Novel Orthodontic Cement Comprising Unique Imidazolium-Based Polymerizable Antibacterial Monomers
Abstract
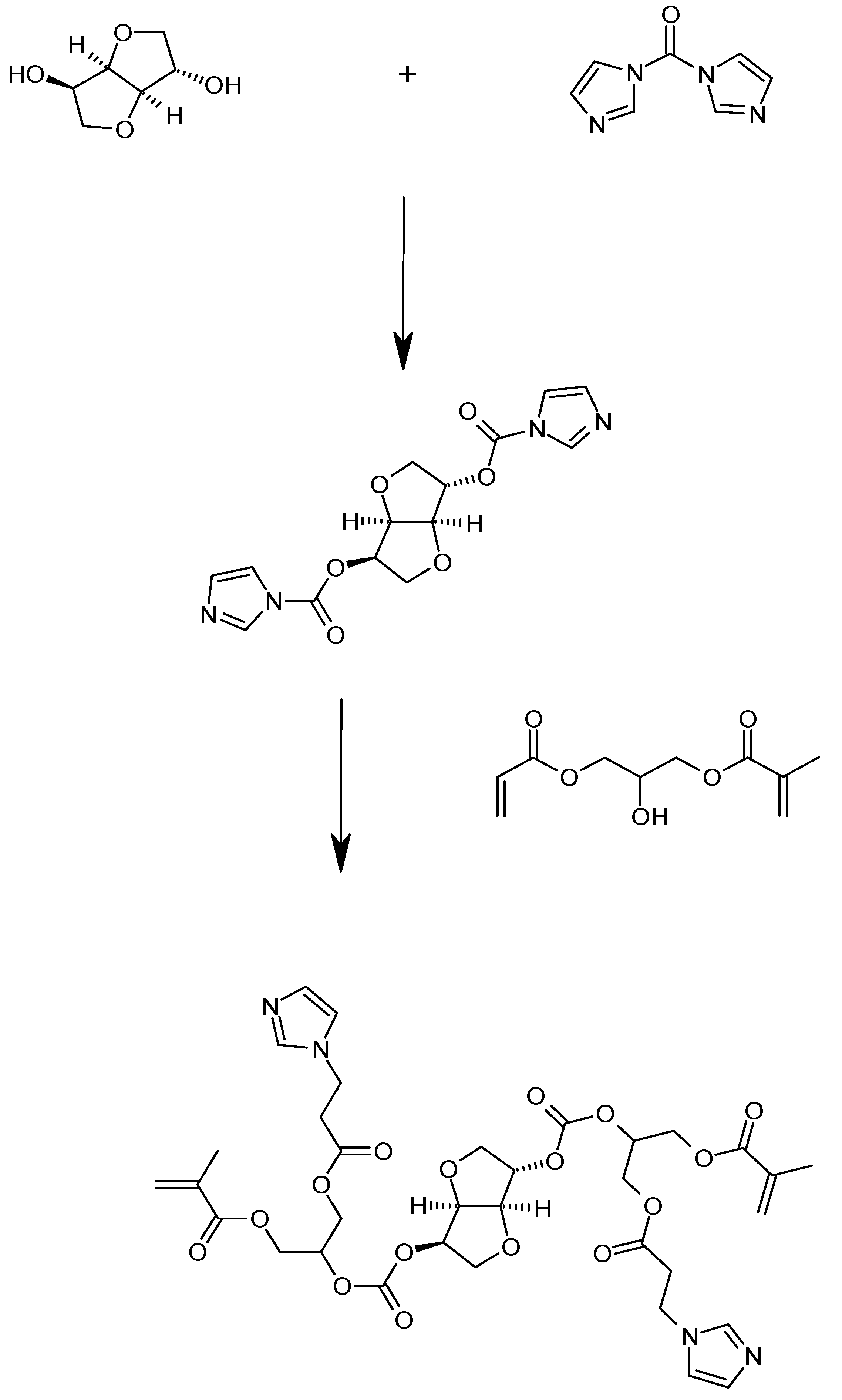
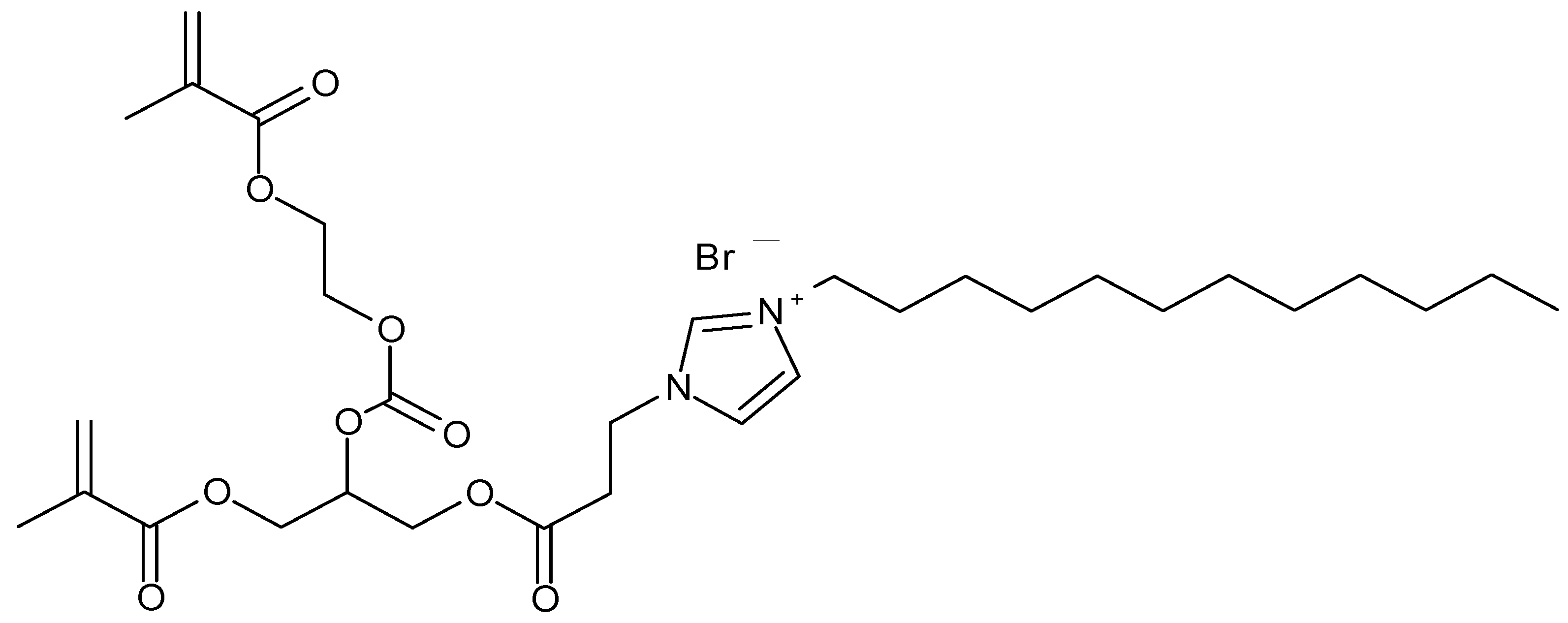
1. Introduction
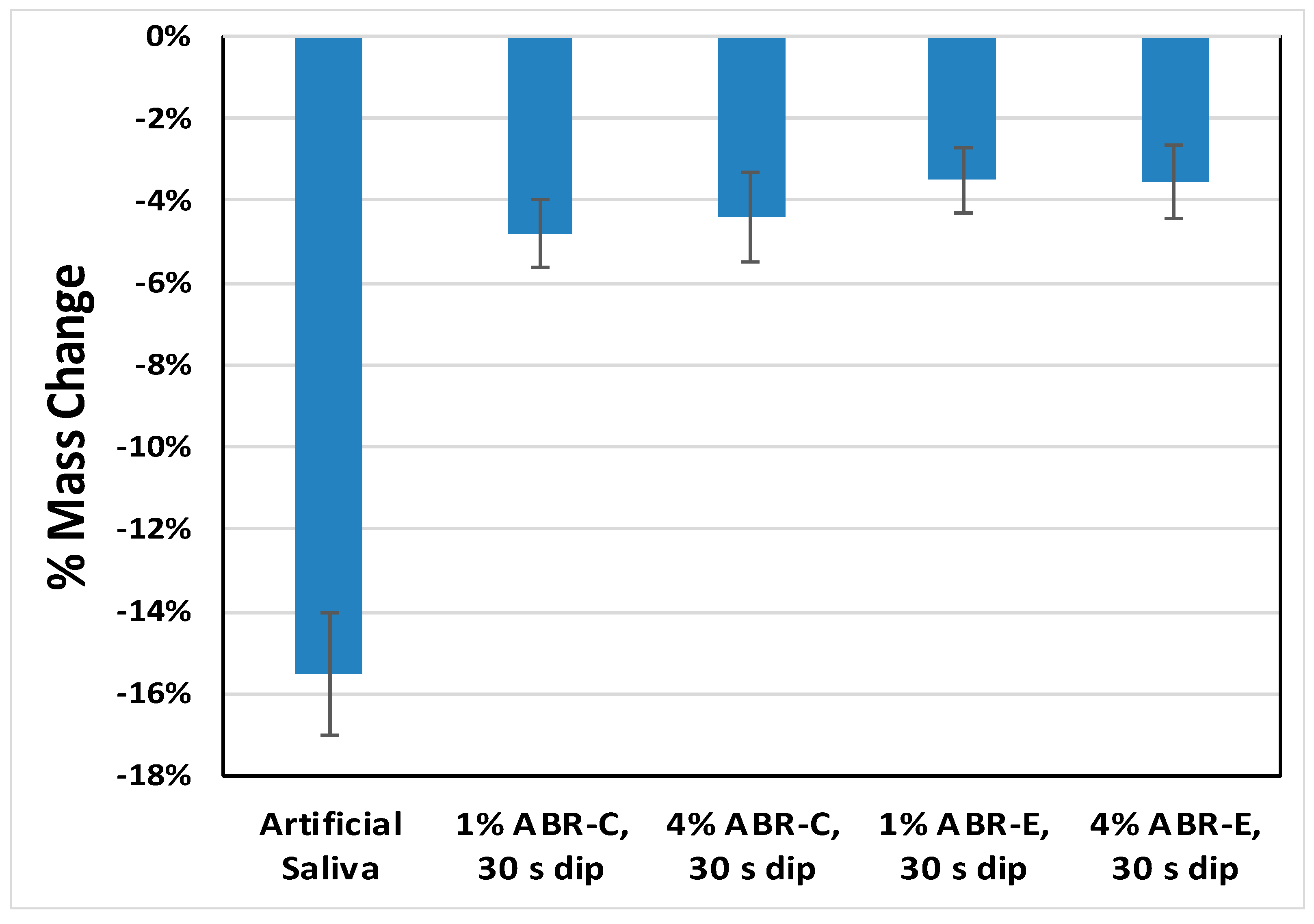
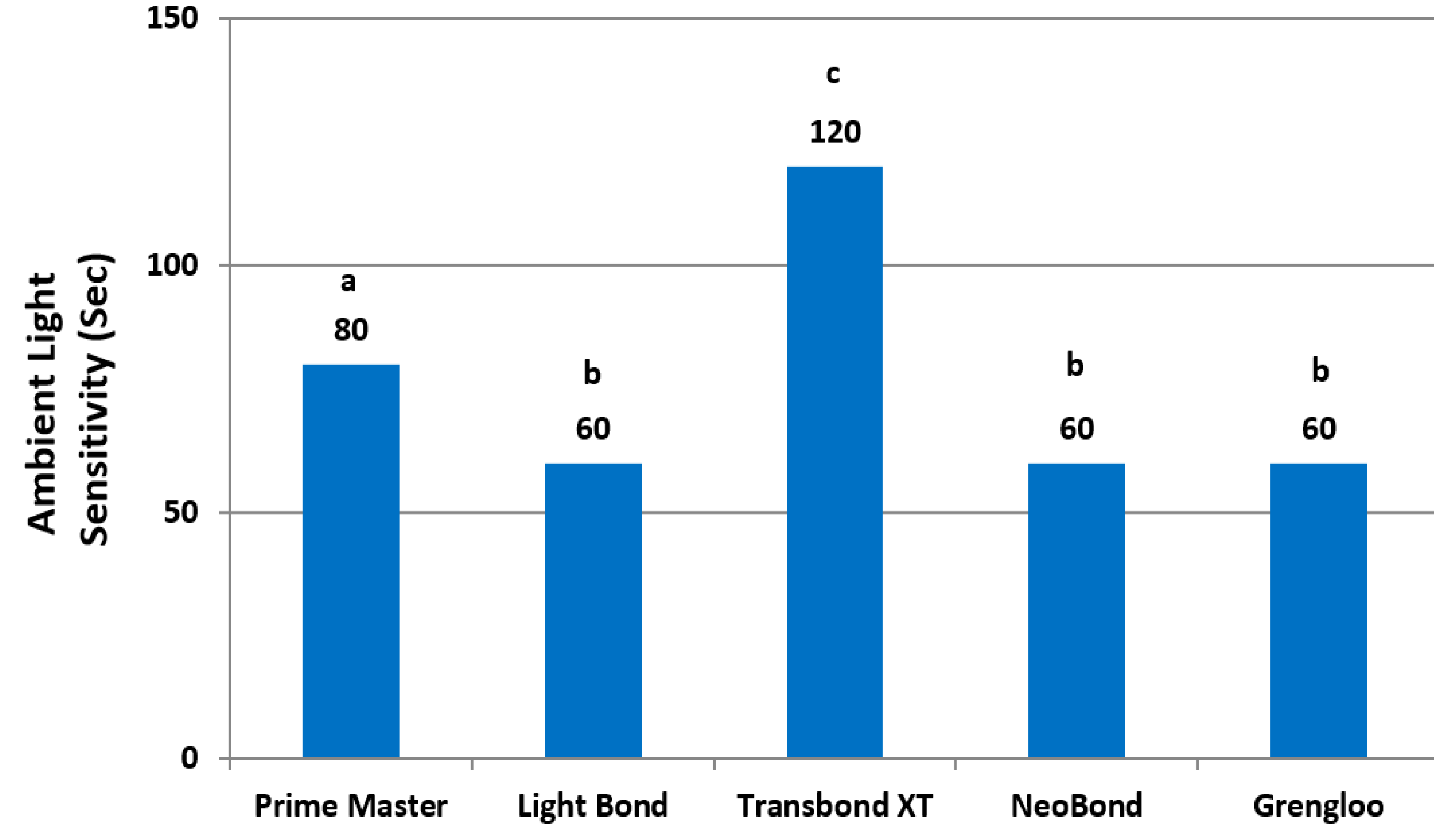
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Evaluation Methods
- B = Average number of viable cells on the control pieces after 24 h.
- C = Average number of viable cells on the test pieces after 24 h.
- B = Number of viable test microorganisms on the control carriers after the contact time.
- A = Number of viable test microorganisms on the test carriers after the contact time.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nascimento, V.; Conti, A.; Cardoso, M.; Valarelli, D.; Almeida-Pedrin, R. Impact of orthodontic treatment on self-esteem and quality of life of adult patients requiring oral rehabilitation. Angle Orthod. 2016, 86, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Grewal, H.; Sapawat, P.; Modi, P.; Aggarwal, S. Psychological impact of orthodontic treatment on quality of life—A longitudinal study. Int. Orthod. 2019, 17, 269–276. [Google Scholar] [CrossRef]
- Wishney, M. Potential risks of orthodontic therapy: A critical review and conceptual framework. Aust. Dent. J. 2017, 62, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Heymann, G.C.; Grauer, D. A contemporary review of white spot lesions in orthodontics. J. Esthet. Restor. Dent. 2013, 25, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.A.; Roberts, W.E.; Eckert, G.J.; Kula, K.S.; González-Cabezas, C. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 188–194. [Google Scholar] [CrossRef]
- Buschang, P.H.; Chastain, D.; Keylor, C.L.; Crosby, D.; Julien, K. Incidence of white spot lesions among patients treated with clear aligners and traditional braces. Angle Orthod. 2019, 89, 359–364. [Google Scholar] [CrossRef]
- Tasios, T.; Papageorgiou, S.N.; Papadopoulos, M.A.; Tsapas, A.; Haidich, A.B. Prevention of orthodontic enamel demineralization: A systematic review with meta-analyses. Orthod. Craniofac. Res. 2019, 22, 225–235. [Google Scholar] [CrossRef]
- Leeper, D.K.; Noureldin, A.; Julien, K.; Campbell, P.M.; Buschang, P.H. Risk assessments in orthodontic patients developing white spot lesions. J. Investig. Clin. Dent. 2019, 10, e12470. [Google Scholar] [CrossRef]
- Tufekci, E.; Dixon, J.S.; Gunsolley, J.C.; Lindauer, S.J. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod. 2011, 81, 206–210. [Google Scholar] [CrossRef]
- Khalaf, K. Factors affecting the formation, severity and location of white spot lesions during orthodontic treatment with fixed appliances. J. Oral Maxillofac. Res. 2014, 5. [Google Scholar] [CrossRef]
- Joen, D.; An, J.; Lim, B.; Ahn, S. Orthodontic bonding procedures significantly influence biofilm composition. Prog. Orthod. 2020, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, H.A.; Rangmar, S.; Kjellberg, H.; Birkhed, D. Dental Caries and Risk Factors in Swedish Adolescents about to Start Orthodontic Treatment with Fixed Appliances. J. Contemp. Dent. Pract. 2019, 20, 538–562. [Google Scholar] [CrossRef]
- Srivastava, K.; Tikku, T.; Khanna, R.; Sachan, K. Risk factors and management of white spot lesions in orthodontics. J. Orthod. Sci. 2013, 2, 43–49. [Google Scholar] [CrossRef]
- Sonesson, M.; Svensäter, G.; Wickström, C. Glucosidase activity in dental biofilms in adolescent patients with fixed orthodontic appliances—A putative marker for white spot lesions—A clinical exploratory trial. Arch. Oral Biol. 2019, 102, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, P.M.; Berry, C.W.; Bennett, C.L.; Israelson, H. Changes in gingival and gingival flora with bonding and banding. Angle Orthod. 1987, 57, 271–278. [Google Scholar] [PubMed]
- Ristic, M.; Svabic, M.V.; Sasic, M.; Zelic, O. Clinical and microbiological effects of fixed orthodontic appliances on periodontal tissues in adolescents. Orthod. Craniofac. Res. 2007, 10, 187–195. [Google Scholar] [CrossRef]
- Paolantonio, M.; Festa, F.; Di Placido, G.; D’Attilio, M.; Catamo, G.; Piccolomini, R. Site-specific subgingival colonization by Actinobacillus actinomycetencomitans in orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 423–428. [Google Scholar] [CrossRef]
- Lucchese, A.; Bondemark, L.; Marcolina, M.; Manuelli, M. Changes in oral microbiota due to orthodontic appliances: A systematic review. J. Oral Microbiol. 2018, 10, 1476645. [Google Scholar] [CrossRef]
- Freitas, A.; Marquezan, M.; Nojima, M.; Alviano, D.; Maia, L. The influence of orthodontic fixed appliances on the oral microbiota: A systematic review. Dent. Press J. Orthod. 2014, 19, 46–55. [Google Scholar] [CrossRef]
- Bourbia, M.; Ma, D.; Cvitkovitch, D.; Santerre, J.P.; Finer, Y. Cariogenic Bacteria Degrade Dental Resin Composites and Adhesives. J. Dent. Res. 2013, 92, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Finer, Y. Biostable, antidegradative and antimicrobial restorative systems based on host-biomaterials and microbial interactions. Dent. Mater. 2019, 35, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef]
- Bergstrand, F.; Twetman, S. A review on prevention and treatment of post-orthodontic white spot Lesions—Evidence-based methods and emerging technologies. Open Dent. J. 2011, 5, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Sandra, C.D.; Maria, D.; Ingrid, M.D.; Vanessa, H.D.; Katia, V.R.; Leandro, C. Preventing and Arresting the Appearance of White Spot Lesions around the Bracket by applying Fluoride Varnish: A Systematic Review. Dentistry 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Aghoutan, H.; Alami, S.; Quars, F.E.; Diouny, S.; Bourzgui, F. White Spots Lesions in Orthodontic Treatment and Fluoride—Clinical Evidence. In Emerging Trends in Oral Health Sciences and Dentistry, 1st ed.; Virdi, M., Ed.; IntechOpen Limited: London, UK, 2015. [Google Scholar] [CrossRef]
- Sonesson, M.; Brechter, A.; Abdulraheem, S.; Lindman, R.; Twetman, S.S. Fluoride varnish for the prevention of white spot lesions during orthodontic treatment with fixed appliances: A randomized controlled trial. Eur. J. Orthod. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Höchli, D.; Hersberger-Zurfluh, M.; Papageorgiou, S.N.; Eliades, T. Interventions for orthodontically induced white spot lesions: A systematic review and meta-analysis. Eur. J. Orthod. 2017, 39, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.B.; Fernandes, A.R.; Coelho, A.; Marto, C.M.; Marques-Ferreira, M.; Caramelo, F.; Vale, F.; Carrilho, E. Therapies for White Spot Lesions-A Systematic Review. J. Evid. Based Dent. Pract. 2017, 17, 23–38. [Google Scholar] [CrossRef]
- Beyth, N.; Yudovin-Farber, I.; Perez-Davidi, M.; Domb, A.J.; Weiss, E.I. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 22038–22043. [Google Scholar] [CrossRef]
- Zaltsman, N.; Shvero, N.D.; Polak, D.; Weiss, E.I.; Beyth, N. Antibacterial orthodontic adhesive incorporating polyethyleneimine nanoparticles. Oral Health Prev. Dent. 2017, 15, 245–250. [Google Scholar] [CrossRef]
- Varon-Shahar, E.; Sharon, E.; Zabrovsky, A.; Houri-Haddad, Y.; Beyth, N. Orthodontic cements and adhesives: A possible solution to streptococcus mutans outgrowth adjacent to orthodontic appliances. Oral Health Prev. Dent. 2019, 17, 49–56. [Google Scholar] [CrossRef]
- Imazato, S.; Ehara, A.; Torii, M.; Ebisu, S. Antibacterial activity of dentine primer containing MDPB after curing. J. Dent. 1998, 26, 267–271. [Google Scholar] [CrossRef]
- Oz, A.Z.; Oz, A.A.; Yazicioglu, S.; Sancaktar, O. Effectiveness of an antibacterial primer used with adhesive-coated brackets on enamel demineralization around brackets: An in vivo study. Prog. Orthod. 2019, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, N.; Xu, H.H.K.; Weir, M.D.; Melo, M.A.S.; Bai, Y.; Zhang, K. Novel orthodontic cement containing dimethylaminohexadecyl methacrylate with strong antibacterial capability. Dent. Mater. J. 2017, 36, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, N.; Wang, B.; Park, S.R.; Weir, M.D.; Xu, H.H.K.; Bai, Y. Novel self-etching and antibacterial orthodontic adhesive containing dimethylaminohexadecyl methacrylate to inhibit enamel demineralization. Dent. Mater. J. 2018, 37, 555–561. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.; Xu, H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Wu, J.; Weir, M.D.; Xu, H.H.K. Novel antibacterial orthodontic cement containing quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2014, 42, 1193–1201. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Zhou, X.; Xu, N.; Xu, H.H.K.; Weir, M.D.; Ge, Y.; Wang, S.; Li, M.; Li, Y.; et al. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of Streptococcus mutans biofilm. Int. J. Mol. Sci. 2014, 15, 12791–12806. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, Y.H.; Xing, X.D.; Li, F.; Ma, S.; Qi, L.L.; Chen, J.H. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Arch. Oral Bio 2011, 56, 367–373. [Google Scholar] [CrossRef]
- Yu, F.; Dong, Y.; Yu, H.-H.; Lin, P.-T.; Zhang, L.; Sun, X.; Liu, Y.; Xia, Y.-N.; Huang, L.; Chen, J.-H. Antibacterial Activity and Bonding Ability of an Orthodontic Adhesive Containing the Antibacterial Monomer 2-Methacryloxylethyl Hexadecyl Methyl Ammonium Bromide. Sci. Rep. 2017, 7, 41787. [Google Scholar] [CrossRef]
- Jin, X. Method and Antibacterial/Antimicrobial Compositions in Dental Compositions. U.S. Patent 8747831, 2014. [Google Scholar]
- Jin, X. Imidazole and Imidazolium Resins and Methods for Preparing Curable Imidazolium Antimicrobial Resins. U.S. Patent RE47512, 2016. [Google Scholar]
- De Almeida, C.M.; Da Rosa, W.L.O.; Meereis, C.T.W.; Ribeiro, J.S.; Da Silva, A.F.; Lund, R.G.; De Almeida, S.M. Efficacy of antimicrobial agents incorporated in orthodontic bonding systems: A systematic review and meta-analysis. J. Orthod. 2018, 45, 79–93. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Perez, L.; Manresa, A.; Comelles, F. Aggregation Behavior and Antimicrobial Activity of Ester-Functionalized Imidazolium-and Pyridinium-Based Ionic Liquids in Aqueous Solution. Langmuir 2013, 29, 2536–2545. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V. A profile of the in vitro anti-tumor activity of imidazolium-based ionic liquids. Bioorganic Med. Chem. Lett. 2010, 20, 581–585. [Google Scholar] [CrossRef]
- Mazzoni, A.; Tjäderhane, L.; Checchi, V.; Di Lenarda, R.; Salo, T.; Tay, F.; Pashley, D.; Breschi, L. Role of dentin MMPs in caries progression and bond stability. J. Dent. Res. 2015, 94, 241–251. [Google Scholar] [CrossRef]
- Sadek, F.T.; Braga, R.R.; Muench, A.; Liu, Y.; Pashley, D.H.; Tay, F.R. Ethanol wet-bonding challenges current anti-degradation strategy. J. Dent. Res. 2010, 89, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Gostemeyer, G.; Schwendicke, F. Inhibition of hybrid layer degradation by cavity pretreatment: Meta- and trial sequential analysis. J. Dent. 2016, 49, 14–21. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, S.; Zhou, X.; Wang, H.; Xu, H.H.K.; Cheng, L. The use of quaternary ammonium to combat dental caries. Materials 2015, 8, 3532–3549. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Koltisko, B.; Jin, X.; Koo, H. Nonleachable imidazolium-incorporated composite for disruption of bacterial clustering, exopolysaccharide-matrix assembly, and enhanced biofilm removal. ACS Appl. Mater. Interfaces 2017, 9, 38270–38280. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
- Riduan, S.N.; Zhang, Y. Imidazolium salts and their polymeric materials for biological applications. Chem. Soc. Rev. 2013, 42, 9055–9070. [Google Scholar] [CrossRef]



| Test Compound | Stock Solution | Tested Concentrations | Bacterial Inoculum Size (CFU/mL) | MIC | MBC |
|---|---|---|---|---|---|
| ABR-E | 2.5% | 0–0.1% | 9 × 107 | 4 µg/mL (0.0004%) | 8 µg/mL (0.0008%) |
| ABR-C | 1% | 0–0.1% | 9 × 107 | 4 µg/mL (0.0004%) | 8 µg/mL (0.0008%) |
| SDR Resin | 0.25% | 0–0.1% | 9 × 107 | No bacterial growth inhibition | |
| TEGDMA | 100% | 0–0.1% | 9 × 107 | No bacterial growth inhibition | |
| Chlorhexidine | 2.5 mg/mL | 0–125 mg/mL | 9 × 107 | 2 mg/mL | 4 mg/mL |
| Resin Sample | Control | 4%_ABR-C | 8%_ABR-C | 12%_ABR-C | 16%_ABR-C |
|---|---|---|---|---|---|
| Antibacterial Monomer in Resin | 0 | 4 wt% | 8 wt% | 12 wt% | 16 wt% |
| Flexural Strength, MPa (s.d.) | 97 (8) A | 88 (5) A,B | 91 (4) A | 95 (3) A | 81 (4) B |
| Flexural Modulus, MPa (s.d.) | 2443 (87) a | 2388 (183) a | 2394 (139) a | 2293 (82) a,b | 2125 (125) b |
| Orthodontic Cement | Control | 1%_ABR-C | 2%_ABR-C | 3%_ABR-C | 4%_ABR-C |
|---|---|---|---|---|---|
| Antibacterial Monomer in Cement | 0 | 1 wt% | 2 wt% | 3 wt% | 4 wt% |
| Resin Conc. | 25.0% | 25.0% | 25.0% | 25.0% | 25.0% |
| Ambient Light Sensitivity | 2:35" | 2:00" | 2:05" | 2:00" | 2:15" |
| Compressive Strength, MPa (s.d.) | 373 (25) A | 361 (12) A,B | 346 (10) B | 352 (5) A,B | 307 (15) C |
| Flexural Strength, MPa (s.d.) | 150 (14) A | 139 (6) A | 114 (10) B | 114 (10) B | 88 (7) C |
| Flexural Modulus, MPa (s.d.) | 11165 (531) a | 10848 (383) a,b | 10319 (549) a,b | 10607 (629) a,b | 10066 (445) b |
| NE-SBS to Etched Enamel, MPa (s.d.) | 32.8 (2.7) A,B | 36.7 (4.5) A | 28.8 (4.3) B,C | 25.4 (3.5) C | 31.0 (5.8) A,B,C |
| SBS to Enamel Range, MPa | 29.5 ~ 37.2 | 31.9 ~ 43.4 | 21.9 ~ 32.7 | 21.5 ~ 29.5 | 23.1 ~ 38.0 |
| SBS to Enamel C.V. | 8.1% | 12.1% | 14.8% | 14.0% | 18.7% |
| Test Micro-Organism | Contact Time | Carrier Type | Anti-Bacterial Monomer in Cement | CFU/Carrier | Percent Reduction Compared to Control at Contact Time | Log10 Reduction Compared to Control at Contact Time |
|---|---|---|---|---|---|---|
| S. aureus 6538 | Time Zero | Control | 0 | 1.00 × 106 | N/A | |
| 24 h | ATL Control | 0 | 8.50 × 105 | |||
| 2%_ABR-C | 2 wt% | 1.00 × 10 | 99.9988% | 4.93 | ||
| 4%_ABR-C | 4 wt% | 1.50 × 10 | 99.998% | 4.75 | ||
| 1%_ABR-C | 1 wt% | 4.72 × 103 | 99.44% | 2.26 | ||
| 3%_ABR-C | 3 wt% | <5.00 | > 99.9994% | > 5.23 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Jin, X. Novel Orthodontic Cement Comprising Unique Imidazolium-Based Polymerizable Antibacterial Monomers. J. Funct. Biomater. 2020, 11, 75. https://doi.org/10.3390/jfb11040075
Lu H, Jin X. Novel Orthodontic Cement Comprising Unique Imidazolium-Based Polymerizable Antibacterial Monomers. Journal of Functional Biomaterials. 2020; 11(4):75. https://doi.org/10.3390/jfb11040075
Chicago/Turabian StyleLu, Hui, and Xiaoming Jin. 2020. "Novel Orthodontic Cement Comprising Unique Imidazolium-Based Polymerizable Antibacterial Monomers" Journal of Functional Biomaterials 11, no. 4: 75. https://doi.org/10.3390/jfb11040075
APA StyleLu, H., & Jin, X. (2020). Novel Orthodontic Cement Comprising Unique Imidazolium-Based Polymerizable Antibacterial Monomers. Journal of Functional Biomaterials, 11(4), 75. https://doi.org/10.3390/jfb11040075




