Abstract
The current emerging COVID-19 pandemic has caused a global impact on every major aspect of our societies. It is known that SARS-Cov-2 can endure harsh environmental conditions for up to 72 h, which may contribute to its rapid spread. Therefore, effective containment strategies, such as sanitizing, are critical. Nanotechnology can represent an alternative to reduce the COVID-19 spread, particularly in critical areas, such as healthcare facilities and public places. Nanotechnology-based products are effective at inhibiting different pathogens, including viruses, regardless of their drug-resistant profile, biological structure, or physiology. Although there are several approved nanotechnology-based antiviral products, this work aims to highlight the use of nanomaterials as sanitizers for the prevention of the spread of mainly SARS-Cov-2. It has been widely demonstrated that nanomaterials are an alternative for sanitizing surfaces to inactivate the virus. Also, antimicrobial nanomaterials can reduce the risk of secondary microbial infections on COVID-19 patients, as they inhibit the bacteria and fungi that can contaminate healthcare-related facilities. Finally, cost-effective, easy-to-synthesize antiviral nanomaterials could reduce the burden of the COVID-19 on challenging environments and in developing countries.
Keywords:
COVID-19; nanotechnology; nanomaterials; antiviral; sanitizers; nanomedicine; infectious diseases 1. Introduction
Other than a natural disaster of cataclysmic proportions, or an all-out man-made nuclear war, infectious diseases pose the only global threat to human life on Earth. Infectious diseases have plagued humanity and represented the major cause of morbidity and mortality through the millennia, and in the process shaped human evolution. They still constitute the leading cause of premature death in the developing world. However, our modern society has become extremely complacent about the threat posed by infectious diseases, mostly due to the availability and increased access to hygiene and sanitization techniques, vaccines, and antibiotics.
Despite this unjustified level of complacency and the false sense of security, epidemiological experts and sentinel organizations around the world have provided ample warning signs, often ignored, of our global vulnerability against infectious diseases and their broad and borderless impact. Microorganisms do not need a passport and do not understand artificial geopolitical boundaries. This is further aggravated by globalization and climate change. In particular, the newest epidemics caused by a virus such as SARS, MERS, Ebola, and H1N1 since the beginning of the century have raised the awareness of the major threat that viral diseases still pose to humanity as a whole. However, these preoccupations were often short-lived and fast forgotten once the resulting epidemics were seemingly under control, which left us completely unprepared despite our conviction and anticipation that it was only a matter of time for the next epidemic or pandemic to come. Sure enough, we did not have to wait too much longer, and as of early 2020, humanity is confronting a pandemic in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease, abbreviated as COVID-19.
2. The SARS-CoV-2 Virus and the Current COVID-19 Pandemic
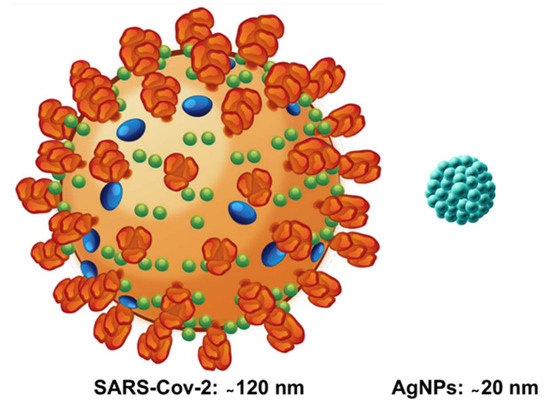
The SARS-CoV-2 is a large, enveloped, positive-stranded RNA virus, with a nucleocapsid, with a similar structure to the SARS-CoV-1; and a diameter size range from 80 to 140 nm [1]. This zoonotic coronavirus has become a major cause of emerging respiratory disease and has rapidly spread around the globe, affecting billions of people, and triggering unexpected changes in the healthcare system, global economy, and interactions in societies worldwide.
So far, as of 23th July, there are over 14.6 million confirmed cases with COVID-19 worldwide, with an estimated death toll of over 607,000 people [2]. The costs related to this communicable disease are expected to be in the trillions of dollars globally, but the real cost cannot be estimated [3]. Implementing strategies to reduce its spread requires the participation of the whole society, as well as the development of innovative ways of social, work, and health policies. Several measures have been implemented to control COVID-19, from campaigns aimed at improving personal hygiene practices to community approaches such as social distancing and quarantines. Among the suggested public health activities to address this pandemic are (1) efficient stay-at-home implementation, (2) fast SARS-CoV-2 testing, and (3) efficient health care response. The last point considers protecting the health care professionals by providing tools to prevent nosocomial infections, from making appropriate personal protective equipment (PPE) available to ensuring sanitizing procedures.
Proper sanitizing measures are critical. A recent study revealed that SARS-CoV-2 can be detected on plastic and stainless-steel surfaces up to 72 h, whereas on copper, no viable SARS-CoV-2 was measured after 4 h of application [4]. The inactivation of the virus by copper is not surprising, as it is known that transition metals can inactivate viral particles [5]. Thus, nanotechnology may provide an alternative for sanitizing surfaces, via antimicrobial and antiviral nanomaterials.
4. Current Nanotechnology Applications that Can Be Used to Combat COVID-19
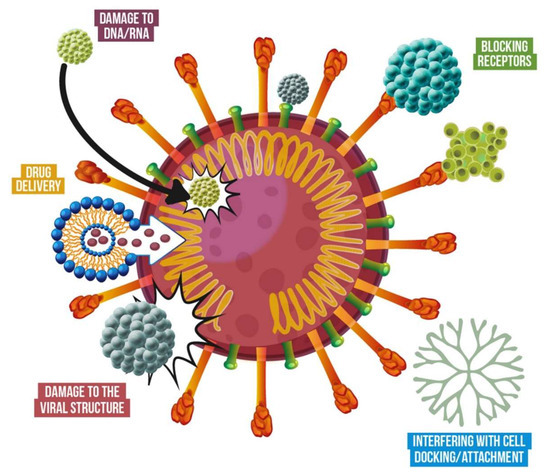
As mentioned earlier, nanomaterials are currently used in multiple commercially available products, including cosmetics and healthcare. A detailed, updated list of products can be consulted at the Nanowerk databases [9]. The wide range of physical and chemical properties of nanomaterials provides numerous advantages to combat the SARS-Cov-2; from reducing its spread to future treatments. When antiviral nanomaterials are fixed in support, their stability and reactivity are increased, as well as the surface area of the support. Usually, nanomaterials are adsorbed by textiles [55] and polymers [56] for diverse applications, such as personal protective equipment (PPE), medical textiles, packaging, and filters. Nanomaterials in suspensions can be used for sanitizing, coatings, and therapeutic applications. Finally, multiple nanomaterials can be used for nanocarriers, diagnosis, and detection. Some of the current applications that can be used to prevent and treat the COVID-19 are described below and summarized in Figure 3.
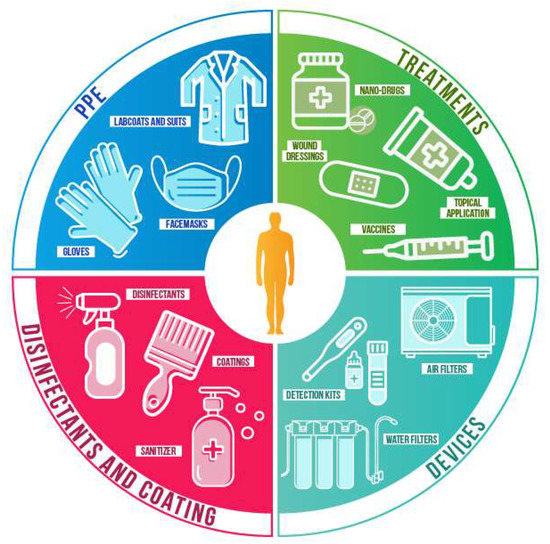
Figure 3.
Representative uses of nanotechnology to combat viruses and other pathogens.
4.1. Personal Protective Equipment (PPE)
The application of nanomaterials-embedded textiles has been intensely researched, as they improve the physicochemical properties of textiles, such as fire-retardant, self-cleaning, antimicrobial, and UV-protection, among others [57,58]. Fibers embedded with metallic nanoparticles, such as copper and silver nanoparticles, display antimicrobial and antiviral properties [11,59]. Mao recently reviewed the use of nanotechnology for protective clothing—including PPE such as lab coats, aprons, and facemasks—as nanomaterials provide novel traits to the fabrics [60]. Nowadays, the use of nanoparticles in textiles has been increasing rapidly, with a current global consumption of around 35 tons just for silver nanoparticles [21]. Moreover, wearable smart textiles and electronics for sensing have been explored, particularly for health-related applications, as described by Libertino et al., as the early detection of pathogens is of particular interest [61]. Jayathilaka et al., suggest that wearables and textiles may be also used for drug delivery [62].
4.2. Surface Coatings
Nanomaterials-based coatings are currently used for several applications, and different products are now available, particularly from metallic elements such as silver, bismuth, copper, or titanium [11,58,63,64]. Also, nanostructured surfaces can physically reduce the attachment of pathogens [65] and even disrupt the structure of the pathogens due to the nanoscale topography organization [66]. Nanomaterials can be embedded in paints or coatings for medical instrumentation, walls, and other highly-touched surfaces, such as doorknobs, handrails, etc. for reducing the presence and viability of viruses and other pathogens. A recent review by Rai et al., describes multiple nanocoatings and their potential use in public places for reducing the presence of pathogens [67].
4.3. Disinfectants and Sanitizing Procedures
Disinfectants with silver salts are currently used [68], as silver is deemed safe for sanitizing purposes [69]. In hospitals and other healthcare-related facilities, sanitizing with nanotechnology-based products could decrease the viral presence on highly-touched surfaces and maintain them virus-free for longer, including the SARS-Cov-2 virus. Metallic nanoparticles may be a viable option, as the viability of the SARS-Cov-2 virus is reduced on a metallic (copper) surface [4].
4.4. Other Current and Potential Applications
Among other uses, nanomaterials can be used to improve the function of air filters in healthcare facilities or in other places that use recirculated air. Nanotechnology-improved devices can reduce the spread of viral particles, such as air filters [70,71,72] and for the removal of pathogens and viruses from water [73]. Also, the use of nanotechnology for producing wound dressings has been thoroughly explored [51,74,75], due to their ability to protect against infections and increase the healing speed rate. Future applications may include the development of improved detection kits, as it has been one of the major needs for the current strategies to contain and follow the virus spread. In a recent review, Hameed et al., describe the role of nanotechnology for emerging detection against pathogens [76]. Besides detection, there have been recent advances for nanotechnology-based smart packaging to prevent pathogens [77]. Although the reviews from Hameed et al., and Zhong et al., focus on food-related applications, these can be used in the clinical sector.
5. A Silent Risk: The Microbial Secondary Infections
The current COVID-19 pandemic has raised several health concerns beyond the disease itself, such as secondary infections, which has been an underestimated risk. Secondary infections may appear during or after treatment of the COVID-19, due to the burden on health caused by the virus. Patients infected with the novel strain of coronavirus frequently show microbial dysbiosis and a decrease of probiotic bacteria [78]. Also, it is not uncommon for patients with COVID-19 to suffer from complications of acute organ injury and secondary infection [79], which may be of microbial origin. Recently, Rawson et al., reviewed the current literature regarding microbial co-infections in patients with different coronaviruses; although the co-infection rate is low (8%), they still pose a threat for COVID-19 patients [80].
A major risk associated with microbial infections is drug-resistance, which leads to failures in the treatments of the diseases. Currently, several health institutions worldwide point out that multidrug-resistant pathogens represent an urgent threat, as they cause thousands of deaths and their cost is incalculable. The CDC, in its latest Antibiotic Resistance Threats in the United States, lists current clinically microbial pathogens and describes the challenges associated with drug resistance [81]. Moreover, an additional threat is the formation of microbial biofilms (layers of microbial cells protected by an extracellular matrix), as they allow the microbial pathogens to survive sanitizing procedures harsh conditions. Biofilms allow microbial pathogens to have a constant presence in health-related facilities. Among the alternatives to combat pathogens, antimicrobial nanomaterials (nanoantibiotics) have been rising as a viable option.
Nanoantibiotics display a broad range antimicrobial activity against bacteria [82,83], protozoa [84,85], and fungi [86,87,88,89]. Metallic nanoparticles have been extensively studied, as metals have intrinsic antimicrobial properties [90] and they display antimicrobial activity regardless of the drug-susceptibly profile of microorganisms [15,86]. Additionally, nanomaterials may enhance the potency of current antimicrobial drugs; Vazquez-Munoz et al. recently described a potential mode of action between AgNPs and antibiotics that lead to synergistic effects [91]. Moreover, nanomaterials can reduce the microbial biofilms leading to a reduced risk of secondary microbial infections [87,88,92]. In Table 3 are listed examples of antimicrobial nanomaterials, with their properties and applications.

Table 3.
Examples of types of nanoantibiotics with current and expected antimicrobial applications.
An additional advantage is that there are a plethora of methods for synthesizing nanomaterials [100]; some methods can be easily reproduced in non-specialized facilities under unfavorable environments [101], therefore they could be produced for sanitizing in rural areas, mobile medical units, or in medical posts for the military on the field.
6. Perspectives
What should be expected in the near future? Although more research is needed, antiviral nanomaterials represent an alternative to reduce the spread of COVID-19, as they have been effective against other viruses. Based on the current knowledge, nanomaterials may be used in the present or the short-term for preventing the spread of the SARS-Cov-2 virus using antiviral sanitizers, nanotechnology-upgraded materials for PPE and medical instrumentation, and nanotechnology-enhanced fabrics for facemasks for general use. Particularly, nanotechnology-based sanitizers also display potent and broad antiviral and antimicrobial activity, therefore they can improve safety in healthcare-related facilities and public spaces, particularly in developing countries.
Regarding upcoming applications, nanotechnology can be used for detection kits, vaccines, and treatments. Currently, the therapeutic use of antiviral nanotechnology-based drugs has proven to be effective and it will be expanded in the future as more research becomes available. Also, nanomaterials display a broad range of activity and they may enhance the antimicrobial potency of some drugs, therefore they can be used for preventing or treating secondary infections, particularly those with multidrug-resistant profiles.
Finally, protocols that favor facile, rapid, cost-effective syntheses of antimicrobial and antiviral nanomaterials produce potent nanomaterials that could reduce the spread of viruses and microorganisms. Affordable nanotechnology may benefit anti-COVID-19 sanitizing procedures in developing countries, where access to advanced materials is limited. Also, easy-to-synthesize nanomaterials may be used under non-favorable conditions, such as medical mobile-units, rural health care facilities, and highly transited public spaces.
Author Contributions
Both authors equally contributed to the conceptualization, writing-original draft preparation, and writing-review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
R.V.-M. acknowledges support from a postdoctoral fellowship from the Mexican Council of Science and Technology (CONACYT). J.L.L.-R. acknowledges support from the Margaret Batts Tobin Foundation. The authors would like to thank the graphic designer Salma Carballo for designing the images.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation and Treatment Coronavirus (COVID-19); StatPearls Publishing: Petersburg, FL, USA, 2020. [Google Scholar]
- Coronavirus Resource Center COVID-19 Map. Available online: https://coronavirus.jhu.edu/map.html (accessed on 8 June 2020).
- Orlik, T.; Rush, J.; Cousin, M.; Hong, J. Coronavirus Could Cost the Global Economy $2.7 Trillion. Here’s How. Available online: https://www.bloomberg.com/graphics/2020-coronavirus-pandemic-global-economic-risk/ (accessed on 8 April 2020).
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Juarez, J.I.; Pierzchła, K.; Sienkiewicz, A.; Kohn, T. Inactivation of MS2 coliphage in Fenton and Fenton-like systems: Role of transition metals, hydrogen peroxide and sunlight. Environ. Sci. Technol. 2010, 44, 3351–3356. [Google Scholar] [CrossRef] [PubMed]
- National Nanotechnology Initiative What’s So Special about the Nanoscale? Available online: https://www.nano.gov/nanotech-101/special (accessed on 14 July 2020).
- Brydson, R.M.; Hammond, C. Generic Methodologies for Nanotechnology: Classification and Fabrication. In Nanoscale Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 1–55. ISBN 9780470850862. [Google Scholar]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.; Hull, D.R. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Nanowerk Database Nanoparticle Database—Single-Element Nanoparticles. Available online: https://www.nanowerk.com/nanoparticle_database.php (accessed on 20 April 2020).
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Dilnawaz, F.; Acharya, S.; Sahoo, S.K. Recent trends of nanomedicinal approaches in clinics. Int. J. Pharm. 2018, 538, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef]
- Romero-Urbina, D.G.; Lara, H.H.; Velázquez-Salazar, J.J.; Arellano-Jiménez, M.J.; Larios, E.; Srinivasan, A.; Lopez-Ribot, J.L.; Yacamán, M.J. Ultrastructural changes in methicillin-resistant Staphylococcus aureus induced by positively charged silver nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2396–2405. [Google Scholar] [CrossRef]
- Baptista, P.V.; Mccusker, M.P.; Carvalho, A.; Ferreira, D.A. Nano-Strategies to Fight Multidrug Resistant Bacteria —“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1–26. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Carbone, C.; Sousa, M.C.; Espina, M.; Garcia, M.L.; Sanchez-Lopez, E.; Souto, E.B. Nanomedicines for the delivery of antimicrobial peptides (Amps). Nanomaterials 2020, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent Development of Inorganic Nanoparticles for Biomedical Imaging. ACS Cent. Sci. 2018, 4, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Zottel, A.; Videtič Paska, A.; Jovčevska, I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials (Basel). 2019, 12, 1588. [Google Scholar] [CrossRef] [PubMed]
- Syafiuddin, A.; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.V.; Parkinson, C.V.; Choi, Y.W.; Speshock, J.L.; Hussain, S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008, 3, 129–133. [Google Scholar] [CrossRef]
- Lu, L.; Sun, R.W.Y.; Chen, R.; Hui, C.K.; Ho, C.M.; Luk, J.M.; Lau, G.K.K.; Che, C.M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 252–262. [Google Scholar]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 1–9. [Google Scholar] [CrossRef]
- Borrego, B.; Lorenzo, G.; Mota-Morales, J.D.; Almanza-Reyes, H.; Mateos, F.; López-Gil, E.; de la Losa, N.; Burmistrov, V.A.; Pestryakov, A.N.; Brun, A.; et al. Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1185–1192. [Google Scholar] [CrossRef]
- Xiang, D.; Zheng, C.; Zheng, Y.; Li, X.; Yin, J.; O’ Conner, M.; Marappan, M.; Miao, Y.; Xiang, B.; Duan, W.; et al. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 4103. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-N.; Hsueh, Y.-H.; Hsieh, C.-T.; Tzou, D.-Y.; Chang, P.-L. Antiviral Activity of Graphene–Silver Nanocomposites against Non-Enveloped and Enveloped Viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jiang, J.; Gu, W.; Sun, C.; Wu, D.; Yang, T.; Yang, G. Photocatalytic Inactivation Efficiency of Anatase Nano-TiO2 Sol on the H9N2 Avian Influenza Virus. Photochem. Photobiol. 2010, 86, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Yang, Z.; Jiang, W.; Geng, C.; Gong, M.; Xiao, H.; Wang, Z.; Cheng, L. Antiviral activity of nano carbon fullerene lipidosome against influenza virus in vitro. J. Huazhong Univ. Sci. Technol. 2008, 28, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Lauster, D.; Glanz, M.; Bardua, M.; Ludwig, K.; Hellmund, M.; Hoffmann, U.; Hamann, A.; Böttcher, C.; Haag, R.; Hackenberger, C.P.R.; et al. Multivalent Peptide–Nanoparticle Conjugates for Influenza-Virus Inhibition. Angew. Chem. Int. Ed. 2017, 56, 5931–5936. [Google Scholar] [CrossRef] [PubMed]
- Surnar, B.; Kamran, M.Z.; Shah, A.S.; Basu, U.; Kolishetti, N.; Deo, S.; Jayaweera, D.T.; Daunert, S.; Dhar, S.; Macdonald, J.T. Orally Administrable Therapeutic Synthetic Nanoparticle for Zika Virus. ACS nano 2019, 13, 11034–11048. [Google Scholar] [CrossRef]
- Chan, W.C.W. Nano Research for COVID-19. ACS Nano 2020. [CrossRef]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef]
- Kovarova, M.; Council, O.D.; Date, A.A.; Long, J.M.; Nochii, T.; Belshan, M.; Shibata, A.; Vincent, H.; Baker, C.E.; Thayer, W.O.; et al. Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission. PLoS Pathog. 2015, 11, e1005075. [Google Scholar] [CrossRef]
- Donovan, B.W.; Reuter, J.D.; Cao, Z.; Myc, A.; Johnson, K.J.; Baker, J.R. Prevention of murine influenza A virus pneumonitis by surfactant nano-emulsions. Antivir. Chem. Chemother. 2000, 11, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Silva, P.J.; Mueller, M.; Galloux, M.; Le Goffic, R.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018, 17, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Bogdanchikova, N.; Muñoz, R.V.; Saquero, A.H.; Jasso, A.P.; Uzcanga, G.A.; Díaz, P.L.P.; Pestryakov, A.; Burmistrov, V.; Martynyuk, O.; Gómez, R.L.V.; et al. Silver nanoparticles composition for treatment of distemper in dogs. Int. J. Nanotechnol. 2016, 13, 227. [Google Scholar] [CrossRef]
- Nazaktabar, A.; Lashkenari, M.S.; Araghi, A.; Ghorbani, M.; Golshahi, H. In vivo evaluation of toxicity and antiviral activity of polyrhodanine nanoparticles by using the chicken embryo model. Int. J. Biol. Macromol. 2017, 103, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Ufaz, S.; Balter, A.; Tzror, C.; Einbender, S.; Koshet, O.; Shainsky-Roitman, J.; Yaari, Z.; Schroeder, A. Anti-viral RNAi nanoparticles protect shrimp against white spot disease. Mol. Syst. Des. Eng. 2018, 3, 38–48. [Google Scholar] [CrossRef]
- Kim, S.S.; Peer, D.; Kumar, P.; Subramanya, S.; Wu, H.; Asthana, D.; Habiro, K.; Yang, Y.G.; Manjunath, N.; Shimaoka, M.; et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol. Ther. 2010, 18, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Tatham, L.M.; Savage, A.C.; Dwyer, A.; Siccardi, M.; Scott, T.; Vourvahis, M.; Clark, A.; Rannard, S.P.; Owen, A. Towards a Maraviroc long-acting injectable nanoformulation. Eur. J. Pharm. Biopharm. 2019, 138, 92–98. [Google Scholar] [CrossRef]
- Herzog, C.; Hartmann, K.; Künzi, V.; Kürsteiner, O.; Mischler, R.; Lazar, H.; Glück, R. Eleven years of Inflexal® V—A virosomal adjuvanted influenza vaccine. Vaccine 2009, 27, 4381–4387. [Google Scholar] [CrossRef]
- Alconcel, S.N.S.; Baas, A.S.; Maynard, H.D. FDA-approved poly(ethylene glycol)-protein conjugate drugs. Polym. Chem. 2011, 2, 1442–1448. [Google Scholar] [CrossRef]
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef]
- Seto, W.K.; Yuen, M.F. New pharmacological approaches to a functional cure of hepatitis B. Clin. Liver Dis. 2016, 8, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, F.D.; Botezat, D.; Gardikiotis, I.; Uritu, C.M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.; Tamba, B.I.; Mihai, C.T. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics 2020, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Prociak, J.; Banach, M. Silver nanoparticles—A material of the future...? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Donalisio, M.; Civra, A.; Argenziano, M.; Cavalli, R. Nanomedicine formulations for the delivery of antiviral drugs: A promising solution for the treatment of viral infections. Expert Opin. Drug Deliv. 2018, 15, 93–114. [Google Scholar] [CrossRef]
- Singh, L.; Kruger, H.G.; Maguire, G.E.M.; Govender, T.; Parboosing, R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017, 4, 105–131. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. P T 2017, 42, 742–755. [Google Scholar]
- Emam, H.E.; Manian, A.P.; Široká, B.; Duelli, H.; Redl, B.; Pipal, A.; Bechtold, T. Treatments to impart antimicrobial activity to clothing and household cellulosic-textiles—Why “nano”-silver? J. Clean. Prod. 2013, 39, 17–23. [Google Scholar] [CrossRef]
- Massella, D.; Giraud, S.; Guan, J.; Ferri, A.; Salaün, F. Textiles for health: A review of textile fabrics treated with chitosan microcapsules. Environ. Chem. Lett. 2019, 17, 1787–1800. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in Textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Brabazon, D.; Pellicer, E.; Zivic, F.; Sort, J.; Baró, M.D.; Grujovic, N.; Choy, K.L. Commercialization of Nanotechnologies-A Case Study Approach; Brabazon, D., Pellicer, E., Zivic, F., Sort, J., Dolors Baró, M., Grujovic, N., Choy, K.-L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319569796. [Google Scholar]
- Suryaprabha, T.; Sethuraman, M.G. Fabrication of copper-based superhydrophobic self-cleaning antibacterial coating over cotton fabric. Cellulose 2017, 24, 395–407. [Google Scholar] [CrossRef]
- Mao, N. Textile Materials for Protective Textiles. In High Performance Technical Textiles; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 107–157. [Google Scholar]
- Libertino, S.; Plutino, M.R.; Rosace, G. Design and development of wearable sensing nanomaterials for smart textiles. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 1990, p. 020016. [Google Scholar]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-García, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W.; et al. Significance of Nanomaterials in Wearables: A Review on Wearable Actuators and Sensors. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef] [PubMed]
- Hebalkar, N.Y.; Acharya, S.; Rao, T.N. Preparation of bi-functional silica particles for antibacterial and self cleaning surfaces. J. Colloid Interface Sci. 2011, 364, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Elbourne, A.; Crawford, R.J.; Ivanova, E.P. Nano-structured antimicrobial surfaces: From nature to synthetic analogues. J. Colloid Interface Sci. 2017, 508, 603–616. [Google Scholar] [CrossRef]
- Rai, P.K.; Usmani, Z.; Thakur, V.K.; Gupta, V.K.; Mishra, Y.K. Tackling COVID-19 pandemic through nanocoatings: Confront and exactitude. Curr. Res. Green Sustain. Chem. 2020, 3, 100011. [Google Scholar] [CrossRef]
- Ku, T.S.N.; Walraven, C.J.; Lee, S.A. Candida auris: Disinfectants and Implications for Infection Control. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Culver, A.; Geiger, C.; Simon, D. Safer Products and Practices for Disinfecting and Sanitizing Surfaces; SF Environment: San Francisco, CA, USA, 2014.
- Vaze, N.; Pyrgiotakis, G.; McDevitt, J.; Mena, L.; Melo, A.; Bedugnis, A.; Kobzik, L.; Eleftheriadou, M.; Demokritou, P. Inactivation of common hospital acquired pathogens on surfaces and in air utilizing engineered water nanostructures (EWNS) based nano-sanitizers. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 234–242. [Google Scholar] [CrossRef]
- Joe, Y.H.; Park, D.H.; Hwang, J. Evaluation of Ag nanoparticle coated air filter against aerosolized virus: Anti-viral efficiency with dust loading. J. Hazard. Mater. 2016, 301, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montelongo, J.H.; Medina-Ramírez, I.E.; Romo-Lozano, Y.; Zapien, J.A. Development of a sustainable photocatalytic process for air purification. Chemosphere 2020. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Herrera, G.; Medina-Ramírez, I.E.; Lozano-Álvarez, J.A.; Rodil, S.E. Evaluation of the Photocatalytic Activity of Copper Doped TiO 2 nanoparticles for the Purification and/or Disinfection of Industrial Effluents. Catal. Today 2018. [Google Scholar] [CrossRef]
- Mishra, M.; Kumar, H.; Tripathi, K. Diabetic Delayed Wound Healing and the Role of Silver. Dig. J. Nano 2008, 3, 49–54. [Google Scholar] [CrossRef]
- Zivic, F.; Grujovic, N.; Mitrovic, S.; Ahad, I.U.; Brabazon, D. Characteristics and applications of silver nanoparticles. In Commercialization of Nanotechnologies-A Case Study Approach; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 227–273. ISBN 9783319569796. [Google Scholar]
- Hameed, S.; Xie, L.; Ying, Y. Conventional and emerging detection techniques for pathogenic bacteria in food science: A review. Trends Food Sci. Technol. 2018, 81, 61–73. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of corona virus disease-19 (COVID-19): The Zhejiang experience. Zhejiang Da Xue Xue Bao. Yi Xue Ban 2020, 49. [Google Scholar] [CrossRef]
- Luo, X.; Xia, H.; Yang, W.; Wang, B.; Guo, T.; Xiong, J.; Jiang, Z.; Liu, Y.; Yan, X.; Zhou, W.; et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. MedRxiv 2020. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States; U.S. Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Vazquez-Muñoz, R.; Arellano-Jimenez, M.J.; Lopez, F.D.; Lopez-Ribot, J.L. Protocol optimization for a fast, simple and economical chemical reduction synthesis of antimicrobial silver nanoparticles in non-specialized facilities. BMC Res. Notes 2019, 12, 773. [Google Scholar] [CrossRef]
- Vega-Jiménez, A.L.; Almaguer-Flores, A.; Flores-Castaneda, M.; Camps, E.; Uribe-Ramirez, M.; Aztatzi-Aguilar, O.G.; De Vizcaya-Ruiz, A. Bismuth subsalicylate nanoparticles with anaerobic antibacterial activity for dental applications. Nanotechnology 2017, 28, 435101. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, M.R.; Mady, R.F.; Diab, R.G.; Shalaby, T.I. Chitosan and silver nanoparticles: Promising anti-toxoplasma agents. Exp. Parasitol. 2014, 143, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kurvet, I.; Juganson, K.; Vija, H.; Sihtmäe, M.; Blinova, I.; Syvertsen-Wiig, G.; Kahru, A. Toxicity of nine (doped) rare earth metal oxides and respective individual metals to aquatic microorganisms Vibrio fischeri and Tetrahymena thermophila. Materials 2017, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Guisbiers, G.; Mendoza, J.; Mimun, L.C.; Vincent, B.A.; Lopez-Ribot, J.L.; Nash, K.L. Synergistic antifungal effect of chitosan-stabilized selenium nanoparticles synthesized by pulsed laser ablation in liquids against Candida albicans biofilms. Int. J. Nanomed. 2018, 13, 2697–2708. [Google Scholar] [CrossRef]
- Montes, M.; Pierce, C.G.; Lopez-Ribot, J.L.; Bhalla, A.S.; Guo, R. Properties of silver and copper nanoparticle containing aqueous suspensions and evaluation of their in vitro activity against candida albicans and staphylococcus aureus biofilms. J. Nano Res. 2016, 37, 109–121. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Jose Yacaman, M.; Lopez-Ribot, J. Inhibition of Candida auris Biofilm Formation on Medical and Environmental Surfaces by Silver Nanoparticles. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural Analysis of Candida albicans When Exposed to Silver Nanoparticles. PLoS ONE 2014, 9, e108876. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Lopez, F.D.; Lopez-Ribot, J.L. Silver nanoantibiotics display strong antifungal activity against the emergent multidrug-resistant yeast Candida auris under both planktonic and biofilm growing conditions. Front. Microbiol. 2020, 11, 1673. [Google Scholar] [CrossRef]
- Möhler, J.S.; Sim, W.; Blaskovich, M.A.T.; Cooper, M.A.; Ziora, Z.M. Silver bullets: A new lustre on an old antimicrobial agent. Biotechnol. Adv. 2018, 36, 1391–1411. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, P.; Singh, D.K.; Poddar, P. Advances in the Experimental and Theoretical Understandings of Antibiotic Conjugated Gold Nanoparticles for Antibacterial Applications. ChemistrySelect 2019, 4, 6719–6738. [Google Scholar] [CrossRef]
- Murakami, N.; Wakabayashi, N.; Matsushima, R.; Kishida, A.; Igarashi, Y. Effect of high-pressure polymerization on mechanical properties of PMMA denture base resin. J. Mech. Behav. Biomed. Mater. 2013, 20, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Edis, Z.; Haj Bloukh, S.; Ashames, A.; Ibrahim, M. Copper-Based Nanoparticles, Their Chemistry and Antibacterial Properties: A Review. In Chemistry for a Clean and Healthy Planet; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 401–428. [Google Scholar]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.; Silva, Í.C.; Pais do Amaral, C.; Gonçalves, S.; Santos, N.C. Advances in Lipid and Metal Nanoparticles for Antimicrobial Peptide Delivery. Pharmaceutics 2019, 11, 588. [Google Scholar] [CrossRef]
- Bui, V.; Park, D.; Lee, Y.-C. Chitosan Combined with ZnO, TiO2 and Ag Nanoparticles for Antimicrobial Wound Healing Applications: A Mini Review of the Research Trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef]
- Nikam, A.V.; Prasad, B.L.V.; Kulkarni, A.A. Wet chemical synthesis of metal oxide nanoparticles: A review. CrystEngComm 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Arellano-Jimenez, M.J.; Lopez-Ribot, J.L. Fast, facile synthesis method for BAL-mediated PVP-bismuth nanoparticles. MethodsX 2020, 7, 100894. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).