A Review of the Calcium Sulphoaluminate Cement Mixed with Seawater: Hydration Process, Microstructure, and Durability
Abstract
1. Background
2. Fundamentals of Calcium Sulphoaluminate Cement (CSAs)
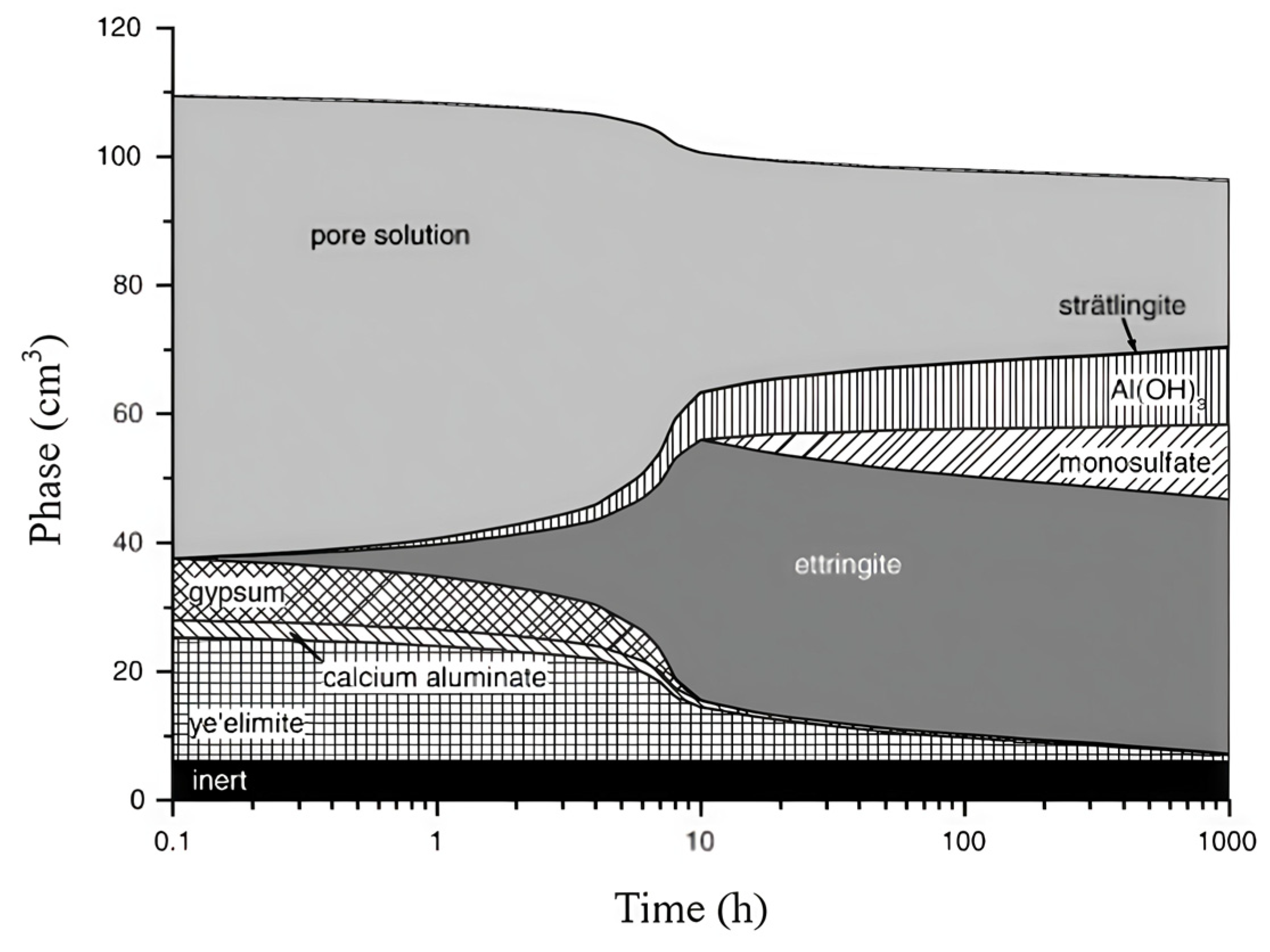
2.1. Chemical Composition and Reaction Mechanisms
2.2. Setting Time and Workability
2.3. Mechanical Properties
2.4. Durability
3. Role of Seawater in CSA Hydration and Microstructures
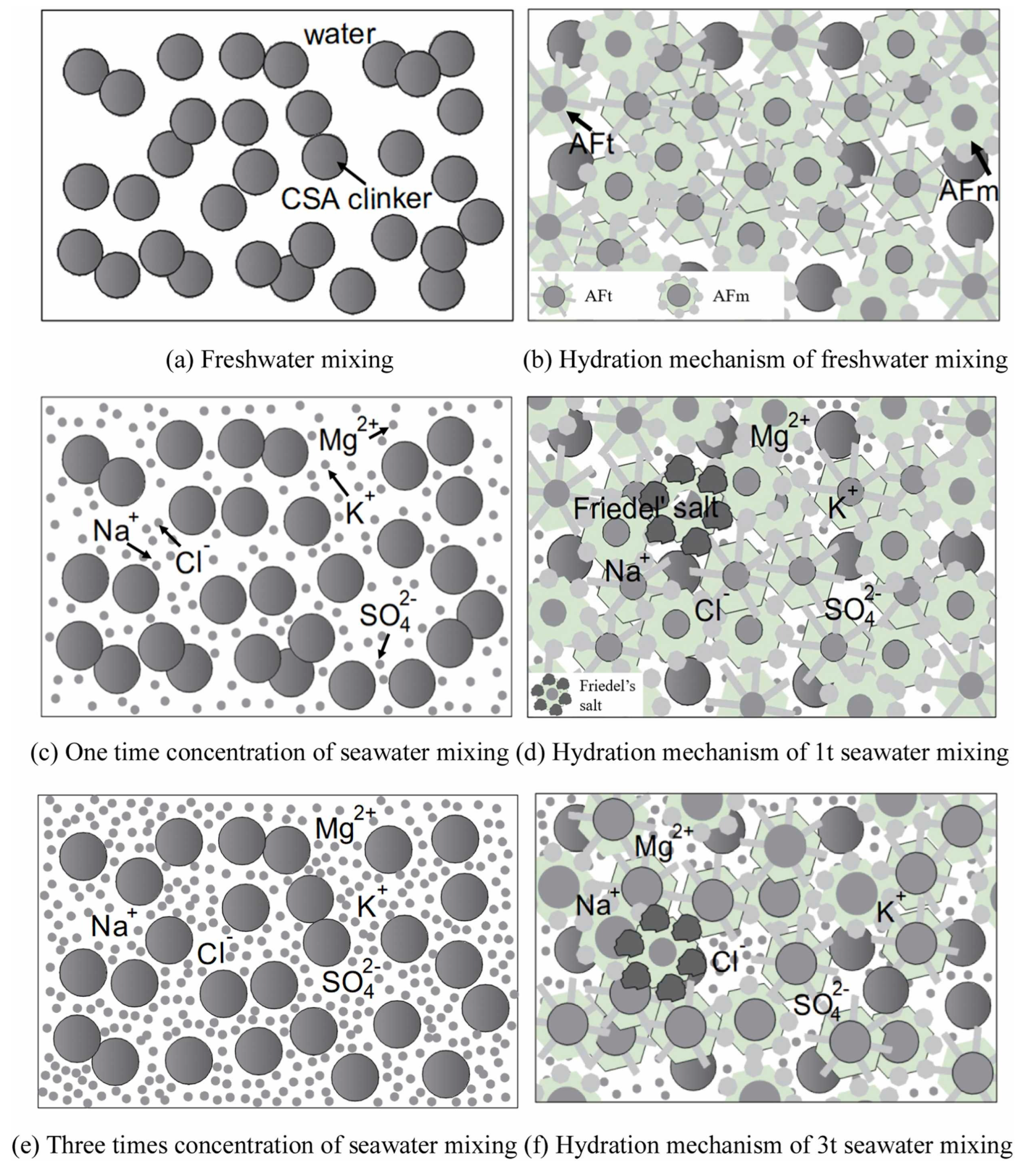
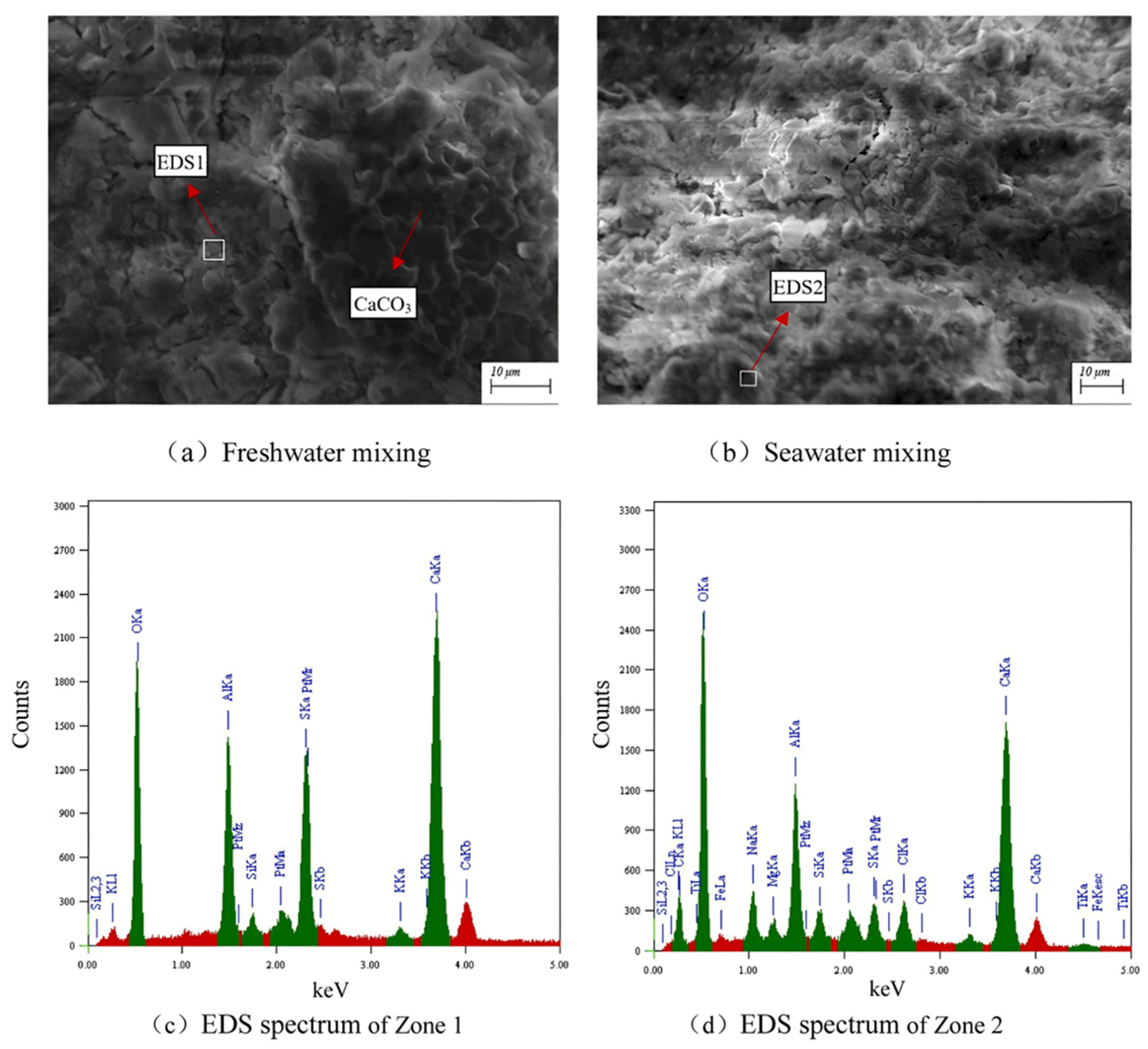
3.1. Hydration Process and Microstructure Evolution with Impacts of Seawater Ions
3.2. Curing Conditions
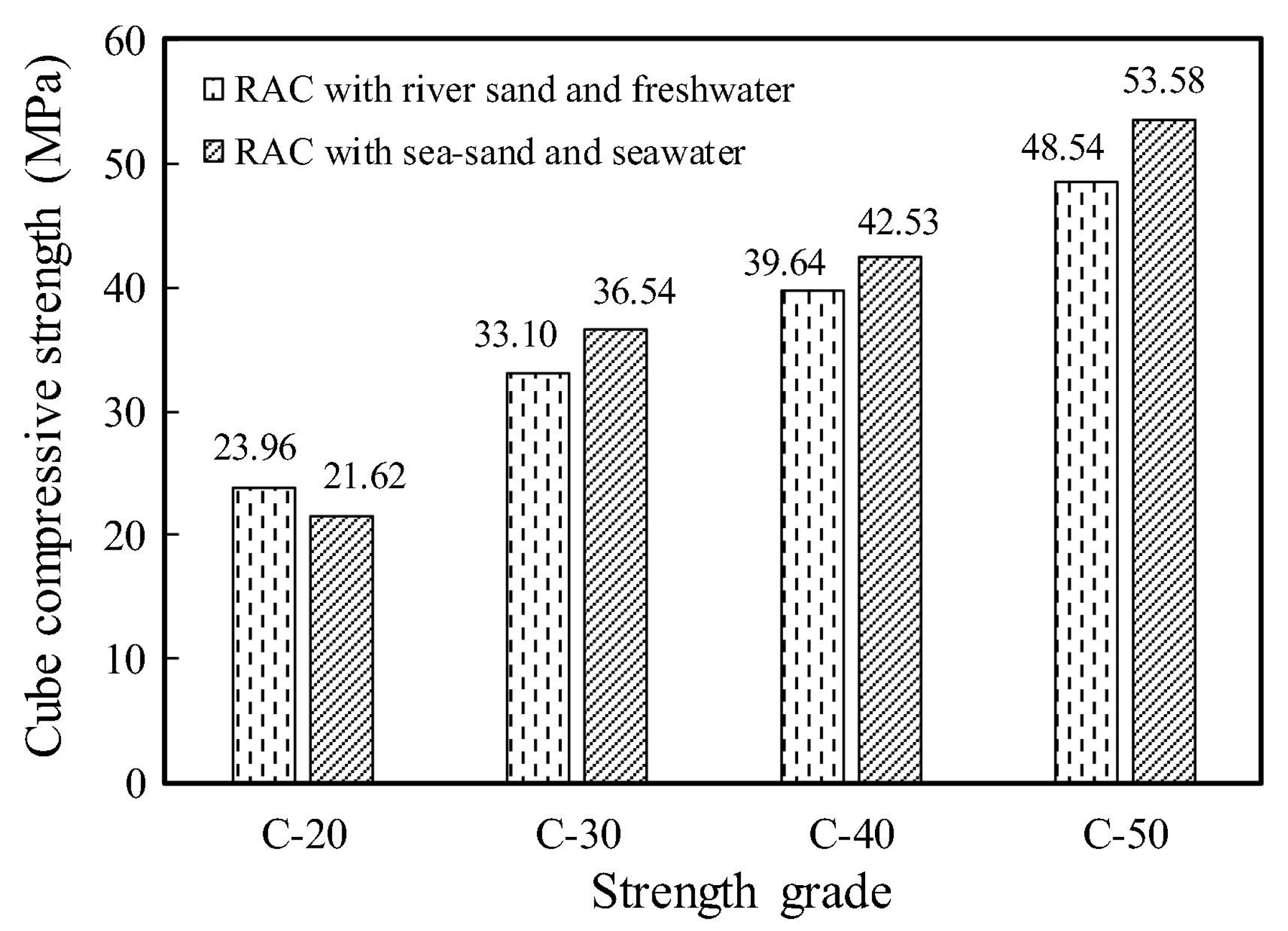
3.3. Mechanical Properties
3.4. Durability Concerns Related to Seawater Ions
3.4.1. Chloride Ingress
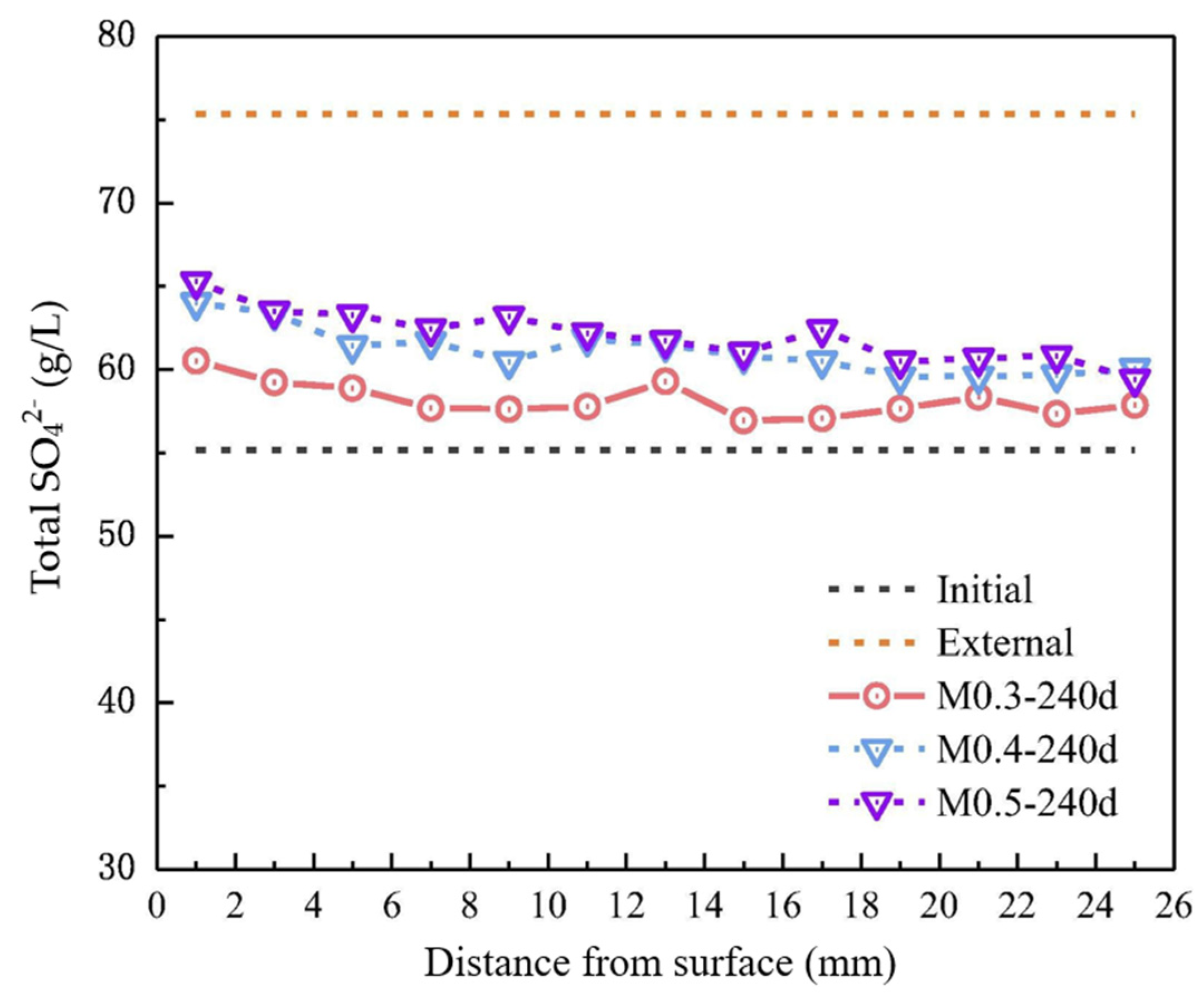
3.4.2. Sulfate Attack
3.4.3. Carbonation Resistance
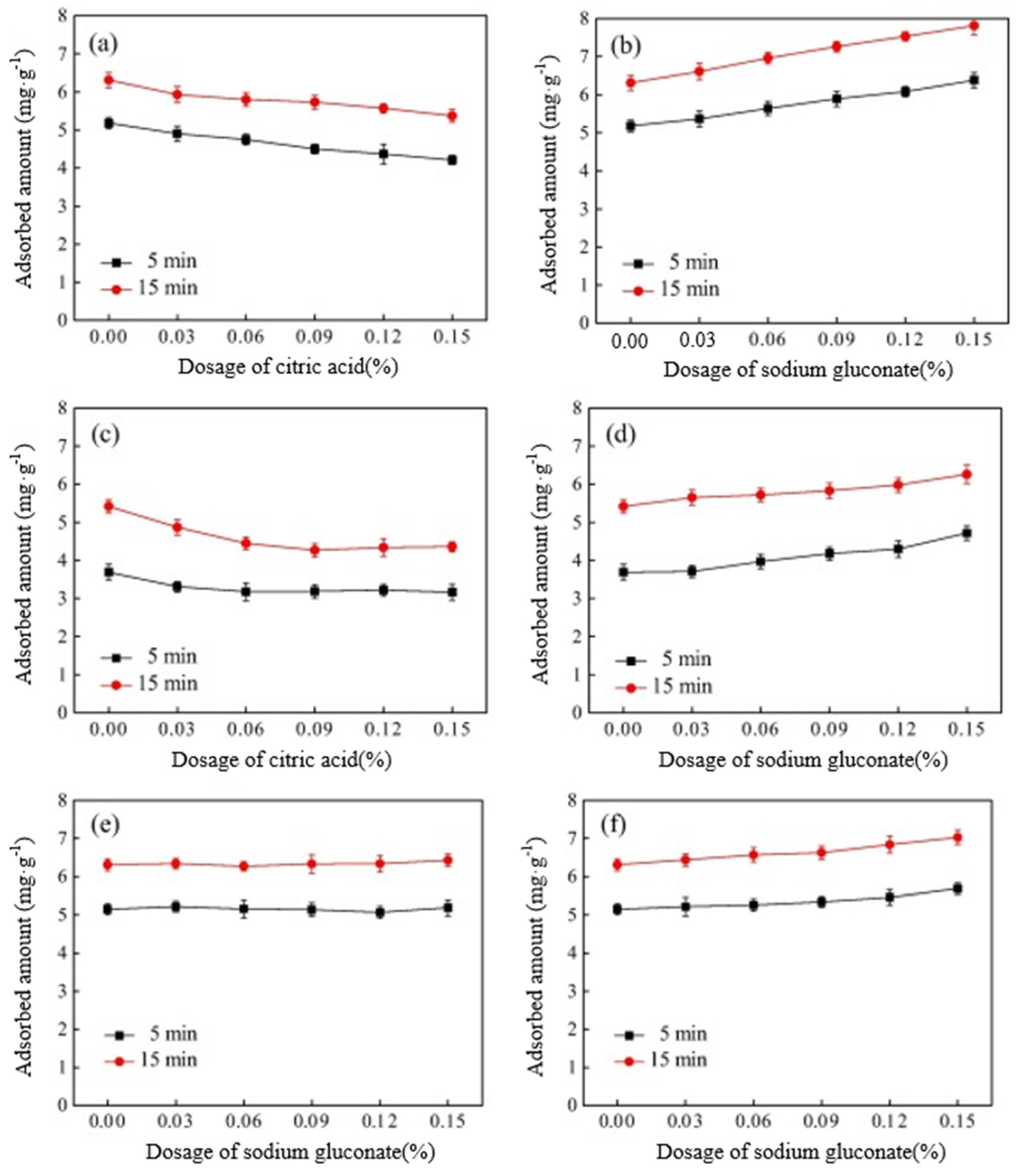
3.4.4. Potential Solutions
4. Environmental and Economic Considerations
4.1. Sustainability Benefits
4.2. Cost Analysis
5. Current Research Gaps, Challenges, and Future Trends
5.1. Combinations of Seawater and Chemical Admixtures
5.2. LCA of CSA Mixed with Seawater
5.3. Modification Strategies via Nanotechnology
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Damtoft, J.S.; Lukasik, J.; Herfort, D.; Sorrentino, D.; Gartner, E.M. Sustainable development and climate change initiatives. Cem. Concr. Res. 2008, 38, 115–127. [Google Scholar] [CrossRef]
- Li, L.; Gong, W.; Li, J. Service life of prestressed high-strength concrete pile in marine environment considering effects of concrete stratification and temperature. Constr. Build. Mater. 2020, 253, 119233. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Impacts of booming concrete production on water resources worldwide. Nat. Sustain. 2018, 1, 69–76. [Google Scholar] [CrossRef]
- Kasselouri, V.; Tsakiridis, P.; Malami, C.; Georgali, B.; Alexandridou, C. A Study on the Hydration Products of a Non-Expansive Sulfoaluminate Cement. Cem. Concr. Res. 1995, 25, 1726–1736. [Google Scholar] [CrossRef]
- Gastaldi, D.; Paul, G.; Marchese, L.; Irico, S.; Boccaleri, E.; Mutke, S.; Buzzi, L.; Canonico, F. Hydration products in sulfoaluminate cements: Evaluation of amorphous phases by XRD/solid-state NMR. Cem. Concr. Res. 2016, 90, 162–173. [Google Scholar] [CrossRef]
- Belhadi, R.; Govin, A.; Grosseau, P. Influence of polycarboxylate superplasticizer, citric acid and their combination on the hydration and workability of calcium sulfoaluminate cement. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Li, L.; Wang, R.; Lu, Q. Influence of polymer latex on the setting time, mechanical properties and durability of calcium sulfoaluminate cement mortar. Constr. Build. Mater. 2018, 169, 911–922. [Google Scholar] [CrossRef]
- Fu, X.; Yang, C.; Liu, Z.; Tao, W.; Hou, W.; Wu, X. Studies on effects of activators on properties and mechanism of hydration of sulphoaluminate cement. Cem. Concr. Res. 2003, 33, 317–324. [Google Scholar] [CrossRef]
- Tang, S.W.; Zhu, H.G.; Li, Z.J.; Chen, E.; Shao, H.Y. Hydration stage identification and phase transformation of calcium sulfoaluminate cement at early age. Constr. Build. Mater. 2014, 75, 11–18. [Google Scholar] [CrossRef]
- Liao, Y.; Wei, X.; Li, G. Early hydration of calcium sulfoaluminate cement through electrical resistivity measurement and microstructure investigations. Constr. Build. Mater. 2011, 25, 1572–1579. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, D.; Cheng, X.; Chen, W. Carbonation resistance of sulphoaluminate cement-based high performance concrete. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2009, 24, 663–666. [Google Scholar] [CrossRef]
- Zhang, G.; Li, G.; Li, Y. Effects of superplasticizers and retarders on the fluidity and strength of sulphoaluminate cement. Constr. Build. Mater. 2016, 126, 44–54. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Song, Z.; Shi, C.; Zhang, A. Improvement of workability and early strength of calcium sulphoaluminate cement at various temperature by chemical admixtures. Constr. Build. Mater. 2018, 160, 427–439. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.-M.; Lecomte, A.; Roux, A.; Rolland, B.L. Hydration of ordinary Portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Compos. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Quillin, K. Performance of belite–sulfoaluminate cements. Cem. Concr. Res. 2001, 31, 1341–1349. [Google Scholar] [CrossRef]
- Bernardo, G.; Telesca, A.; Valenti, G.L. A porosimetric study of calcium sulfoaluminate cement pastes cured at early ages. Cem. Concr. Res. 2006, 36, 1042–1047. [Google Scholar] [CrossRef]
- Shang, C.; Wu, C.; Wang, J.; Lu, L.; Fu, Q.; Zhang, Y.; Song, X. Investigating mechanical properties and microstructure of reactive powder concrete blended with sulfoaluminate cement. Case Stud. Constr. Mater. 2022, 17, e01552. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Z.; He, F.; Huang, J.; Zhou, J. Sulfate diffusion in calcium sulphoaluminate mortar. Constr. Build. Mater. 2019, 234, 117312. [Google Scholar] [CrossRef]
- Song, M.; Wang, C.; Cui, Y.; Li, Q.; Gao, Z. Mechanical performance and microstructure of ultra-high-performance concrete modified by calcium sulfoaluminate cement. Adv. Civ. Eng. 2021, 9, 4002536. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv. Cem. Res. 2002, 14, 141–155. [Google Scholar] [CrossRef]
- Rungchet, A.; Poon, C.S.; Chindaprasirt, P.; Pimraksa, K. Synthesis of low-temperature calcium sulfoaluminate-belite cements from industrial wastes and their hydration: Comparative studies between lignite fly ash and bottom ash. Cem. Concr. Compos. 2017, 83, 10–19. [Google Scholar] [CrossRef]
- He, W.; Li, R.; Nie, D.; Zhang, J.; Wang, Y.; Zhang, Y.; Chen, Q. Belite-calcium sulphoaluminate cement prepared by EMR and BS: Hydration characteristics and microstructure evolution behavior. Constr. Build. Mater. 2022, 333, 127415. [Google Scholar] [CrossRef]
- Filani, I.; Butt, A.A.; Harvey, J. Life cycle cost and environmental impacts of portland limestone cement and calcium sulfoaluminate cement as alternative binders in concrete. In Pavement, Roadway, and Bridge Life Cycle Assessment; Springer Nature: Cham, Switzerland, 2024; pp. 61–68. [Google Scholar]
- Song, Y.-J.; Kim, S.-H.; Kim, J.-S.; Yun, H.-D.; Tera, M.; Shin, M. Influence of CSA expansive admixture on mechanical properties of strain-Hardening Cement-Based Composite (SHCC). Adv. Sci. Lett. 2012, 13, 727–731. [Google Scholar] [CrossRef]
- Li, Z.; Guo, T.; Chen, Y.; Fang, C.; Chang, Y.; Nie, J. Influence of basalt fiber and polypropylene fiber on the mechanical and durability properties of cement-based composite materials. J. Build. Eng. 2024, 90, 109335. [Google Scholar] [CrossRef]
- Bescher, E.; Rice, E.K.; Ramseyer, C. Sulfate resistance of calcium sulphoaluminate cement. J. Struct. Integr. Maint. 2016, 1, 131–139. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Huang, Y.; Yang, D. Synthesis of belite sulfoaluminate-ternesite cements with phosphogypsum. Cem. Concr. Compos. 2015, 63, 67–75. [Google Scholar] [CrossRef]
- Shang, C.; Wu, C.; Liu, Y.; Wang, J.; Fu, Q.; Lu, L.; Sheng, Z.; Xing, F. Impact of sulfoaluminate cement modification on corrosion resistance of reactive powder concrete subjected to ammonium sulfate-rich sewer environment. Constr. Build. Mater. 2024, 411, 134652. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Tang, W.; Wen, L.; Yu, B.; Tao, W.; Xu, H.; Li, J. Study on the anti-corrosion and barrier ability of modified sulfoaluminate cement mortar cutoff wall against sulfate. Constr. Build. Mater. 2023, 409, 134035. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Hu, W.; Wu, K. Durability and microstructure of CSA cement-based materials from MSWI fly ash. Cem. Concr. Compos. 2014, 46, 26–31. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, G.; Gao, D.; Zhao, S. Influences of freeze–thaw cycle and curing time on chloride ion penetration resistance of Sulphoaluminate cement concrete. Constr. Build. Mater. 2014, 53, 305–311. [Google Scholar] [CrossRef]
- Ke, G.; Zhang, J.; Liu, Y. Shrinkage characteristics of calcium sulphoaluminate cement concrete. Constr. Build. Mater. 2022, 337, 127627. [Google Scholar] [CrossRef]
- Duan, P.; Chen, W.; Ma, J.; Shui, Z. Influence of layered double hydroxides on microstructure and carbonation resistance of sulphoaluminate cement concrete. Constr. Build. Mater. 2013, 48, 601–609. [Google Scholar] [CrossRef]
- Dillard, R.J.; Murray, C.D.; Deschenes, R.A. Belitic calcium sulfoaluminate cement subjected to sulfate attack and sulfuric acid. Constr. Build. Mater. 2022, 343, 128089. [Google Scholar] [CrossRef]
- Damion, T.; Chaunsali, P. Evaluating acid resistance of Portland cement, calcium aluminate cement, and calcium sulfoaluminate based cement using acid neutralization. Cem. Concr. Res. 2022, 162, 107000. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Lian, S.; Tang, X. Drying-wetting correlation analysis of chloride transport behavior and mechanism in calcium sulphoaluminate cement concrete. Materials 2024, 17, 4600. [Google Scholar] [CrossRef]
- Zhang, W.; Du, H.; Pang, S.D. Sulfate resistance of cement paste to internal and external seawater. Constr. Build. Mater. 2024, 447, 138101. [Google Scholar] [CrossRef]
- Ting, M.Z.Y.; Yi, Y. Durability of cementitious materials in seawater environment: A review on chemical interactions, hardened-state properties and environmental factors. Constr. Build. Mater. 2023, 367, 130224. [Google Scholar] [CrossRef]
- Wang, C.; Song, M. Influence of water-cement ratio and type of mixing water on the early hydration performance of calcium sulphoaluminate (CSA) cement. Adv. Mater. Sci. Eng. 2021, 1, 5557763. [Google Scholar] [CrossRef]
- Park, S.; Lee, N.; Park, K.G. Early-age hydration behavior of calcium sulfoaluminate (CSA) cement/ordinary portland cement-blended ultra-high performance concrete. J. Build. Eng. 2024, 87, 109058. [Google Scholar] [CrossRef]
- Jen, G.; Stompinis, N.; Jones, R. Chloride ingress in a belite-calcium sulfoaluminate cement matrix. Cem. Concr. Res. 2017, 98, 130–135. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Zhang, T.; Zhang, Y.; Liu, Z.; Zhao, X. Influence of the Concentration of Seawater on the Early Hydration Properties of Calcium Sulphoaluminate (CSA) Cement: A Preliminary Study. Buildings 2021, 11, 243. [Google Scholar] [CrossRef]
- Li, G.; Zhang, A.; Song, Z.; Shi, C.; Wang, Y.; Zhang, J. Study on the resistance to seawater corrosion of the cementitious systems containing ordinary Portland cement or/and calcium aluminate cement. Constr. Build. Mater. 2017, 157, 852–859. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.; Xue, G.; Niu, J. Acoustic emission behavior of polyvinyl alcohol (PVA) fiber reinforced calcium sulphoaluminate cement mortar under flexural load. J. Build. Eng. 2021, 40, 102734. [Google Scholar] [CrossRef]
- Tadesse, D.M.; Jeon, J.; Kim, S.; Park, S. The effect of seawater on the phase assemblage of hydrated cement paste: A study on PC, CAC and CSA. Dev. Built Environ. 2023, 15, 100183. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, Z.; Accornero, F.; Zhou, S.; Ou, Q. Influence of seawater and salt ions on the properties of calcium sulfoaluminate cement. J. Mater. Civ. Eng. 2025, 37, 6. [Google Scholar] [CrossRef]
- Li, G.; Bai, Z.; Zhang, G.; Wu, Y. Study on properties and degradation mechanism of calcium sulphoaluminate cement-ordinary Portland cement binary repair material under seawater erosion. Case Stud. Constr. Mater. 2022, 17, e01440. [Google Scholar] [CrossRef]
- Moffatt, E.G.; Thomas, M.D.A. Performance of rapid-repair concrete in an aggressive marine environment. Constr. Build. Mater. 2017, 132, 478–486. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, Z.; Accornero, F.; Wang, C. Multi-technique analysis of seawater impact on the performance of calcium sulphoaluminate cement mortar. Constr. Build. Mater. 2023, 443, 137717. [Google Scholar] [CrossRef]
- Acarturk, B.C.; Burris, L.E. Investigations of the optimal requirements for curing of calcium sulfoaluminate cement systems. Cement 2023, 12, 100072. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Jörg Müller, C. Beneficial use of Limestone filler with calcium sulphoaluminate cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- Wang, P.; Li, N.; Xu, L. Hydration evolution and compressive strength of calcium sulphoaluminate cement constantly cured over the temperature range of 0 to 80 °C. Cem. Concr. Res. 2017, 100, 203–213. [Google Scholar] [CrossRef]
- Berger, S.; Coumes, C.C.D.; Bescop, P.L.; Damidot, D. Influence of a thermal cycle at early age on the hydration of calcium sulphoaluminate cements with variable gypsum contents. Cem. Concr. Res. 2011, 41, 149–160. [Google Scholar] [CrossRef]
- Xu, L.; Liu, S.; Li, N.; Peng, Y.; Wu, K.; Wang, P. Retardation effect of elevated temperature on the setting of calcium sulfoaluminate cement clinker. Constr. Build. Mater. 2018, 178, 112–119. [Google Scholar] [CrossRef]
- Li, L.; Wang, R.; Zhang, S. Effect of curing temperature and relative humidity on the hydrates and porosity of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 213, 627–636. [Google Scholar] [CrossRef]
- Xiao, J.; Qiang, C.; Nanni, A.; Zhang, K. Use of sea-sand and seawater in concrete construction: Current status and future opportunities. Constr. Build. Mater. 2017, 155, 1101–1111. [Google Scholar] [CrossRef]
- Tang, X. Effects of cyclic seawater exposure on the mechanical performance and chloride penetration of calcium sulfoaluminate concrete. Constr. Build. Mater. 2021, 303, 124139. [Google Scholar] [CrossRef]
- Gu, P.; Beaudoin, J.J.; Quinn, E.G.; Myers, R.E. Early strength development and hydration of ordinary portland cement/Calcium Aluminate cement pastes. Adv. Cem. Based Mater. 1997, 6, 53–58. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Shi, C.; Fernández Jiménez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Y.; Kang, M.; Ju, Y.; Zeng, C. Durability of reactive powder concrete containing mineral admixtures in seawater erosion environment. Constr. Build. Mater. 2021, 306, 124863. [Google Scholar] [CrossRef]
- Soares, S.; Freitas, N.; Pereira, E.; Nepomuceno, E.; Pereira, E.; Sena-Cruz, J. Assessment of GFRP bond behaviour for the design of sustainable reinforced seawater concrete structures. Constr. Build. Mater. 2020, 231, 117277. [Google Scholar] [CrossRef]
- Cheng, S.; Shui, Z.; Gao, X.; Lu, J.; Sun, T.; Yu, R. Degradation progress of Portland cement mortar under the coupled effects of multiple corrosive ions and drying-wetting cycles. Cem. Concr. Compos. 2020, 111, 103629. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, X.; Ji, T.; Liang, Y.; Pan, W. Comparison of corrosion resistance mechanism between ordinary Portland concrete and alkali-activated concrete subjected to biogenic sulfuric acid attack. Constr. Build. Mater. 2019, 228, 117071. [Google Scholar] [CrossRef]
- Paul, G.; Boccaleri, E.; Buzzi, L.; Canonico, F.; Gastaldi, D. Friedel’s salt formation in sulfoaluminate cements: A combined XRD and 27Al MAS NMR study. Cem. Concr. Res. 2015, 67, 93–102. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, T.; Yang, Z.; Ye, Y. Preliminary investigation of artificial reef concrete with sulphoaluminate cement, marine sand and sea water. Constr. Build. Mater. 2019, 211, 837–846. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, W.; Yuan, J.; Zhang, Z.; Wu, G. Study on seawater corrosion resistance of calcium sulfoaluminate cement-based novel grouting materials. Mater. Lett. 2024, 366, 136509. [Google Scholar] [CrossRef]
- Ming, X.; Liu, Q.; Wang, M.; Cai, Y.; Chen, B.; Li, Z. Improved chloride binding capacity and corrosion protection of cement-based materials by incorporating alumina nano particles. Cem. Concr. Compos. 2023, 136, 104898. [Google Scholar] [CrossRef]
- Rivera-Gonzalez, N.; Bajpayee, A.; Nielsen, J.; Zakira, U.; Zaheer, W.; Handy, J.; Sill, T.; Birgisson, B.; Bhatia, M.; Banerjee, S. Textured ceramic membranes for desilting and deoiling of produced water in the Permian Basin. iScience 2022, 25, 105063. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Li, Q.; Wu, K.; Dou, Y. Electrochemical performance of steel embedded in CSA concrete and its interfacial microstructure. Adv. Mater. Sci. Eng. 2020, 8, 4761854. [Google Scholar] [CrossRef]
- Ma, J.; Wang, H.; Yu, Z.; Shi, H.; Wu, Q.; Shen, X. A systematic review on durability of calcium sulphoaluminate cement-based materials in chloride environment. J. Sustain. Cem.-Based Mater. 2023, 12, 687–698. [Google Scholar] [CrossRef]
- Hargis, C.W.; Lothenbach, B.; Müller, C.J.; Winnefeld, F. Carbonation of calcium sulfoaluminate mortars. Cem. Concr. Compos. 2017, 80, 123–134. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Z.; Hou, P.; Fu, X.; Qu, Y.; Li, Q.; Cheng, X.; Zhu, X. The influence of surface treatment on the transport properties of hardened calcium sulfoaluminate cement-based materials. Cem. Concr. Compos. 2020, 114, 103784. [Google Scholar] [CrossRef]
- Park, S. Simulating the carbonation of calcium sulfoaluminate cement blended with supplementary cementitious materials. J. CO2 Util. 2020, 41, 101286. [Google Scholar] [CrossRef]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, C.; Tan, H.; Liu, X. Potential application of Portland cement-sulfoaluminate cement system in precast concrete cured under ambient temperature. Constr. Build. Mater. 2020, 251, 118869. [Google Scholar] [CrossRef]
- Tanguler-Bayramtan, M.; Aktas, C.B.; Yaman, I.O. Environmental assessment of calcium sulfoaluminate cement: A monte carlo simulation in an industrial symbiosis framework. Buildings 2024, 14, 3673. [Google Scholar] [CrossRef]
- Ren, C.; Wang, W.; Mao, Y.; Yuan, X.; Song, Z.; Sun, J.; Zhao, X. Comparative life cycle assessment of sulfoaluminate clinker production derived from industrial solid wastes and conventional raw materials. J. Clean. Prod. 2017, 167, 1314–1324. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Yu, T.; Qu, F.; Tam, V.W.Y. Investigation on early-age hydration, mechanical properties and microstructure of seawater sea sand cement mortar. Constr. Build. Mater. 2020, 249, 118776. [Google Scholar] [CrossRef]
- Wang, J.; Liu, E.; Li, L. Multiscale investigations on hydration mechanisms in seawater OPC paste. Constr. Build. Mater. 2018, 191, 891–903. [Google Scholar] [CrossRef]
- Kang, C.; Kim, T. Influence of brine on hydration reaction of calcium sulfoaluminate and slag blended cement. Case Stud. Constr. Mater. 2023, 18, e02159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Meng, J.; Liu, Y.; Yang, L.; Wang, Y.; Xie, N.; Ou, J.; Zhou, G. A Review of the Calcium Sulphoaluminate Cement Mixed with Seawater: Hydration Process, Microstructure, and Durability. J. Mar. Sci. Eng. 2025, 13, 1076. https://doi.org/10.3390/jmse13061076
Li H, Meng J, Liu Y, Yang L, Wang Y, Xie N, Ou J, Zhou G. A Review of the Calcium Sulphoaluminate Cement Mixed with Seawater: Hydration Process, Microstructure, and Durability. Journal of Marine Science and Engineering. 2025; 13(6):1076. https://doi.org/10.3390/jmse13061076
Chicago/Turabian StyleLi, Han, Jing Meng, Yang Liu, Lilin Yang, Yukai Wang, Ning Xie, Jinping Ou, and Guoxiang Zhou. 2025. "A Review of the Calcium Sulphoaluminate Cement Mixed with Seawater: Hydration Process, Microstructure, and Durability" Journal of Marine Science and Engineering 13, no. 6: 1076. https://doi.org/10.3390/jmse13061076
APA StyleLi, H., Meng, J., Liu, Y., Yang, L., Wang, Y., Xie, N., Ou, J., & Zhou, G. (2025). A Review of the Calcium Sulphoaluminate Cement Mixed with Seawater: Hydration Process, Microstructure, and Durability. Journal of Marine Science and Engineering, 13(6), 1076. https://doi.org/10.3390/jmse13061076








