Abstract
This study aimed to elucidate the effects of different photoperiods (0 L:24 D, 6 L:18 D, 12 L:12 D, 18 L:6 D, 24 L:0 D, “Light (L) and Dark (D)”) on the growth performance and physiological responses of the juvenile scalloped spiny lobster (Panulirus homarus). Over a period of 56 days, parameters such as growth rate, digestive enzyme, immune enzyme, and antioxidant enzyme were meticulously evaluated in 90 lobsters subjected to these varying light conditions. The present study found no significant differences in survival rate (SR), molting frequency (MF), and meat yield production (MYP) among the various photoperiod treatments (p > 0.05). Notably, the highest weight gain rate (WGR) and specific growth rate (SGR) were observed under a 12 L:12 D photoperiod. In the continuous dark phase (0 L:24 D), pepsin (PEP) activity remained high in gastric tissues, while trypsin (TRYP) and chymotrypsin (CHT) activities reached the highest in hepatopancreas tissues. The α-amylase (AMS) activity in the hepatopancreas was most elevated under 18 L:6 D, and the optimal lipase (LPS) activity was recorded under 12 L:12 D. The activity of acid phosphatase (ACP) in the hepatopancreas was highest in the absence of light (0 L:24 D), whereas the activities of alkaline phosphatase (AKP) and lysozyme (LZM) were most effective under the 12 L:12 D photoperiod. The total antioxidant capacity (T-AOC), along with catalase (CAT) and superoxide dismutase (SOD) activities of the hepatopancreas reached the highest at 12 L:12 D. The highest activity of glutathione peroxidase (GSH-Px) was seen under 18 L:6 D. The concentration of malondialdehyde (MDA), a marker of oxidative stress, was found to be highest under 12 L:12 D. Consequently, this specific photoperiod is essential for achieving optimal growth and maintaining appropriate physiological balance in the scalloped spiny lobster during aquaculture. These findings provide a foundational guideline for establishing the lighting environment in the farming of the juvenile scalloped spiny lobster.
1. Introduction
The scalloped spiny lobster (Panulirus homarus Linnaeus, 1878), a large warm-water crustacean, inhabits tropical and subtropical coasts [1]. Its distribution and aquaculture are primarily concentrated in the Indo-West Pacific region [2,3]. Recognized as one of the most valuable seafood products [4], the scalloped spiny lobster is highly sought after in international markets due to its exquisite taste and rich nutritional profile [5]. However, with the development of fishery, the natural resources of lobsters have decreased dramatically [4,5,6,7,8,9,10,11]. In order to meet the market demand, larvae are captured from the wild and reared artificially [6,7,8,9,10,11]. Current research on this species encompasses a range of topics, including breeding practices [6], nutritional analysis [7], capture techniques [8,9], resource assessment [9,10,11], habitat studies [12], physiological characteristics [13,14,15], disease management [16,17], and genomics [18,19,20]. Despite these advancements, the impact of lighting conditions on scalloped spiny lobsters remains relatively underexplored. Presently, there is a notable gap in standardized breeding protocols that account for light conditions.
Light serves as a critical ecological factor within aquatic ecosystems [21]. It exerts a profound influence on various physiological processes and feeding behaviors in aquatic animals [22], modulating growth performance and developmental stages [23,24,25]. Notably, light impacts the growth and development of diverse species, including fish [26,27], shrimp [28], crabs [29], other crustaceans [22], and turtles [30], along with various physiological responses. As a pivotal environmental element, light is instrumental in regulating the circadian rhythms of these organisms [31]. Light possesses three fundamental characteristics: intensity, spectrum (or light quality), and photoperiod [21]. Among these, the photoperiod is particularly crucial for managing biological rhythms in aquatic life [32]. The diverse array of life on Earth is profoundly influenced by the varying light conditions resulting from the planet’s rotation around the Sun [32,33]. To cope with these consistent changes, terrestrial and aquatic organisms have developed intricate internal circadian rhythm systems [33]. Consequently, the photoperiod is recognized as a critical environmental cue impacting these biological rhythms [21]. In crustaceans, the photoperiod can directly or indirectly affect the circadian system, influencing growth, molting, and reproductive processes [34,35]. Optimizing photoperiods in aquaculture can enhance the growth and development rates of aquatic animals, thereby potentially shortening their production cycles [36].
Extensive research illustrates the impact of the photoperiod on vital activities and physiological responses in various aquatic species. In eastern rock lobsters (Sagmariasus verreauxi), longer photoperiods (18 L:6 D and 24 L:0 D) significantly improved survival and growth rates during larval metamorphosis [37]. Photoperiods of 6 L:18 D, 12 L:12 D, and 18 L:6 D have been shown to promote early larval growth, molting, and feeding in spiny lobsters (Jasus edwardsii) [38]. In juvenile goldfish (Carassius auratus), a light duration exceeding 16 h enhanced fat formation, lipolysis, and fatty acid oxidation [33]. Long photoperiods (16 L:8 D) induced stress in blunt snout bream (Megalobrama amblycephala), increasing plasma cortisol levels and causing oxidative stress [39]. Similarly, long photoperiods (18 L:6 D) significantly elevated plasma gonadotropins in greater amberjack (Seriola dumerili) [40] and affected osmoregulation and hepatic energy metabolism in cultured olive flounder (Paralichthys olivaceus), triggering stress responses [41]. Shorter photoperiods (2 L:22 D and 4 L:20 D) enhanced innate immune and antioxidant responses in white leg shrimp (Penaeus vannamei), reducing mortality in adult white leg shrimp [34]. Continuous darkness (0 L:24 D) was found to increase reproductive efficiency and sperm production in male narrow-clawed crayfish (Pontastacus leptodactylus) [42] and to elevate molt frequency and growth rate in juvenile mud crabs (Scylla paramamosain) [29]. Persian sturgeon (Acipenser persicus) exhibited the lowest stress levels in a no-light environment (0 L:24 D) [43]. These findings underscore the importance of the photoperiod in influencing the growth and behavior of aquatic animals. Adjusting the photoperiod in aquacultural settings may offer a means to shorten production cycles and enhance overall production efficiency. However, the specific effects of the photoperiod on the growth, development, and physiology of the scalloped spiny lobster remain to be fully determined.
Altered photoperiods have been shown to significantly influence the functioning of digestive and non-specific immune enzymes in aquatic animals. Several studies have suggested that external environmental factors, including light, can modulate the activity of digestive enzymes, which are critical indicators of digestive performance in aquatic species [22]. For instance, a 13 L:11 D photoperiod significantly alters the activities of pepsin (PEP), lipase (LPS), α-amylase (AMS), trypsin (TRYP), and chymotrypsin (CHT) in the tiger shrimp (Macrobrachium tenellum) [44]. Total darkness (0 L:24 D) has been reported to maximize LPS and AMS activities in the fairy shrimp (Branchinecta orientalis) [45,46], while a prolonged photoperiod (18 L:6 D) affects LPS and TRYP activities in spotted sea bass (Lateolabrax maculatus) [23]. Similarly, the highest TRYP and LPS activities in the Chinese soft-shell turtle were observed in complete darkness (0 L:24 D) [30].
Key barrier enzymes like alkaline phosphatase (AKP), acid phosphatase (ACP), and lysozyme (LZM), which protect aquatic animals from pathogenic bacteria, are widely distributed in their organs and tissues, serving as vital indicators of immune levels [47,48,49]. LZM can release hydrolytic enzymes that break down pathogens, thus playing an immune defense role [48,49,50]. ACP can catalyze the hydrolysis of organophosphorus; participate in phagocytosis, nodules, and envelope formation; regulate the activity of immune cells; help the immune system to better recognize and remove foreign substances; and maintain health [47,49]. The role of AKP in the immune system is mainly reflected in promoting the activation and proliferation of immune cells and enhancing the intercellular immune response [47]. It can enhance immunity by promoting the activity of immune cells, helping cells to recognize and resist [47,50,51]. Optimal ACP and LZM activities were recorded in freshwater shrimp (Macrobrachium rosenbergii) under complete darkness [50] and in tiger puffer (Takifugu rubripes) larvae under a long photoperiod (16 L:8 D) [51]. In brown frogs (Rana dybowskii), the best LZM activity was noted under light-free conditions [52]. The activity level of total antioxidant capacity (T-AOC) reflects the aquatic organisms’ antioxidant enzymes and non-enzymatic antioxidants, crucial for stress resistance and overall health and growth [53]. Reactive oxygen species (ROS) overproduction can impair physiological functions and cause oxidative damage to essential biomolecules [39]. Enzymes like superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) form the core of the organism’s antioxidant system, scavenging ROS and playing a pivotal role in their elimination [54]. Malondialdehyde (MDA), a marker of ROS levels and a byproduct of lipid peroxidation, can indicate cellular and tissue damage [35]. In white leg shrimp, MDA levels increased with longer light durations [35], while blunt snout bream exhibited higher SOD, CAT, and GSH activities with light durations exceeding 12 h [39]. Additionally, abalones showed a gradual increase in T-AOC, SOD, GSH-Px activity, and GSH contents with increasing light duration [55]. These findings underscore the significance of the photoperiod in determining the activities of digestive and immune enzymes in aquatic animals. Regulating the photoperiod can, thus, enhance digestive and immune functions in these species. However, the specific impacts of the photoperiod on the digestive, immunological, and antioxidant enzymes in the scalloped spiny lobster remain to be elucidated.
The primary objective of this study was to examine the physiological responses of the scalloped spiny lobster to various photoperiods (0 L:24 D, 6 L:18 D, 12 L:12 D, 18 L:6 D, 24 L:0 D). Specifically, the research focused on assessing the impact of these photoperiods on the lobster’s digestive performance, immune function, and antioxidant capacity. The outcomes of this study aim to establish an optimal photoperiod for the scalloped spiny lobster aquaculture. This would not only contribute to formulating a standardized system for regulating light duration in lobster farming but also optimize the production cycle and enhance the economic efficiency of this valuable seafood resource.
2. Materials and Methods
2.1. Experimental Materials
Scallop spiny lobsters are lobsters that have been farmed for six months after being caught in the wild. The experiment was conducted in the circulating water system of an indoor factory in Lingshui Experimental Station, Sanya Tropical Fisheries Research Institute (Sanya, China). For the experiment, ninety healthy lobsters of uniform size were selected, each averaging a weight of 171.28 ± 23.12 g. Initially, six samples were randomly placed in fifteen 15-L fiberglass canisters for a week of domestication under experimental photoperiodic conditions. Feeding was conducted every evening at 18:00 using the satiation method, where 8 to 12% of the lobsters’ body mass in chilled crab bait was provided. Subsequently, at approximately 8:00 am the following day, tank maintenance involved cleaning the bottom of the tanks to remove residual feed and feces.
2.2. Experimental Design
The scalloped spiny lobsters were accurately weighed and allocated into fifteen 15-L experimental tanks, part of a recirculating water system. Each treatment group consisted of three replicates, with six lobsters per replicate. The experiment utilized natural seawater, which was sedimented, sand-filtered, and continuously aerated for 24 h. To ensure optimal water quality, a daily 100% water change was implemented. Monitored water quality parameters included the following: temperature between 28 and 32 °C, salinity from 28 to 32‰, pH levels ranging from 7.5 to 8.5, dissolved oxygen concentration of at least 7.0 mg/L, ammonia nitrogen concentration below 0.02 mg/L, and nitrite levels not exceeding 0.02 mg/L. Based on previous studies [21,56], five photoperiod groups were established: 24 L:0 D, 18 L:6 D, 12 L:12 D, 6 L:18 D, and 0 L:24 D (L: light, D: dark). Photoperiods were controlled using a programmable timer (Gongniu Group Co., Ltd., Ningbo, China). The experiment spanned 56 days, with a maintained light intensity of 100 lx and a spectrum range of white light (λ = 400–770 nm) (Opple Lighting Co., Ltd., Shanghai, China). Shade cloths were employed to achieve total darkness in each experimental group, with lights scheduled to turn on daily at 6:30 am.
2.3. Calculation of Growth Performance
Growth indicators such as weight gain rate (WGR), specific growth rate (SGR), survival rate (SR), molting frequency (MF), hepatopancreatic index (HSI), and meat yield production (MYP) of the scalloped spiny lobster were measured and calculated using the following equations:
where WGR is weight gain rate (%), SGR is specific growth rate (%/d), SR is survival rate (%), MF is molting frequency (%), HSI is hepatopancreas index (%), and MYP is meat yield production (%). W0 is the initial body mass of the experimental lobster (g), Wt is the final body mass of the experimental lobster (g), t is the experimental time (d), Nf is the initial number of experimental lobsters (only), Ni is the final number of experimental lobsters (only), Nm is the number of molted experimental lobsters in a single bucket (only), Ns is the total number of experimental lobsters in a single bucket (only), Ws is the net meat weight (g), and Wt is the final body mass of the experimental lobster (g).
WGR = (Wt − W0)/W0 × 100%
SGR = (lnWt − lnW0)/t × 100%
SR = Nf/Ni × 100%
MF = (Nm/Ns) × 100%
HSI = (Wg/W0) × 100%
MYP = (WS/Wt) × 100%
SGR = (lnWt − lnW0)/t × 100%
SR = Nf/Ni × 100%
MF = (Nm/Ns) × 100%
HSI = (Wg/W0) × 100%
MYP = (WS/Wt) × 100%
2.4. Sample Collection and Processing
At the end of the experiment, lobsters were anesthetized in an ice bath for two minutes. Before dissection, their body surfaces were carefully dried using absorbent paper. The dissection was conducted using sterilized tools and performed in a consistently cold environment to preserve tissue integrity. Appropriate amounts of lobster stomach and hepatopancreas tissues were collected in 2 mL freezing tubes, frozen in liquid nitrogen, and stored at −80 °C in a refrigerator. To minimize sampling error, samples from each treatment group were stored separately, and then samples with different duplicates from the same treatment group were mixed and extracted.
For tissue analysis, a 10% homogenized tissue solution was prepared. Specified amounts of tissue samples were weighed and added to a pre-cooled homogenization medium, followed by thorough grinding. The resulting tissue homogenate was then transferred to a high-speed centrifuge, maintained at 4 °C, and centrifuged at 3500 rpm for 10 min. Post-centrifugation, the supernatant of the homogenized tissue was collected and stored at −80 °C for subsequent analysis.
Prior to enzyme activity assays, the supernatant was diluted to the optimal concentration as per the requirements of each specific enzyme assay. Enzyme activities were then quantitatively determined according to the standardized procedures provided in the respective assay kits.
2.5. Determination of Indicators of Enzyme Activity
All biochemical assays were conducted using kits provided by the Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China), strictly following the manufacturer’s instructions. For the analysis of digestive enzymes and related indicators, gastric tissues were specifically harvested to measure PEP activity and total protein (TP) content. Similarly, hepatopancreatic tissues were collected for a comprehensive assessment of various enzymes and biochemical markers. These included LPS, AMS, TRYP, and CHT activities, alongside assays for ACP, AKP, and LZM activities. Additionally, key oxidative stress markers and antioxidant enzymes were quantified in hepatopancreas, including MDA content, SOD, CAT, GSH-Px, and T-AOC. Concurrently, protein TP was also performed on these samples.
The protein content was determined using the Coomassie Brilliant Blue method with bovine serum protein as the standard used in the protein quantitative kit (Catalog No. A045-4, Nanjing, China), incubated at 37 °C for 30 min at 562 nm wavelength, and the protein concentration was measured using microplate colorimetry. A PEP detection kit (catalog No. A080-1-1, Nanjing, China) was used to determine the activity of PEP in animal tissue samples. PEP can hydrolyze protein to produce phenol-containing amino acids, and phenol reagents can be reduced to blue substances by phenol-containing amino acids. The absorbance value at the zero setting point of distilled water at a 37 °C water bath for 20 min was determined via colorimetry. The unit of activity is defined as follows: 1 μg tyrosine generated by decomposing protein per milligram at 37 °C per minute is equivalent to 1 unit of enzyme activity (U/mgprot). A TRYP assay kit (Catalog No. A080-2-2, Nanjing, China) was used to determine the activity of TRYP in the animal tissue samples. TRYP can catalyze the hydrolysis of the ester chain of ethyl arginine and increase its absorbance at 253 nm. The activity of the enzyme can be calculated according to the change in absorbance. The activity unit was defined as follows: under the condition of pH 8.0, 37 °C, the absorbance change of 0.003 per minute by TRYP contained in each milliliter of serum (pulp) is an enzyme activity unit (U/mgprot). The AMS assay kit (catalog No. C016-1-1, Nanjing, China) was used to determine the activity of AMS in animal tissue samples. AMS can hydrolyze starch to produce glucose, maltose, and dextrin. When the concentration of a substrate was known and excessive, iodine solution was added to combine with unhydrolyzed starch to form a blue complex. The amount of hydrolyzed starch was calculated according to the depth of blue, so as to calculate the activity of AMS. The unit of activity was defined as follows: each milligram of protein in the tissue reacted with the substrate at 37 °C for 30 min, and hydrolyzed 10 mg of starch was defined as 1 unit of amylase activity (U/mgprot). A CHT assay kit (Catalog No. A080-3-1, Nanjing, China) was used to determine CHT activity in animal tissue samples. Using casein as a substrate, CHT hydrolyzed protein to produce phenol-containing amino acids, phenol reagents were reduced to blue substances by phenol-containing amino acids, and CHT activity was determined via colorimetry. The unit of activity was defined as follows: 1μg amino acid generated by decomposing protein per milligram at 37 °C per minute was equivalent to 1 unit of enzyme activity (U/mgprot). The activity of LPS in animal tissue samples was determined with an LPS detection kit (catalog No. A054-2-1, Nanjing, China). 1, 2-o-dilaurin-racemic glycerol-3-valerate-(6-methylhalide) ester +H2O→1, 2-o-dilaurin-racemic glycerol + valerate-(6-methylhalide) ester, valerate-(6-methylhalide) ester→valerate + 6-methylhalide (color development), at 580 nm wavelength. The activity of LPS was determined according to the production rate of the red product. The activity unit was defined as follows: at 37 °C, each gram of hiprotein reacted with the substrate in this reaction system for 1 min, and each consumption of 1 μmol of the substrate was an enzyme activity unit (U/gprot).
An ACP assay kit (Catalog No. A060-2-2, Nanjing, China) was used to determine the activity of ACP in the animal tissue samples. ACP decomposes disodium phenyl phosphate to produce free phenol and phosphoric acid. Phenol reacts with 4-amino-antipyrine in alkaline solution to oxidize red quinone derivatives by potassium ferricyanide. The activity of enzyme was measured according to the red intensity. The unit of activity was defined as follows: 100 mL of serum or liquid at 37 °C with the matrix for 30 min to produce 1 mg of phenol as 1 Gold unit/mgprot. An AKP assay kit (Catalog No. A059-2-2, Nanjing, China) was used to determine AKP activity in the animal tissue samples. AKP decomposed phenylene disodium phosphate to produce free phenol and phosphoric acid. Phenol reacted with 4-amino-antipyrine in alkaline solution to oxidize red quinone derivatives by potassium ferricyanide. The activity of enzyme was measured according to the red intensity. The activity unit was defined as one Gold unit/mgprot for 1 mg of phenol produced per gram of hiprotein interacting with the matrix at 37 °C for 15 min. The LZM assay kit (Catalog No. A050-1-1, Nanjing, China) was used to determine the activity of LZM in the animal tissue samples. LZM can hydrolyze peptidoglycan on the cell wall of bacteria, resulting in bacterial lysation with decreased concentration and increased transmittance, so the content of LZM was estimated according to the change in transmittance. The activity unit was defined as follows: accurate water bath for 15 min per milliliter of liquid at 37 °C, ice water bath for 3 min below 0 °C, removed tube-by-tube, poured into the light diameter of a 1 cm colorimetric dish, at a 530 nm wavelength, double steaming water regulation light transmission rate of 100%, and the colorimetric determination of the light value of each tube is a unit of vitality (U/mL).
A T-AOC assay kit (Catalog No. A015-1, Nanjing, China) was used to determine the activity of T-AOC in the animal tissue samples. Under the action of appropriate oxidants, ABTS is oxidized to green ABTS+, and in the presence of antioxidants, the production of ABTS·+ is inhibited. The T-AOC of the samples was determined by measuring the absorbance of ABTS+ at 405 nm. The activity unit was defined as follows: reaction at room temperature for 6 min, wavelength of 405 nm, and absorbance (OD) value of the reaction system could be directly used as T-AOC activity unit (mM). A CAT test kit (Catalog No.:A007-1-1, Nanjing, China) was used to determine the activity of CAT in the animal tissue samples. The decomposition reaction of H2O2 by CAT could be quickly stopped by adding ammonium molybdate. The remaining H2O2 reacted with ammonium molybdate to produce a light-yellow complex. The activity of CAT was calculated by measuring its change at 405 nm. The unit is defined as the decomposition of 1 µmol of H2O2 per milligram of histone per second as one unit of activity (U/gHb). The GSH-Px activity in the tissues was measured with a GSH-Px determination kit (Catalog No. A005-1, Nanjing, China). The GSH-Px activity was expressed by the consumption rate of GSH in the enzymatic reaction, while the more stable yellow substance formed by GSH and dithiodinitrobenzoic acid was determined through colorimetry to calculate the GSH-Px activity. Through the colorimetric method, a 1 cm optical path cuvette was used at a 412 nm wavelength, the distilled water was adjusted to zero, the absorbance value was measured, and its activity was calculated. The activity unit U indicates that the GSH concentration in the reaction system is reduced by 1% per milligram of protein per minute by deducting a non-enzymatic reaction in μmol·L−1. The SOD test kit (Catalog No. A001-3, Nanjing, China) was used to measure the activity of the SOD in the animal tissue samples. The activity of the SOD was determined using the xanthine oxidase method. The absorbance value was measured at the wavelength of 550 nm through colorimetry to calculate its activity. The activity unit was defined as follows: when the SOD inhibition rate reached 50% per milligram of tissue protein in 1 mL of the reaction solution, the corresponding amount of SOD was 1 SOD activity unit (U·mgprot−1). The MDA determination kit (Catalog No. A003-1, Nanjing, China) was used to measure the content of MDA in the animal tissues. The MDA was condensed with thiobarbituric acid to form a red substance, and MDA was determined through colorimetry at 532 nm. All samples were processed in triplicate.
2.6. Data Analysis
All collected data underwent a normality test to confirm their distribution patterns. Subsequent statistical analyses were conducted using one-way ANOVA, followed by Duncan’s multiple range test to identify significant differences among the groups. The levels of significance were set at p < 0.05 for significant differences and p < 0.01 for highly significant differences. These analyses were performed using the SPSS 26.0 (SPSS, Chicago, IL, USA) statistical software package. For data presentation, all values were expressed as mean ± standard deviation (mean ± SD). Graphical representations of the data were generated using Origin 2022 (OriginLab Corporation, Northampton, MA, USA) software.
3. Results
3.1. Growth Performance
According to the data in Table 1 and the ANOVA results in Table 2, the WGR and SGR were significantly higher in lobsters exposed to a 12 h light and 12 h dark cycle (12 L:12 D) compared to those under 6 L:18 D and continuous darkness (0 L:24 D) conditions. While longer photoperiods (24 L:0 D and 18 L:6 D) facilitated faster growth than shorter ones (6 L:18 D and 0 L:24 D), the differences between these longer photoperiod groups were not statistically significant. Notably, the HSI was significantly greater in the 6 L:18 D group than in other experimental conditions. However, the photoperiod did not significantly influence (p > 0.05) the SR, MF, and MYP of the scalloped spiny lobsters, as detailed in Table 1.

Table 1.
Influence of photoperiod on growth performance of Panulirus homarus.

Table 2.
The ANOVA results of WGR, SGR, SR, MF, his, and MYP.
3.2. Digestive Properties
According to the results of the variance analysis shown in Figure 1 and Table 3, PEP activity was found to be significantly higher (p < 0.05) in the 0 L:24 D (10.71 ± 0.56 U·mgprot−1), 6 L:18 D (9.49 ± 0.56 U·mgprot−1), and 18 L:6 D (10.23 ± 0.99 U·mgprot−1) photoperiod groups compared to the 24 L:0 D (7.04 ± 0.35 U·mgprot−1) and 12 L:12 D (7.47 ± 0.43 U·mgprot−1) groups, as shown in Figure 1A. Similarly, TRYP and CHT activities were significantly elevated (p < 0.05) in the 0 L:24 D (1173.83 ± 536.76 U·mgprot−1 and 3.15 ± 0.18 U·mgprot−1) group relative to the other experimental groups, as depicted in Figure 1B,D, respectively. For AMS activity, the highest levels were observed in the 24 L:0 D (11.70 ± 2.15 U·mgprot−1) and 18 L:6 D (13.86 ± 2.77 U·mgprot−1) groups, significantly surpassing those in other groups (p < 0.05), as indicated in Figure 1C. Additionally, LPS activity was significantly higher (p < 0.05) in the 0 L:24 D (0.70 ± 0.09 U·gprot−1) and 12 L:12 D (1.07 ± 0.18 U·gprot−1) photoperiod groups compared to the rest, as illustrated in Figure 1E.
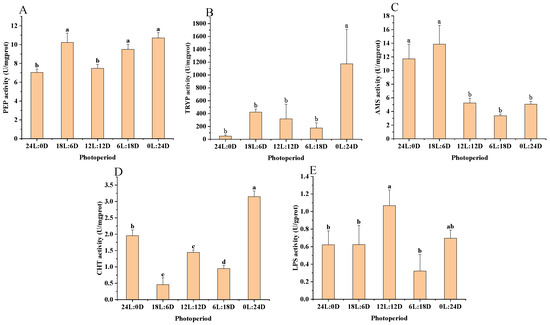
Figure 1.
Effect of photoperiod on digestive enzyme activities in scalloped spiny lobsters (n = 18). Pepsin (A), trypsin (B), amylase (C), chymotrypsin (D), and lipase (E). Different superscripts letters indicate statistically significant differences between treatments (p < 0.05).

Table 3.
The ANOVA results of PEP, TRYP, AMS, CHT, and LPS.
3.3. Immune Function
According to the results of variance analysis in Figure 2 and Table 4, ACP activity showed no significant difference between the 0 L:24 D (0.16 ± 0.02 gold unit·gprot−1) and 12 L:12 D (0.12 ± 0.02 gold unit·gprot−1) photoperiods (p > 0.05). However, its activity was significantly higher in the 0 L:24 D photoperiods compared to 18 L:6 D (0.10 ± 0.01 gold unit·gprot−1), 24 L:0 D (0.12 ± 0.02 gold unit·gprot−1), and 6 L:18 D (0.11 ± 0.02 gold unit·gprot−1) (p < 0.05), as shown in Figure 2A. In terms of AKP activity, the levels of the 18 L:6 D group (0.04 ± 0.00 gold unit·gprot−1) and 12 L:12 D group (0.04 ± 0.00 gold unit·gprot −1) were significantly higher than other groups, as shown in Figure 2B. For LZM activity, the 12 L:12 D (76.77 ± 7.01 U·mL−1) and 6 L:18 D (85.30 ± 5.64 U·mL−1) groups had significantly higher activity compared to the 0 L:24 D (58.4 ± 3.71 U·mL−1), 18 L:6 D (67.59 ± 6.50 U·mL−1), and 24 L:0 D (64.96 ± 1.61 U·mL−1) groups (p < 0.05). However, there was no significant difference in LZM activity between the 12 L:12 D and 6 L:18 D groups (p > 0.05), as indicated in Figure 2C.
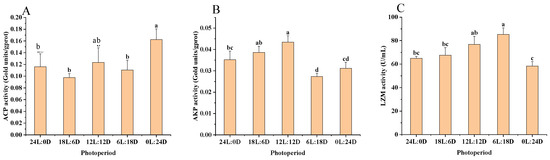
Figure 2.
Effect of photoperiod on the immune enzyme activity of scalloped spiny lobsters (n = 18). Acid phosphatase (A), alkaline phosphatase (B), lysozyme (C). Different superscripts letters indicate statistically significant differences between treatments (p < 0.05).

Table 4.
The results of ANOVA for ACP, AKP, and LZM.
3.4. Antioxidant Capacity
According to the results of the variance analysis shown in Figure 3 and Table 5, the activities of SOD were significantly higher in the 24 L:0 D (15.69 ± 1.91 U·mgprot−1), 18 L:6 D (18.85 ± 3.38 U·mgprot−1), and 12 L:12 D (17.87 ± 3.67 U·mgprot−1) groups compared to the 0 L:24 D (8.12 ± 2.17 U·mgprot −1) group (p < 0.05). However, these SOD levels did not show a significant difference from those in the 6 L:18 D group (p > 0.05), as illustrated in Figure 3A. In terms of CAT activity, 18 L:6 D (0.01 ± 0.00 U·gHb−1) was significantly lower than other experimental groups (p < 0.05) (p < 0.05), as shown in Figure 3B. The GSH-Px activity was significantly greater in the 18 L:6 D (404.20 ± 13.70 activity unit) group compared to all other experimental groups (p < 0.05), as depicted in Figure 3C. Additionally, the T-AOC activity and MDA content were notably higher in the 12 L:12 D (0.12 ± 0.00 mM and 2.02 ± 0.10 nmol·mgprot−1) group than in the other groups (p < 0.05), as indicated in Figure 3D,E, respectively.
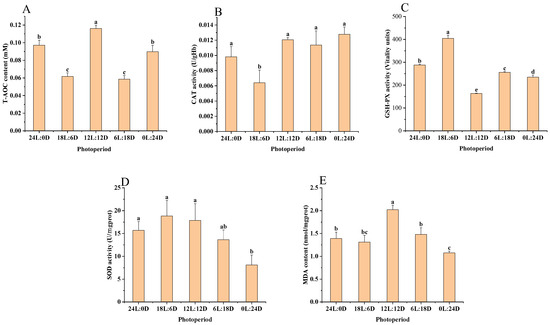
Figure 3.
Effect of photoperiod on antioxidant capacity of scalloped spiny lobsters (n = 18). Superoxide dismutase (A), peroxidase (B), glutathione peroxidase (C), total antioxidant capacity (D), malondialdehyde (E). Different superscripts letters indicate statistically significant differences between treatments (p < 0.05).

Table 5.
The results of ANOVA for T-AOC, CAT, GSH-Px, SOD, and MDA.
4. Discussion
4.1. Effects of the Photoperiod on the Growth Performance of the Animals
In this study, the scalloped spiny lobster exhibited optimal WGR and SGR under 12 L:12 D. Although no significant difference was observed in WGR and SGR between long (>12 L) and short (<12 L) photoperiods, lobsters under longer photoperiods showed higher growth rates, suggesting that extended light periods may more effectively promote growth in aquatic animals. This finding aligns with observations in other species, including larval coconut crabs (Birgus latro) [57], mud crabs [21], narrow-clawed crayfish (Astacus leptodactylus) [46], blue swimming crab (Portunus pelagicus) [58], and juvenile winter flounder (Pseudopleuronectes americanus) [59]. Contrarily, horsehair crab (Erimacrus isenbeckii) larvae showed optimal survival and growth under continuous darkness (0 L:24 D) [60].
Similarly, short photoperiods have been found to enhance growth in other species, such as the African catfish (Clarias gariepinus) [61], abalone (Haliotis discus hannai) [61,62], and largemouth bass (Micropterus salmoides) [36], all of which displayed improved growth rates under limited light conditions. These differences in photoperiod adaptation across species may be attributed to their unique species characteristics and ecological behaviors. Comparative analysis with other aquatic species such as spiny lobster [63], swimming crab (Portunus trituberculatus) [64], spanner crab (Ranina ranina) [65], neotropical fish (Hoplias intermedius) [66], and banded cichlid (Heros severus) [67] revealed similar optimal growth under a 12 L:12 D photoperiod. This suggests the possibility of a universal optimal photoperiod for different species, likely influenced by their ecological habits. Consequently, for scalloped spiny lobster aquaculture, 12 L:12 D is recommended to enhance growth performance, shorten production cycles, and improve economic efficiency.
4.2. Effects on the Digestive Performance of Animals Due to the Photoperiod
In this study, the activities of PEP, TRYP, and CHT in scalloped spiny lobsters were highest under continuous darkness (0 L:24 D). This finding suggests that scalloped spiny lobsters are more efficient at protein digestion and absorption in a dark environment. Conversely, LPS and AMS activities peaked in 12 L:12 D and 18 L:6 D light conditions, respectively. This pattern indicates a shift from protein digestion to the utilization of stored nutrients in the hepatopancreas after protein sources are consumed.
The observed differences in enzyme activities may be attributed to the unique ecological and feeding habits of scalloped spiny lobsters. Being nocturnal marine crustaceans, they predominantly feed at night [68] and exhibit a distinct behavior of burrowing and hiding in coral crevices or reefs during the day [69]. Post feeding, the lobsters retreat to their burrows, aiding in the digestion and absorption of proteins. In the absence of daytime feeding, the stored nutrients in the hepatopancreas are utilized [70]. Consequently, photoperiods exceeding 12 h appear to favor the breakdown of fats and starches. Previous research indicates that specific photoperiods can stimulate or inhibit the activity of certain digestive enzymes [71]. Thus, in aquacultural practices, adjusting the photoperiod can be a strategic approach to facilitate the digestion and absorption of specific nutrients in scalloped spiny lobsters.
4.3. Effects of Photoperiod on Animals’ Immune Function
In this study, ACP activity in scalloped spiny lobsters was found to be optimal under continuous darkness (0 L:24 D). This suggests that ACP secretion is more favorable in such light conditions, aligning with observations in freshwater shrimp [50]. In contrast, the highest AKP activity in scalloped spiny lobsters was recorded under 12 L:12 D. This difference implies distinct immune response mechanisms to photoperiods in the two types of phosphatases.
The variation in phosphatase activities might be associated with the lobsters’ nocturnal feeding behavior [5,68]. Post feeding at night, the lobsters generate energy, which, in turn, could promote the production of these phosphatases, thereby enhancing their immune function [47]. LZM activity reached its peak at 6 L:18 D, and an increase in LZM activity was noted under photoperiods longer than 6 h (24 L:0 D, 18 L:6 D, 12 L:12 D, and 6 L:18 D). This pattern indicates that light exposure positively influences LZM secretion in scalloped spiny lobsters, a finding consistent with studies on tiger puffer [51].
The increased LZM activity under certain photoperiods might be indicative of a photoperiod-induced innate immune response in scalloped spiny lobsters. Therefore, a 12 L:12 D photoperiod is recommended in aquacultural practices to boost their immune function. Such immune enhancement, through the suppression of pathogenic bacteria, could lead to heightened specific immunity in these organisms [72]. The results of this study are in line with findings in white leg shrimp [34], freshwater prawn [50], and red claw crayfish (Cherax quadricarinatus) [56], suggesting that an appropriate photoperiod (12 L:12 D) can effectively improve the immune function of scalloped spiny lobsters.
4.4. Effect of Photoperiod on Animals’ Antioxidant Capacity
In this study, T-AOC activity and MDA levels in scalloped spiny lobsters were observed to be highest under 12 L:12 D. Similarly, the activities of SOD and CAT were also elevated under these light conditions. These results indicate that the antioxidant capacity of scalloped spiny lobsters is responsive to varying levels of oxidative stress in the external environment. Under the 12 L:12 D photoperiod, the lobsters are likely exposed to external stressors that generate a significant amount of ROS, potentially causing damage to the organisms. MDA is a crucial biomarker for assessing ROS levels in aquatic animals [35], and the activity of T-AOC reflects the overall level of antioxidant enzymes and non-enzymatic antioxidants in an organism, which are vital for combating oxidative stress [53]. Therefore, the activities of T-AOC, SOD, and CAT may fluctuate in response to the changes in MDA levels induced by external stressors. These findings align with research conducted on South American white shrimp [35], bluntmouth bream [39], and abalone [55].
Interestingly, the GSH-Px activity in scalloped spiny lobsters was found to be optimal under a longer photoperiod of 18 L:6 D. This may suggest that the antioxidant system of the lobsters under excessively long or short photoperiods struggles to effectively scavenge excess ROS, resulting in increased GSH-Px activity. In practical aquaculture settings, adjusting the photoperiod could be a strategic measure to mitigate oxidative stress and enhance the antioxidant capacity in scalloped spiny lobsters.
5. Conclusions
This study explored the impact of five different light–dark cycles (0 L:24 D, 6 L:18 D, 12 L:12 D, 18 L:6 D, and 24 L:0 D) on the growth and physiological aspects of scalloped spiny lobsters. Notably, under a 12 L:12 D light–dark cycle, the lobsters demonstrated optimal growth performance, along with enhanced levels of digestive and immune enzymes, and improved antioxidant capacity. These findings suggest that adopting a 12 L:12 D lighting regimen could effectively boost the growth rate and shorten the production cycle in scalloped spiny lobster aquaculture. This research provides valuable insights for creating an ideal lighting environment, contributing significantly to the optimization and enhancement of scalloped spiny lobster cultivation.
Author Contributions
Conceptualization, Z.M. and Z.B.; Methodology, Y.W. and R.Y.; Software, R.Y.; Validation, Z.F.; Formal Analysis, Z.F.; Investigation, R.Y.; Resources, Z.B.; Data Curation, Y.W.; Writing—Original Draft Preparation, Y.W.; Writing—Review and Editing, Z.M. and Z.F.; Visualization, R.Y.; Supervision, Z.M.; Project Administration, R.Y. and Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Central Public-Interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (NO. 2021SD09), Central Public—interest Scientific Institution Basal Research Fund, CAFS (No. 2023TD58).
Institutional Review Board Statement
The animal study was reviewed and approved by the Animal Care and Use Committee of South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. The ethical code is CAFS (2020TD55).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the confidentiality of the project.
Conflicts of Interest
Author Zemin Bai was employed by the Yazhou Bay Agriculture and Aquaculture Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Macdonald, A.H.H.; Reddy, M.M.; Groeneveld, J.C.; Schleyer, M.H. Phylogeography of the scalloped spiny-lobster Panulirus homarus rubellus in the southwest Indian Ocean. J. Crustac. Biol. 2014, 34, 773–781. [Google Scholar] [CrossRef]
- Singh, S.P.; Groeneveld, J.C.; Willows-Munro, S. Between the current and the coast: Genetic connectivity in the spiny lobster Panulirus homarus rubellus, despite potential barriers to gene flow. Mar. Biol. 2019, 166, 36. [Google Scholar] [CrossRef]
- Marzouqi, A.; Al-Nahdi, A.; Jayabalan, N.; Groeneveld, J.C. An Assessment of the Spiny Lobster Panulirus homarus Fishery in Oman—Another Decline in the Western Indian Ocean? West. Indian Ocean J. Mar. Sci. 2009, 6, 1183. [Google Scholar] [CrossRef]
- Tirtadanu; Suman, A.; Chodrijah, U.; Kang, B.; Zhang, C. Stock assessment and management implications of three lobster species in Gunungkidul waters, Indonesia. Ocean. Coast. Manag. 2021, 211, 105780. [Google Scholar] [CrossRef]
- Devi, N.R.; Rasheeq, A.A.; Preethi, B.A.; Anand, M.; Titus, C.; Subbiah, S.; Rangesh, K.; Dineshkumar, R.; Arumugam, A. Assessment of Lobster Resources in Coastal Region of Gulf of Mannar, Southeast Coast of India. Thalass. Int. J. Mar. Sci. 2023, 39, 1169–1186. [Google Scholar] [CrossRef]
- Duy Mai, M.; Thi Tran, L. Growth Performance of Scalloped Spiny Lobster Panulirus homarus (Linaeus) Fed Formulated Diet in Recirculating System. Agric. For. Fish. 2022, 11, 1. [Google Scholar] [CrossRef]
- Ihsan, M.; Suhirman, S.; Jayadi, E.M.; Sagista, R.; Hardianti, Y.E.; Ilahi, W.B.; Muliasari, H.; Tantilar Wangsajati Sukmaring Kalih, L.A. Analisis makanan alami dalam lambung dan mikrohabitat lobster pasir (Panulirus homarus) fase puerulus di teluk awang. J. Ris. Akuakultur 2019, 14, 183–191. [Google Scholar] [CrossRef]
- Suman, A.; Hasanah, A.; Pane, A.R.P.; Panggabean, A.S. Penangkapan, parameter populasi serta tingkat pemanfaatan lobster pasir (Panulirus homarus) dan lobster batu (Panulirus penicillatus) di perairan gunung kidul, dan sekitarnya. J. Penelit. Perikan. Indones. 2020, 25, 147–160. [Google Scholar] [CrossRef]
- Priyambodo, B.; Jones, C.M.; Sammut, J. Improved collector design for the capture of tropical spiny lobster, Panulirus homarus and P. ornatus (Decapoda: Palinuridae), pueruli in Lombok, Indonesia. Aquaculture 2017, 479, 321–332. [Google Scholar] [CrossRef]
- Nurfiarini, A.; Wijaya, D. Estimasi potensi DAN tingkat pemanfaatan sumberdaya lobster pasir (Panulirus homarus) di perairan prigi kabupanen trenggalek. J. Penelit. Perikan. Indones. 2020, 25, 169–178. [Google Scholar] [CrossRef]
- Priyambodo, B.; Jones, C.M.; Sammut, J. Assessment of the lobster puerulus (Panulirus homarus and Panulirus ornatus, Decapoda: Palinuridae) resource of Indonesia and its potential for sustainable harvest for aquaculture. Aquaculture 2020, 528, 735563. [Google Scholar] [CrossRef]
- Junaidi, M.; Cokrowati, N.; Diniarti, N.; Setyono, B.D.H.; Mulyani, L.F. Identifying the Environmental Factors Affecting Puerulus Settlement of the Spiny Lobster, Panulirus homarus in Awang Bay, Lombok Island. Asian J. Fish. Aquat. Res. 2022, 18, 1–14. [Google Scholar] [CrossRef]
- Chen, H.; Pan, J.; Wang, Y.; Qiao, Y.; Han, F.; Xu, C.; Farhadi, A.; Li, E. Growth, health status and gut microbiota of the scalloped spiny lobster (Panulirus homarus) at different salinities. Aquaculture 2023, 562, 738779. [Google Scholar] [CrossRef]
- Adiputra, Y.T.; Zairin Jr, M.; Suprayudi, M.A.; Manalu, W.; Widanarni; Brite, M. The effects of thyroxine hormone on gonadal maturation and growth of male spiny lobster (Panulirus homarus). Malays. J. Sci. 2020, 39, 30–40. [Google Scholar] [CrossRef][Green Version]
- Adiputra, Y.T.; Zairin, M., Jr.; Suprayudi, M.A.; Manalu, W.; Widanarni. Identification of steroid hormones and fatty acids during gonadal maturation of spiny lobster Panulirus homarus. Invertebr. Reprod. Dev. 2019, 63, 77–87. [Google Scholar] [CrossRef]
- Sudewi, S.; Widiastuti, Z.; Slamet, B.; Mahardika, K. Experimental inffctions of milky hemolymph disease in spiny lobster Panulirus homarus. Indones. Aquac. J. 2018, 13, 31–40. [Google Scholar] [CrossRef]
- Syed Musthaq, S.; Sudhakaran, R.; Balasubramanian, G.; Sahul Hameed, A.S. Experimental transmission and tissue tropism of white spot syndrome virus (WSSV) in two species of lobsters, Panulirus homarus and Panulirus ornatus. J. Invertebr. Pathol. 2006, 93, 75–80. [Google Scholar] [CrossRef]
- Farhadi, A.; Jeffs, A.G.; Lavery, S.D. Genome-wide SNPs in the spiny lobster Panulirus homarus reveal a hybrid origin for its subspecies. BMC Genom. 2022, 23, 750. [Google Scholar] [CrossRef]
- Zhuo, H.B.; Liang, H.F.; Cai, C.X.; Luo, J.J.; Liang, F.S.; Wen, C.Q. Molecular cloning, characterization and expression analysis of the ecdysone receptor from the spiny lobster Panulirus homarus (Linnaeus, 1758) (Decapoda, Palinuridae). Crustaceana 2020, 93, 769–783. [Google Scholar] [CrossRef]
- Delghandi, M.; Saif Nasser Al Hinai, M.; Afzal, H.; Khalfan Al-Wahaibi, M. Parentage analysis of tropical spiny lobster (Panulirus homarus) by microsatellite markers. Aquac. Res. 2017, 48, 4718–4724. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.; Shi, C.; Migaud, H.; Ye, Y.; Song, C.; Mu, C.; Ren, Z.; Wang, C. Effect of photoperiod on growth, survival, and lipid metabolism of mud crab Scylla paramamosain juveniles. Aquaculture 2023, 567, 739279. [Google Scholar] [CrossRef]
- Gao, X.L.; Pang, G.W.; Luo, X.; You, W.W.; Ke, C.H. Effects of light cycle on circadian feeding activity and digestive physiology in Haliotis discus hannai. Aquaculture 2021, 539, 736642. [Google Scholar] [CrossRef]
- Mirko Bögner, C.S.T.G. Effect of ambient light intensity on growth performance and diurnal stress response of juvenile starry flounder (Platichthys stellatus) in recirculating aquaculture systems (RAS). Aquacult Eng. 2018, 83, 20–26. [Google Scholar] [CrossRef]
- Hou, Z.; Wen, H.; Li, J.; He, F.; Li, Y.; Qi, X.; Zhao, J.; Zhang, K.; Tao, Y. Effects of photoperiod and light Spectrum on growth performance, digestive enzymes, hepatic biochemistry and peripheral hormones in spotted sea bass (Lateolabrax maculatus). Aquaculture 2019, 507, 419–427. [Google Scholar] [CrossRef]
- Wang, K.; Li, K.; Liu, L.; Tanase, C.; Mols, R.; van der Meer, M. Effects of light intensity and photoperiod on the growth and stress response of juvenile Nile tilapia (Oreochromis niloticus) in a recirculating aquaculture system. Aquac. Fish. 2023, 8, 85–90. [Google Scholar] [CrossRef]
- Bapary, M.A.J.; Amin, M.N.; Takeuchi, Y.; Takemura, A. The stimulatory effects of long wavelengths of light on the ovarian development in the tropical damselfish, Chrysiptera cyanea. Aquaculture 2011, 314, 188–192. [Google Scholar] [CrossRef]
- Kim, B.; Jung, S.J.; Choi, Y.J.; Kim, N.N.; Choi, C.Y.; Kim, J. Effects of different light wavelengths from LEDs on oxidative stress and apoptosis in olive flounder (Paralichthys olivaceus) at high water temperatures. Fish Shellfish. Immun. 2016, 55, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Reis, W.G.; Wasielesky, W., Jr.; Abreu, P.C.; Brandão, H.; Krummenauer, D. The influence of different light wavelengths in the culture of the Pacific white shrimp Litopenaeus vannamei reared in BFT using LED lights. Aquaculture 2023, 563, 738924. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.; Zhou, Y.; Ma, H.; Saqib, H.S.A.; Su, Q.; Cui, W.; Ma, H. The effects of different diet, salinity and light condition on growth performance and moulting cycle of juvenile mud crab, Scylla paramamosain. Aquac. Res. 2022, 53, 6333–6342. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Ji, B.; Zhang, Y.; Ye, Z.; Zhu, S. Effects of Photoperiod on Growth, Digestive Enzyme Activity, Stress, and Oxidative Status of Juvenile Chinese Soft-Shelled Turtles (Pelodiscus sinensis) in a Greenhouse. Trans. ASABE 2020, 63, 1787–1793. [Google Scholar] [CrossRef]
- Boeuf, G.; Le Bail, P. Does light have an influence on fish growth? Aquaculture 1999, 177, 129–152. [Google Scholar] [CrossRef]
- Mel Nikova, Y.B.; Melnikov, A.V. Solar radiation as a synchronizing factor of circadian and ultradian biological rhythms of planktonic communities. IOP Conf. Ser. Earth Environ. Sci. 2021, 853, 12001. [Google Scholar] [CrossRef]
- Wei, H.; Cai, W.; Liu, H.; Han, D.; Zhu, X.; Yang, Y.; Jin, J.; Xie, S. Effects of photoperiod on growth, lipid metabolism and oxidative stress of juvenile gibel carp (Carassius auratus). J. Photochem. Photobiol. B Biol. 2019, 198, 111552. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, B.; Gao, X.; Wang, X.; Li, H.; Xu, L.; Wang, G.; Zhao, K.; Huang, B. The Effects of Different UVA Photoperiods on the Growth Performance, Immune Responses, Antioxidant Status and Apoptosis-Related Gene Expression of the Pacific White Shrimp (Penaeus vannamei). Antibiotics 2022, 11, 37. [Google Scholar] [CrossRef]
- Bembe, S.; Zmora, N.; Williams, E.; Place, A.R.; Liang, D.; Chung, J.S. Effects of temperature and photoperiod on hemolymph vitellogenin levels during spawning events of the blue crab, Callinectes sapidus, in captivity. Aquac. Res. 2018, 49, 2201–2209. [Google Scholar] [CrossRef]
- Malinovskyi, O.; Rahimnejad, S.; Stejskal, V.; Boňko, D.; Stará, A.; Velíšek, J.; Policar, T. Effects of different photoperiods on growth performance and health status of largemouth bass (Micropterus salmoides) juveniles. Aquaculture 2022, 548, 737631. [Google Scholar] [CrossRef]
- Fitzgibbon, Q.P.; Battaglene, S.C. Effect of photoperiod on the culture of early-stage phyllosoma and metamorphosis of spiny lobster (Sagmariasus verreauxi). Aquaculture 2012, 368–369, 48–54. [Google Scholar] [CrossRef]
- Bermudes, M.; Ritar, A.J. Response of early stage spiny lobster Jasus edwardsii phyllosoma larvae to changes in temperature and photoperiod. Aquaculture 2008, 281, 63–69. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, D.; Li, X.; Jiang, G.; Liu, W. Photoperiod affects blunt snout bream (Megalobrama amblycephala) growth, diel rhythm of cortisol, activities of antioxidant enzymes and mRNA expression of GH/IGF-I. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 233, 4–10. [Google Scholar] [CrossRef]
- Nyuji, M.; Hamada, K.; Kazeto, Y.; Mekuchi, M.; Gen, K.; Soyano, K.; Okuzawa, K. Photoperiodic regulation of plasma gonadotropin levels in previtellogenic greater amberjack (Seriola dumerili). Gen. Comp. Endocr. 2018, 269, 149–155. [Google Scholar] [CrossRef]
- Zou, H.; Bai, X.; Feng, Y.; Zhang, Y.; Wang, Y.; Lu, W. Influence of long (16L:8D) and short (8L:16D) photoperiods on blood metabolites and hepatic metabolism in Olive flounder, Paralichthys olivaceus. SpringerPlus 2016, 5, 924. [Google Scholar] [CrossRef]
- Farhadi, A.; Harlıoğlu, M.M. Photoperiod affects gamete production, and protein and lipid metabolism in male narrow-clawed Crayfish Pontastacus leptodactylus (Eschscholtz, 1823). Anim. Reprod. Sci. 2019, 211, 106204. [Google Scholar] [CrossRef] [PubMed]
- Falahatkar, B.; Poursaeid, S.; Efatpanah, I.; Meknatkhah, B.; Biswas, A. Effect of Photoperiod Manipulation on Growth Performance, Physiological and Hematological Indices in Juvenile Persian Sturgeon, Acipenser persicus. J. World Aquacult. Soc. 2012, 43, 679–687. [Google Scholar] [CrossRef]
- Espinosa-Chaurand, D.; Vega-Villasante, F.; Carrillo-Farnés, O.; Nolasco-Soria, H. Effect of circadian rhythm, photoperiod, and molt cycle on digestive enzymatic activity of Macrobrachium tenellum juveniles. Aquaculture 2017, 479, 225–232. [Google Scholar] [CrossRef]
- Farhadi, S.; Atashbar Kangarloei, B.; Imani, A.; Sarvi Moghanlou, K. Biological Impact of Photoperiod on Fairy Shrimp (Branchinecta orientalis): Life History and Biochemical Composition. Biology 2021, 10, 695. [Google Scholar] [CrossRef]
- Farhadi, A.; Jensen, M.A. Effects of photoperiod and stocking density on survival, growth and physiological responses of narrow clawed crayfish (Astacus leptodactylus). Aquac. Res. 2016, 47, 2518–2527. [Google Scholar] [CrossRef]
- Ray, G.W.; Liang, D.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S.; Rimei, L. Effects of replacing fishmeal with dietary soybean protein concentrate (SPC) on growth, serum biochemical indices, and antioxidative functions for juvenile shrimp Litopenaeus vannamei. Aquaculture 2020, 516, 734630. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, Y.; Li, T.; Zhao, H.; Li, X.; Li, J.; Sun, T. Effects of lysozyme combined with cinnamaldehyde on storage quality of olive flounder (Paralichthys olivaceus) fillets. J. Food Sci. 2020, 85, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tian, S.; Luo, K.; Zhang, Y.; Pan, H.; Zhang, W.; Mai, K. Dietary recombinant human lysozyme improves the growth, intestinal health, immunity and disease resistance of Pacific white shrimp Litopenaeus vannamei. Fish Shellfish. Immun. 2022, 121, 39–52. [Google Scholar] [CrossRef]
- Wei, J.; Tian, L.; Wang, Y.; Yu, L.; Zhu, X. Effects of salinity, photoperiod, and light spectrum on larval survival, growth, and related enzyme activities in the giant freshwater prawn, Macrobrachium rosenbergii. Aquaculture 2021, 530, 735794. [Google Scholar] [CrossRef]
- Ma, H.; Wei, P.; Li, X.; Liu, S.; Tian, Y.; Zhang, Q.; Liu, Y. Effects of photoperiod on growth, digestive, metabolic and non-special immunity enzymes of Takifugu rubripes larvae. Aquaculture 2021, 542, 736840. [Google Scholar] [CrossRef]
- Hu, N.; Yu, C.; Jin, J.; Zhao, X.; Zhao, Y.; Wei, H.; Li, Y. Impact of photoperiods on the specific activities of immune and antioxidant enzymes in different tissues of Dybowski’s frog (Rana dybowskii). Biol. Rhythm. Res. 2022, 53, 1790–1799. [Google Scholar] [CrossRef]
- Hao, Z.L.; Tang, X.J.; Ding, J.; Ben, Y.; Chang, Y.Q. Effect of high temperature on survival, oxygen consumption, behavior, ammonia-N excretion, and related immune indicators of the Japanese scallop Mizuhopecten yessoensis. Aquacult. Int. 2014, 22, 1863–1876. [Google Scholar] [CrossRef]
- Xu, H.; Shi, C.; Ye, Y.; Mu, C.; Wang, C. Effects of different photoperiods and feeding regimes on immune response, oxidative status, and tissue damage in juvenile rainbow trout (Oncorhynchus mykiss). Front. Mar. Sci. 2022, 9, 1036289. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, M.; Li, X.; Song, C.; Liu, Y. Physiological metabolism of Haliotis discus hannai Ino under different light qualities and cycles. Aquac. Res. 2017, 48, 3340–3355. [Google Scholar] [CrossRef]
- Cheng, S.; Zheng, J.; Jia, Y.; Chi, M.; Jiang, W.; Liu, S.; Li, F.; Liu, Y.; Gu, Z.; Wang, D. Effects of light color, photoperiod, and growth-related gene interference or overexpression on the survival, growth, or physiological and biochemical indices of red claw crayfish juveniles. Aquaculture 2023, 562, 738740. [Google Scholar] [CrossRef]
- Hamasaki, K.; Ogiso, Y.; Dan, S.; Kitada, S. Survival, development and growth of larvae of the coconut crab, Birgus latro, cultured under different photoperiod conditions. Aquac. Res. 2016, 47, 2506–2517. [Google Scholar] [CrossRef]
- Andrés, M.; Rotllant, G.; Zeng, C. Survival, development and growth of larvae of the blue swimmer crab, Portunus pelagicus, cultured under different photoperiod conditions. Aquaculture 2010, 300, 218–222. [Google Scholar] [CrossRef]
- Litvak, M.K.; Zadmajid, V.; Butts, I.A.E. Growth and survival of winter flounder (Pseudopleuronectes americanus) larvae reared on different photoperiod regimes from hatch to metamorphosis. Aquac. Res. 2020, 51, 2314–2321. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hamasaki, K.; Murakami, K. Larval survival, development and growth in the horsehair crab, Erimacrus isenbeckii, cultured under different photoperiod conditions. Aquac. Res. 2018, 49, 2511–2517. [Google Scholar] [CrossRef]
- Indriastuti, C.E.; Junior, M.Z.; Suprayudi, M.A.; Supriyono, E.; Alimuddin, A. Cannibalism, survival and growth performance of juvenile African catfish Clarias gariepinus in relation to photoperiod and 17β-Oestradiol treatment. Aquac. Res. 2022, 53, 4437–4448. [Google Scholar] [CrossRef]
- Gao, X.L.; Mo, Z.; Li, X.; Wu, F.C.; Song, C.B.; Liu, Y. Light cycle effects on Haliotis discus hannai Ino growth, energy budget, and related gene expression. Aquaculture 2018, 483, 213–222. [Google Scholar] [CrossRef]
- Crear, B.J.; Hart, P.R.; Thomas, C.W. The effect of photoperiod on growth, survival, colour and activity of juvenile southern rock lobster, Jasus edwardsii. Aquac. Res. 2003, 34, 439–444. [Google Scholar] [CrossRef]
- Xu, H.; Dou, J.; Wu, Q.; Ye, Y.; Wang, C.; Song, C.; Mu, C.; Ren, Z.; Shi, C. Photoperiod affects the survival rate but not the development of larval swimming crab Portunus trituberculatus. Aquacult. Int. 2022, 30, 1769–1778. [Google Scholar] [CrossRef]
- Minagawa, M. Effects of photoperiod on survival, feeding and development of larvae of the red frog crab, Ranina ranina. Aquaculture 1994, 120, 105–114. [Google Scholar] [CrossRef]
- Ramos, S.E.; Carvalho, A.F.S.D.; Castro, T.F.D.; Vasconcelos, A.C.N.; Veras, G.C.; Mourão Júnior, C.A.; Murgas, L.D.S. Cannibalism, growth performance, and body composition of giant trahira juveniles under different photoperiods. Pesqui. Agropecuária Bras. 2018, 53, 664–672. [Google Scholar] [CrossRef]
- Veras, G.C.; Paixão, D.J.D.M.; Brabo, M.F.; Soares, L.M.O.; Sales, A.D. Influence of photoperiod on growth, uniformity, and survival of larvae of the Amazonian ornamental Heros severus (Heckel, 1840). Rev. Bras. Zootec. 2016, 45, 422–426. [Google Scholar] [CrossRef]
- Lesmana, D.; Supriyono, E.; Junior, M.Z.; Nirmala, K.; Jusadi, D. The colour preference of scalloped spiny lobster, Panulirus homarus. IOP Conf. Ser. Earth Environ. Sci. 2021, 744, 12039. [Google Scholar] [CrossRef]
- Lavery, S.D.; Farhadi, A.; Farahmand, H.; Chan, T.; Azhdehakoshpour, A.; Thakur, V.; Jeffs, A.G. Evolutionary Divergence of Geographic Subspecies within the Scalloped Spiny Lobster Panulirus homarus (Linnaeus 1758). PLoS ONE 2014, 9, e97247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, B.; Yu, C.; Xu, Y.; Huang, Z.; Cai, Y.; Li, Y. Hepatopancreas immune response during different photoperiods in the Chinese mitten crab, Eriocheir sinensis. Fish Shellfish. Immun. 2023, 132, 108482. [Google Scholar] [CrossRef]
- Zhou, S.J.; Hu, J.; Yu, G.; Yang, Q.B.; Yang, R.; Liu, Y.J.; Ma, Z.H. Effects of photoperiod on digestive enzyme activity in larval and juvenile barramundi Lates calcarifer (Bloch). Mar. Sci. 2018, 42, 63–69. [Google Scholar] [CrossRef]
- Lee, M.; Kovacs-Nolan, J.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. Hen Egg Lysozyme Attenuates Inflammation and Modulates Local Gene Expression in a Porcine Model of Dextran Sodium Sulfate (DSS)-Induced Colitis. J. Agr. Food Chem. 2009, 57, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).