Abstract
Sulfate and chloride in the marine environment threaten the lifespan of concrete structures. Predicting the strength of concrete under different degrees of ion erosion is essential for marine structure design and maintenance. In this paper, a novel method was developed to predict the compressive strength evolution due to sulfate and chloride attack. The degradation and ion diffusion behavior of cement-based materials was investigated by analyzing the visual appearance, compressive strength, porosity, and ion distributions of mortar soaked in sulfate and chloride solutions with different concentrations. The damage degree was observed to increase with sulfate concentration and decrease with chloride concentration. Additionally, it was discovered that chloride and sulfate ions inhibited the diffusion of each other, and a higher concentration resulted in a more substantial inhibition effect. The total effective sulfate and chloride intrusions were proposed to describe the erosion degree of mortar based on the evaluation of the ion distributions with Fick’s second law. A compressive strength assessment method was established based on the analysis of the correlation between the strength contribution of sulfate reaction and the total effective chloride and sulfate intrusions. This method exhibits the potential for estimating the concrete strength of actual marine structures damaged by sulfate and chloride with accelerated laboratory tests.
1. Introduction
Sulfate and chloride are the predominant sources of aggressive chemical corrosion for concrete structures in marine environments [1,2,3]. Upon ingress into concrete, sulfate ions undergo a chemical reaction with cement hydration products, forming expansive products that may cause expansion, cracking, and spalling. Furthermore, chloride ions not only accelerate steel corrosion but also produce Friedel’s salt when binding with the aluminate phase contained in cement. The intricate interplay between chloride and sulfate in diffusion and reaction processes adds complexity to the topic of combined sulfate and chloride attack [4].
A comprehensive understanding of the mechanical property evolution of concrete exposed to corrosion environments is essential for designing and maintaining marine concrete structures [5]. The degradation of elastic modulus, compressive strength, and flexural strength in sulfate-attacked concrete has been reported to be influenced by diverse factors, including sulfate concentration, concrete composition, and temperature [6,7,8,9]. In contrast, the influence of chloride alone on concrete mechanical properties is deemed negligible [10]. Moreover, most existing research indicates that chloride mitigates the damage caused to concrete by sulfate reactions [11,12]. Several prediction methods for mechanical properties have been proposed based on laboratory tests. Yu et al. [7] established the relationship between compressive strength and sulfate immersion duration for different sulfate concentrations and concrete compositions. Diab et al. [13] presented a method to predict the compressive strength of concrete with various cement types from expansion strain. Meanwhile, González-Taboada et al. [14] utilized genetic programming methodology to predict the mechanical properties of concrete based on a database developed with 1831 mixes from 81 papers. Nevertheless, it should be noted that these studies employed accelerated corrosion and small-scale standard specimens, resulting in significant discrepancies from the environments and dimensions of in situ concrete structures. Furthermore, the damage inflicted on concrete in sulfate and chloride solutions is non-uniform, with the external portion experiencing severe deterioration, while the internal part may remain healthy [15]. Consequently, these methods may not be directly applicable to predicting the mechanical properties of in situ concrete structures.
Previous studies have devoted considerable attention to investigating the diffusion behavior of sulfate and chloride. Results in the literature have confirmed the inhibitory effect of chloride on the transportation of sulfate ions [16]. However, both the promoting and inhibiting impacts of sulfate on chloride transportation have been clarified by different authors [17,18]. The discrepancy could be ascribed to the diverse corrosion environments, materials, and corrosion degrees in these studies. In addition, some chemo-mechanical models describing the diffusion and damage behavior of sulfate and chloride have been developed [4,19], thereby addressing the problem of ion distribution prediction after long-term erosion. The studies by Cheng et al. [8] and Liao et al. [20] suggest that the degradation of mechanical properties is closely linked to the invasion of sulfate ions. However, evaluating the mechanical properties under different degrees of ion erosion remains challenging. A consensus is still needed regarding how sulfate ions deteriorate concrete and how chloride ions impact sulfate-induced damage [16,21,22,23].
The current literature indicates that it is imperative to introduce a novel approach for predicting the mechanical properties of marine concrete structures based on simultaneous ion erosion levels. Therefore, this paper aims to establish the association between compressive strength and ion distributions in cases of marine sulfate and chloride attack with accelerated laboratory tests. The mechanical and physical properties and ion distributions of samples immersed in different sulfate and chloride solutions were first investigated. Then, the total effective sulfate and chloride intrusions were introduced to describe the erosion degree of the attacked mortar based on the evaluation of the ion distributions with Fick’s second law. Finally, a compressive strength assessment method was established based on the relationship between the contribution of sulfate reaction to strength and total effective sulfate and chloride intrusions.
2. Experimental Procedure
2.1. Materials and Specimens
P.O.42.5 cement, with the characteristics presented in Table 1, was used as the binding material. The mineral composition of the cement was calculated using the Bogue method, yielding a composition of 57.7% C3S, 13.7% C2S, 7.27% C3A, and 8.97% C4AF. River sand with a fineness modulus of about 2.63 and a particle size smaller than 5 mm was chosen as the aggregate. Mortar was prepared according to the Chinese code SL/T 352-2020 [24]. The w/c and s/c of the mortar are 0.55 and 3, respectively. The cast samples were 70.7 mm cubes. All mortar specimens were cured in saturated lime water for 56 d before immersion into the corrosion solutions.

Table 1.
Chemical compositions and physical properties of the cement.
After demolding, the cubes were coated with paraffin, and only two opposite faces were left in contact with the immersion solution to achieve one-dimensional diffusion. Prior to conducting the mortar property tests, the paraffin layer was removed, and the surface was clean. In order to study the damage process of mortar under sulfate and chloride attack within a limited period, the specimens were fully immersed in high-concentration solutions of chloride and sulfate to accelerate corrosion. However, existing research [25] indicated that the erosion mechanism underwent alteration once the solution concentration reached 15%. Therefore, the concentrations listed in Table 2 were selected referring to standards and the literature [11,24,26,27]. The solutions were periodically replaced to maintain a consistent pH and concentration.

Table 2.
Composition of corrosion solutions.
2.2. Test Methods
2.2.1. X-ray Diffraction Tests
X-ray diffraction (XRD) analysis was employed to identify the products formed by chloride and sulfate attack. The experiment was performed on powders obtained from the erosion surface of the samples with a particle size smaller than 75 μm. The samples immersed in LL, ML, LM, and MM solutions for 360 d presented in Table 2 were used for the tests. A Bruker D8 Advance X-ray powder diffractometer with a Cu-Kα source at a scanning speed of 6°/min and scanned two-theta ranging from 5 to 55° was used.
2.2.2. Compressive Strength
This investigation involved the evaluation of the compressive strength of mortar following the Chinese code SL/T 352-2020 [24]. The rate of the axial compressive load was 2 kN/s. Each group comprised three specimens.
2.2.3. Porosity
This study examined porosity by measuring the volume of permeable voids according to ASTM C642-21 [28]. To obtain the porosity values, three specimens were examined for each immersion period, and the mean value was reported. After the specified immersion time, the samples were baked to a constant weight at 110 °C in an electric oven. The dry weight (A) was tested when cooled down to ambient temperature. Subsequently, the samples were soaked in room-temperature water for 48 h and boiled for 5 h. The mass of the samples (C) was tested after cooling and drying the surface moisture. Finally, the apparent mass in water (D) was determined by hanging the specimens in water. The porosity P can be calculated as follows:
2.2.4. Ion Content
After the samples were immersed in the solutions for 30, 90, 150, 210, 270, and 330 d, powders were produced by drilling along the erosion direction at depths of 5, 10, 15, 20, and 25 mm. Subsequently, the powders were sieved to ensure a particle size smaller than 0.075 mm and baked at 60 °C for 24 h. The resulting powders were weighed and dissolved in dilute nitric acid for 24 h to extract the ions. Later, the dissolved solution was filtered to eliminate the solids. Finally, the chloride and sulfate ion contents in the filter liquor were analyzed with a ZDJ-4A automatic potentiometric titrator and a UV755B spectrophotometer, respectively. The ion content of chloride and sulfate at different depths was determined according to Equation (2):
where cp is the ion content, Vfl is the total volume of the filter liquor, cfl is the ion concentration in the filter liquor, and mp is the mass of the powder.
3. Results and Discussion
3.1. Visual Examination
Figure 1 and Figure 2 illustrate typical surface damages for mortar exposed to the 6 groups of solutions outlined in Table 2 for 180, 270, and 360 d. Calcium leaching was observed in samples immersed in all solutions after 180 d, with the visible dissolution of hydration products around the pores on the mortar surface. Little damage was detected in the samples immersed in the LL and ML solutions except for calcium leaching. Notably, the dissolution of hydration products was more pronounced in specimens exposed to the ML solution, suggesting that chloride may accelerate calcium leaching in cement-based materials [29,30]. This phenomenon was also supported by the XRD results presented in Figure 3, where weaker peaks for portlandite were detected in the ML solution than in the LL solution. Mortar immersed in the LM solution presented little evidence of sulfate attack before 180 d. However, the reaction between sulfate ions and cement paste resulted in portlandite consumption and ettringite formation, which was examined in Figure 3. The reduction in cement hydration products weakened the binding property of the cement paste, and the accumulation of excessive ettringite led to pore cracking [7]. As the sulfate erosion intensified, severe cracking and spalling were observed on the samples immersed in the LM solution up to 360 d.
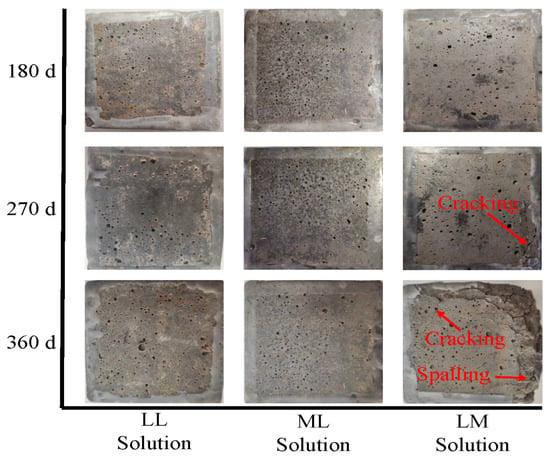
Figure 1.
Visual appearance of mortar exposed to water, sulfate solution, and chloride solution.
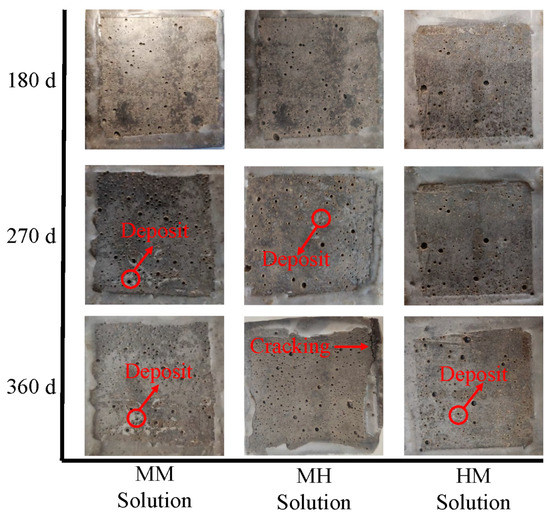
Figure 2.
Visual appearance of mortar exposed to composite chloride and sulfate solutions.
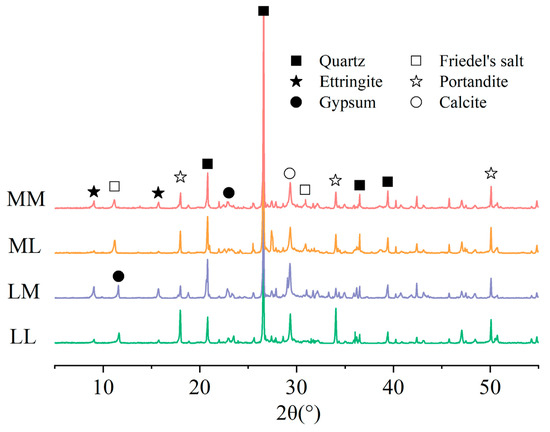
Figure 3.
Phase variation under sulfate and chloride attack.
A white powdery material was observed around the pores on the erosion surface of the specimens after 270 d of immersion in the MM solution, which was also found by Lee et al. [31] in their research about mortar exposed to sulfate solutions. Although the area of the white powder increased with time, no visible cracks were detected on the mortar immersed in the MM solution until 360 d. Analysis of the phase composition of the samples exposed to MM and LM solutions revealed that the composite solution resulted in less portlandite consumption and ettringite production, thereby causing less damage. Furthermore, the white powder area decreased for mortar exposed to the HM solution. Specimens immersed in a composite solution with a higher chloride concentration exhibited less visual damage. For mortar immersed in the MH solution, cracks were detected along the edges of the samples at 360 d, but no spalling occurred. Interestingly, mortar in the MH solution exhibited less deterioration than in the LM solution, despite exposure to a solution with a higher sulfate concentration.
3.2. Compressive Strength
The strength evolution in mortar samples subjected to various environments is presented in Figure 4. The specimens submerged in pure water exhibited a considerable rise in strength within the initial 90 d, followed by a slight increase in the subsequent 270 d. Throughout the immersion for 360 d, there was a growth in the compressive strength by a magnitude of 25.52%. This enhancement in strength was attributed to the uninterrupted hydration of residual unhydrated cement, which gradually decelerated over time [10].
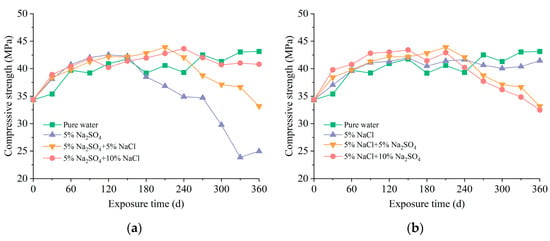
Figure 4.
Compressive strength of mortar exposed to different solutions: (a) effect of chloride concentration; (b) effect of sulfate concentration.
Resembling the variation of visual appearances, the compressive strength in the case of pure sulfate attack changed significantly as the duration of immersion progressed. Initially, the strength increased with time and was marginally higher compared to the pure water case for the same erosion duration. It was observed that the compressive strength of samples in pure sulfate solution was 7.20% higher than in pure water at 90 d. This phenomenon was caused by the filling action of corrosion products [7]. Subsequently, the compressive strength deteriorated rapidly, since a substantial quantity of ettringite accumulated in the pores and caused cracks in the samples [32]. After immersion in pure sulfate solution for 360 d, the compressive strength is only 57.90% of that in pure water. With the addition of chloride to the corrosion solutions, the duration of strength growth increased, and the strength at 360 d was 32.73% higher compared to samples under pure sulfate attack. Moreover, the damage to compressive strength was reduced as the chloride concentration increased. There was a significant decrease of 36.69% in compressive strength degradation at 360 d upon elevating the chloride concentration from 0 to 10%. Evidently, the existence of chloride ions in the composite solutions delayed the onset of sulfate attack. A higher chloride concentration often corresponded to a higher level of inhibitory effect.
As demonstrated in Figure 4b, the specimens soaked in chloride solution had a comparable strength growth to that of pure-water-immersed mortar, which meant that the long-term exposure to chloride solution caused minor damage. Nonetheless, the strength of samples exposed to composite chloride and sulfate environments underwent a reduction, with higher sulfate concentration resulting in more significant strength deterioration. When the sulfate concentration increased from 0 to 10%, the compressive strength decreased by 21.70%. The outcome mentioned above was similar to pure sulfate attack, where the extent of strength degradation also increased with sulfate concentration [32].
3.3. Porosity
The porosity of the 70.7 mm mortar specimens after long-term immersion in corrosion solutions is shown in Figure 5. It is pertinent to mention that the porosity measured in this research solely pertained to the volumetric fraction of pores permeable by water. A similar porosity variation was observed in different solutions, characterized by a decline that was succeeded by an increase with prolonged erosion duration. The initial reduction in porosity for specimens exposed to pure water could be explained by the continuous cement hydration, which coincided with the increasing compressive strength during this period. The later growth of porosity was traced to the dissolution of cement hydration products stemming from calcium leaching, which was found on the sample surfaces during the visual examination experiment. When immersed in pure chloride solution, the mortar showed a greater reduction in porosity before 180 d and a faster increase in porosity from 180 d to 360 d compared to pure water. The porosity of the samples in both solutions was very close at 330 d, indicating an accelerated calcium leaching of the samples in a chloride-rich environment.
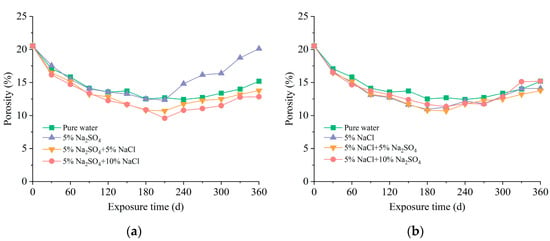
Figure 5.
Porosity of mortar exposed to different solutions: (a) effect of chloride concentration; (b) effect of sulfate concentration.
During the initial exposure stage, the samples in the sulfate solution had almost the same decrease in porosity as in pure water. However, the specimens experienced a significantly accelerated porosity increase during the later stage of sulfate attack, which could be explained by the dissolution of portlandite and C-S-H in cement paste and excessive deposition of ettringite [33]. This phenomenon was also evidenced by the variation in phase composition in Figure 3. The addition of chloride into the immersion solution effectively reduced the porosity of the mortar specimens, and the samples in the MM solution even had lower porosity than those in pure water. As shown in Figure 3, the rise in chloride concentration within the pore solution stimulated cement hydration and facilitated the production of Friedel’s salt, ultimately bringing about more compact mortar structures.
3.4. Distributions of Chloride Ions
Figure 6 shows the chloride content profiles of mortar soaked in chloride and composite solutions at diverse immersion periods. The research findings illustrated a decrease in the content of chloride ions with increasing depth in all examined environments. Additionally, a positive relationship between chloride content and immersion time at the identical erosion depth was found. The chloride diffusion behavior in pure chloride solution and composite chloride and sulfate solution was analogous, in line with the previously reported experimental results [16,34,35].
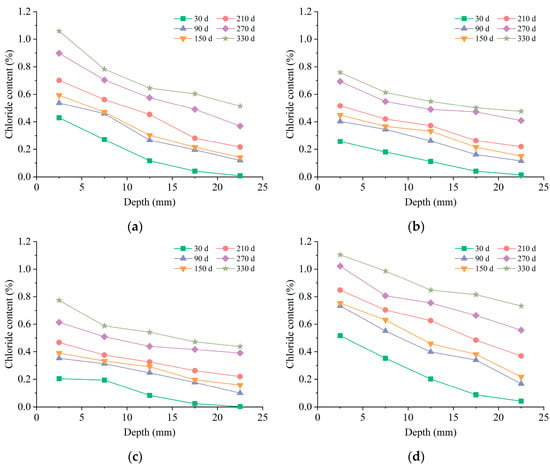
Figure 6.
Distributions of chloride ions: (a) ML solution; (b) MM solution; (c) MH solution; (d) HM solution.
The chloride content of specimens soaked in the ML solution exhibited a decline in growth rate from 30 d to 150 d, which was related to the decrease in concentration gradient and porosity. Subsequently, the growth rate of chloride content increased from 150 d to 270 d. The development of porosity during this stage might be the leading cause. The growth rate experienced a decrease at 330 d, which was speculated to be due to the relatively small concentration gradient at high concentrations.
The research findings presented in Figure 6b,c demonstrated that the introduction of sulfate exerted a considerable inhibitory impact on the diffusion behavior of chloride ions in cementitious materials. Moreover, this effect became more prominent with increasing sulfate concentrations. The impact of sulfate was primarily observed at the erosion depth of 0–10 mm. In contrast, the chloride content profiles at a depth of 10–25 mm remained similar between the samples in composite solutions and those in pure chloride solution, which could be attributed to the low levels of sulfate content at the same depth. Comparing the content profiles depicted in Figure 6b,d revealed that elevating the chloride concentration in the immersion solution had a notable influence on the chloride ion content at various depths.
3.5. Distributions of Sulfate Ions
The sulfate content profiles in mortar samples exposed to various environments are shown in Figure 7. It was observed that, akin to chloride, the sulfate content of the mortar gradually decreased from the surface towards the core in all solutions. At the identical erosion depth, the sulfate content increased with immersion time. However, the ion transportation rate of sulfate was lower than that of chloride. After 330 d of external sulfate attack, the sulfate content increased significantly only at a depth of 0–10 mm, while the content development was very weak at 10–25 mm. This observation was similar to those in previous studies by Wang et al. [36] and Min et al. [37]. The XRD results in Figure 3 also evidenced that large amounts of sulfate-rich phases, such as ettringite, gypsum, and Friedel’s salt, were also generated on the surface.
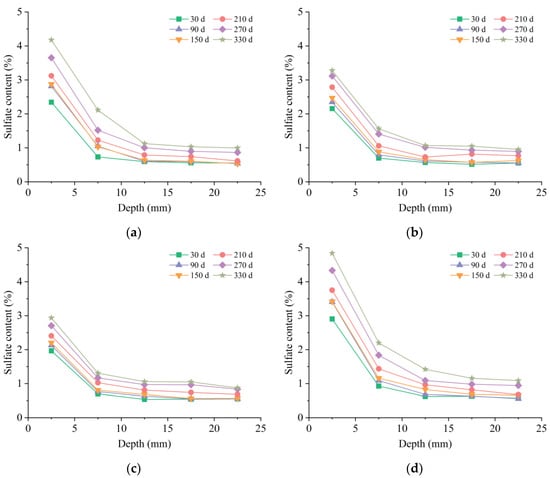
Figure 7.
Distributions of sulfate ions: (a) LM solution; (b) MM solution; (c) HM solution; (d) MH solution.
Figure 7a shows the ion distributions of sulfate in mortar samples soaked in the LM solution. At 30 d, a high sulfate content was observed at a depth of 0–5 mm. The increase rate in sulfate content decreased rapidly from 30 d to 150 d, which could be attributed to the reduction in porosity during this stage. Subsequently, the growth of sulfate content accelerated again, with an increase in porosity induced by calcium leaching and cracking.
As per the experimental results, the samples immersed in the MM solution exhibited a lower sulfate content than those in the LM solution, suggesting that adding chloride to the immersion solution inhibited the sulfate diffusion. Although the difference in sulfate content between the two solutions was minimal at 30 d, it increased to over 1% at 330 d. Therefore, the inhibition effect of chloride on sulfate diffusion was seen to increase with prolonged immersion time. Moreover, a more pronounced inhibition effect was observed at higher chloride concentrations, as indicated by the lower ion content in the specimens submersed in the HM solution, compared to those in the LM and MM solutions.
As depicted in Figure 7d, increasing the sulfate concentration in composite solutions resulted in a more extensive intrusion of sulfate ions into the mortar. Despite the coexistence of chloride in the immersion environment, the sulfate content of mortar in the MH case was higher than that in the LM case at 330 d. Conversely, the strength degradation of specimens subjected to the MH solution was less pronounced than those in the LH solution. This observation suggested that sulfate ions with higher content did not cause more damage to the samples when coexisting with chloride ions.
4. Evaluation of Ion Distributions with Fick’s Second Law
4.1. Sulfate Ions
To determine the distributions of chloride and sulfate at various depths with limited test results, the profile data of chloride and sulfate in concrete were fitted with a one-dimensional unsteady state diffusion solution of Fick’s law [38,39]:
where cb is the ion content at the boundary between the corrosion solution and test sample ascertained by the correlation analysis, c(x,t) is the ion content at time t and depth x, erf() is the error function, c0 is the initial ion content for the test material antecedent to immersion in the corrosion solution, and Da is the apparent diffusion coefficient.
The boundary ion content between the corrosion solution and test sample (cb)s and apparent diffusion coefficient (Da)s calculated from the measured sulfate content for different solutions and durations are listed in Table 3. The initial sulfate content was set at 0.55%, referring to the experimental results. The high correlation coefficient (R2 > 0.95) observed between most corrosion solutions and exposure time confirmed the accuracy of Equation (3) in capturing the diffusion behavior of sulfate ions. The interfacial sulfate content increased with time in the pure sulfate solution but experienced a decline during the intermediate corrosion stage in composite solutions. The apparent sulfate diffusion coefficient of the sample initially decreased and then increased with time for all the environments presented in Table 3. The similarity between the variation in the sulfate apparent diffusion coefficient and porosity further confirmed the close relationship between the ion diffusion capacity and the porosity of the material [40].

Table 3.
Fitting results of sulfate profile data.
In this study, a criterion for the sulfate diffusion depth was established, defined as the depth where the sulfate content in the mortar surpassed 5% of the initial value, considering the measurement error. With the parameters provided in Table 3, the sulfate diffusion depth was computed using Equation (3). As shown in Figure 8, the diffusion depth exhibited an increase with time in all solutions. It was found that the development of diffusion depth over time could be fitted to an exponential function. Furthermore, the results indicated that an augmentation of the sulfate concentration within the corrosion solution resulted in a larger diffusion depth, whereas an elevation of the chloride concentration produced a smaller diffusion depth.
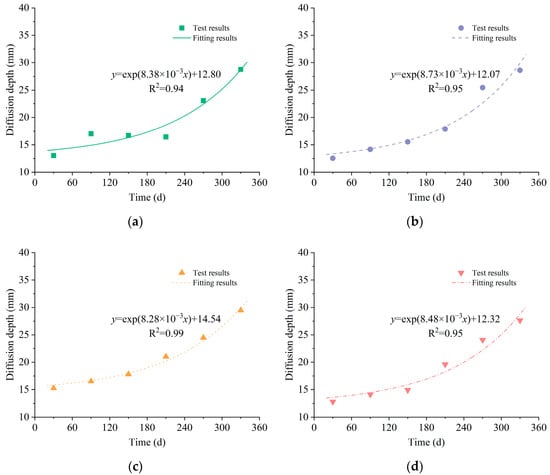
Figure 8.
Sulfate diffusion depth: (a) LM solution; (b) MM solution; (c) MH solution; (d) HM solution.
As per the investigations conducted previously [8,20], it has been concluded that ion distribution is a more precise indicator of cement-based material degradation caused by sulfate, in comparison to sulfate erosion depth. The concept of total effective sulfate intrusion was defined as the integral of sulfate content along erosion depth, as represented in Figure 9. Table 4 demonstrates the calculation outcomes of the total effective sulfate intrusion for diverse corrosion solutions and immersion durations. The data indicated that the total effective sulfate intrusion increased with the duration of exposure to all solutions. Additionally, the concentration of sulfate in the corrosion solution was found to have a positive relationship with the total effective sulfate intrusion, while the concentration of chloride had an inverse correlation. It was evident that the total effective sulfate intrusion yielded meaningful insights into the corrosion process associated with external sulfate attack.
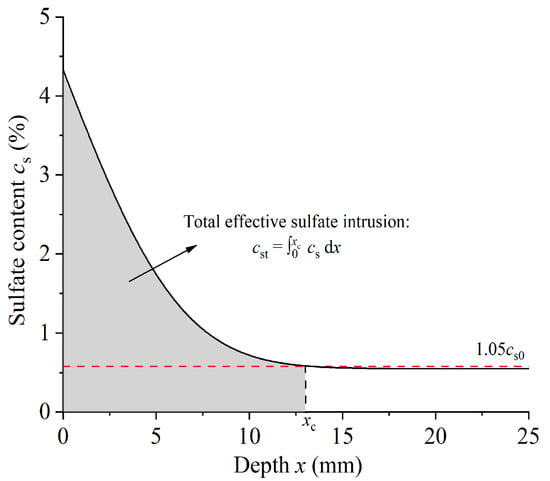
Figure 9.
Definition of total effective sulfate intrusion.

Table 4.
The total effective sulfate intrusion (mm%).
4.2. Chloride Ions
The interfacial content between the corrosion solution and test sample (cb)cl and apparent diffusion coefficient (Da)cl of chloride ions were also fitted with Equation (3). The initial content was established as 0 since there was only a negligible amount of chloride present in the uncorroded mortar. The accuracy of the fit was assured because the correlation coefficient R2 was larger than 0.90 under all conditions (Table 5). The results revealed an increase in chloride content at the exposure surface over time in all solutions. The apparent diffusion coefficient of chloride ions exhibited an initial decrease, followed by an increase with time, similar to sulfate ions.

Table 5.
Fitting results of chloride profile data.
In consideration of the strength and ion concentration data, it was evident that chloride ions had a substantial influence on sulfate-induced mortar degradation. Furthermore, the influence increased with an escalation in chloride content. Hence, the concept of total effective chloride intrusion was introduced for assessing the material impairment under combined chloride and sulfate attack, which was defined as the integral of chloride content along sulfate diffusion depth:
where cclt is the total effective chloride intrusion, xc is the sulfate diffusion depth, and ccl is the chloride content at location x.
The calculation outcomes of the total effective chloride intrusion under various immersion solutions and exposure durations are presented in Table 6. The results indicated that the total effective chloride intrusion augmented with the progression of immersion time.

Table 6.
The total effective chloride intrusion (mm%).
5. Strength Assessment Method Based on Ion Distributions
Non-uniform damage was observed in the samples subjected to external salt erosion at different depths [41]. The outer regions exposed to the salt solution exhibited severe deterioration, while the damage in the inner regions was relatively weak. Consequently, the cross-section of the attacked samples was segmented into two distinct regions: the deteriorated outer region and the intact inner region. The relationship between the homogenized strength of the whole sample, the damaged region, and the healthy region was investigated through Taylor-type homogenization analysis [42]:
where ft, fd, and fh are the average strength of the entire sample, the damaged region, and the healthy region, respectively, Vt, Vd, and Vh are the volumes of the entire sample, the damaged area, and the healthy area, respectively.
It was difficult to determine the location of the corrosion boundary and measure ion content in two-dimensional and three-dimensional external salt corrosion. Thus, one-dimensional diffusion tests were suggested to determine the parameters of the compressive strength assessment method. In one-dimensional conditions, the average strength of the damaged region could be described as
where lt and ld are the length of the whole sample and the damaged region along the erosion direction, respectively.
In this study, the strength in the pure water case for the same duration was assumed to be the average strength of the healthy region. Since the sulfate reaction was the principal motive for the strength deterioration of the samples exposed to composite solutions, the sulfate diffusion depth given in Figure 8 was used as the length of the damaged region.
All samples experienced a strength increase due to cement hydration regardless of salt corrosion, which could be seen from the results depicted in Figure 4. Therefore, the development of the average strength of the damaged region was attributed to both sulfate reaction and cement hydration. Assuming the same degree of hydration throughout the sample, the contribution of the sulfate reaction to the strength development of the damaged region fsc could be expressed as
The contribution of the sulfate reaction to the compressive strength of the damaged region is displayed in Figure 10. The results illustrated a favorable effect of the sulfate reaction in all solutions at 30 d but an adverse effect at later stages. Notably, the degradation of compressive strength in the damaged region was mitigated by increasing the chloride concentration in the solution. Conversely, an increase in sulfate concentration accelerated the damage within this area.
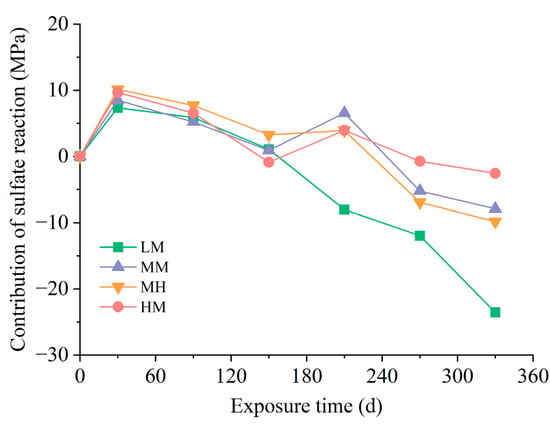
Figure 10.
Contribution of the sulfate reaction to the compressive strength of the damaged region.
Drawing upon the examination conducted in the preceding sections, the decline in the strength of test specimens subjected to composite chloride and sulfate environments was related to the total effective sulfate and chloride intrusions. In light of the influence law of the total effective sulfate and chloride intrusion on strength development, Equation (8) was formulated to depict the connection between the strength contribution of the sulfate reaction, fsc, the total effective sulfate intrusion, cst, and the total effective chloride intrusion, cclt:
where a1, a2, a3, and a4 are parameters determined from the tests.
The experimental data may contain outliers due to possible errors in the sampling and determination of strength and ion content. Thus, as depicted in Figure 11, the random sample consensus [43] was employed to fit Equation (8) to the test results in this study. The parameters a1, a2, a3, and a4 were estimated to be −1.06 MPa/(%·mm), 1.05 MPa/(%·mm), −25.26 MPa, and 28.01 MPa when the threshold in the random sample consensus was established at 5 MPa.
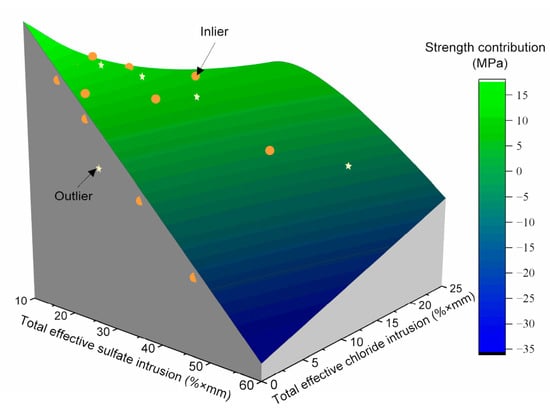
Figure 11.
The relationship between the strength contribution of the sulfate reaction and the total effective chloride and sulfate intrusions.
The process of utilizing the method proposed in this study to predict compressive strength is illustrated in Figure 12. Upon acquiring the ion distribution data of a concrete structure through experiments or numerical simulation, the diffusion depth of sulfate ions and the length of the damaged region can be determined by Equation (3). Subsequently, the total effective intrusion of sulfate and chloride ions is calculated using the formula in Figure 8 and Equation (4). The contribution of the sulfate reaction to the average compressive strength of the damaged region can be deduced from the total invasion amount of sulfate and chloride ions using Equation (8). Finally, Equation (7) can be used to obtain the average compressive strength of the concrete structure from the contribution of the sulfate reaction to the compressive strength of the damaged region and the compressive strength of the uncorroded standard samples at the same age.
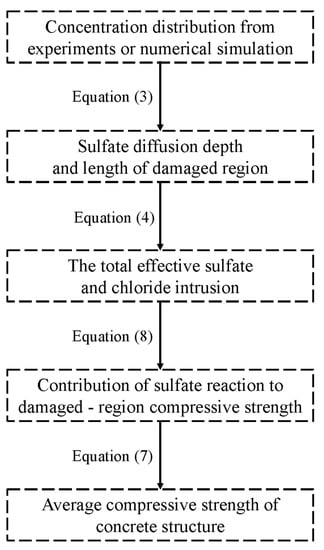
Figure 12.
The process of compressive strength prediction.
6. Conclusions
This paper explored the degradation of mortar properties and ion transport behavior under marine sulfate and chloride attack, and a compressive strength assessment method was established based on the ion distributions of chloride and sulfate. The conclusions can be encapsulated as follows:
- (1)
- The damage caused by the sulfate reaction was observed in the test results of visual examination, compressive strength, and porosity of the specimens submersed in pure sulfate and composite sulfate and chloride solutions. The strength degradation increased by 21.69% with the sulfate concentrations and decreased by 36.69% with the chloride concentrations.
- (2)
- Chloride and sulfate ions were found to inhibit the diffusion of each other in mortar, and the more elevated the ion concentration, the more intensified the retarding effect. The apparent diffusion coefficient of sulfate and chloride exhibited a similar variation pattern to porosity.
- (3)
- The damage degree of the mortar was related to both sulfate and chloride ion distributions. The total effective sulfate and chloride intrusions were proposed to reflect the material deterioration triggered by sulfate and chloride attack based on the analysis of ion distributions with Fick’s second law.
- (4)
- The degradation of compressive strength exhibited a positive correlation with total effective sulfate intrusion and an inverse relationship with total effective chloride intrusion. The established formula between compressive strength and total effective sulfate and chloride intrusions can serve as a reliable predictor of the strength development of cement-based materials confronted with marine sulfate and chloride attack, given the distributions of ion concentrations.
Author Contributions
Conceptualization, writing—original draft preparation, S.L.; methodology, writing—review and editing, X.Y.; investigation, W.Z.; writing—review and editing, Y.L.; supervision, writing—original draft preparation, D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Jiangsu Province (grant number BK20220981); the Jiangsu Provincial Key Research and Development Program (grant number BE2020715); the Outstanding Postdoctoral Research Program of Jiangsu Province (grant number 2022ZB183); and the China Scholarship Council (grant number 202006710058).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, J.; Wang, K.; Qi, C. Determining the Mineral Admixture and Fiber on Mechanics and Fracture Properties of Concrete under Sulfate Attack. J. Mar. Sci. Eng. 2021, 9, 251. [Google Scholar] [CrossRef]
- Dong, M.; Elchalakani, M.; Karrech, A.; Yang, B. Long-Term Strength of Alkali-Activated Mortars with Steel Fibres Cured in Various Conditions. J. Mar. Sci. Eng. 2020, 8, 278. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Menezes, I.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. EIS Investigation of the Corrosion Behavior of Steel Bars Embedded into Modified Concretes with Eggshell Contents. Metals 2022, 12, 417. [Google Scholar] [CrossRef]
- Sun, D.; Cao, Z.; Huang, C.; Wu, K.; De Schutter, G.; Zhang, L. Degradation of concrete in marine environment under coupled chloride and sulfate attack: A numerical and experimental study. Case Stud. Constr. Mater. 2022, 17, e01218. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H. A review on effects of limestone powder on the properties of concrete. Constr. Build. Mater. 2018, 192, 153–166. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Zhao, Y.; Yu, X.; Li, C.; Chen, D. Effect of initial curing period on the behavior of mortar under sulfate attack. Constr. Build. Mater. 2022, 326, 126852. [Google Scholar] [CrossRef]
- Yu, X.; Chen, D.; Feng, J.; Zhang, Y.; Liao, Y. Behavior of mortar exposed to different exposure conditions of sulfate attack. Ocean Eng. 2018, 157, 1–12. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, T.; Zou, D.; Zhou, A. Compressive strength assessment of sulfate-attacked concrete by using sulfate ions distributions. Constr. Build. Mater. 2021, 293, 123550. [Google Scholar] [CrossRef]
- Liang, N.; Mao, J.; Yan, R.; Liu, X.; Zhou, X. Corrosion resistance of multiscale polypropylene fiber-reinforced concrete under sulfate attack. Case Stud. Constr. Mater. 2022, 16, e01065. [Google Scholar] [CrossRef]
- Du, J.; Tang, Z.; Li, G.; Yang, H.; Li, L. Key inhibitory mechanism of external chloride ions on concrete sulfate attack. Constr. Build. Mater. 2019, 225, 611–619. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Shi, M.; Cui, J.; Xie, F. Degradation of cast-in-situ concrete subjected to sulphate-chloride combined attack. Constr. Build. Mater. 2020, 241, 117995. [Google Scholar] [CrossRef]
- Wu, J.; Wei, J.; Huang, H.; Hu, J.; Yu, Q. Effect of multiple ions on the degradation in concrete subjected to sulfate attack. Constr. Build. Mater. 2020, 259, 119846. [Google Scholar] [CrossRef]
- Diab, A.M.; Awad, A.E.M.; Elyamany, H.E.; Abd Elmoaty, A.E.M. Guidelines in compressive strength assessment of concrete modified with silica fume due to magnesium sulfate attack. Constr. Build. Mater. 2012, 36, 311–318. [Google Scholar] [CrossRef]
- González-Taboada, I.; González-Fonteboa, B.; Martínez-Abella, F.; Pérez-Ordóñez, J.L. Prediction of the mechanical properties of structural recycled concrete using multivariable regression and genetic programming. Constr. Build. Mater. 2016, 106, 480–499. [Google Scholar] [CrossRef]
- Xie, F.; Li, J.; Li, L.; Zhao, G.; Yao, M. Numerical solution and damage evaluation for cast-in-situ piles exposed to external sulfate attack. Constr. Build. Mater. 2019, 214, 269–279. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, L.; Bindiganavile, V.; Yi, C. Coupled models to describe the combined diffusion-reaction behaviour of chloride and sulphate ions in cement-based systems. Constr. Build. Mater. 2020, 243, 118232. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Jin, Z.; Chen, F.; Zhong, P.; Zhang, L. Real-time strain monitoring of reinforced concrete under the attacks of sulphate and chloride ions based on XCT and DIC methods. Cem. Concr. Compos. 2022, 125, 104314. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Mu, S.; Šavija, B.; Liu, Q. Numerical investigation of external sulfate attack and its effect on chloride binding and diffusion in concrete. Constr. Build. Mater. 2021, 285, 122806. [Google Scholar] [CrossRef]
- Wang, P.; Mo, R.; Li, S.; Xu, J.; Jin, Z.; Zhao, T.; Wang, D. A chemo-damage-transport model for chloride ions diffusion in cement-based materials: Combined effects of sulfate attack and temperature. Constr. Build. Mater. 2021, 288, 123121. [Google Scholar] [CrossRef]
- Liao, K.; Zhang, Y.; Zhang, W.; Wang, Y.; Zhang, R. Modeling constitutive relationship of sulfate-attacked concrete. Constr. Build. Mater. 2020, 260, 119902. [Google Scholar] [CrossRef]
- Idiart, A.E.; López, C.M.; Carol, I. Chemo-mechanical analysis of concrete cracking and degradation due to external sulfate attack: A meso-scale model. Cem. Concr. Compos. 2011, 33, 411–423. [Google Scholar] [CrossRef]
- Bary, B. Simplified coupled chemo-mechanical modeling of cement pastes behavior subjected to combined leaching and external sulfate attack. Int. J. Numer. Anal. Methods Geomech. 2008, 32, 1791–1816. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, W.; Feng, Y.; Castel, A.; Chen, X.; Liu, A. On the competitive antagonism effect in combined chloride-sulfate attack: A numerical exploration. Cem. Concr. Res. 2021, 144, 106406. [Google Scholar] [CrossRef]
- SL/T 352-2020; Test Code for Hydraulic Concrete. China Water Conservancy and Hydropower Press: Beijing, China, 2020.
- Drimalas, T. Laboratory and Field Evaluations of External Sulfate Attack. Ph.D. Thesis, University of Texas at Austin, Austin, TX, USA, 2007. [Google Scholar]
- Zhang, M.; Chen, J.; Lv, Y.; Wang, D.; Ye, J. Study on the expansion of concrete under attack of sulfate and sulfate–chloride ions. Constr. Build. Mater. 2013, 39, 26–32. [Google Scholar] [CrossRef]
- ASTM C642-21; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C1012/C1012M-18b; Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution. ASTM International: West Conshohocken, PA, USA, 2018.
- Nakarai, K.; Ishida, T.; Maekawa, K. Modeling of calcium leaching from cement hydrates coupled with micro-pore formation. J. Adv. Concr. Technol. 2006, 4, 395–407. [Google Scholar] [CrossRef]
- Machner, A.; Bjørndal, M.H.; Justnes, H.; Hanžič, L.; Šajna, A.; Gu, Y.; Bary, B.; Ben Haha, M.; Geiker, M.R.; De Weerdt, K. Effect of leaching on the composition of hydration phases during chloride exposure of mortar. Cem. Concr. Res. 2022, 153, 106691. [Google Scholar] [CrossRef]
- Lee, S.T.; Swamy Ramnath, N.; Kim, S.S.; Park, Y.G. Durability of Mortars Made with Recycled Fine Aggregates Exposed to Sulfate Solutions. J. Mater. Civ. Eng. 2008, 20, 63–70. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, Y.; Liao, Y.; Chen, D. Study of the evolution of properties of mortar under sulfate attack at different concentrations. Adv. Cem. Res. 2016, 28, 617–629. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, L.; Chen, B.; Fei, X. Modeling early age hydration kinetics and the hydrated phase of cement paste blended with chloride and sulfate. Constr. Build. Mater. 2020, 261, 120537. [Google Scholar] [CrossRef]
- Cheng, S.; Shui, Z.; Sun, T.; Gao, X.; Guo, C. Effects of sulfate and magnesium ion on the chloride transportation behavior and binding capacity of Portland cement mortar. Constr. Build. Mater. 2019, 204, 265–275. [Google Scholar] [CrossRef]
- Jin, Z.; Sun, W.; Zhang, Y.; Jiang, J.; Lai, J. Interaction between sulfate and chloride solution attack of concretes with and without fly ash. Cem. Concr. Res. 2007, 37, 1223–1232. [Google Scholar]
- Wang, K.; Guo, J.; Yang, L. Effect of dry–wet ratio on sulfate transport-reaction mechanism in concrete. Constr. Build. Mater. 2021, 302, 124418. [Google Scholar] [CrossRef]
- Min, H.; Sui, L.; Xing, F.; Tian, H.; Zhou, Y. An effective transport model of sulfate attack in concrete. Constr. Build. Mater. 2019, 216, 365–378. [Google Scholar] [CrossRef]
- Tumidajski, P.J.; Chan, G.W.; Philipose, K.E. An effective diffusivity for sulfate transport into concrete. Cem. Concr. Res. 1995, 25, 1159–1163. [Google Scholar] [CrossRef]
- ASTM C1556-22; Standard Test Method for Determining the Apparent Chloride Diffusion Coefficient of Cementitious Mixtures by Bulk Diffusion. ASTM International: West Conshohocken, PA, USA, 2022.
- Garboczi, E.; Bentz, D. Computer simulation of the diffusivity of cement-based materials. J. Mater. Sci. 1992, 27, 2083–2092. [Google Scholar] [CrossRef]
- Chu, H.; Wang, T.; Han, L.; Cao, L.; Guo, M.; Liang, Y.; Jiang, L. Vickers hardness distribution and prediction model of cement pastes corroded by sulfate under the coexistence of electric field and chloride. Constr. Build. Mater. 2021, 309, 125119. [Google Scholar] [CrossRef]
- Klusemann, B.; Svendsen, B. Homogenization modeling of thin-layer-type microstructures. Int. J. Solids Struct. 2012, 49, 1828–1838. [Google Scholar] [CrossRef]
- Fischler, M.A.; Bolles, R.C. Random sample consensus: A paradigm for model fitting with applications to image analysis and automated cartography. Commun. ACM 1981, 24, 381–395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).