies involving animal models, which present their own set of limitations. As noted in the tables, these limitations include a lack of mechanical and characterisation tests, the application of materials for short periods, and insufficient comprehensive mechanical, biological, or bioactive tests in some in vitro experiments. To facilitate the translation of these materials to clinical trials and human studies, it is crucial to examine their long-term immunogenicity to evaluate potential adverse reactions and immune responses over extended periods. Assessing their efficacy and safety in practical medical settings is also essential. Addressing these challenges is vital for overcoming the current evidence base limitations and ensuring the successful clinical application of CB biomaterials.
Translating findings into clinical practice presents significant challenges. Regulatory approval requires extensive preclinical and clinical data to meet safety and efficacy standards. Clinical validation demands robust trials with adequate sample sizes and follow-up durations to establish reliability and effectiveness. The long-term monitoring of patients with CB implants is crucial for assessing their safety, efficacy, and potential complications over extended periods. Additionally, understanding CB’s degradation kinetics and biostability in physiological conditions is essential for predicting its durability and performance in vivo. Despite the promising mechanical properties of CB biomaterials, such as their high porosity and stiffness, optimising these characteristics for specific biomedical applications, like load-bearing implants or tissue engineering scaffolds, remains challenging. Standardising processing methods is essential to ensuring reproducibility and consistency of results across studies. Addressing these research gaps and overcoming translational hurdles are critical steps towards fully realising the potential of CB biomaterials in medical applications. Future research efforts should prioritise clinical validation, standardisation of protocols, and compliance with regulatory requirements to facilitate the broader adoption of these innovative biomaterials in clinical practice.
5. Future Directions
CB stands poised for avant-garde implementations, capitalising on its unique attributes. Leveraging the advantageous features of CB, such as its porosity, microstructures, and crystallography, has paved the way for the synthesis and enhancement of bone tissue materials. The synthesis of HAp and the innovation of scaffold structures with diverse geometries have ushered in new horizons for medical applications. CB can find utility in the surgical arena as a graft material replacement, offering fresh options as a “smart” biomaterial tailored for craniofacial bone reconstruction and regenerative procedures. Furthermore, injectable HAp synthesis holds promise for treating irregular bone defects.
Given the compelling mechanical, biological, and physical properties demonstrated by HAp in previous literature, it presents a fitting biological solution for a spectrum of challenges in restorative dentistry. For instance, CB-HAp, enriched with calcium and phosphate ions, emerges as a compelling, biocompatible, and cost-effective alternative for the treatment of demineralised dental tissues.
In the context of endodontic practice, the use of irrigant solutions with bleaching or chelating actions within root canals can inadvertently soften dentinal walls post mineral depletion. Herein lies the hypothesis that incorporating CB-HAp into the final irrigant solution could effectively surmount this challenge. The rationale behind this hypothesis is attributed to the potential ability of CB-HAp to enhance mechanical and physical properties, coupled with its innate outstanding biocompatibility. Moreover, CB-HAp’s potent antimicrobial properties against certain bacterial strains suggest its potential use in treating root canal infections [83,84]. This opens avenues for incorporating this material into root canal sealers, irrigant solutions, intercanal medicaments, implant coatings, and drug delivery systems. CB-derived HAp materials could also increase the microhardness of the internal walls of the roots. Interestingly, since CB-derived materials are rich in calcium ions [4], they could be incorporated into restorative materials; these encompass cement, liners, luting agents, capping materials for dental implants, sealants, and adhesives. However, such hypotheses need to be confirmed through conducting more laboratory and clinical studies.
Notably, tissue engineering and nanotechnology convergence can yield natural tissue-mimicking designs, culminating in structures akin to dental tissues, including dentin, pulp, periodontal ligament, cementum, and alveolar bone. This confluence holds immense potential for transformative dental science and clinical practice advancements.
For future research, large-scale randomised controlled trials (RCTs) with sufficient sample sizes, robust study designs, and adequate follow-up periods to generate high-quality evidence are crucial. In addition, long-term biocompatibility studies with standardisation for the protocol will enhance result reproducibility, reliability, and comparability across different research studies and clinical trials. Innovative approaches can be applied to improve the use of CB in regenerative medicine, such as by incorporating bioactive molecules or growth factors to promote tissue regeneration and accelerate healing processes [102]. Utilising 3D printing techniques to create patient-specific implants or scaffolds, and investigating the synergistic effects of combining CB with other biomaterials, cells, or therapeutic agents for tailored regenerative treatments, is also promising. Adopting these strategies makes it possible to overcome current limitations, advance scientific understanding, and accelerate the clinical application of CB biomaterials in regenerative medicine and dentistry. These efforts have the potential to pave the way for innovative treatments that improve patient outcomes and quality of life.
6. Conclusions
The exploration of CB has revealed its myriad applications across tissue engineering, regenerative medicine, and dentistry. In vitro and in vivo studies have demonstrated CB’s exceptional mechanical, biological, and chemical properties, paving the way for innovative biomedical applications. Its inherent biocompatibility, availability, and cost-effectiveness position CB as a promising therapeutic agent derived from waste materials. This not only underscores the sustainable aspect of utilising byproducts but also signifies a valuable advancement in the field of biomedical science. Additionally, the green synthesis of CB biomaterials highlights their eco-friendly nature, further enhancing their appeal as sustainable and innovative solutions in biomedical applications.
Large-scale RCTs are required to evaluate the efficacy and safety of CB biomaterials in various clinical applications, such as for bone grafts and dental implants. Additionally, extended follow-up studies should be performed to assess CB biomaterials’ long-term biocompatibility and immunogenicity. These suggestions will help improve the utilisation of CB biomaterials in biomedical applications.
Author Contributions
R.A.A.-R.: conceptualisation, investigation, project administration, writing—original draft. H.M.A.-R.: data curation, formal analysis, resources. A.C.: conceptualisation, methodology, validation, supervision, writing—review and editing. A.M.: formal analysis, supervision, writing—review and editing. Y.M.L.: formal analysis, supervision, writing—review and editing. P.S.: supervision, investigation, methodology, validation. W.N.W.H.: conceptualisation, supervision, funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial support from the Universiti Malaya Impact-Oriented Interdisciplinary Research Grant (IIRG002B-2022HWB).
Data Availability Statement
Data will be made available on request.
Acknowledgments
We would like to express our sincere gratitude to Nozimjon Tuygunov for his invaluable assistance in creating the figures for this review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Venkatesan, J.; Anil, S. Hydroxyapatite Derived from Marine Resources and their Potential Biomedical Applications. Biotechnol. Bioprocess Eng. 2021, 3, 312–324. [Google Scholar] [CrossRef]
- Antoniac, I.; Lesci, I.G.; Blajan, A.; Vitioanu, G.; Antoniac, A. Bioceramics and biocomposites from marine sources. Key Eng. Mater. 2015, 672, 276–292. [Google Scholar] [CrossRef]
- Monzack, E.L.; Rodriguez, K.J.; McCoy, C.M.; Gu, X.; Masters, K.S. Natural materials in tissue engineering applications. In Biomaterials for Tissue Engineering Applications; Burdick, J.A., Mauck, R.L., Eds.; Springer: Vienna, Austria, 2011; pp. 209–241. [Google Scholar] [CrossRef]
- Pu’ad, N.M.; Koshy, P.; Abdullah, H.; Idris, M.; Lee, T. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef]
- Venkatesan, S. Introduction to marine biomaterials. In Marine Biomaterials: Characterization, Isolation and Applications, 1st ed.; Kim, S.-K., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 3–16. [Google Scholar]
- Khrunyk, Y.; Lach, S.; Petrenko, I.; Ehrlich, H. Progress in modern marine biomaterials research. Mar. Drugs 2020, 18, 589. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Valente, S.; Parchi, G.; Pasquinelli, G.; Taddei, P.; Prati, C. Green Hydrogels Composed of Sodium Mannuronate/Guluronate, Gelatin and Biointeractive Calcium Silicates/Dicalcium Phosphate Dihydrate Designed for Oral Bone Defects Regeneration. Nanomaterials 2021, 11, 3439. [Google Scholar] [CrossRef]
- Yan, J.; Miao, Y.; Tan, H.; Zhou, T.; Ling, Z.; Chen, Y.; Xing, X.; Hu, X. Injectable alginate/hydroxyapatite gel scaffold combined with gelatin microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 274–284. [Google Scholar] [CrossRef]
- Adnen, N.A.; Halim, N.A.; Nor, M.A. Development of hydroxyapatite from Setiu coral via hydrothermal method. AIP Conf. Proc. 2017, 1885, 020151. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.; Ali, A.; Sun, Z. Synthesis of nano-hydroxyapatite (nHA) from waste mussel shells using a rapid microwave method. Mater. Chem. Phys. 2015, 149, 607–616. [Google Scholar] [CrossRef]
- Gheysari, H.; Mohandes, F.; Mazaheri, M.; Dolatyar, B.; Askari, M.; Simchi, A. Extraction of hydroxyapatite nanostructures from marine wastes for the fabrication of biopolymer-based porous scaffolds. Mar. Drugs 2020, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Rujitanapanich, S.; Kumpapan, P.; Wanjanoi, P. Synthesis of hydroxyapatite from oyster shell via precipitation. Energy Procedia 2014, 56, 112–117. [Google Scholar] [CrossRef]
- Raya, I.; Mayasari, E.; Yahya, A.; Syahrul, M.; Latunra, A. Synthesis and characterizations of calcium hydroxyapatite derived from crabs shells (Portunus pelagicus) and its potency in safeguard against to dental demineralizations. Int. J. Biomater. 2015, 2015, 469176. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.; Walsh, P.; Maggs, C.; Buchanan, F. Designs from the deep: Marine organisms for bone tissue engineering. Biotechnol. Adv. 2011, 29, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Macha, I.J.; Ben-Nissan, B. Marine Skeletons: Towards Hard Tissue Repair and Regeneration. Mar. Drugs 2018, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Griesshaber, E.; Schmahl, W.W.; Neuser, R.; Pettke, T.; Blüm, M.; Mutterlose, J.; Brand, U. Crystallographic texture and microstructure of terebratulide brachiopod shell calcite: An optimized materials design with hierarchical architecture. Am. Mineral. 2007, 92, 722–734. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.J.; Lee, K.S. Evaluation of biocompatibility of porous hydroxyapatite developed from edible cuttlefish bone. Key Eng. Mater. 2007, 361–363, 155–158. [Google Scholar] [CrossRef]
- Manoli, F.; Dalas, E. Calcium carbonate crystallization on xiphoid of the cuttlefish. J. Cryst. Growth 2000, 4, 422–428. [Google Scholar] [CrossRef]
- Sarin, P.; Lee, S.J.; Apostolov, Z.D.; Kriven, W.M. Porous Biphasic Calcium Phosphate Scaffolds from Cuttlefish Bone. J. Am. Ceram. Soc. 2011, 94, 2362–2370. [Google Scholar] [CrossRef]
- Rocha, J.H.G.; Lemos, A.F.; Agathopoulos, S.; Kannan, S.; Valério, P.; Ferreira, J.M.F. Hydrothermal growth of hydroxyapatite scaffolds from aragonitic cuttlefish bones. J. Biomed. Mater. Res. Part A 2006, 77, 160–168. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, H.J.; Lee, J. Improvement of the compressive strength of a cuttlefish bone-derived porous hydroxyapatite scaffold via polycaprolactone coating. J. Biomed. Mater. Res. B 2013, 101, 1302–1309. [Google Scholar] [CrossRef]
- Venkatesan, J.; Rekha, P.D.; Anil, S.; Bhatnagar, I.; Sudha, P.N.; Dechsakulwatana, C.; Kim, S.-K.; Shim, M.S. Hydroxyapatite from Cuttlefish Bone: Isolation, Characterizations, and Applications. Biotechnol. Bioprocess. Eng. 2018, 23, 383–393. [Google Scholar] [CrossRef]
- Jia, X.; Qian, W.; Wu, D.; Wei, D.; Xu, G. Cuttlebone-derived organic matrix as a scaffold for assembly of silver nanoparticles and application of the composite films in surface-enhanced Raman scattering. Colloids Surf. B 2009, 68, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Cadman, J.; Zhou, S.; Chen, Y.; Li, W.; Appleyard, R.; Li, Q. Characterization of cuttlebone for a biomimetic design of cellular structures. Acta Mech. Sin. 2010, 26, 27–35. [Google Scholar] [CrossRef]
- Ben-Nissan, B. Natural bioceramics: From coral to bone and beyond. Curr. Opin. Solid State Mater. Sci. 2003, 7, 283–288. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Cadman, J.; Zhou, S.; Chen, Y.; Li, Q. Cuttlebone: Characterisation, Application and Development of Biomimetic Materials. J. Bionic Eng. 2012, 9, 367–376. [Google Scholar] [CrossRef]
- Rajesh, P.; Kalita, K.; Gao, X. Process Modelling and Experimental Analysis of Optimal Specimen Selection in Organic CMCs. Comput. Mater. Contin. 2022, 70, 2415–2433. [Google Scholar] [CrossRef]
- Cadman, J.; Chang, C.; Chen, J.; Chen, Y.; Zhou, S.; Li, W.; Li, Q. Bioinspired lightweight cellular materials-Understanding effects of natural variation on mechanical properties. Mater. Sci. Eng. C 2013, 33, 3146–3152. [Google Scholar] [CrossRef]
- Zhang, X.; Vecchio, K.S. Conversion of natural marine skeletons as scaffolds for bone tissue engineering. Front. Mater. Sci. 2013, 7, 103–117. [Google Scholar] [CrossRef]
- Ivankovic, H.; Tkalcec, E.; Orlic, S.; Gallego Ferrer, G.; Schauperl, Z. Hydroxyapatite formation from cuttlefish bones: Kinetics. J. Mater. Sci. Mater. Med. 2010, 21, 2711–2722. [Google Scholar] [CrossRef]
- Kamalbabu, P.; Mohankumar, G. Sea coral-derived cuttlebone reinforced epoxy composites: Characterization and tensile properties evaluation with mathematical models. J. Compos. Mater. 2015, 50, 807–823. [Google Scholar]
- Wan, M.C.; Qin, W.; Lei, C.; Li, Q.; Meng, M.; Fang, M.; Song, W.; Chen, J.; Tay, F.; Niu, L. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Sathish, L.; Govindan, R.; Girija, E.K. Utilization of snail shells to synthesise hydroxyapatite nanorods for orthopaedic applications. RSC Adv. 2015, 5, 39544–39548. [Google Scholar] [CrossRef]
- Bee, S.L.; Hamid, Z.A. Hydroxyapatite derived from food industry bio-wastes: Syntheses, properties and its potential multifunctional applications. Ceram. Int. 2020, 46, 17149–17175. [Google Scholar] [CrossRef]
- Rocha, J.H.G.; Lemos, A.F.; Agathopoulos, S.; Valério, P.; Kannan, S. Scaffolds for bone restoration from cuttlefish. Bone 2005, 37, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: The QUIN. J. Prosthet. Dent. 2022, 131, 1038–1042. [Google Scholar] [CrossRef]
- Wei, Y.; Ju, M.; Zheng, F.; Wei, S.; Han, S.; Lu, S.; Liu, R.; Wu, H. Cuttlebone-Derived Organic Matrix: A Facile Periosteum Substitute for Bone Regeneration. Adv. Funct. Mater. 2023, 33, 2214095. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Chen, X.; Jiang, L. Biomimetic cuttlebone polyvinyl alcohol/carbon nanotubes/hydroxyapatite aerogel scaffolds enhanced bone regeneration. Colloids Surf. B Biointerfaces 2022, 210, 112221. [Google Scholar] [CrossRef]
- Irianto, K.A.; Limbang, S. Cytotoxic effect of natural cuttlefish bone xenograft: In vitro and in vivo study. Med. J. Indones. 2020, 29, 136–142. [Google Scholar]
- Aminatun, A.; Handayani, F.D.E.; Widiyanti, P.; Winarni, D.; Siswanto, S. In vivo approach on femur bone regeneration of white rat (Rattus norvegicus) with the use of hydroxyapatite from cuttlefish bone (Sepia spp.) as bone filler. Vet. World 2019, 12, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Fattahian, H.; Mansouri, N.; Mostafavi, P.G.; Kajbafzadeh, A. The role of cuttlebone and cuttlebone derived hydroxyapatite with platelet-rich plasma on tibial bone defect healing in rabbit: An experimental study. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 107–115. [Google Scholar] [CrossRef]
- Lambert, F.; Bacevic, M.; Layrolle, P.; Schüpbach, P.; Drion, P.; Rompen, E. Impact of biomaterial microtopography on bone regeneration: Comparison of three hydroxyapatites. Clin. Oral Implants Res. 2017, 28, e201–e207. [Google Scholar] [CrossRef] [PubMed]
- Kloping, L.P.; Purwati, P.; Edward, M. The Healing Effect of Cuttlefish Bone on Fractured Bone in Rat Model, Bali. Med. J. 2016, 5, 193–196. [Google Scholar]
- Karthik, R.; Manigandan, V.; Saravanan, R.; Rajesh, R.P.; Chandrika, B. Structural characterization and in vitro biomedical activities of sulfated chitosan from Sepia pharaonis. Int. J. Biol. Macromol. 2016, 84, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, W.; Yan, X.; Wei, J.; Geng, W.; Cui, J.; Xi, X.; Fulin, C. Osteoinductive nanohydroxyapatite bone substitute prepared via in situ hydrothermal transformation of cuttlefish bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 816–824. [Google Scholar]
- Won, S.; Lee, J.M.; Cheong, J.; Park, H.; Seo, J. Evaluation of the biocompatibility of cuttlebone in mouse. J. Vet. Clin. 2015, 32, 417–421. [Google Scholar] [CrossRef]
- Won, S.; Lee, J.M.; Park, H.; Seo, J.; Cheong, J. Evaluation of the Bone Defect Regeneration after Implantation with Cuttlebone in Rabbit. J. Vet. Clin. 2015, 3, 410–416. [Google Scholar] [CrossRef]
- Kim, B.; Hyo, J.; Sun, S.; Lee, J. Comparison of in vitro and in vivo bioactivity: Cuttlefish-bone-derived hydroxyapatite and synthetic hydroxyapatite granules as a bone graft substitute. Biomed. Mater. 2014, 9, 025004. [Google Scholar] [CrossRef]
- Dogan, E.; Okumus, Z. Cuttlebone used as a bone xenograft in bone healing. Vet. Med. 2014, 59, 254–260. [Google Scholar]
- Li, X.; Zhao, Y.; Bing, Y.; Li, Y.; Gan, N.; Guo, Z.; Peng, Z.; Zhu, Y. Biotemplated syntheses of macroporous materials for bone tissue engineering scaffolds and experiments in vitro and vivo. ACS Appl. Mater. Interfaces 2013, 5, 5557–5562. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.K.; Lee, O.S.; Kang, T.J.; Lim, S.C. Wound healing effect of cuttlebone extract in burn injury of rat. Food Sci. Biotechnol. 2013, 22, 99–105. [Google Scholar] [CrossRef]
- Tattanon, T.; Pongprayoon, T.; Arpornmaeklong, P.; Ummartyotin, S. Development of hydroxyapatite from cuttlebone and gelatin-based hydrogel composite for medical materials. J. Polym. Res. 2022, 29, 364. [Google Scholar] [CrossRef]
- Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials 2021, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Balu, S.K.; Sampath, V.; Andra, S.; Alagar, S.; Manisha Vidyavathy, S. Fabrication of carbon and silver nanomaterials incorporated hydroxyapatite nanocomposites: Enhanced biological and mechanical performances for biomedical applications. Mater. Sci. Eng. C 2021, 128, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Arjama, M.; Mehnath, S.; Rajan, M.; Jeyaraj, M. Injectable cuttlefish HAP and macromolecular fibroin protein hydrogel for natural bone mimicking matrix for enhancement of osteoinduction progression. React. Funct. Polym. 2021, 160, 104841. [Google Scholar] [CrossRef]
- Cestari, F.; Chemello, G.; Galotta, A.; Sglavo, V.M. Low-temperature synthesis of nanometric apatite from biogenic sources. Ceram. Int. 2020, 46, 23526–23533. [Google Scholar] [CrossRef]
- Ivankovic, H.; Gallego Ferrer, G.; Tkalcec, E.; Orlic, S.; Ivankovic, M. Preparation of highly porous hydroxyapatite from cuttlefish bone. J. Mater. Sci. Mater. Med. 2009, 20, 1039–1046. [Google Scholar] [CrossRef]
- Gang, F.; Ye, W.; Ma, C.; Wang, W.; Xiao, Y.; Liu, C.; Sun, X. 3D Printing of PLLA/Biomineral Composite Bone Tissue Engineering Scaffolds. Materials 2022, 15, 4280. [Google Scholar] [CrossRef]
- Curti, F.; Serafim, A.; Olaret, E.; Dinescu, S.; Samoila, I. Development of Biocomposite Alginate-Cuttlebone-Gelatin 3D Printing Inks Designed for Scaffolds with Bone Regeneration Potential. Mar. Drugs. 2022, 20, 670. [Google Scholar] [CrossRef] [PubMed]
- Cestari, F.; Yang, Y.; Wilbig, J.; Günster, J.; Motta, A.; Sglavo, V.M. Powder 3D Printing of Bone Scaffolds with Uniform and Gradient Pore Sizes Using Cuttlebone-Derived Calcium Phosphate and Glass-Ceramic. Materials 2022, 15, 5139. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.; Zhao, N.; Liang, Y.; Bai, H. Mechanically Efficient Cellular Materials Inspired by Cuttlebone. Adv. Mater. 2021, 33, e2007348. [Google Scholar] [CrossRef] [PubMed]
- Curti, F.; Dragusin, D.M.; Serafim, A.; Sorescu, A.; Stancu, I.C. Cuttlefish bone-based ink for 3D printing of scaffolds for orthopedic applications. UPB Sci. Bull. B Chem. Mater. Sci. 2021, 83, 3–14. [Google Scholar]
- Bauer, L.; Antunović, M.; Rogina, A.; Ivanković, M.; Ivanković, H. Bone-mimetic porous hydroxyapatite/whitlockite scaffolds: Preparation, characterization and interactions with human mesenchymal stem cells. J. Mater. Sci. 2021, 56, 3947–3969. [Google Scholar] [CrossRef]
- Palaveniene, A.; Songailiene, K.; Baniukaitiene, O.; Tamburaci, S.; Kimna, C. The effect of biomimetic coating and cuttlebone microparticle reinforcement on the osteoconductive properties of cellulose-based scaffolds. Int. J. Biol. Macromol. 2020, 152, 1194–1204. [Google Scholar] [CrossRef]
- Rogina, A.; Antunović, M.; Milovac, D. Biomimetic design of bone substitutes based on cuttlefish bone-derived hydroxyapatite and biodegradable polymers. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Palaveniene, A.; Tamburaci, S.; Kimna, C.; Glambaite, K.; Baniukaitiene, O. Osteoconductive 3D porous composite scaffold from regenerated cellulose and cuttlebone-derived hydroxyapatite. J. Biomater. Appl. 2019, 33, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.S.; Fonseca, A.C.; Abrantes, J.C.C.; Coelho, J.F.J.; Ferreira, J.M.F. Surface functionalization of cuttlefish bone-derived biphasic calcium phosphate scaffolds with polymeric coatings. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110014. [Google Scholar] [CrossRef]
- Palaveniene, A.; Glambaite, K.; Jaskunas, A.; Baniukaitiene, O.; Liesiene, J. Biomimetic mineralisation on cellulose/cuttlebone scaffolds. Chemija 2017, 28, 148–153. [Google Scholar]
- Sukul, M.; Min, Y.K.; Lee, B.T. Collagen-hydroxyapatite coated unprocessed cuttlefish bone as a bone substitute. Mat. Lett. 2016, 181, 156–160. [Google Scholar] [CrossRef]
- Siddiqi, S.A.; Manzoor, F.; Jamal, A.; Tariq, M.; Ahmad, R. Mesenchymal stem cell (MSC) viability on PVA and PCL polymer coated hydroxyapatite scaffolds derived from cuttlefish. RSC Adv. 2016, 6, 32897–32904. [Google Scholar] [CrossRef]
- Aksakal, B.; Demire, M. Synthesis and fabrication of novel cuttlefish (Sepia officinalis) backbone biografts for biomedical applications. Ceram. Int. 2015, 41, 4531–4537. [Google Scholar]
- Milovac, D.; Ferrer, G.G.; Ivankovic, M.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: Morphology, mechanical properties and bioactivity. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lim, Y.M.; Youn, M.H.; Gwon, H.J.; Nho, Y.C. Biodegradable polycaprolactone/cuttlebone scaffold composite using salt leaching process. Korean J. Chem. Eng. 2012, 29, 931–934. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, J.S.; Sung, H.M.; You, H.K.; Lee, J. Cellular attachment and osteoblast differentiation of mesenchymal stem cells on natural cuttlefish bone. J. Biomed. Mater. Res. A 2012, 100, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Battistella, E.; Mele, S.; Foltran, I.; Lesci, I.G.; Roveri, N.; Sabatino, P. Cuttlefish bone scaffold for tissue engineering: A novel hydrothermal transformation, chemical-physical, and biological characterization. J. Appl. Biomater. Funct. Mater. 2012, 10, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ivanišević, A.; Rajić, V.B.; Pilipović, A.; Par, M.; Ivanković, H. Compressive Strength of Conventional Glass Ionomer Cement Modified with TiO2 Nano-Powder and Marine-Derived HAp Micro-Powder. Materials 2021, 14, 4964. [Google Scholar] [CrossRef] [PubMed]
- Bilic-Prcic, M.; Salinovic, I.; Gurgan, S.; Koc Vural, U.; Krmek, S.J.; Miletic, I. Effects of Incorporation of Marine-Derived Hydroxyapatite on the Microhardness, Surface Roughness, and Fluoride Release of Two Glass-Ionomer Cements. Appl. Sci. 2021, 11, 11027. [Google Scholar] [CrossRef]
- Bilić-Prcić, M.; Rajić, V.B.; Ivanišević, A.; Pilipović, A.; Gurgan, S. Mechanical Properties of Glass Ionomer Cements after Incorporation of Marine-Derived Porous Cuttlefish Bone Hydroxyapatite. Materials 2020, 13, 3542. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Javid, N.; Honarmandrad, Z.; Amirmahani, N.; Nasiri, A. Evaluation of antimicrobial activities of powdered cuttlebone against Klebsiella oxytoca, Staphylococcus aureus, and Aspergillus flavus. Environ. Health Eng. Manag. 2021, 8, 39–45. [Google Scholar] [CrossRef]
- Balu, S.; Sundaradoss, M.V.; Andra, S.; Jeevanandam, J. Facile biogenic fabrication of hydroxyapatite nanorods using cuttlefish bone and their bactericidal and biocompatibility study. Beilstein J. Nanotechnol. 2020, 11, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Mostoufi, A.; Bavarsad, N.; Aryanfar, S.; Akhgari, A. New Natural Marine Antacid Drug from Cuttlebone. Pharm. Sci. 2018, 24, 227–234. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Stoecker, H.; Bazhenov, V.V.; Langer, E. An extreme biomimetic approach: Hydrothermal synthesis of beta-chitin/ZnO nanostructured composites. J. Mater. Chem. B. 2013, 1, 16469–16476. [Google Scholar] [CrossRef] [PubMed]
- North, L.; Labonte, D.; Oyen, M.L.; Coleman, M.P.; Caliskan, H.B.; Johnston, R.E. Interrelated chemical-microstructural-nanomechanical variations in the structural units of the cuttlebone of Sepia officinalis. Appl. Mater. 2017, 5, 116100–116107. [Google Scholar] [CrossRef]
- Salem, A.; Bensalah, W.; Mezlini, S. Tribological Investigation of HDPE-cuttlebone and HDPE-red Coral Composites. J. Bionic Eng. 2019, 16, 1068–1079. [Google Scholar] [CrossRef]
- Khazri, H.; Ghorbel-Abid, I.; Kalfat, R.; Trabelsi-Ayadi, M. Extraction of clarithromycin and atenolol by cuttlefish bone powder. Environ. Technol. 2018, 39, 2662–2668. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.S.; Osman, D.I.; Mohammed, H.; A Attia, S.K. Cuttlefish bone biowaste for production of holey aragonitic sheets and mesoporous mayenite-embedded Ag2CO3 nanocomposite: Towards design high-performance adsorbents and visible-light photocatalyst for detoxification of dyes wastewater and waste oil recovery. J. Photochem. Photobiol. A 2021, 421, 113523. [Google Scholar] [CrossRef]
- Vajrabhaya, L.; Korsuwannawong, S.; Surarit, R. Cytotoxic and the proliferative effect of cuttlefish bone on MC3T3-E1 osteoblast cell line. Eur. J. Dent. 2017, 11, 503–507. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 1–27. [Google Scholar] [CrossRef]
- Brown, P.W.; Constantz, B. Hydroxyapatite and Related Materials; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Hu, J.; Russell, J.; Ben-Nissan, B.; Vago, R. Production and analysis of hydroxyapatite from Australian corals via hydrothermal process. J. Mater. Sci. 2001, 20, 85–87. [Google Scholar]
- Murugan, R.; Ramakrishna, S.; Rao, K.P. Nanoporous hydroxy-carbonate apatite scaffold made of natural bone. Mater. Lett. 2006, 60, 2844–2847. [Google Scholar] [CrossRef]
- Navarro, M.; Ginebra, M.; Planell, J.; Zeppetelli, S.; Ambrosio, L. Development and cell response of a new biodegradable composite scaffold for guided bone regeneration. J. Mater. Sci. Mater. Med. 2004, 15, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Isikli, C.; Hasirci, V.; Hasirci, N. Development of porous chitosan–gelatin/hydroxyapatite composite scaffolds for hard tissue-engineering applications. J. Tissue Eng. Regen. Med. 2012, 6, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Nicholson, J.W. A review of glass-ionomer cement for clinical dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Venhoven, B.A.; de Gee, A.J.; Werner, A.; Davidson, C.L. Influence of filler parameters on the mechanical coherence of dental restorative resin composites. Biomaterials 1996, 17, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Arcís, R.; López-Macipe, A.; Osorio, R.; Rodríguez-Clemente, R. Dental composites reinforced with hydroxyapatite: Mechanical behaviour and absorption/elution characteristics. J. Biomed. Mater. Res. 2001, 56, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Gao, J.; Wang, M.; Fu, J.; Wang, D. Ultra-trace silver-doped hydroxyapatite with non-cytotoxicity and effective antibacterial activity. Mater. Sci. Eng. 2015, 55, 497–505. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2019, 7, 469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).