Are Fe-Based Stenting Materials Biocompatible? A Critical Review of In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Iron Corrosion and Toxicity
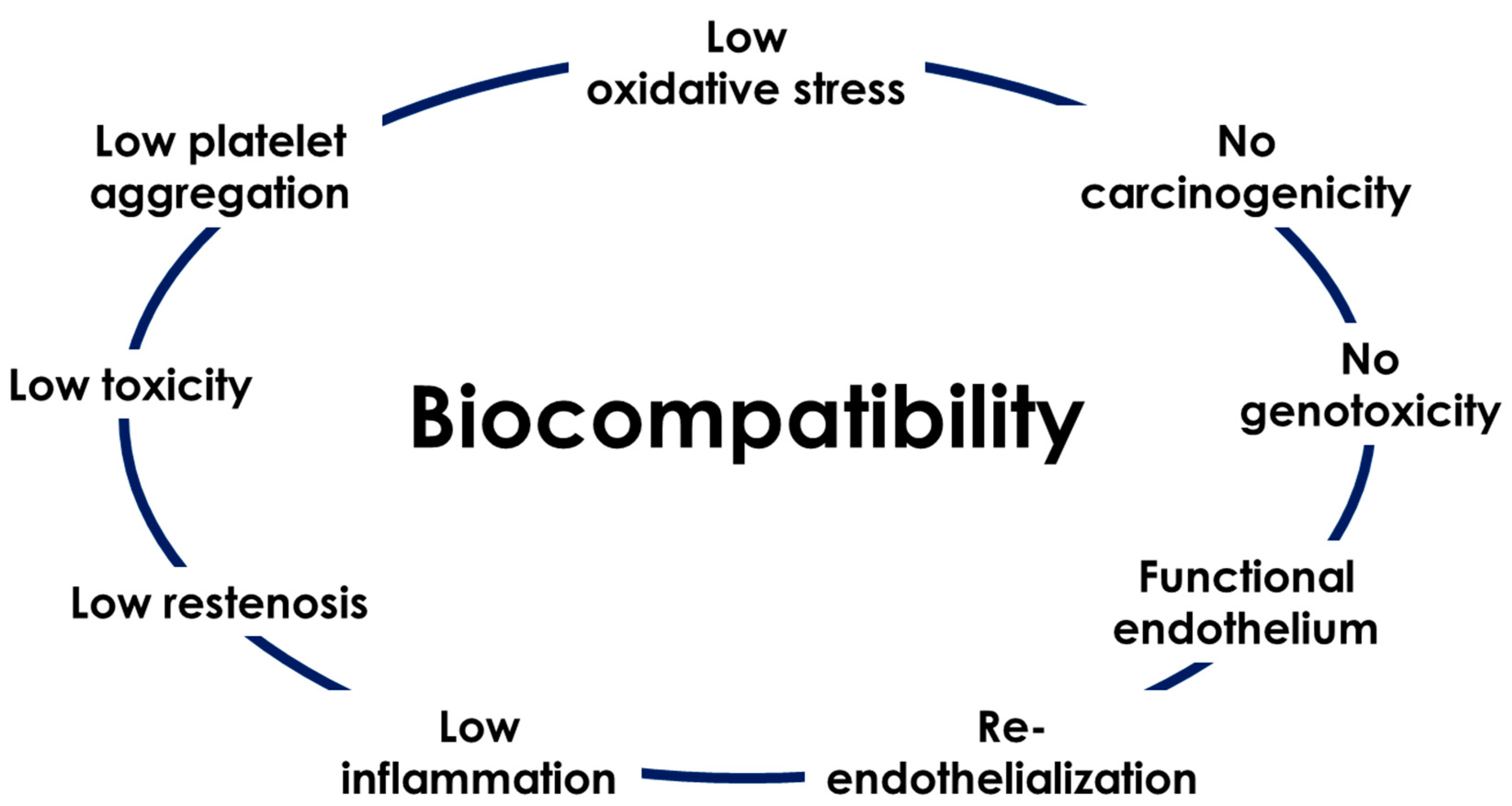
3. Assessing the Biocompatibiliy of Fe-Based Materials
3.1. In Vitro Experiments
- The majority of cell viability studies were performed only by exposure to extracts after centrifugation. This simple methodology presents some advantages, e.g., homogeneous concentrations of ion exposure, whereas replicates of insoluble leachates are more problematic to reproduce. However, it assumes that released soluble ions are the main species that can cause cytotoxicity. Few authors compared the consequences of the different procedures on cell cultures. Lin et al. [35] compared the three methods for in vitro cytotoxicity evaluation, i.e., testing of extracts, direct contact, and the indirect contact method, and they reported completely opposite results. They showed a high fibroblast cytotoxicity after the direct exposure to corrosion particles precipitating during extraction or incubation processes, whereas the exposure to extracts (only iron ions) did not induce cytotoxicity. These results suggest that the supernatant and degradation products in the extracts should be assessed separately in order to identify the exact species responsible for toxic effects. Fagali et al. [36] wondered if soluble and insoluble Fe degradation products have different biological impacts, and they concluded that cell toxicity is mainly associated with the presence of insoluble products. The corrosion of Fe-containing materials in a biological environment involves both soluble and insoluble Fe species, stressing the importance of distinguishing the impact of all the components individually. Moreover, as described above, ROIs such as hydroxyl radicals are released during corrosion, which could react with surrounding cells. Due to their short half-life, their possible cytotoxic activity is completely missed in the indirect contact test. We demonstrated in our previous work that only the direct contact between the Fe and cells, and not degradation products, caused cytotoxicity and oxidative stress through HO• release, as confirmed by the protective role of catalase [37].
- The surface of bulk materials is often pretreated prior to cellular testing. Grinding processes differ from 1000 to 4000 mesh papers and are only seldom followed by diamond paste polishing. Surface treatments such as mechanical polishing or electrolytic polishing enhance the corrosion resistance, while an increased surface roughness amplitude with a low surface organization increases the corrosion rate [38]. It has been previously demonstrated that surface roughness amplitude has an influence on the corrosion rate of biodegradable materials and consequently the concentration of released species [39,40]. Moreover, Martin et al. [41] demonstrated that the surface roughness of Ti implants alters osteoblast proliferation, differentiation, and matrix production in vitro. Therefore, to simulate the biodegradation process of implants and the related released species, the surface treatment of test samples should be as close as possible to clinical products [42].Cells used for cytotoxicity assays are not always relevant for endovascular implants (e.g., BALB/3T3 fibroblasts). The stent is mainly in contact with endothelial and smooth muscle cells during its lifetime, simultaneously during the initial wound-healing phase, or exclusively with SMCs after neointima formation. Selecting a given cell type implies a specific scenario for the remodeling of the artery as each cell type has a different role and sensitivity. Some authors examined the response of cell types separately and found preferential cellular sensitivity [35,43,44,45]. As restenosis is one of the principal adverse effects of stent implantation, some authors argue that elective cytotoxicity to VSMCs could antagonize restenosis by reducing excessive vascular cell proliferation [34]. However, this probably represents oversimplification because neointimal proliferation is a complex process involving the interaction with different cell types, including endothelial cells, platelets and macrophages. Additionally, it should be demonstrated that VSMC cytotoxicity is not associated with other damage such as oxidative stress, or consequent dysfunction.Few authors have used human cell lines, highly relevant for the development of human endovascular implants, or primary cell lines, instead of animal cells, which are easier to handle and give more consistent results but are less closely related to the clinical situation [7,18,35,45]. Finally, so far, Fe-based materials have not been tested in co-culture or 3-dimensional models, which might better predict the in vivo response in humans.
- Cell viability is often assessed by a single assay. Interference and disturbances in viability assays are, however, likely to happen as previously reported for materials other than Fe [46,47,48]. Only a few authors have checked for possible interference, and efforts have been limited to merely absorbance interference, rarely exploring deeply into the possible mechanism of the interaction [18]. Multiple assays should be combined for an overall cytocompatibility assessment of bioabsorbable Fe-based materials, as well as for any material.
- To investigate the mechanism of toxicity in depth, other endpoints, such as the cell cycle and gene expression profile, have to be assessed in order to define a material as biocompatible. Indeed, at the early stage, ROIs released from the material could induce oxidative stress with an increase of oxidative stress genes such as HO-1 [37], or induce genomic DNA mutation. Carcinogenesis may be caused by depletion of antioxidant defenses, nuclear transcription factor, such as NF-kB, activation, or cell growth regulation alterations [49]. Assessing DNA alterations or damage, using a simple method such as the comet assay, can thus be fundamental for defining the potential genotoxicity of a material.
- Assessing blood compatibility is a fundamental part of defining a material as biocompatible. Blood flow across the stent surface could induce erythrocyte rupture, adsorption of plasma proteins leading to platelet activation, and finally, activation of the intrinsic coagulation pathway, resulting in thrombin activation [50]. Some studies have defined Fe-based alloys as biocompatible degradable biomaterials exclusively based on cytotoxicity results, without assessing hemolysis, platelet adhesion or coagulation [18,51]. Even if in vitro assays enable one to reproduce the physiological environment and the endothelium plays a key role in platelet activation, the blood compatibility test is a first step for prescreening a material. Few authors performed platelet adhesion or haemolysis assays, as indicated in Table 1. Overall, investigators showed a good in vitro blood compatibility for Fe-based materials, with a hemolysis rate less than 5%, according to the ISO standard ISO 10993-4, and an anti-platelet adhesion property in comparison with 316 L stainless steel.
- Finally, all authors have used healthy cell lines. As the target tissue is by definition diseased when a stent is implanted, the impact of Fe corrosion on cells from patients with coronary disease or on cells from ApoE mice, that develop atherosclerosis, might be more appropriate [52]. Messer et al. [53] showed, moreover, that the presence of monocytes in vitro, as an indicator of inflammatory disease, decreased the corrosion rate of stainless steel, demonstrating the importance of addressing the interaction of candidate implant materials with multiple components of atheromatous tissue.
3.2. In Vivo Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Purnama, A.; Hermawan, H.; Mantovani, D. Biodegradable Metal Stents: A Focused Review on Materials and Clinical Studies. J. Biomater. Tissue Eng. 2014, 4, 868–874. [Google Scholar] [CrossRef]
- Waksman, R.O.N.; Pakala, R.; Baffour, R.; Seabron, R.; Hellinga, D.; Fermin, O.; Tio, F.O. Short-term effects of biocorrodible iron stents in porcine coronary arteries. J. Interv. Cardiol. 2008, 21, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Zhao, S.; Guillory, R.J.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Pierson, D.; Edick, J.; Tauscher, A.; Pokorney, E.; Bowen, P.; Gelbaugh, J.; Stinson, J.; Getty, H.; Lee, C.H.; Drelich, J.; et al. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res. Part B 2012, 100, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Yang, Y.; Virtanen, S.; Boccaccini, A.R. Boccaccini, Iron and iron-based alloys for temporary cardiovascular applications. J. Mater. Sci. Mater. Med. 2015, 26, 138. [Google Scholar] [CrossRef] [PubMed]
- Peuster, M.; Wohlsein, P.; Brügmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal—Results 6–18 months after implantation into New Zealand white rabbits. Heart (Lond. UK) 2001, 86, 563–569. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, N.; Xu, L.; Zhang, Y.; Liu, H.; Sun, H.; Leng, Y. Biocompatibility of pure iron: In vitro assessment of degradation kinetics and cytotoxicity on endothelial cells. Mater. Sci. Eng. C 2009, 29, 1589–1592. [Google Scholar] [CrossRef]
- Levesque, J.; Hermawan, H.; Dube, D.; Mantovani, D. Design of a pseudo-physiological test bench specific to the development of biodegradable metallic biomaterials. Acta Biomater. 2008, 4, 284–295. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable Fe-Mn-C(-Pd) alloys. Mater. Sci. Eng. C 2013, 33, 1882–1893. [Google Scholar] [CrossRef]
- Borhani, S.; Hassanajili, S.; Tafti, S.H.A.; Rabbani, S. Cardiovascular stents: Overview, evolution, and next generation. Prog. Biomater. 2018, 7, 175–205. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharm. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Ponka, P.; Tenenbein, M.; Eaton, J.W. Chapter 41—Iron, in Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 879–902. [Google Scholar]
- Maines, M.D. The heme oxygenase system: Past, present, and future. Antioxid. Redox Signal. 2004, 6, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C. Molecular control of iron metabolism. Best Prac. Res. Clin. Haematol. 2005, 18, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.; Enns, C.; Wessling-Resnick, M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001, 33, 940–959. [Google Scholar] [CrossRef]
- Prousek, J.; Palacková, E.; Priesolová, S.; Marková, L.; Alevová, A. Fenton- and Fenton-Like AOPs for Wastewater Treatment: From Laboratory-To-Plant-Scale Application. Sep. Sci. Technol. 2007, 42, 1505–1520. [Google Scholar] [CrossRef]
- Schinhammer, M.; Gerber, I.; Hänzi, A.C.; Uggowitzer, P.J. On the cytocompatibility of biodegradable Fe-based alloys. Mater. Sci. Eng. C 2013, 33, 782–789. [Google Scholar] [CrossRef]
- Huang, X. Iron overload and its association with cancer risk in humans: Evidence for iron as a carcinogenic metal. Mutat. Res. 2003, 533, 153–171. [Google Scholar] [CrossRef]
- Weinberg, E.D. The Development of Awareness of the Carcinogenic Hazard of Inhaled Iron. Oncol. Res. 1999, 11, 109–113. [Google Scholar]
- Lison, D.; Carbonnelle, P.; Mollo, L.; Lauwerys, R.; Fubini, B. Physicochemical mechanism of the interaction between cobalt metal and carbide particles to generate toxic activated oxygen species. Chem. Res. Toxicol. 1995, 8, 600–606. [Google Scholar] [CrossRef]
- Tsaryk, R.; Kalbacova, M.; Hempel, U.; Scharnweber, D.; Unger, R.E.; Dieter, P.; Kirkpatrick, C.J.; Peters, K. Response of human endothelial cells to oxidative stress on Ti6Al4V alloy. Biomaterials 2007, 28, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Tsaryk, R.; Peters, K.; Barth, S.; Unger, R.E.; Scharnweber, D.; Kirkpatrick, C.J. The role of oxidative stress in pro-inflammatory activation of human endothelial cells on Ti6Al4V alloy. Biomaterials 2013, 34, 8075–8085. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Chen, K.; Keaney, J.F., Jr. Evolving concepts of oxidative stress and reactive oxygen species in cardiovascular disease. Curr. Atheroscler. Rep. 2012, 14, 476–483. [Google Scholar] [CrossRef]
- Williams, D.F. There is no such thing as a biocompatible material. Biomaterials 2014, 35, 10009–10014. [Google Scholar] [CrossRef]
- Khan, W.; Muntimadugu, E.; Jaffe, M.; Domb, A.J. Domb, Implantable Medical Devices. In Focal Controlled Drug Delivery; Springer: Boston, MA, USA, 2014; pp. 33–59. [Google Scholar] [CrossRef]
- Onuki, Y.; Bhardwaj, U.; Papadimitrakopoulos, F.; Burgess, D.J. A Review of the Biocompatibility of Implantable Devices: Current Challenges to Overcome Foreign Body Response. J. Diabetes Sci. Technol. 2008, 2, 1003–1015. [Google Scholar] [CrossRef]
- ISO. ISO 10993-5-2009 International Organisation for Standardization, 3rd ed.; International Organization for Standardization (ISO): London, UK, 2009. [Google Scholar]
- Purnama, A.; Hermawan, H.; Couet, J.; Mantovani, D. Assessing the biocompatibility of degradable metallic materials: State-of-the-art and focus on the potential of genetic regulation. Acta Biomater. 2010, 6, 1800–1807. [Google Scholar] [CrossRef]
- Korybalska, K.; Kawka, E.; Breborowicz, A.; Witowski, J. Atorvastatin does not impair endothelial cell wound healing in an in vitro model of vascular injury. J. Physiol. Pharmacol. 2012, 63, 389–395. [Google Scholar] [CrossRef]
- Ivanova, L.; Varinska, L.; Pilatova, M.; Gal, P.; Solar, P.; Perjesi, P.; Smetana, K.; Ostro, A., Jr.; Mojzis, J. Cyclic chalcone analogue KRP6 as a potent modulator of cell proliferation: An in vitro study in HUVECs. Mol. Biol. Rep. 2013, 40, 4571–4580. [Google Scholar] [CrossRef]
- Zhu, T.; Yao, Q.; Hu, X.; Chen, C.; Yao, H.; Chao, J. The Role of MCPIP1 in Ischemia/Reperfusion Injury-Induced HUVEC Migration and Apoptosis. Cell. Physiol. Biochem. 2015, 37, 577–591. [Google Scholar] [CrossRef]
- Mueller, P.P.; May, T.; Perz, A.; Hauser, H.; Peuster, M. Control of smooth muscle cell proliferation by ferrous iron. Biomaterials 2006, 27, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, G.; Cao, P.; Zhang, D.; Zheng, Y.; Wu, R.; Qin, L.; Wang, G.; Wen, T. Cytotoxicity and its test methodology for a bioabsorbable nitrided iron stent. J. Biomed. Mater. Res. Part B 2015, 103, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Fagali, N.S.; Grillo, C.A.; Puntarulo, S.; de Mele, M.A.F.L. Is there any difference in the biological impact of soluble and insoluble degradation products of iron-containing biomaterials? Colloids Surf. B 2017, 160, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Scarcello, E.; Herpain, A.; Tomatis, M.; Turci, F.; Jacques, P.J.; Lison, D. Hydroxyl radicals and oxidative stress: The dark side of Fe corrosion. Colloids Surf. B 2019, 185, 110542. [Google Scholar] [CrossRef] [PubMed]
- Giljean, S.; Bigerelle, M.; Anselme, K. Roughness statistical influence on cell adhesion using profilometry and multiscale analysis. Scanning 2014, 36, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Bigerelle, M. On the relation between surface roughness of metallic substrates and adhesion of human primary bone cells. Scanning 2014, 36, 11–20. [Google Scholar] [CrossRef]
- Gawlik, M.M.; Wiese, B.; Desharnais, V.; Ebel, T.; Willumeit-Romer, R. The Effect of Surface Treatments on the Degradation of Biomedical Mg Alloys-A Review Paper. Materials (Basel) 2018, 11, 2561. [Google Scholar] [CrossRef]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Zhen, Z.; Xi, T.-F.; Zheng, Y.-F. A review on in vitro corrosion performance test of biodegradable metallic materials. Trans. Nonferrous Met. Soc. China 2013, 23, 2283–2293. [Google Scholar] [CrossRef]
- Nie, F.L.; Zheng, Y.F.; Wei, S.C.; Hu, C.; Yang, G. In vitro corrosion, cytotoxicity and hemocompatibility of bulk nanocrystalline pure iron. Biomed. Mater. 2010, 5, 065015. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.F.; Ruan, L. In vitro investigation of Fe30Mn6Si shape memory alloy as potential biodegradable metallic material. Mater. Lett. 2011, 65, 540–543. [Google Scholar] [CrossRef]
- Huang, T.; Cheng, J.; Zheng, Y.F. In vitro degradation and biocompatibility of Fe-Pd and Fe-Pt composites fabricated by spark plasma sintering. Mater. Sci. Eng. C 2014, 35, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Wörle-Knirsch, J.M.; Pulskamp, K.; Krug, H.F. Oops They Did It Again! Carbon Nanotubes Hoax Scientists in Viability Assays. Nano Lett. 2006, 6, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Riviere, N.A.; Inman, A.O.; Zhang, L.W. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol. Appl. Pharm. 2009, 234, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Prosenc, M.H.; Wolff, M.; Hort, N.; Willumeit, R.; Feyerabend, F. Interference of magnesium corrosion with tetrazolium-based cytotoxicity assays. Acta Biomater. 2010, 6, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.S.; Estrela, J.M. Oxidative stress in environmental-induced carcinogenesis. Mutat. Res. 2009, 674, 36–44. [Google Scholar] [CrossRef]
- Verhaegen, C.; Lepropre, S.; Octave, M.; Brusa, D.; Bertrand, L.; Beauloye, C.; Jacques, P.J.; Kefer, J.; Horman, S. Bioreactivity of Stent Material: In Vitro Impact of New Twinning-Induced Plasticity Steel on Platelet Activation. J. Biomater. Nanobiotechnol. 2019, 10, 175–189. [Google Scholar] [CrossRef]
- Hermawan, H.; Purnama, A.; Dube, D.; Couet, J.; Mantovani, D. Fe–Mn alloys for metallic biodegradable stents: Degradation and cell viability studies. Acta Biomater. 2010, 6, 1852–1860. [Google Scholar] [CrossRef]
- Meir, K.S.; Leitersdorf, E. Atherosclerosis in the apolipoprotein-E-deficient mouse: A decade of progress. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1006–1014. [Google Scholar] [CrossRef]
- Messer, R.L.; Mickalonis, J.; Lewis, J.B.; Omata, Y.; Davis, C.M.; Brown, Y.; Wataha, J.C. Interactions between stainless steel, shear stress, and monocytes. J. Biomed. Mater. Res. A 2008, 87, 229–235. [Google Scholar] [CrossRef]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Electroformed pure iron as a new biomaterial for degradable stents: In vitro degradation and preliminary cell viability studies. Acta Biomater. 2010, 6, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Chen, H.; Shen, F. Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial. J. Mater. Sci. Mater. Med. 2010, 21, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative in vitro Study on Pure Metals (Fe, Mn, Mg, Zn and W) as Biodegradable Metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Purnama, A.; Hermawan, H.; Champetier, S.; Mantovani, D.; Couet, J. Gene expression profile of mouse fibroblasts exposed to a biodegradable iron alloy for stents. Acta Biomater. 2013, 9, 8746–8753. [Google Scholar] [CrossRef]
- Drynda, A.; Hassel, T.; Bach, F.W.; Peuster, M. In vitro and in vivo corrosion properties of new iron-manganese alloys designed for cardiovascular applications. J. Biomed. Mater. Res B Appl. Biomater. 2015, 103, 649–660. [Google Scholar] [CrossRef]
- Lin, W.; Qin, L.; Qi, H.; Zhang, D.; Zhang, G.; Gao, R.; Qiu, H.; Xia, Y.; Cao, P.; Wang, X.; et al. Long-term in vivo corrosion behavior, biocompatibility and bioresorption mechanism of a bioresorbable nitrided iron scaffold. Acta Biomater. 2017, 54, 454–468. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Wu, Y.; Maitz, M.F.; Wang, Z.; Koo, Y.; Zhao, A.; Sankar, J.; Kong, D.; Huang, N.; et al. Ex vivo blood vessel bioreactor for analysis of the biodegradation of magnesium stent models with and without vessel wall integration. Acta Biomater. 2017, 50, 546–555. [Google Scholar] [CrossRef]
- Engeland, C.G.; Sabzehei, B.; Marucha, P.T. Sex hormones and mucosal wound healing. Brain Behav. Immun. 2009, 23, 629–635. [Google Scholar] [CrossRef]
- Gibney, E.R.; Milenkovic, D.; Combet, E.; Ruskovska, T.; Greyling, A.; Gonzalez-Sarrias, A.; de Roos, B.; Tomas-Barberan, F.; Morand, C.; Rodriguez-Mateos, A. Factors influencing the cardiometabolic response to (poly)phenols and phytosterols: A review of the COST Action POSITIVe activities. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef]
- Wnek, G.E.; Bowlin, G.L. Encyclopedia of Biomaterials and Biomedical Engineering; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Leopold, J.A. Neoatherosclerosis: Another Consequence of Endothelial Dysfunction? Circ. Cardiovasc. Interv. 2014, 7, 635–637. [Google Scholar] [CrossRef][Green Version]
- Apostolos, K.; Tassiopoulos, H.P.G. Angiogenic mechanisms of endothelialization of cardiovascular implants: A review of recent investigative strategies. J. Biomater. Sci. Polym. Ed. 2000, 11, 1275–1284. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zhang, D.; Xin, C.; Liu, X.; Lin, W.; Zhang, W.; Chen, S.; Sun, K. Characterization and in vivo evaluation of a bio-corrodible nitrided iron stent. J. Mater. Sci. Mater. Med. 2013, 24, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.U.; Hong, Q.I.; Hu, X.Y.; Ruan, Y.M.; Yi, T.I.; Yan, C.H.; Xu, X.L.; Liang, X.U.; Yue, T.A.; Gao, R.L. Short-term safety and efficacy of the biodegradable iron stent in mini-swine coronary arteries. Chin. Med. J. 2013, 126, 4752–4757. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M.; et al. Biodegradable Fe-based alloys for use in osteosynthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef]
- Hong, D.; Chou, D.T.; Velikokhatnyi, O.I.; Roy, A.; Lee, B.; Swink, I.; Issaev, I.; Kuhn, H.A.; Kumta, P.N. Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomater. 2016, 45, 375–386. [Google Scholar] [CrossRef]
- Peuster, M.; Beerbaum, P.; Bach, F.W.; Hauser, H. Are resorbable implants about to become a reality? Cardiol. Young 2006, 16, 107–116. [Google Scholar] [CrossRef]
- Hu, T.; Yang, C.; Lin, S.; Yu, Q.; Wang, G. Biodegradable stents for coronary artery disease treatment: Recent advances and future perspectives. Mater. Sci. Eng. C 2018, 91, 163–178. [Google Scholar] [CrossRef]
- Waksman, R.; Pakala, R.; Kuchulakanti, P.K.; Baffour, R.; Hellinga, D.; Seabron, R.; Tio, F.O.; Wittchow, E.; Hartwig, S.; Harder, C.; et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheter. Cardiovasc. Interv. 2006, 68, 607–617. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
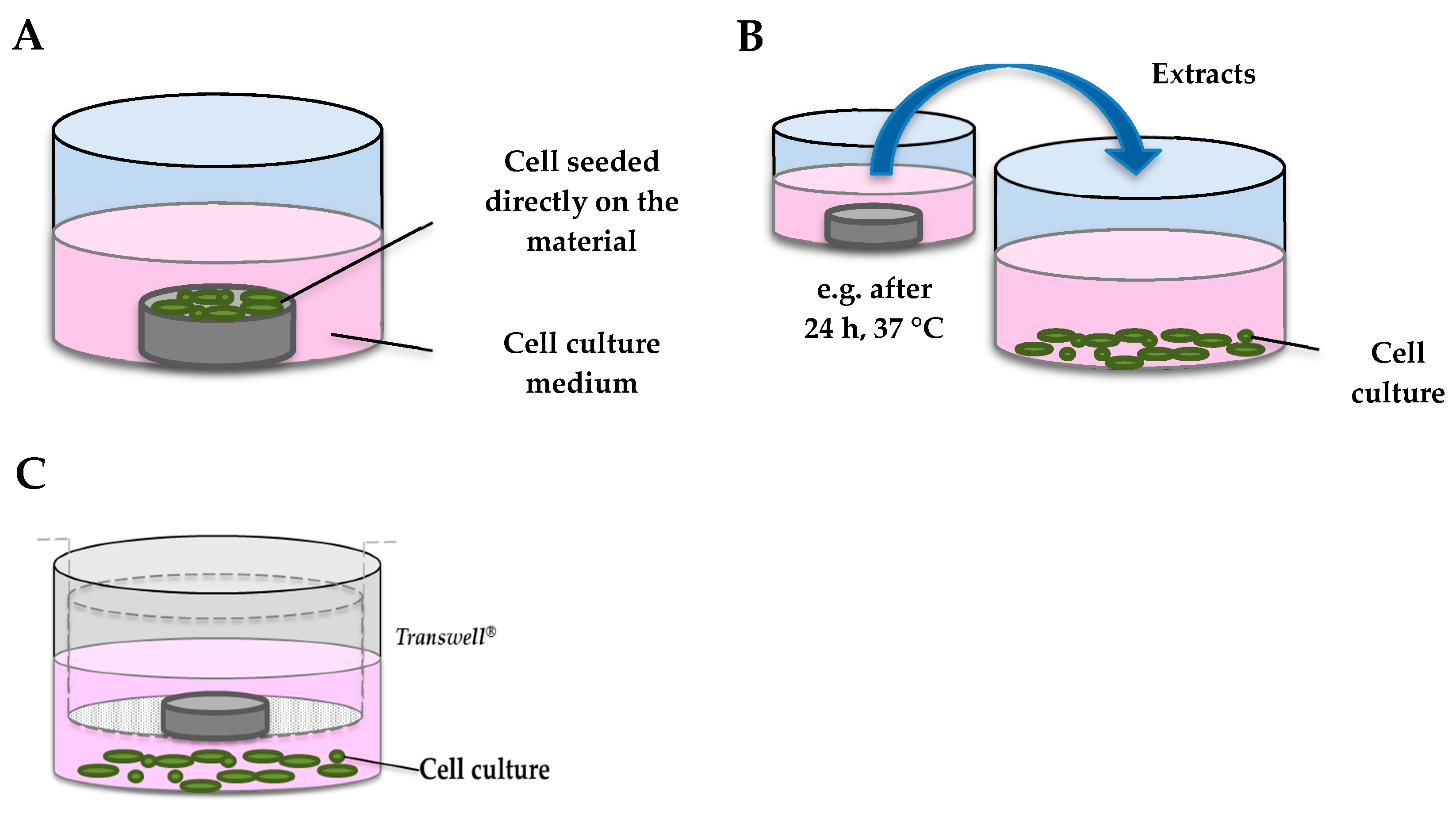
| Metallic Materials | Form of the Material | Surface State | Cells Type | Direct/Extracts Test | Viability/Metabolic Activity Test | Blood Compatibility | References |
|---|---|---|---|---|---|---|---|
| Pure Fe | Massive samples | Mechanically polished | HUVECs | Extracts | WST-8 | NA | S. Zhu, 2009 [7] |
| Fe; Mn; Fe35Mn | Particles | / | 3T3 | Insert * | WST-1 | NA | H. Hermawan, 2010 [51] |
| As-electroformed Fe, annealed E-Fe and annealed CTT-Fe | Massive samples | Polished with SiC 1000–4000 & 0.05 µm alumina paste | Rat VSMCs | Extracts | WST-1 | NA | M. Moravej, 2010 [54] |
| Pure Fe | Massive samples | Polished up to 1 µm SiC | Mouse bone marrow stem cells | Extracts | MTT | Platelet adhesion/haemolysis assays | E. Zhang, 2010 [55] |
| Bulk nanocrystalline pure Fe | Massive samples | Polished up to 2000 grit | L-929, rodent VSMC, ECV304 | Extracts | MTT | Haemolysis assay | F.L. Nie, 2010 [43] |
| Fe alloyed by different elements (Mn, Co, Al, W, Sn, B, C & S): as cast | Massive samples | Polished up to 2000 grit | L-929, rodent VSMC, ECV304 | Extracts | MTT | Platelet adhesion/haemolysis assays | B. Liu, 2011 [44] |
| Fe–21Mn–0.7C; Fe–21Mn–0.7C–1Pd | Massive samples | Polished with 2400 grit SiC | HUVECs | Extracts | NR; MTT | NA | M. Schinhammer, 2013 [15] |
| Pure Fe | Massive samples | Polished to 2000 grit | L929, ECV304 | Extracts | MTT | Platelet adhesion/haemolysis assays | J. Cheng, 2013 [56] |
| Pure Fe | Particles | / | BALB/3T3 | Insert * | WST-1 | NA | A. Purnama, 2013 [57] |
| Pure Fe, Fe–Pd and Fe–Pt composites | Massive samples | Polished to 2000 grit | L-929, human VSMC and ECV304 | Extracts | MTT | Platelet adhesion/haemolysis assays | T. Huang, 2014 [45] |
| Pure Fe; nitrited pure Fe | Stent; foils | Stent electrochemically polished, foils mechanically polished | L-929, human VSMC and HUVECs | Direct/Indirect/Extracts | MTT | NA | W. Lin, 2015 [35] |
| FeMn 0.5 wt %, FeMn 2.7 wt %, and FeMn 6.9 wt %; pure Fe | Massive samples | Polished with 2500 grit | Primary human ECs and SMCs from umbilical cord veins | Direct | Live/Dead | NA | A. Drynda, 2015 [58] |
| Pure Fe | Massive samples and particles | / | BALB/c 3T3 | Direct/Extracts | Acridine orange dye | NA | N.S. Fagali, 2017 [36] |
| Pure Fe | Particles | / | HUVECs, HAoECs, HAoSMCs, HCASMCs | Direct/Extracts | WST-1; ATP | NA | E. Scarcello, 2019 [37] |
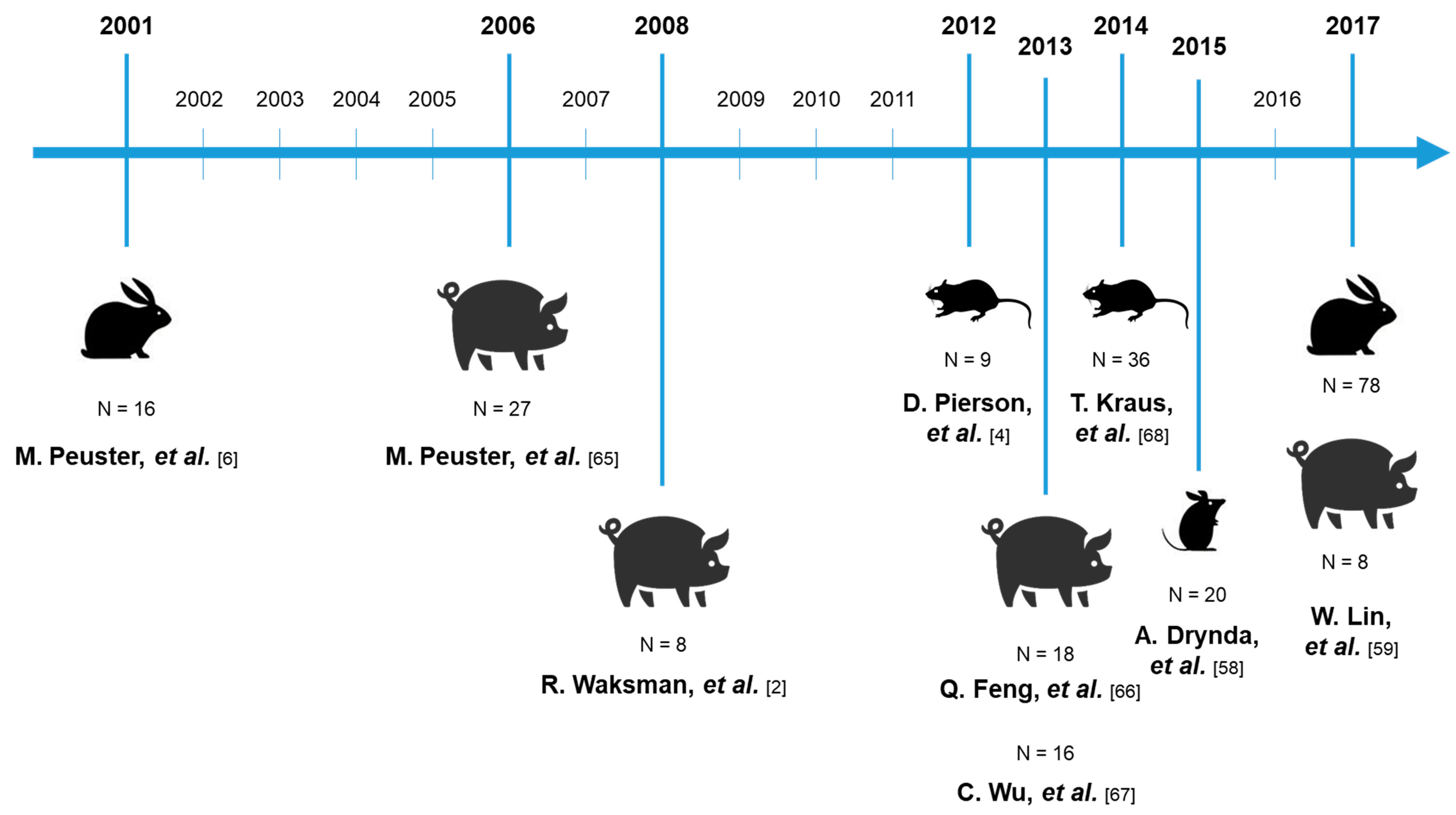
| Material | Form of the Material | Surface State | Dimension of the Material (Diameter/Length; mm) | Animal Model | Number of Animal | Implantation Site | Duration of the Study | Application | Analysis | Results | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pure Fe (ARMCO quality) | Stent | Polished to achieve a strut thickness of 100–120 µm | 3–6/16 | New Zealand white rabbits | 16 | Descending aorta | 6, 12, 18 months | Coronary stent | Angiography | No thromboembolic complications, no significant neointimal proliferation, no pronounced inflammatory response, and no systemic toxicity | M. Peuster, 2001 [6] |
| Pure Fe (ARMCO quality) | Stent | Electropolished to achieve a strut thickness of 120 µm | 8/20 | Minipigs | 27 | Descending aorta | 1–360 days | Coronary stent | Histomorphometry and quantitative angiography analysis | No signs of iron overload or iron-related organ toxicity, no evidence for local toxicity | M. Peuster, 2006 [66] |
| Pure Fe | Stent | / | 1.1:1 to 1:1.2 stent/artery diameter ratio | Juvenile domestic pigs | 8 | Proximal left anterior descending, left circumflex artery, or right coronary artery | 28 days | Coronary stent | Histochemistry, vessel morphometry | No adverse effects in the persistent areas | R. Waksman, 2008 [2] |
| Pure Fe | Wire | / | 0.25/20 | Male Sprague Dawley rats | 9 | Abdominal aorta | 22 days; 1.5, 3, 4.5, or 9 months | Coronary stent | Histological examination | Critical role of the arterial environment in directing the corrosion behavior of biodegradable metals | D. Pierson, 2012 [4] |
| Pure Fe and nitrided Fe | Stent | Electrochemically polished | 8/20 | Minipigs | 18 | Left and right iliac arteries | 1, 3, 6 and 12 months | Coronary stent | Histological examination | No thrombosis or local tissue necrosis; decreased inflammation from 3-6 to 12 months post-operation | Q. Feng, 2013 [67] |
| Nitriding Fe | Stent | / | 3/18 | Minipigs | 8 Fe, 8 Co-Cr | Coronary artery | 28 days | Coronary stent | Coronary angiography, endothelialization and histological observation | No signs of organ toxicity | C. Wu, 2013 [68] |
| FeMn 0.5 wt %, FeMn 2.7 wt %, and FeMn 6.9 wt %; pure Fe | Cylindrical plate | Polished with abrasive papers 800, 1200, and 2500 grains | 3/1.4 (height) | NMRI mice | 20 | Subcutis resting on the fascia of the gluteal muscle | 3, 6, 9 months | Cardiovascular application | Histological examination | No significant corrosion was detectable, not possible to make serious predictions | A. Drynda, 2015 [58] |
| Fe 0.074 wt%N; pure Fe; 316L stainless steel | Scaffold | Electrochemically polished | 3/18 | New Zealand white rabbits | 78 | Abdominal aorta | 7 days; 1, 4, 6, 9, 12, 24, 36 months | Coronary stent | Endothelialization and histopathologic observation | No adverse effects, homogeneous endothelial coverage, slight inflammatory response | W. Lin, 2017 [59] |
| Fe 0.074 wt%N | Scaffold | Electrochemically polished | 3/18 | Tibet minipigs | 8 | Left anterior descending, coronary artery and right coronary artery | 33, 53 months | Coronary stent | Gross observation and histopathology analysis on the organs and tissue | No abnormalities found for the organs and no pathologic changes | W. Lin, 2017 [59] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarcello, E.; Lison, D. Are Fe-Based Stenting Materials Biocompatible? A Critical Review of In Vitro and In Vivo Studies. J. Funct. Biomater. 2020, 11, 2. https://doi.org/10.3390/jfb11010002
Scarcello E, Lison D. Are Fe-Based Stenting Materials Biocompatible? A Critical Review of In Vitro and In Vivo Studies. Journal of Functional Biomaterials. 2020; 11(1):2. https://doi.org/10.3390/jfb11010002
Chicago/Turabian StyleScarcello, Eleonora, and Dominique Lison. 2020. "Are Fe-Based Stenting Materials Biocompatible? A Critical Review of In Vitro and In Vivo Studies" Journal of Functional Biomaterials 11, no. 1: 2. https://doi.org/10.3390/jfb11010002
APA StyleScarcello, E., & Lison, D. (2020). Are Fe-Based Stenting Materials Biocompatible? A Critical Review of In Vitro and In Vivo Studies. Journal of Functional Biomaterials, 11(1), 2. https://doi.org/10.3390/jfb11010002





