Abstract
Seasonal and long-term variability of phytoplankton in the Middle Caspian was studied based on remote sensing data of the sea by SeaWiFS and MODIS-Aqua scanners in 1998–2021 and the results of field observations in 2004–2021. The seasonal variability of chlorophyll “a” (CHL) calculated from satellite data using a regional algorithm indicated that the autumn and winter seasons were the main phytoplankton production periods of the year. In summer, a period of stagnation was observed in phytoplankton growth in the surface layer. However, according to satellite data in the first months of each year, winter blooms were observed recurrently in the Middle Caspian Sea, as confirmed by the results of field observations in 2004–2021. The phytoplankton biomass during the winter vegetation period reached 4.5–5.0 g/m3. In the first decade of the century (2004–2006), winter blooms were almost entirely (as much as 96%) formed through the mass growth of the alien diatom Cerataulina pelagica. In the modern period (2021), the winter bloom was formed both by three endemic diatom species Thalassionema nitzschioides, Cyclotella comta and Dactyliosolen fragilissimus and by two alien species Pseudo-nitzschia seriata and Cerataulina pelagica. In spring, the diatom Cyclotella caspia and the dinoflagellate Prorocentrum micans, both endemics, dominated. In summer, the phytoplankton biomass was composed of the mass growth of small flagellates and dinoflagellates in the seasonal thermocline layer, which current remote sensing methods cannot record. The diatoms’ contribution to the community’s total biomass in summer did not exceed 3%. In the autumn phytoplankton, the main role was played by a diatom component represented by alien species, mainly Chaetoceros peruvianus.
1. Introduction
The Caspian Sea is a highly productive basin with unique biological resources. In the first decades of the 21st century, structural deformations are noted at all trophic levels in the ecosystem of the Caspian Sea, which are caused by both global climate change and anthropogenic factors [1,2,3,4,5,6,7]. As the primary production link, phytoplankton determines the functioning of higher trophic levels and is the most sensitive and vulnerable component of the marine ecosystem. In recent decades, the anthropogenic impact associated with expanding hydrocarbon resource exploitation has increased [8]. In addition, biological pollution by invasive alien organisms plays a no less important role in transforming the Caspian ecosystem [9,10,11,12,13,14,15,16,17,18,19,20,21]. The functioning of the ecosystem of the Caspian Sea is largely determined by the river flow regime, the volume and the chemical composition of river runoff affecting the primary production link of the marine ecosystem by determining the structure and functional characteristics of phytoplankton. This influence is especially pronounced in the Northern Caspian Sea and the western part of the Middle Caspian Sea [22,23,24,25,26]. Phytoplankton research in the Caspian Sea has a long history. In the period from 1955 to 2000, regularities in the formation of the structure of Caspian phytoplankton were studied, dominant forms were identified, the level of productivity of communities was determined, and the role of invasive species and of potentially toxic algae was determined [27,28,29,30,31,32,33,34,35,36,37,38].
From 2000, phytoplankton has been one of the least studied components of the Caspian ecosystem. Studies of the seasonal dynamic of phytoplankton in recent decades are extremely few and insufficiently supported by field observations. Modern experience shows that the use of remote-sensing chlorophyll “a” (CHL) data can fill significant gaps in the study of the dynamics of marine phytoplankton and makes it possible to link the results of local observations to the regional and general ecological situation in the sea [39,40,41,42]. The reliable interpretation of CHL satellite data is possible, provided that the results of remote sensing are verified, and synchronous field observations of the state of phytoplankton are undertaken. However, with rare exceptions [43,44], the remote sensing of CHL in the Caspian Sea [45,46,47,48] has been conducted without in situ data validation. One of the previous studies has pointed to a progressive eutrophication of the Caspian Sea [46]. Remote studies based on regional algorithms for the calculation of chlorophyll concentrations only make it possible to reveal generalized patterns of spatial and seasonal development of phytoplankton across the entire area of the sea [49]. Moreover, to date, the dynamics of production processes in the Caspian Sea, as extrapolated from satellite data on the chlorophyll variability, have not been compared with the quantitative and structural characteristics of the Caspian phytoplankton. The current work is one of the first steps towards solving this problem by analyzing the seasonal dynamic of phytoplankton in the Middle Caspian Sea by integrating satellite data and field observations.
Addressing these tasks clarifies the scope of satellite data for analyzing phytoplankton variability. Furthermore, it creates a scientific basis for developing approaches to the remote assessment of the quantitative characteristics of phytoplankton and the identification of mass algae species that form the structure of communities in crucial periods of seasonal succession.
2. Materials and Methods
2.1. Satellite Data
In order to assess the variability of phytoplankton according to satellite data and identify the main production periods in the development of phytoplankton of the Middle Caspian Sea, data from the second-level color scanners SeaWiFS (1998–2002) and MODIS-Aqua (2002–2021) were used [50] (https://oceancolor.gsfc.nasa.gov) (accessed on 20 December 2022).
The initial bio-optical characteristics, used for calculations, had a discreteness of one day and a 1 km spatial resolution. Therefore, a bank of bio-optical characteristics of the sea surface was created for 1998–2021. To estimate the concentration of chlorophyll “a” in the waters of the Caspian Sea, a regional algorithm developed at the IO RAS [48] was used. This regional algorithm was verified based on data from previous and synchronous expeditionary studies. The averaged data allowed the identification of the main and most stable patterns of seasonal variability of phytoplankton in the Caspian Sea.
For the solution of the study tasks, phytoplankton samples were analyzed, which were collected at different seasons of the year during the research periods of 2004–2008 and 2019–2022 at 25 stations in the deep-water areas of the Middle Caspian Sea (Figure 1).
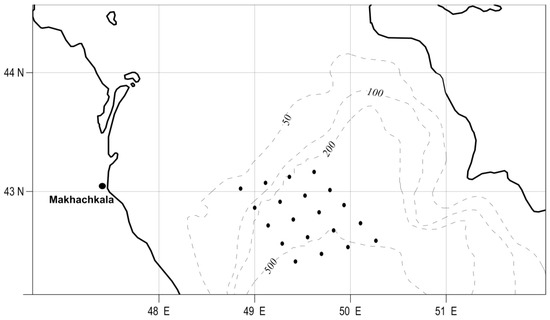
Figure 1.
Stations of studies of phytoplankton in the Middle Caspian Sea.
2.2. Sampling
Water samples for phytoplankton analysis were collected using 5 L Niskin bottles mounted on a carousel water sampler (SBE 32). Samples were taken from the surface, the middle of the upper mixed layer, the seasonal thermocline, and below the thermocline. Phytoplankton samples (0.5 l volume) were fixed with neutralized formaldehyde (final concentration 1.0%). A lower final concentration of formaldehyde was used for a better preservation of cells, including flagellates, while considering their processing within one month from the moment of sampling. The samples were stored in the dark, at room temperature, for two weeks. After that, the samples were slowly decanted. An Ergoval light microscope (Karl Zeiss, Jena, Germany) with a magnification of 160× and 400× was used to identify species and cell counting. Nano-phytoplankton: cells with linear dimensions <20 μm were counted using a Naujott chamber (0.05 mL). The counts were considered statistically sufficient if at least 100 cells of each species were counted. A Nauman chamber (1 mL) was used to count larger cells; usually, the entire chamber volume was counted. In matters of nomenclature [51,52], WoRMS (http://www.marinespecies.org) (accessed on 5 October 2022) served as a guide. Cells with an unknown taxonomic affiliation ranging in size from 4 to 10 µm were classified as “small flagellates”, assuming that this was a size class. Biomass was determined by the “true volume” method, equating the cell configuration to a geometric figure [53]. The total biomass was calculated as the sum of the biomass of all species. The dominant species was the one whose biomass was the highest at the station. When converting raw biomass units into carbon units, formulas for a specific systematic group were used [54] (http://www.marinespecies.org) (accessed on 5 October 2022). A total of 300 phytoplankton samples were processed and analyzed.
3. Results
3.1. Remote Research
Maps of average monthly CHL values were constructed based on color scanner data and diagrams of chlorophyll variability in the Middle Caspian Sea for the entire period of the remote observations. The average climatic distribution of chlorophyll concentrations in the sea by months and diagrams of seasonal variability of chlorophyll in the Middle Caspian are shown in Figure 2 and Figure 3.
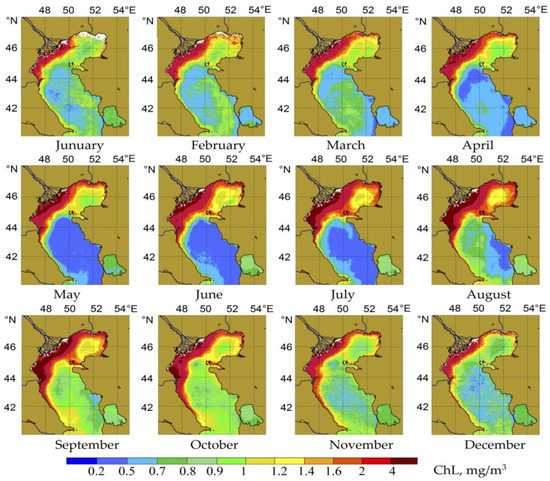
Figure 2.
“Average climatic” distribution of chlorophyll in the Caspian Sea in 1998–2021.
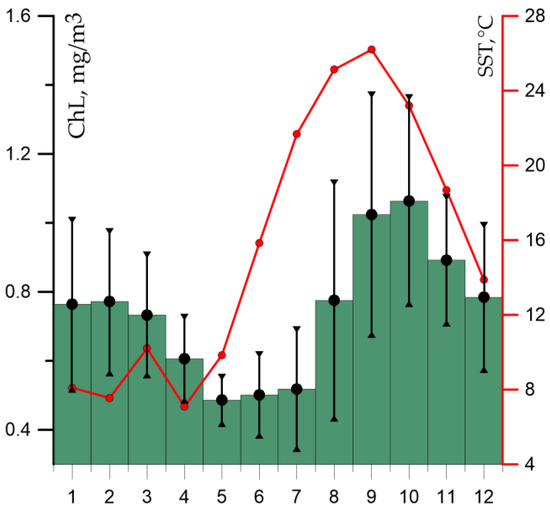
Figure 3.
Average monthly values of chlorophyll “a” concentration (mg/m3 green columns), standard deviation (vertical black lines) and temperature in °C (red line) in the Middle Caspian (average values for the period from 1998 to 2021).
Considered in its entirety, the autumn season was the most productive for phytoplankton in the Middle Caspian Sea (Figure 3 and Figure 4). The chlorophyll content varied in the deep part of the Middle Caspian between 0.25 and 1.0 mg/m3, and rarely exceeded 2.0 mg/m3. According to satellite data, the values of chlorophyll concentrations increased in open waters from August to October and peaked during the weakening insolation period and the beginning of active wind mixing. As to its spatial aspect, the zone of active development of phytoplankton in the waters of the Middle Caspian extended from west to east from summer to autumn (see Figure 2). According to remote sensing data, a winter period of active phytoplankton growth was also noticeable (see Figure 2 and Figure 3). A detailed analysis of satellite data further showed that winter blooms of phytoplankton repeatedly occurred in the Middle Caspian over twenty years, as reflected in maps of the average climatic variability of CHL (see Figure 2). It should be noted that winter blooms in the Middle Caspian Sea were irregular and had a localized, patchy character. Nevertheless, in terms of CHL concentrations >1.5/m3 in these patches, some of the winter-spring blooms, for example, those of 2004, 2012, and 2021 (Figure 4), were comparable to the autumn peaks of phytoplankton development.
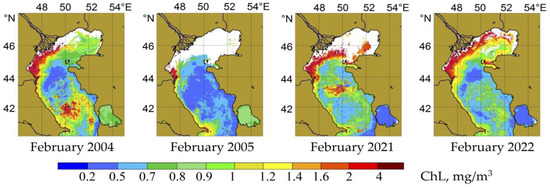
Figure 4.
Chlorophyll “a” concentrations (mg/m3) in the surface layer of the Caspian Sea during the winter blooms of phytoplankton in 2004, 2021, and the immediately succeeding years.
A characteristic feature of the annual succession of phytoplankton in the surface layer of the Middle Caspian, especially in the deep part of this subregion, was a period of summer stagnation, which lasted from May to July (see Figure 2 and Figure 3). According to remote sensing data, minimal concentrations of chlorophyll were observed in the surface layer of the sea in the summer season.
The western part of the Middle Caspian, especially its coastal zone, was characterized throughout the year by a higher productivity and higher chlorophyll concentrations compared to the deep part of the waters. This was most pronounced from August to November when a band of high CHL concentrations (1.0–2.0 mg/m3) formed along the entire west coast from north to south and reached the South Caspian Sea.
According to satellite data, at the same time, in the eastern part of the Middle Caspian, the formation of a band of anomalously low CHL concentrations was recorded (see Figure 2). Geographically, this coincided with the zone of seasonal upwelling, which is formed annually along the eastern coast of the Caspian Sea. The intensity of upwelling could be judged by a drop in the temperature of the surface layer by more than 10 degrees during the period of its most significant growth (May–July). This hydrological situation negatively affects the development of phytoplankton in the surface layer in the east of the Middle Caspian. As a result, in some years, the band of low CHL concentrations (0.2–0.4 mg/m3) significantly expanded from the east to the west of the Middle Caspian. It covered a significant part of the subregion.
Based on field observations, the quantitative and structural characteristics of phytoplankton corresponding to the main stages of its seasonal succession were further studied. In addition, based on the available field data, attention was also paid to the factor of long-term variability of the phytoplankton structure during distinct periods of seasonal succession.
3.2. Phytoplankton Community Structure in Winter
The structural and taxonomic characteristics of phytoplankton during the winter were studied based on field observations conducted in February 2004 and 2021 (Table 1, Table 2 and Table 3). Figure 4 shows the chlorophyll distribution in the Caspian Sea’s water area in the winter season corresponding to the dates of field observations and the state of phytoplankton. Data from neighboring years are also presented, illustrating the interannual variability in the development of phytoplankton in winter.

Table 1.
Taxonomic composition of phytoplankton (N—number of species; % of the total number).

Table 2.
Dominant species of phytoplankton.

Table 3.
Maximum total biomass of phytoplankton (B mg/m3), biomass of the main systematic and dimensional groups (B mg/m3), contribution to the total biomass (%), and the depth of the maximum biomass (H max).
During the cold period of the year, the development of the phytoplankton community in the Caspian Sea occurred under the conditions of temperature convection and an active enrichment of the surface layer with nutrients. Over twenty years, winter phytoplankton blooms were repeatedly observed in the Middle Caspian through satellite data (see Figure 4). Their role in the seasonal cycle of phytoplankton growth was also reflected in the average climatic maps of chlorophyll distribution (see Figure 2). However, in about half the cases, the winter development of phytoplankton was weakly expressed (see Figure 4). Field observations showed that the phytoplankton biomass in a winter bloom patch could reach 4.8–5.0 g/m3 (Table 3) and exceed the corresponding values for the autumn production period. The mass growth of diatom algae during winter blooming accounted for more than 50% of the community’s biomass. In February 2021, the bloom was formed both by the endemic marine species Thalassionema nitschioides, Cyclotella comta, and Dactyliosolen fragilissimus and by the alien species Pseudo-niztschia seriata and Cerataulina pelagica. The large centric diatom Coscinodiscus perforatus was a subdominant species (Table 1 and Table 2).
In winter, phytoplankton blooms occupied the water layer from the surface to a depth of 50 m, sometimes extending to 100 m. Representatives of dinoflagellates and green and blue-green algae were only observed in small numbers in the surface water layer. A more significant abundance of small flagellates of undetermined taxonomy was noted at the lower boundary of the mixed layer. In February 2004, the winter bloom in the Middle Caspian Sea was formed exclusively by the alien species Cerataulina pelagica and occupied the entire upper 50-metre water layer (Table 2 and Table 3). An analysis of the species structure of this phytoplankton indicated that the winter period was characterized by the most remarkable species diversity of the community, primarily constituted by diatoms (Table 3).
3.3. Phytoplankton and Community Structure in the Early Summer
According to satellite data, the development of phytoplankton throughout the year was characterized by a summer period of stagnation (Figure 5), which began in May and continued until July (see Figure 1 and Figure 2). The level of chlorophyll concentrations and the nature of its distribution in the waters of the Middle Caspian during the summer stagnation period had characteristic features. The surface layer became biologically impoverished during these months, and pigment concentrations decreased to 0.2–0.3 mg/m3. Field observation material of the development of phytoplankton for the early summer period is presented here with data collected in May 2004 and May 2021.
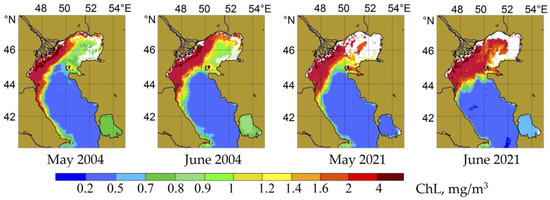
Figure 5.
Distribution of chlorophyll “a” in the Caspian Sea during the summer stagnation period in the phytoplankton growth.
In May 2004, the structure of phytoplankton in the study area was determined by the formation of a seasonal thermocline that prevented the vertical mixing of waters and the replenishment of the surface layer with biogenic elements. The endemic small-celled diatom Cyclotella caspia, which forms winter-spring blooms, settled in May–June in the lower layers (45–50 m), where the maximum abundance of this species was observed (1.6 × 104 cells/L. Diatoms and dinoflagellates dominated in terms of the number of species in the community (see Table 1). In May 2004, the dominant phytoplankton species were diatoms, the alien Pseudo-nitzschia seriata and Cerataulina pelagica, which formed the winter-spring diatom bloom. In addition, the large diatom (4.0 × 104 cells/L) was among the biomass dominants. Most of these algae were recorded in the layer of the seasonal thermocline at a depth of 25 m. Dinoflagellates (4.0 × 105 cells/L) predominated in the surface water layer, primarily the endemic species Prorocentrum cordatum (see Table 2). The high abundance of this species, at 50%, constituted the maximum phytoplankton biomass (Table 3).
At the beginning of the 2021 summer season, a later stage of phytoplankton succession was noted in the surface water layer with the dominance of Diplopsalis lenticula and the massive development of the large diatoms Coscinodiscus perforatus and Cyclotella comta (Table 2). Diatoms and dinoflagellates predominated, regarding the number of species in the community (see Table 1). In May 2021, the maximum biomass level was recorded in the surface water layer and was formed by dinoflagellates (Table 3). The maximum phytoplankton biomass was two times less than in the corresponding period of 2004. Studies conducted in May 2021 also indicated the dominance of two endemic marine forms in early summer phytoplankton, the diatom Cyclotella caspia and the dinoflagellate Prorocetrum micans. The maximum abundance of C. caspia (5.0 × 104 cells/L), as in 2021, was recorded at depths of 35–40 m.
3.4. Phytoplankton and Community Structure in the Late Summer
According to satellite data, the summer–autumn period of phytoplankton growth began in August. It was reflected in the chlorophyll’s distribution in the sea’s surface layer (Figure 6). The increase in CHL concentrations gradually spread from the western part of the Middle Caspian Sea to its open areas. The timing and intensity of the summer–autumn growth of phytoplankton differed from year to year (see Figure 3).
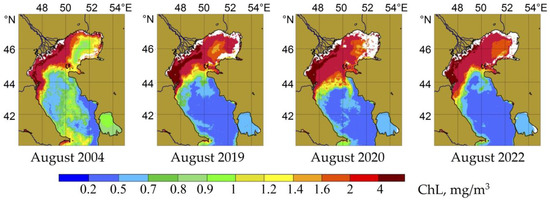
Figure 6.
Monthly averaged distribution of chlorophyll “a” in the Caspian Sea during the summer–autumn period of phytoplankton growth.
In August 2004, dinoflagellates and, above all, Gonyaulax spinifera, were the principal phytoplankton components. The maximum abundance of G. spinifera was noted near the water surface, where the species created up to 65% of the total biomass. The maximum phytoplankton biomass was noted in the seasonal thermocline layer (2.2 g/m3, Table 3) and was formed by small flagellates and dinoflagellates. Blue-green algae in phytoplankton developed locally, constituted from a single species, Microcystis aeruginosa. In the lower horizons, below the thermocline, diatoms were found to a depth of 75 m; first of all, the alien species Chaetoceros peruvianus (Table 2). Moreover, the contribution of diatoms to the total biomass was minimal and did not exceed 3% (Table 3).
At present (2020–2022), in the northwestern part of the Middle Caspian, the leading role in forming the structure of summer phytoplankton belongs to the large dinoflagellate Gonyaulax polygramma (Table 2). This species’ abundance and biomass maxima (up to 4.8 × 104 cells/L; 2.3 g/m3) were recorded at a depth of 30 m. However, in the surface layer, the community’s biomass was low. It was determined by small flagellates, the green algae Binuclearia lauterbornii, and diatoms, including P. calcar-avis (Table 2 and Table 3).
3.5. Phytoplankton and Community Structure in Autumn
According to averaged satellite data, the autumn season was the most productive period for the Middle Caspian Sea. However, the intensity and timing of the autumn growth of phytoplankton varied from year to year. In general, October 2004 and 2005 were characterized by higher phytoplankton production rates than the period 2019–2021 (Figure 7).
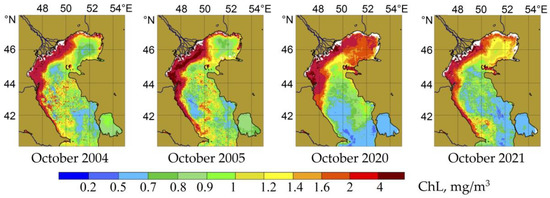
Figure 7.
The chlorophyll “a” concentration (mg/m3) in the surface layer of the Caspian Sea during the autumn production period.
According to the data obtained at the end of October in 2004 and 2005, the phytoplankton biomass in the Middle Caspian reached 1 g/m3 or more. During these periods, it was based on diatoms (Table 2). Among them were observed the two endemics Cyclotella caspia (up to 2.7 × 105 cells/L) and Cylindrotheca closterium (1.0 × 105 cells/L), as well as the alien species Chaetoceros peruvianus (1.7 × 105 cells/L). These species dominated the upper 25 m water layer. In deeper horizons, the role of small flagellates and, in some cases, dinoflagellates, was significant. At the same time, the species diversity of diatoms and dinoflagellates continued to be relatively high (see Table 1). Compared to 2020, dinoflagellates’ role in forming the total biomass in October 2004 and 2005 was small (Table 3). Blue greens were practically absent in phytoplankton composition in autumn 2004 and 2005.
Recent data on the quantitative characteristics of phytoplankton were obtained at the beginning of October 2020, when the level of quantitative development of phytoplankton did not exceed 300 mg/m3, which was consistent with satellite data. The leading role in forming the biomass of the surface water layer was played by blue-green algae, primarily Lyngbya limnetica, small flagellates, and the dinoflagellate Prorocentrum micans. At the same time, in the seasonal thermocline layer, the role of the diatom community component increased, represented by Thalassionema nitzschioides and Cyclotella comta, both endemic to the Caspian, and the alien species Chaetoceros peruvianus (Table 2 and Table 3). The data from the beginning of October 2020 reflected the early stage of the community’s transition from summer to autumn. A community restructuring from summer to autumn occurred in the study area at the end of October, as was observed in autumn 2004 and 2005. It was associated with increased wind mixing and the influx of nutrients into the upper producing layer. The growth of biomass to the blooming stage in autumn occurred due to the diatom component. A specific role in the different states of phytoplankton in October 2004, 2005, and 2020 could also be played by an interannual variability in the seasonal development of phytoplankton, as confirmed by satellite data (see Figure 4).
4. Discussion
Based on the analysis of averaged data on chlorophyll dynamics, at least three primary and two transitional periods of phytoplankton development in the Middle Caspian Sea could be distinguished: winter (December–March), spring (April), summer (May–July), late summer (August), and autumn (October–November). The actual calendar terms of the periods selected may vary from year to year within the framework of the long-term variability of the hydrometeorological characteristics of the marine environment, which determine the succession of phytoplankton. During transitional periods, the hydrological structure of the waters is restructured, which, in turn, determines the availability of biogenic elements for phytoplankton.
The results indicated that in the study area, phytoplankton developed most actively in autumn and winter, with summer stagnating in phytoplankton development. Satellite data also showed that winter blooms were often patchy. The supply of nutrients from the lower layers of water to the upper producing layer due to active wind mixing and winter convection are the main mechanisms that ensure a high level of quantitative development of the community in the Middle Caspian Sea.
The growth of the exclusive diatom component of the community ensured a high biomass level in these seasons. An interesting fact is that, while in 2008, the winter bloom was formed by the alien species Cerataulina pelagica, in 2021, the role of endemic species increased: this could be associated with changes in environmental conditions and, possibly, salinity. Since all alien species were representatives of the Mediterranean–Black Sea flora, it could be assumed that their mass development in the Caspian Sea would be facilitated by an increase in salinity and, conversely, the development of endemic species may be associated with its decrease. Thus, in addition to the spring and autumn maxima of the abundance and biomass of phytoplankton characteristic of the boreal seas, which were formed due to diatoms, a significant winter maximum was established for the Middle Caspian. The maximum biomass level in the winter season exceeded the corresponding values in autumn and was also formed due to the diatom component. Above the northern slope of the Derbent Basin and in the area of the ancient riverbed of the Volga River, this maximum was formed by a single species. This centric diatom, Cerataulina pelagica, usually determines the development of autumn blooms in this sea area.
In the summer seasons of 2020 and 2022, the species diversity of diatoms and dinoflagellates remained at the same level. From spring to summer, the number of blue-green and green algae species slightly increased. In the summer period of 2022, as compared to the summer of 2004, there was a sharp decrease in the species diversity of the community due to a decrease in the number of species of diatoms and dinoflagellates (Table 1). At the same time, the total biomass of the community remained at a similar level due to the massive development of dinoflagellates. Their contribution to the total biomass has increased from 65% to 98% since 2004 (Table 3). In 2004, at this stage of seasonal succession, the species diversity of phytoplankton was approximately two times higher than in 2020–2022 (see Table 1). At the end of October, with active wind mixing and temperature convection, biogenic elements began to enter the upper producing layer again, initiating the autumn blooming of diatoms. It should be noted that in 2004, one of the dominant species of autumn blooms was the alien species Chaetoceros peruvianus, while in 2020, small flagellates dominated. At the same time, the level of quantitative development of C. peruvianus was an order of magnitude lower and did not exceed the abundance of each of the subdominant species, Thalassionema nitzschioides and Cyclotella comta, species endemic to the Caspian Sea. During our research in the spring, autumn, and summer periods of 2019–2021, a decrease in the species diversity of algae and a decrease in the maximum total phytoplankton biomass in spring and autumn were recorded compared to 2004 (see Table 1 and Table 3). Different stages of the succession cycle could also explain these differences (before “blooming”, during “blooming”, after “blooming”). Like other authors [17,18], we determined a sharp decrease in the abundance of the main Caspian species, the large diatom Pseudosolenia calcar-avis, which formed the principal component of the phytoplankton biomass until the 2000s. According to our observations, from 2004 to 2021, the maximum abundance of that species in the study area decreased by about an order of magnitude. In addition, over the same period, the abundance of Chaetoceros peruvianus, the main dominant of autumn phytoplankton bloom in the Middle Caspian Sea in previous years, sharply decreased. A similar species structure and composition of the leading species complex in the spring–summer period were also given by other authors [17,20,55]. Our data confirmed the sharp decrease in the number of the main dominant species observed in recent years.
The analysis of field observation data indicated that the highly productive phytoplankton status of the study area was fully confirmed for the winter and autumn periods. We showed for the first time that in winter, in the deep part of the Middle Caspian Sea, diatom blooms occurred near the northern and western periphery of the Derbent Basin, as confirmed by satellite data. In the satellite images, the distribution of chlorophyll in the studied water area of the Middle Caspian adequately reflected the situation observed in situ. Alien species continued to participate in the formation of the leading complex of species in the phytoplankton of the Middle Caspian.
However, over the past decades, their role in forming the structure of spring and autumn phytoplankton has decreased against the background of a simultaneous increase in the abundance of large dinoflagellates of the genera Diplopsalis and Gonyaulax, which could result from an increase in the proportion of dissolved organic matter in the sea area studied. In other seasons of the year, the structure of phytoplankton was determined by the presence of a seasonal thermocline and a deficiency of nutrients in the surface layer. In May, with the establishment of density stratification, the spring blooming of diatoms ceased, as confirmed by satellite data. Its remains, which formed the first stage of succession, settled into the lower horizons, concentrating on the thermocline. During that period, mixotrophic dinoflagellates belonging to the mass Caspian forms (Prorocentrum cordatum, Proprocentrum micans and Diplopsalis lenticula) and large-celled diatoms from the genus Coscinidiscus (the second stage of succession) developed in the upper producing layer, impoverished in terms of biogenic elements. A similar species structure and composition of the leading species complex in the summer period were also reported by other authors [54]. This situation usually continued until August. In some years, the hydrological conditions that determined the summer state of the phytoplankton could persist until early October, as was noted in the earlier work of the authors, who demonstrated the critical role of the large dinoflagellate Gonyaulax spinifera in the formation of summer phytoplankton of the surface layer of the Middle Caspian Sea [35].
The annual formation of a band of low concentrations of chlorophyll and phytoplankton along the eastern coast of the subregion in the zone of seasonal upwelling is of interest. The reasons for this anomalous phenomenon have yet to be fully elucidated and require additional hydrobiological and hydrochemical studies. Obviously, in the zone of intense eastern upwelling, the waters of the surface layer are replaced by cold deep waters depleted in phytoplankton. A high turbulence in areas of water rise can prevent cells from fixing in the optimum light zone. The abnormally low temperature of rising deep waters also limits the growth of that phytoplankton adapted to summer conditions. The chemical composition of rising deep waters is practically unknown. In addition, phytoplankton in the area of upwelling can develop in deep layers in the thermocline zone and not be detected by remote sensing methods, as evidenced by single observations made during the upwelling relaxation period [20]. No less interesting are the little-studied upwelling phenomena that manifested in summer in the western part of the Middle Caspian [56,57]. Unlike the eastern part of the sea, western upwelling in the summer of 2017 caused phytoplankton blooming, which was recorded by satellite observations [58].
5. Conclusions
This work represents the first instance of studying the structure of the Caspian Sea phytoplankton community using long-term remote sensing data in the periods identified. Based on the analysis of these data, it was shown that the autumn and winter seasons were the main production periods of the year. According to satellite data, winter blooms were recurrently observed in the Middle Caspian. The evidence of winter blooms was confirmed by the results of field observations in 2004–2021. Phytoplankton biomass during the winter vegetation period at the beginning of the 2000s (2006) reached 4.5–5.0 g/m3. It was almost completely (up to 96%) engendered by the mass development of the alien diatom species Cerataulina pelagica. In the current period (2021), the winter bloom was formed by the endemic diatom species Thalassionema nitzschioides, Cyclotella comta, and Dactyliosolen fragilissimus, and by the alien species Pseudo-nitzschia seriata and Cerataulina pelagica. In spring, the diatom Cyclotella caspia and the dinoflagellate Prorocentrum micans, endemic to the Caspian Sea, dominated. In summer, a period of stagnation was observed in the development of the phytoplankton community in the surface layer. In summer, the phytoplankton biomass was engendered by the mass development of small flagellates and dinoflagellates in the seasonal thermocline layer, which remote methods cannot currently record. The diatoms’ contribution to the community’s total biomass in summer did not exceed 3%. In the autumn phytoplankton, the leading role was played by the diatom component represented mainly by alien species, Chaetoceros peruvianus. Autumn blooms, as a rule, covered the entire water area of the Middle Caspian, while winter blooms often had a patchy character.
The results obtained deepen the understanding of the dynamics of production processes in the Caspian Sea and are of both fundamental and applied importance. In addition, they clarify the scope of remote sensing methods for studying marine phytoplankton and create a scientific basis for a reliable interpretation of satellite monitoring data and the development of new approaches to the remote identification of dominants, including toxic, algal species that form the structure of the phytoplankton community in crucial periods of seasonal succession.
Author Contributions
S.V.V.: Conceptualization, investigation, writing—original draft preparation, supervision; L.A.P.: methodology, formal analysis, writing—original draft preparation; I.V.S.: resources software, validation, visualization; A.S.V.: data curation, visualization, writing—review and editing; A.A.G.: project administration, writing—review and editing; G.P.: writing—review and editing; E.N.L., investigation, data curation; B.A.: supervision, writing—review and editing; M.G.S.: funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by RFBR according to the research project no. 20-54-56053, State Assignment, project no. 0128-2021-0004 and also by Iran National Science Foundation (INSF), project number no. 99003103. Analyses of the phytoplankton structure were supported by the Russian Science Foundation project no. 22-17-00066.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data on phytoplankton is unavailable due to privacy and ethical restrictions. Remote sensed Data: NASA Ocean Color. Available online: https://oceancolor.gsfc.nasa.gov (accessed on 20 December 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ginzburg, A.I.; Kostianoy, A.G.; Serykh, I.V.; Lebedev, S.A. Climatic Changes in Hydrometeorological Parameters of the Caspian Sea (1980–2020). Sovrem. Probl. Distantsionnogo Zondirovaniya Zemli Iz Kosm. 2021, 18, 277–291. (In Russian) [Google Scholar] [CrossRef]
- Kosarev, A.N. Physico-Geographical Conditions of the Caspian Sea. Handb. Environ. Chem. 2005, 5, 5–31. [Google Scholar] [CrossRef]
- Ambrosimov, A.K.; Lukashin, V.N.; Burenkov, V.I.; Kravchishina, M.D.; Libina, N.V.; Mutovkin, A.D. Comprehensive studies of the Caspian Sea system in the 32nd voyage of the research vessel Rift. Oceanology 2011, 51, 751–757. (In Russian) [Google Scholar] [CrossRef]
- Panin, G.N.; Solomonova, I.V.; Vyruchalkina, T.Y. Regime of Water Balance Components of the Caspian Sea. Water Resour. 2014, 41, 505–511. [Google Scholar] [CrossRef]
- Chen, J.L.; Pekker, T.; Wilson, C.R.; Tapley, B.D.; Kostianoy, A.G.; Cretaux, J.F.; Safarov, E.S. Long-Term Caspian Sea Level Change. Geophys. Res. Lett. 2017, 44, 6993–7001. [Google Scholar] [CrossRef]
- Kostianoy, A.G.; Ginzburg, A.I.; Lavrova, O.Y.; Lebedev, S.A.; Mityagina, M.I.; Sheremet, N.A.; Soloviev, D.M. Comprehensive Satellite Monitoring of Caspian Sea Conditions. Remote Sens. Asian Seas 2018, 505–521. [Google Scholar] [CrossRef]
- Sapozhnikov, V.V.; Mordasova, N.V.; Metreveli, M.P. Transformations in the Caspian Sea Ecosystem under the Fall and Rise of the Sea Level. Oceanology 2010, 50, 488–497. [Google Scholar] [CrossRef]
- Ivanov, A.Y.; Vostokov, S.V.; Ermoshkin, I.S. Mapping of Oil Films in The Caspian Sea Using Space borne Synthetic Aperture Radar Imagery (on the example of the Caspian Sea). Oceanology 2004, 4, 82–92. (In Russian) [Google Scholar]
- Tatarintseva, T.A.; Ardabyeva, O.V.; Terletskaya, D.H.; Titenkova, L.V.; Malinovskaya, L.I.; Tarasova, E.L.; Petrenko, E.L. Mediterranean invaders in plankton and benthic fauna of the Caspian Sea. Sat. scientific Proceedings "Invader Species in the European Seas of Russia" RAS. Apatity 2000, 169–183. (In Russian) [Google Scholar]
- Ivanov, V.P.; Kamakin, A.M.; Ushivtzev, V.B.; Shiganova, T.A.; Zhukova, O.; Aladin, N.; Susan, I.; Wilson, S.I.; Harbison, G.R.; Dumont, H.J. Simultaneous invasion of the Caspian Sea by two jellies Mnemiopsis and Aurelia. J. Biol. Invasions 2000, 2, 255–258. (In Russian) [Google Scholar] [CrossRef]
- Vostokov, S.V.; Ushivtsev, V.B.; Lisitsyn, B.E.; Soloviev, D.M. The summer state of the ctenophore Mnemiopsis leidyi population in the Caspian Sea and its relationship with habitat conditions. Oceanology 2004, 44, 101–109. (In Russian) [Google Scholar]
- Vostokov, S.V.; Gadzhiev, A.A.; Vostokova, A.S.; Rabazanov, N.I. The Ctenophore Beroe Cf. Ovata in the Caspian Sea. The Beginning of a New Stage in the Evolution of the Caspian Ecosystem? South Russ. Ecol. Dev. 2021, 15, 21–35. [Google Scholar] [CrossRef]
- Pautova, L.A.; Kravchishina, M.D.; Silkin, V.A.; Lisitzin, A.P. The Phenomenon of Bloom Development of the Invasive Potentially Toxic Dinoflagellate Gonyaulax Polygramma in Deep Water Areas of the Caspian Sea. Dokl. Earth Sci. 2017, 474, 657–661. [Google Scholar] [CrossRef]
- Kamakin, A.M.; Chizenkova, O.A.; Zaitzev, V.F. Mnemiopsis Leidyi Impact on Some Trophical Chains of the Caspian Sea. South Russ. Ecol. Dev. 2015, 5, 67–74. (In Russian) [Google Scholar] [CrossRef]
- Vostokov, S.V.; Vostokova, A.S.; Lobachev, E.N.; Reza, R.H.; Behrooz, A.; Tumalaeva, O.M.; Rabazanov, N.I. New Data on the Ctenophore Beroe Ovata, an Invader to the Caspian Sea. South Russ. Ecol. Dev. 2022, 16, 18–26. (In Russian) [Google Scholar] [CrossRef]
- Pautova, L.A.; Kravchishina, M.D.; Vostokov, S.V.; Zernova, V.V.; Silkin, V.A. Features of the Vertical Phytoplankton Structure in the Deep-Sea Parts of the Caspian Sea in Summer. Dokl. Earth Sci. 2015, 462, 604–608. [Google Scholar] [CrossRef]
- Karpinsky, M.G. On Peculiarities of Introduction of Marine Species into the Caspian Sea. Russ. J. Biol. Invasions 2010, 1, 7–10. [Google Scholar] [CrossRef]
- Karpinsky, M.G. Pseudosolenia Calcar-Avis (Bacillariophyta, Centrophyceae) in the Caspian Sea. Russ. J. Biol. Invasions 2010, 1, 81–86. [Google Scholar] [CrossRef]
- Zarbalieva, T.S.; Akhundov, M.M.; Kasimov, A.M.; Nadirov, S.N.; Huseynova, G.G. The impact of invasive species on the native fauna of the Caspian Sea in the coastal waters of Azerbaijan. Russ. J. Biol. Invasions 2016, 2, 33–48. (In Russian) [Google Scholar]
- Vostokov, S.V.; Gadzhiev, A.A.; Lobachev, E.N.; Vostokova, A.S.; Rabazanov, N.I.; Barkhalov, R.M.; Sapojnikov, P.V.; Abtahi, B.; Shojaei, M.G. Development and Interaction of Ctenophores Beroe Ovata Bruguière, 1789 and Mnemiopsis Leidyi A. Agassiz, 1865 in the Coastal Zone of the Middle Caspian Sea. South Russ. Ecol. Dev. 2022, 17, 8–15. [Google Scholar] [CrossRef]
- Pautova, L.A.; Silkin, V.A.; Kravchishina, M.D.; Vostokov, S.V. The domination of invasive species in the present phytoplankton of the Caspian Sea. In Proceedings of the 42 Congress CIESM, Cascais, Portugal, 7–11 October 2019; pp. 37–39. (In Russian). [Google Scholar]
- Makarova, I.V. Diatoms of the plankton of the Middle and Southern Caspian. Bot. J. 1957, 42, 54–58. (In Russian) [Google Scholar]
- Kuhn, M.S. Plankton of the Caspian Sea in conditions of overregulation of the Volga runoff. In Changing Biological Complexes of the Caspian Sea in Recent Decades; 1965; pp. 54–97. [Google Scholar]
- Proshkina-Lavrenko, A.I.; Makarova, I.V. Algae of the plankton of the Caspian Sea; 1968; p. 291. (In Russian) [Google Scholar]
- Babaev, G.B. Composition and distribution of phytoplankton in the Middle and Southern Caspian. In Biology of the Middle and Southern Caspian; 1968; pp. 50–63. [Google Scholar]
- Kiselev, I.A. Plankton of Seas and Continental Reservoirs; 1969; p. 657. (In Russian) [Google Scholar]
- Barsukova, L.A. Long-term biogenic runoff of the Volga River near Astrakhan. Work. Kaspnirkh 1971, 26, 42–53. (In Russian) [Google Scholar]
- Levshakova, V.D. Some ecological features of the phytoplankton of the Northern Caspian. Proc. KaspNIRKh 1971, 26, 67–82. (In Russian) [Google Scholar]
- Levshakova, V.D.; Sanina, L.V. Summer phytoplankton of the Middle Caspian Sea before and after the introduction of rice salting. Proc. VNIRO 1973, 80, 18–27. (In Russian) [Google Scholar]
- Biological Productivity of the Caspian Sea; Nauka Publishers: Moscow, Russia, 1974; p. 875. (In Russian)
- Levshakova, V.D. Phytoplankton. In The Caspian Sea. Fauna and Biological Productivity; Nauka Publishers: Moscow, Russia, 1985; pp. 23–54. (In Russian) [Google Scholar]
- Levshakova, V.D.; Ardabyeva, A.G.; Tatarintseva, T.A. Phytoplankton and primary production of plankton // Fauna and biological productivity. In The Caspian Sea; Nauka Publishers: Moscow, Russia, 1985; pp. 5–54. (In Russian) [Google Scholar]
- Salmanov, M.A. Ecology and Biological Productivity of the Caspian Sea; Publishing House of the Institute of Microbiology of the Academy of Sciences of Azerbaijan: Baku, Azerbaijan, 1991; p. 398. (In Russian)
- Borodin, V.E. Summer phytoplankton of different size groups of the Middle and Southern Caspian//Fisheries research of plankton. In The Caspian Sea; Kuzmichev, V.I., Ed.; Part 2; VNIRO Publishing House: Moscow, Russia, 1991; pp. 102–110. (In Russian) [Google Scholar]
- Sanina, L.V.; Levshakova, V.D.; Tatarintseva, T.A. Summer phytoplankton of the Middle Caspian Sea during sea level rise and in comparison with previous years//Fisheries research of plankton. In The Caspian Sea; Kuzmicheva, V.I., Ed.; Part 2; VNIRO Publishing House: Moscow, Russia, 1991; pp. 77–95. (In Russian) [Google Scholar]
- Tatarintseva, T.A. Finding a new species of Nitzschia seriata Cleve (Bacillariophyta) in the Caspian Sea. Biol. Sci. 1992, 6, 55–77. (In Russian) [Google Scholar]
- Ardabyeva, A.G.; Tatarintseva, T.A. Characteristics of summer phytoplankton of the Caspian Sea. In Marine Hydrobiological Researc; 2000; pp. 22–38. (In Russian) [Google Scholar]
- Fisheries Research in the Caspian Sea; (Research Results for 2000); KaspNIRKh Publishing House: Astrakhan, Russia, 2001; 453p. (In Russian)
- Silkin, V.A.; Pautova, L.A.; Giordano, M.; Chasovnikov, V.K.; Vostokov, S.V.; Podymov, O.I.; Pakhomova, S.V.; Moskalenko, L.V. Drivers of phytoplankton blooms in the northeastern Black Sea. Mar. Pollut. Bull. 2019, 138, 274–284. [Google Scholar] [CrossRef]
- Vostokov, S.V.; Lobkovskiy, L.I.; Vostokova, A.S.; Solov’ev, D.M. Estimation of seasonal and inter-annual variations of phytoplankton in the Black Sea on the base of remote sensed data procession and chlorophyll a in situ measurements. RAS Rep. 2019, 485, 99–103. [Google Scholar] [CrossRef]
- Vostokova, A.S.; Lobkovskiy, L.I.; Vostokov, S.V. Anomalous Phenomena in the Development of Phytoplankton in the Black Sea on the Basis of Remote Sensing Data. RAS Rep. 2021, 497, 261–265. [Google Scholar] [CrossRef]
- Vostokov, S.V.; Vostokova, A.S.; Vazyulya, S.V. Seasonal and Long-Term Variability of Coccolithophores in the Black Sea According to Remote Sensing Data and the Results of Field Investigations. J. Mar. Sci. Eng. 2022, 10, 97. [Google Scholar] [CrossRef]
- Moradi, M. Comparison of the efficacy of MODIS and MERIS data for detecting cyanobacterial blooms in the southern Caspian Sea. Mar. Poll. Bull. 2014, 87, 311–322. [Google Scholar] [CrossRef]
- Kopelevich, O.V.; Sahling, I.V.; Vazyulya, S.V.; Glukhovets, D.I.; Sheberstov, S.V.; Burenkov, V.I.; Karalli, P.G.; Yushmanova, A.V. Bio-Optical Characteristics of the Seas, Surrounding theWestern Part of Russia, from Data of the Satellite Ocean Color Scanners of 1998–2017; Nash Format Publishers: Moscow, Russia, 2018; 140p, Available online: https://optics.ocean.ru/Atlas_2019/8_Monography_2018.pdf (accessed on 5 February 2022).
- Nezlin, N. Patterns of Seasonal and Interannual Variability of Remotely Sensed CHLorophyll. Handb. Environ. Chem. 2005, 5, 143–157. [Google Scholar] [CrossRef]
- Guseynova, N.O.; Bagomaev, A.A.; Akhmedova, L.S.; Kuramagomedov, B.M. Upwelling Study of CHLorophyll a Content in Phytoplankton of the Western Caspian in 2017 Based on Remote Sensing Data. South Russ. Ecol. Dev. 2022, 16, 159–172. [Google Scholar] [CrossRef]
- Ahmadi, B.; Gholamalifard, M.; Kutser, T.; Vignudelli, S.; Kostianoy, A. Spatio-Temporal Variability in Bio-Optical Properties of the Southern Caspian Sea: A Historic Analysis of Ocean Color Data. Remote Sens. 2020, 12, 3975. [Google Scholar] [CrossRef]
- Modabberi, A.; Noori, R.; Madani, K.; Ehsani, A.H.; Danandeh Mehr, A.; Hooshyaripor, F.; Kløve, B. Caspian Sea Is Eutrophying: The Alarming Message of Satellite Data. Environ. Res. Lett. 2020, 15, 124047. [Google Scholar] [CrossRef]
- Vostokov, S.; Saling, I.; Vostokova, A.; Shojaei, M.G. Study of Seasonal and Long-Term Phytoplankton Variability in the Caspian Sea Based on Remote Sensing Data. Proc. SPIE 12341, 28th International Symposium on Atmospheric and Ocean Optics: Atmospheric Physics, 1234151. Available online: https://doi.org/10.1117/12.2645217 (accessed on 7 December 2022).
- NASA Ocean Color. Available online: https://oceancolor.gsfc.nasa.gov (accessed on 20 December 2022).
- World Register of Marine Species. Available online: http://www.marinespecies.org (accessed on 5 October 2022).
- Gogorev, R. Check-List for Caspian Sea Phytoplankton. Caspian Sea Biodiversity Project 2006. Available online: http://www.zin.ru/projects/caspdiv/caspian_phytoplankton.html (accessed on 5 October 2022).
- Hillebrand, H.; Durselen, C.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to Volume Relationships for Dinoflagellates, Diatoms, and Other Protist Plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Gasanova, A.S.; Guseynov, K.M.; Guseynov, M.K. Phytoplankton Species Composition of the Russian Sector of the Caspian Sea. South Russ. Ecol. Dev. 2014, 9, 102–108. (In Russian) [Google Scholar] [CrossRef]
- Bagomaev, A.A.; Guseynova, N.O. Identification of Temperature Anomalies in the Western Caspian Sea in 2017 Based on Remote Sensing Data. South Russ. Ecol. Dev. 2020, 15, 63–74. (In Russian) [Google Scholar] [CrossRef]
- Kuramagomedov, B.M.; Monakhova, G.A.; Gadzhiev, A.A.; Akhmedova, G.A. The Experience of Using of the Geoinformation Technologies in the Investigation of Apwelling in the Caspian Sea. South Russ. Ecol. Dev. 2014, 9, 121–125. (In Russian) [Google Scholar] [CrossRef]
- Vostokov, S.V.; Saling, I.V.; Vostokova, A.S.; Gadzhiev, A.A.; Lobachev, E.N.; Abtahi, B.; Shojaei, M.G. Seasonal and Long-Term Chlorophyll Variability in the Caspian Sea Based on Remote Sensing Data. RAS Rep. 2023, 509, 134–140. (In Russian) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).