Automatic Hemiplegia Type Detection (Right or Left) Using the Levenberg-Marquardt Backpropagation Method
Abstract
1. Introduction
2. Related Work
3. The LM-BP Algorithm
- Randomizing weights and thresholds.
- Setting the maximum number of steps, the initial value of the learning coefficient, and the maximum allowed sum of squares network error.
| Algorithm 1: The Levenberg-Marquardt Algorithm |
| 1: 2: 3: 4: 5: 6: 7: do 8: 9: 10: 11: 12: 13: 14: 15: if 16: if 17: 18: 19: 20: else 21: 22: end if 23: else 24: 25: 26: end if 27: while 28: return |
4. System Architecture
5. Experimental Results
- SCG-BP
- FPCG-BP
- BFGS-BP
- OSS-BP
- GD-BP
- RPROP
- BR-BP
- RMSProp
- Adam
- AdaMax
- AMSGrad
- ELM
- OP-ELM
- SVM
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, P.M. Steps to Follow: The Comprehensive Treatment of Patients with Hemiplegia; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Ruskin, A.P. Understanding stroke and its rehabilitation. Stroke 1983, 14, 438–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donath, L.; Faude, O.; Lichtenstein, E.; Pagenstert, G.; Nüesch, C.; Mündermann, A. Mobile inertial sensor based gait analysis: Validity and reliability of spatiotemporal gait characteristics in healthy seniors. Gait Posture 2016, 49, 371–374. [Google Scholar] [CrossRef] [PubMed]
- HASOMED. RehaGait—Mobile Gait Analysis. Available online: https://hasomed.de/en/products/rehagait/ (accessed on 3 September 2021).
- Schwesig, R.; Fischer, D.; Lauenroth, A.; Becker, S.; Leuchte, S. Can falls be predicted with gait analytical and posturographic measurement systems? A prospective follow-up study in a nursing home population. Clin. Rehabilit. 2013, 27, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.; Shin, H. Detection of Hemiplegic Walking Using a Wearable Inertia Sensing Device. Sensors 2018, 18, 1736. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Zhou, H.; Guo, K.; Samuel, O.W.; Huang, Z.; Xu, L.; Li, G. Appropriate mother wavelets for continuous gait event detection based on time-frequency analysis for hemiplegic and healthy individuals. Sensors 2019, 19, 3462. [Google Scholar] [CrossRef]
- Pauk, J.; Minta-Bielecka, K. Gait patterns classification based on cluster and bicluster analysis. Biocybern. Biomed. Eng. 2016, 36, 391–396. [Google Scholar] [CrossRef]
- Patil, S.; Shah, A.; Dalvi, S.; Sisodia, J. Early Detection of Hemiplegia by Analyzing the Gait Characteristics and Walking Patterns Using. In Proceedings of the Soft Computing and Signal Processing, Proceedings of the 2nd ICSCSP 2019, Hyderabad, India, 21–22 June 2019; Springer: Singapore, 2019; Volume 1118, p. 39. [Google Scholar]
- Padilla, U. Fuzzy Classification of Hemiplegic Gait Using Kinematic Indicators in Knee. In Proceedings of the VI Latin American Congress on Biomedical Engineering CLAIB 2014 Paraná, Argentina, 29–31 October 2014; Springer: Cham, Switzerland, 2014; pp. 596–599. [Google Scholar]
- Manca, M.; Ferraresi, G.; Cosma, M.; Cavazzuti, L.; Morelli, M.; Benedetti, M.G. Gait Patterns in Hemiplegic Patients with Equinus Foot Deformity. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.-I.; Cho, H.; Kim, H.S.; Chon, J.; Lee, W.J.; Shin, J.H.; Ahn, J.Y.; Kim, T.; Han, J.-S.; et al. Gait patterns of chronic ambulatory hemiplegic elderly compared with normal Age-Matched elderly. Int. J. Precis. Eng. Manuf. 2015, 16, 385–392. [Google Scholar] [CrossRef]
- LeMoyne, R.; Kerr, W.; Mastroianni, T.; Hessel, A. Implementation of machine learning for classifying hemiplegic gait disparity through use of a force plate. In Proceedings of the 2014 13th International Conference on Machine Learning and Applications, Detroit, MI, USA, 3–6 December 2014; pp. 379–382. [Google Scholar]
- Jung, S.; Bong, J.; Kim, S.-J.; Park, S. DNN-Based FES Control for Gait Rehabilitation of Hemiplegic Patients. Appl. Sci. 2021, 11, 3163. [Google Scholar] [CrossRef]
- Yardimci, A. Fuzzy Logic Based Gait Classification for Hemiplegic Patients. In International Symposium on Intelligent Data Analysis; Springer: Berlin/Heidelberg, Germany, 2007; pp. 344–354. [Google Scholar]
- Luo, H.; Luo, J. Evaluating the Intra-limb Coordination during Gait in Hemiplegia. In Proceedings of the 2018 IEEE International Conference on Cyborg and Bionic Systems (CBS), Shenzhen, China, 25–27 October 2018; pp. 612–615. [Google Scholar]
- Wong, A.M.; Pei, Y.-C.; Hong, W.-H.; Chung, C.-Y.; Lau, Y.-C.; Chen, C.P. Foot contact pattern analysis in hemiplegic stroke patients: An implication for neurologic status determination. Arch. Phys. Med. Rehabilit. 2004, 85, 1625–1630. [Google Scholar] [CrossRef]
- LeMoyne, R.; Mastroianni, T. Implementation of a smartphone as a wearable and wireless gyroscope platform for machine learning classification of hemiplegic gait through a multi-layer perceptron neural network. In Proceedings of the 2018 17th IEEE International Conference on Machine Learning and Applications (ICMLA), Orlando, FL, USA, 17–20 December 2018; pp. 946–950. [Google Scholar]
- Aguilera, A.; Subero, A. Automatic gait classification patterns in spastic hemiplegia. Adv. Data Anal. Classif. 2020, 14, 897–925. [Google Scholar] [CrossRef]
- Morbidoni, C.; Cucchiarelli, A.; Agostini, V.; Knaflitz, M.; Fioretti, S.; Di Nardo, F. Machine-learning-based prediction of gait events from EMG in cerebral palsy children. IEEE Trans. Neural Syst. Rehabilit. Eng. 2021, 29, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Agostini, V.; Knaflitz, M.; Nascimberi, A.; Gaffuri, A. Gait measurements in hemiplegic children: An automatic analysis of foot-floor contact sequences and electromyographic patterns. In Proceedings of the 2014 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lisboa, Portugal, 11–12 June 2014; pp. 1–4. [Google Scholar]
- Di Nardo, F. EMG-based characterization of walking asymmetry in children with mild hemiplegic cerebral palsy. Biosensors 2019, 9, 82. [Google Scholar] [CrossRef]
- McAloon, M.T.; Hutchins, S.; Twiste, M.; Jones, R.; Forchtner, S. Validation of the activPAL activity monitor in children with hemiplegic gait patterns resultant from cerebral palsy. Prosthet. Orthot. Int. 2014, 38, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Krzak, J.J.; Corcos, D.M.; Graf, A.; Smith, P.; Harris, G.F. Effect of fine wire electrode insertion on gait patterns in children with hemiplegic cerebral palsy. Gait Posture 2013, 37, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y. Gait analysis of children with spastic hemiplegic cerebral palsy. Neural Regen. Res. 2012, 7, 1578–1584. [Google Scholar]
- Aguilera, A.; Subero, A.; Mata-Toledo, R. Application of Data Mining Techniques on EMG Registers of Hemiplegic Patients. In Industrial Conference on Data Mining; Springer: Berlin/Heidelberg, Germany, 2013; pp. 254–265. [Google Scholar]
- Abaid, N.; Cappa, P.; Palermo, E.; Petrarca, M.; Porfiri, M. Gait detection in children with and without hemiplegia using single-axis wearable gyroscopes. PLoS ONE 2013, 8, e73152. [Google Scholar] [CrossRef]
- Watanabe, T.; Miyazawa, T.A. Validation Test of a Simple Method of Stride Length Measurement Only with Inertial Sensors and a Preliminary Test in FES-assisted Hemiplegic Gait. In World Congress on Medical Physics and Biomedical Engineering Toronto, Ontario, Canada; Springer: Cham, Switzerland, 2015; pp. 1111–1114. [Google Scholar]
- Granat, M.; Maxwell, D.; Bosch, C.; Ferguson, A.; Lees, K.; Barbenel, J. A body-worn gait analysis system for evaluating hemiplegic gait. Med. Eng. Phys. 1995, 17, 390–394. [Google Scholar] [CrossRef]
- Ohnishi, T.; Iwasaki, T.; Tanaka, M. Evaluation of hemiplegia caused by stroke by using joint detection of depth sensors-case of SIAS. Electr. Eng. Jpn. 2019, 206, 33–43. [Google Scholar] [CrossRef]
- Kumari, P.; Cooney, N.J.; Kim, T.-S.; Minhas, A.S. Gait analysis in Spastic Hemiplegia and Diplegia cerebral palsy using a wearable activity tracking device-a data quality analysis for deep convolutional neural networks. In Proceedings of the 2018 5th Asia-Pacific World Congress on Computer Science and Engineering (APWC on CSE), Nadi, Fiji, 10–12 December 2018; pp. 1–4. [Google Scholar]
- Li, Q.; Wang, Y.; Sharf, A.; Cao, Y.; Tu, C.; Chen, B.; Yu, S. Classification of gait anomalies from kinect. Vis. Comput. 2018, 34, 229–241. [Google Scholar] [CrossRef]
- Pandit, T.; Nahane, H.; Lade, D.; Rao, V. Abnormal gait detection by classifying inertial sensor data using transfer learning. In Proceedings of the 18th IEEE International Conference On Machine Learning And Applications (ICMLA), Boca Raton, FL, USA, 16–19 December 2019; pp. 1444–1447. [Google Scholar]
- Azlan, W.N.W.; Zakaria, W.N.W.; Othman, N.; Mohd, M.N.H.; Ghani, M.N.A. Evaluation of Leap Motion Controller Usability in Development of Hand Gesture Recognition for Hemiplegia Patients. In Proceedings of the 11th National Technical Seminar on Unmanned System Technology 2019; Springer: Berlin/Heidelberg, Germany, 2021; pp. 671–682. [Google Scholar]
- Cai, S.; Li, G.; Huang, S.; Zheng, H.; Xie, L. Automatic detection of compensatory movement patterns by a pressure distribution mattress using machine learning methods: A pilot study. IEEE Access 2019, 7, 80300–80309. [Google Scholar] [CrossRef]
- Christou, V. Neural network-based approach for hemiplegia detection via accelerometer signals. In Proceedings of the 6th South-East Europe Design Automation, Computer Engineering, Computer Networks and Social Media Conference, Preveza, Greece, 24–26 September 2021. [Google Scholar]
- Priya, S.J.; Rani, A.J.; Subathra, M.; Mohammed, M.A.; Damaševičius, R.; Ubendran, N. Local pattern transformation based feature extraction for recognition of Parkinson’s disease based on gait signals. Diagnostics 2021, 11, 1395. [Google Scholar] [CrossRef] [PubMed]
- Møller, M.F. A scaled conjugate gradient algorithm for fast supervised learning. Neural Netw. 1993, 6, 525–533. [Google Scholar] [CrossRef]
- Levenberg, K. A method for the solution of certain non-linear problems in least squares. Q. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Scales, L. Introduction to Non-Linear Optimization; Macmillan International Higher Education: London, UK, 1985. [Google Scholar]
- Hery, M.A.; Ibrahim, M.; June, L. BFGS method: A new search direction. Sains Malays. 2014, 43, 1591–1597. [Google Scholar]
- Battiti, R. First-and second-order methods for learning: Between steepest descent and Newton’s method. Neural Comput. 1992, 4, 141–166. [Google Scholar] [CrossRef]
- Lemaréchal, C. Cauchy and the gradient method. Doc. Math. Extra. 2012, 251, 10. [Google Scholar]
- Riedmiller, M.; Braun, H. A direct adaptive method for faster backpropagation learning: The RPROP algorithm. In Proceedings of the IEEE International Conference on Neural Networks, San Francisco, CA, USA, 28 March–1 April 1993; pp. 586–591. [Google Scholar]
- MacKay, D.J. Bayesian interpolation. Neural Comput. 1992, 4, 415–447. [Google Scholar] [CrossRef]
- Foresee, F.D.; Hagan, M.T. Gauss-Newton approximation to Bayesian learning. In Proceedings of the International Conference on Neural Networks (ICNN’97), Houston, TX, USA, 12 June 1997; Volume 3, pp. 1930–1935. [Google Scholar]
- Tieleman, T.; Hinton, G. Coursera: Neural Networks for Machine Learning-Lecture 6.5: RMSprop; University of Toronto: Toronto, ON, Canada, 2012. [Google Scholar]
- Kingma, D.P.; Adam, J.B. Adam: A method for stochastic optimizatio. In Proceedings of the International Conference on Learning Representations, San Diego, CA, USA, 7–9 May 2015. [Google Scholar]
- Duchi, J.; Hazan, E.; Singer, Y. Adaptive subgradient methods for online learning and stochastic optimization. J. Mach. Learn. Res. 2011, 12, 7. [Google Scholar]
- Huang, G.-B.; Zhu, Q.-Y.; Siew, C.-K. Extreme learning machine: Theory and applications. Neurocomputing 2006, 70, 489–501. [Google Scholar] [CrossRef]
- Miche, Y.; Sorjamaa, A.; Bas, P.; Simula, O.; Jutten, C.; Lendasse, A. OP-ELM: Optimally pruned extreme learning machine. IEEE Trans. Neural Netw. 2009, 21, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Zahir, N.; Mahdi, H. Snow depth estimation using time series passive microwave imagery via genetically support vector regression (case study urmia lake basin). ISPRS—Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 40, 555–558. [Google Scholar] [CrossRef]
- Farias, F.S., Jr.; Azevedo, R.A.; Rivera, E.C.; Herrera, W.E.; Rubens, F.M.; Lima, L.P., Jr. Product Quality Monitoring Using Extreme Learning Machines and Bat Algorithms: A Case Study in Second-Generation Ethanol Production. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; Volume 33, pp. 955–960. [Google Scholar]
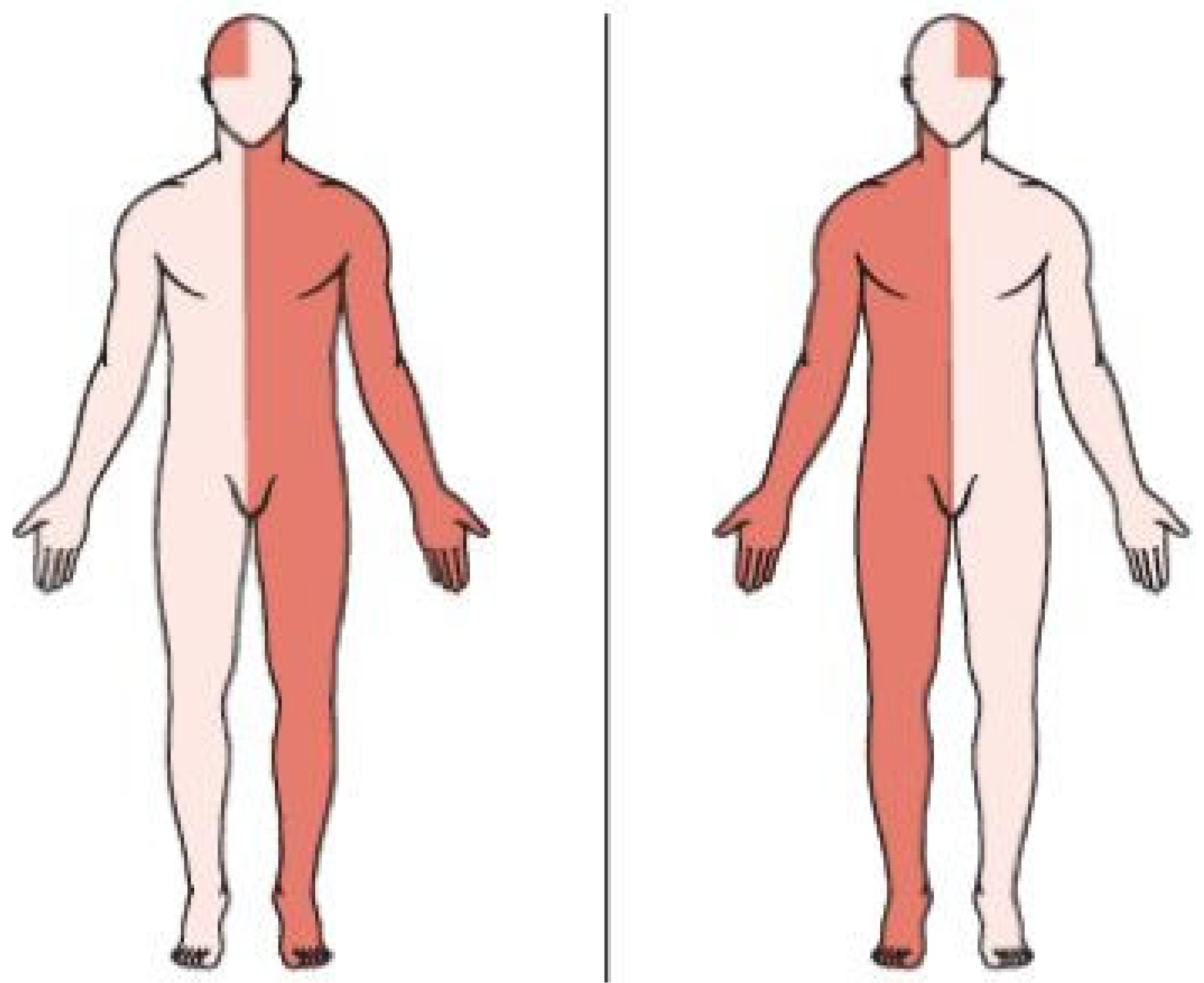





| Parameter Name | Value |
|---|---|
| Experiment Repeats | 10 |
| Hidden Layer Nodes No | 30 |
| Output Layer Nodes No | 3 |
| Input Vector Size | 126 |
| Test Set Size | 20% |
| RMSProp, Adam, AdaMax and AMSGrad Epochs No | 500 |
| Heterogenous OP-ELM Kernel Types | Linear, Gaussian, Sigmoid |
| Parameter Name | Value |
|---|---|
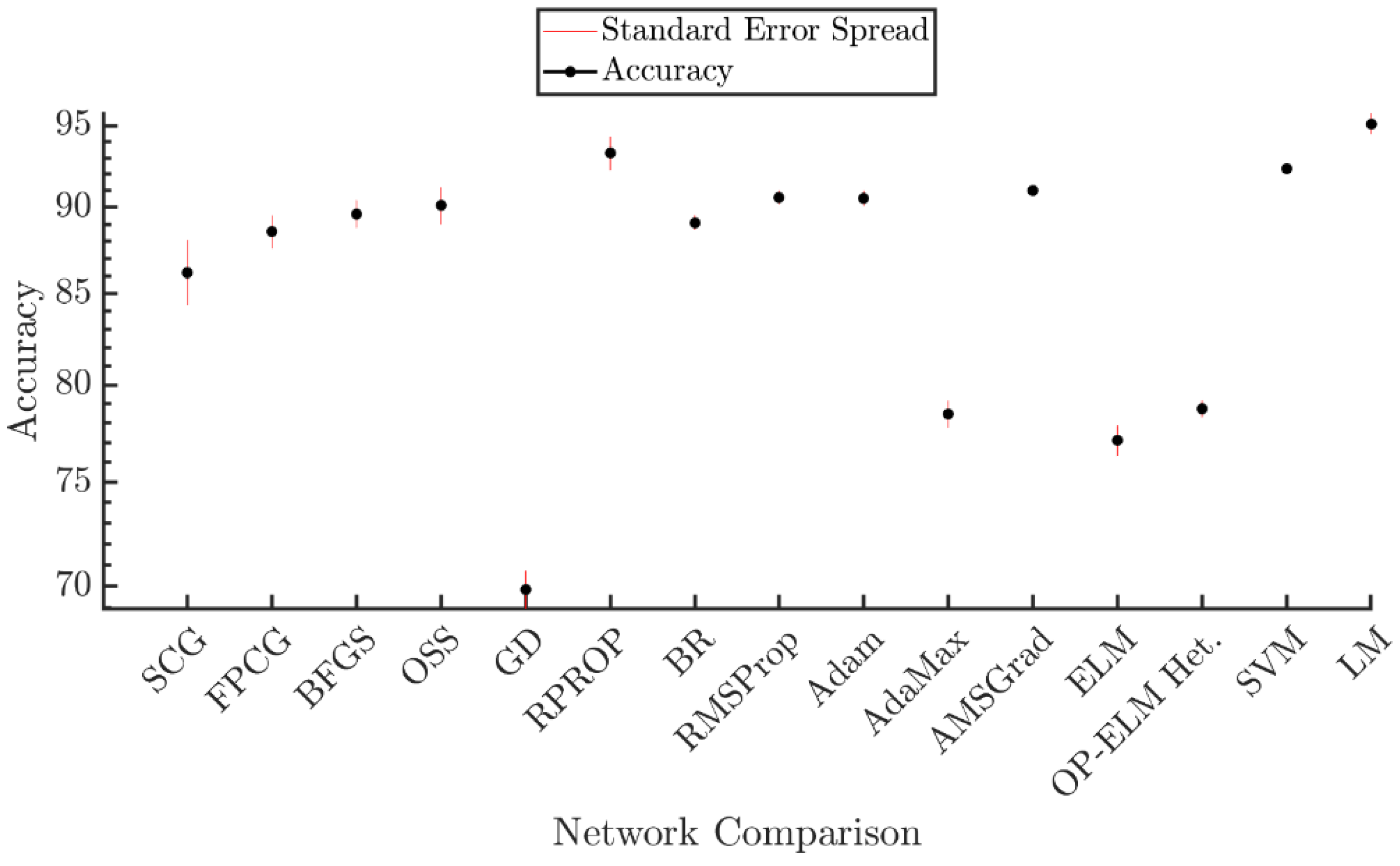
| SCG-BP | 86.21 |
| FPCG-BP | 88.55 |
| BFGS-BP | 89.61 |
| OSS-BP | 90.10 |
| GD-BP | 69.85 |
| RPROP | 93.28 |
| RMSProp | 90.59 |
| Adam | 90.54 |
| AdaMax | 78.47 |
| AMSGrad | 91.01 |
| BR-BP | 89.09 |
| ELM | 77.12 |
| OP-ELM | 78.74 |
| SVM | 92.36 |
| LM-BP | 95.12 |
| Precision | Recall | Specificity | F-Score | |
|---|---|---|---|---|
| Left Hemiplegia | 0.9771 | 0.9697 | 0.9891 | 0.9734 |
| Right Hemiplegia | 0.972 | 0.9498 | 0.9679 | 0.9607 |
| Normal | 0.8689 | 0.9636 | 0.9772 | 0.9138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christou, V.; Arjmand, A.; Dimopoulos, D.; Varvarousis, D.; Tsoulos, I.; Tzallas, A.T.; Gogos, C.; Tsipouras, M.G.; Glavas, E.; Ploumis, A.; et al. Automatic Hemiplegia Type Detection (Right or Left) Using the Levenberg-Marquardt Backpropagation Method. Information 2022, 13, 101. https://doi.org/10.3390/info13020101
Christou V, Arjmand A, Dimopoulos D, Varvarousis D, Tsoulos I, Tzallas AT, Gogos C, Tsipouras MG, Glavas E, Ploumis A, et al. Automatic Hemiplegia Type Detection (Right or Left) Using the Levenberg-Marquardt Backpropagation Method. Information. 2022; 13(2):101. https://doi.org/10.3390/info13020101
Chicago/Turabian StyleChristou, Vasileios, Alexandros Arjmand, Dimitrios Dimopoulos, Dimitrios Varvarousis, Ioannis Tsoulos, Alexandros T. Tzallas, Christos Gogos, Markos G. Tsipouras, Evripidis Glavas, Avraam Ploumis, and et al. 2022. "Automatic Hemiplegia Type Detection (Right or Left) Using the Levenberg-Marquardt Backpropagation Method" Information 13, no. 2: 101. https://doi.org/10.3390/info13020101
APA StyleChristou, V., Arjmand, A., Dimopoulos, D., Varvarousis, D., Tsoulos, I., Tzallas, A. T., Gogos, C., Tsipouras, M. G., Glavas, E., Ploumis, A., & Giannakeas, N. (2022). Automatic Hemiplegia Type Detection (Right or Left) Using the Levenberg-Marquardt Backpropagation Method. Information, 13(2), 101. https://doi.org/10.3390/info13020101












