Abstract
Despite the great utility of nanoparticles (NPs) in water remediation, their effects on marine ecosystems are unknown and unpredictable. The toxicity of the most used nanoparticles, such as ZnO, Ag, and TiO2 on the purple sea urchin, Paracentrotus lividus (Lamarck, 1816), has been demonstrated by several authors. The aim of this study was to evaluate the effects of TiO2 sol-gel and TiO2-rGO nanocompounds on both vitality and motility of spermatozoa of P. lividus. The spermatozoa were exposed at different times (30 and 60 min) and concentrations (10, 20, 40 µg/mL) of both nano-TiO2 compounds. The results clearly showed a decrease in both vitality and motility of P. lividus spermatozoa exposed. In particular, vitality and motility were inversely related to both exposure time and concentration of TiO2 sol-gel and TiO2-rGO nanocompounds.
1. Introduction
A lot of emerging contaminants, such as pharmaceuticals and personal care products, hormones, pesticides, polycyclic aromatic hydrocarbons, alkylphenolic compounds, nanomaterials, and fluorinated substances directly or indirectly reach the aquatic environment [1,2,3]. Depending on the nature of such contaminants (highly polar and acidic/alkaline compounds), they can negatively impact aquatic ecosystems [4,5]. One of the risks related to pollution by these compounds is that they can bioaccumulate in lipid-rich tissues of organisms due to their hydrophobic properties and cause damage to the endocrine systems of humans and other animals [6]. In general, they can influence the growth, reproduction, and evolution of species in the environment.
There are many methods used to remove emerging contaminants from water, and among these, photocatalysis, which requires the use of catalysts to facilitate the transfer of energy from the photon to a water molecule, is one of the most used [7,8]. In recent decades, photocatalysis using TiO2 as a catalyst has proven useful for removing pollutants and microorganisms from wastewater [9]. TiO2 is a versatile compound found in nature in the forms of rutile, anastase, and brookite. This molecule used in numerous pharmaceutical and cosmetic products, and together with ZnO, is a well-known major component of sunscreens, thanks to its proprieties in blocking ultraviolet (UV) radiations [10,11]. Some authors reported that TiO2 nanoparticles (TiO2 NPs) under UV irradiation generated reactive oxygen species (ROS) through a photoactive reaction, which caused the inhibition of the growth of living organisms such as Daphnia and algae [12]. Furthermore, the proliferation of plankton can be suppressed by its presence in the environment and can lead to bleaching of coral reefs [13,14]. However, it is considered an ideal semiconductor for photocatalysis, especially in the form of nanoparticles (NPs), and is one of the most frequent catalytic methods used in water remediation [15].
The advantages of this advanced oxidation treatment are reduced costs, flexibility in the reuse of the catalyst, effectiveness in environmental temperature and pressure, the possibility of using sunlight to irradiate the catalyst, and it guarantees the complete mineralization of aliphatic organic pollutants, aromatics, polymers, dyes, surfactants, pesticides, and herbicides in CO2, water, and mineral acids [3]. However, there are some disadvantages, including difficulty in obtaining uniform radiation over the entire catalyst surface on a larger scale, the ability to absorb only UV light, and the rapid recombination of the charge which decreases its photocatalytic activity [16]. A solution could be a combination with other materials, such as graphene, one of the most promising compounds due to its acidic and basic inertness, good flexibility, large area, and excellent charge carrier mobility and improving its photocatalytic capabilities [17]. Often, graphene oxide (GO) is reduced to rGO to facilitate interaction with the TiO2 surface and the reduction is achieved by heat treatment or solar photoreduction.
The production, consumption, and disposal of engineered nanoparticles in such a quantity and diversity of products will inevitably lead to their release into the environment, where they can also pose a risk to humans. Some studies have been carried out on the specific physicochemical and kinetics properties of the widely used metal nanoparticles, both to observe their dissolution and their bioavailability in the aquatic environment and to describe their implication in controlling toxicity [18]. However, most of the studies on the behaviour of metallic nanomaterials in the aquatic environment to date have focused on freshwater systems. On the other hand, it is known that once in contact with the marine environment, engineered nanoparticles are modified by a series of processes, including at a chemical level through redox reactions (e.g., pH and salinity differences of seawater affect dissolution and aggregation of nanomaterials). To date, their effect on the marine environment and life is still unknown [19,20].
Several studies demonstrated that TiO2 NPs cause toxic effects on marine species. Despite this, existing data are difficult to compare and integrate, limiting hazard risk assessment [21]. A few studies have evaluated the toxicity of the most common nanoparticles, such as ZnO, Ag, and TiO2 NPs on spermatozoa, larvae, and adults of sea urchins [19], in particular on the purple sea urchin, Paracentrotus lividus (Lamarck, 1816), demonstrating toxic effects that dramatically affect the survival rate of embryos [22,23].
An interesting study carried out on P. lividus was performed by Gambardella et al. (2013) [24] whose conclusions highlight the toxic effect of TiO2 NPs exerted on the plutei of this species. However, in the literature there is still no data on the effects caused by these newly synthesized nanomaterials on the spermatozoa of this model organism. This study aims to provide the first data on the toxicity of TiO2 sol-gel and TiO2-rGO NPs on P. lividus spermatozoa in terms of viability and motility. Data obtained were discussed with a particular focus on the effects of newly synthetized materials on marine life and the necessity to test them before introduction in aquatic environments in general.
2. Materials and Methods
2.1. Experimental Section
A total of 10 sea urchins were collected by local fishermen in May 2022 and transported in sea water coolers to the laboratory of the University of Catania. Seven out of all specimens were mature males and were selected after careful stereomicroscopic observation of the genital papillae present on the 5 plates of the aboral surface (non-invasive method) [25,26] (Figure 1a). Female specimens were released alive. A solution of 0.5–1 mL KCl at 0.5 M was inoculated inside their bodies to cause an osmotic shock favouring the release of gametes (Figure 1b). The stock solution was centrifugated at 2000× g rpm for 1 min and subsequently 20 μL of sperm pellets was diluted in 1980 μL (final rate of 1:100) [27] in filtered sea water (temp: 20 ± 1 °C, pH: 8.1 ± 0.05, psu: 37 ± 1‰), obtained using 0.22 μm filters. The gametes were preliminarily observed under a microscope to evaluate their quality and before carrying out the experiments. Both the vitality and the motility of the samples obtained were evaluated, finding a percentage greater than 70%.
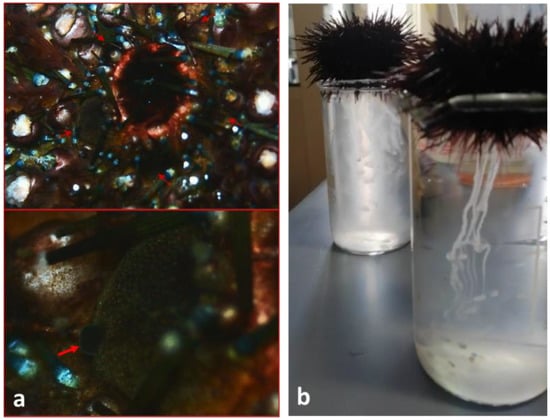
Figure 1.
(a) Genital papillae (red arrows) in mature male of P. lividus; (b) sperm release of P. lividus in 150 mL beaker after 0.5 M KCl inoculation.
2.2. Synthesis and Characterizations of TiO2 Sol-Gel and TiO2-rGO NPs
The TiO2 nanoparticles used in this work were prepared using the sol-gel technique. The samples coded as TiO2 sol-gel were synthetized following the procedure reported in the [28]. Briefly, 2 mL of titanium butoxide (Sigma-Aldrich, Buchs, Switzerland) was added to a solution of acetic acid and ethanol and stirred for 10 min at room temperature. Successively, this solution was mixed with another solution added dropwise containing water, acetic acid, and ethanol. The resultant gel was stirred for further 3 h and aged for 24 h. The obtained slurry-gel was dried at 100 °C for 12 h and calcined in air at 500 °C for 6 h.
The TiO2-rGO was obtained using the as-prepared TiO2 and using a photoreduction method explained in detail in our previous work [17]. In particular, in a Pyrex homemade jacketed reactor, the TiO2 were mixed with ethanol and 4 mL of GO solution (Graphenea, San Sebastián, Spain, to have a final amount of 2 wt.% of rGO) previously sonicated for 30 min. The resultant solution was purged with argon for 2 h to eliminate all the oxygen present inside the reactor. Afterwards, to favour the photoreduction of GO into rGO, the reactor was irradiated at 25 °C for 30 min with a solar lamp (OSRAM Vitalux 300 W, 300–2000 nm; OSRAM Opto Semiconductors GmbH, Leibniz, Regensburg Germany; solar irradiance: 10.7 mW/cm2). Finally, the as-obtained powders were washed with water and dried at 70° C for 24 h.
The samples were characterized by N2 adsorption-desorption measurements, to determine the BET (Brunauer–Emmet–Teller) surface area and the mean pore size, with XRD (X-ray diffraction) to evaluate the crystalline form and by SEM (scanning electron microscopy) for the morphology.
In detail, nitrogen adsorption-desorption measurements were performed with a Micromeritics Tristar II Plus 3020, (Micromeritics Instrument Corp. Norcross, GA, USA). Before the measurements, the samples were outgassed at 100 °C overnight.
The XRD measurements were carried out with a Bruker (Bruker GmbH, Mannheim, Germany) D-500 diffractometer, equipped with a parallel Cu-Kα radiation at 40 kV.
The SEM images were obtained with a (FE-SEM) ZEISS SUPRA 55 VP (Carl Zeiss QEC Gmb, Garching b. München, Germany) microscope.
2.3. Preparation of TiO2 Sol-Gel and TiO2-rGO NPs Solutions
Two stock concentrations were carried out, respectively, with the nanocompounds TiO2-rGO and TiO2 sol-gel in filtered seawater. The solutions obtained were sonicated (3 cycles of 3 min each; frequency 40 kHz) to guarantee a homogeneous dispersion of the nanoparticles and were then resuspended by vortexing before each use. Dilutions were obtained from the stock concentrations and spermatozoa were exposed to 10, 20, and 40 μg/mL of TiO2-rGO and TiO2 sol-gel at two different exposure times: 30 and 60 min. For both spermiotoxicity assays, 3 replicates were performed for each concentration tested.
2.4. Vitality Analysis
For each replicate, the viability of spermatozoa was evaluated using the eosin test [29]. The cell membrane of non-vitality spermatozoa exposed to the dye eosin (ratio 1:1) breaks down thus allowing the dye to pass through, so dead sperm appear fully or partially coloured pink, while live sperm have no colour. The slides were prepared with 10 µL of sperm which was added with 10 µL of eosin Y (0.5% v/v, Bio-Optica) and finally covered with a coverslip. The slides were observed under an optical microscope (Leica DMLB) equipped with a camera at ×100 magnification. At least 200 sperm cells were counted in 5 different fields.
2.5. Motility Analysis
The evaluation of motility was carried out through observations under the optical microscope (Leica DMLB) at ×40 magnification. For each replicate of the time series exposure, 10 µL of sperm sample was placed on a glass slide and covered with a coverslip. Spermatozoa were classified into motile (progressive and non-progressive) and immobile. At least 200 spermatozoa were considered in 5 different observation fields.
2.6. Statistical Analysis
Statistical analysis was performed using GraphPad Prism software (version 9.3.1). The difference between variances was analysed by 2-way ANOVA, followed by Tukey’s post hoc test for differences between groups. The level of significance was set at p < 0.05. All data were represented as mean ± standard deviation (SD).
3. Results
3.1. TiO2 Sol-Gel and TiO2-rGO NPs Characterization
From the XRD patterns reported in Figure 2 it is evident that the adopted synthesis and the thermal treatments led to the formation of only TiO2 anatase crystalline without the presence of anatase or rutile [28]. No signals associated with rGO were detected by XRD due to the low amount (2 wt.%) present in the TiO2-rGO sample. On the contrary, it is possible to note the presence of the rGO sheets from the SEM images (Figure 3b), marked by the red circle, as reported previously by [17], whereas the morphology of TiO2 particles was characterized to quasi-spherical particles [30] (Figure 3a).
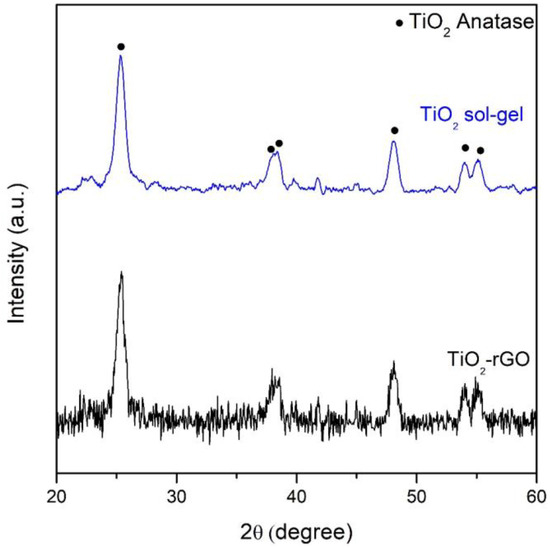
Figure 2.
XRD patterns of the examined samples.
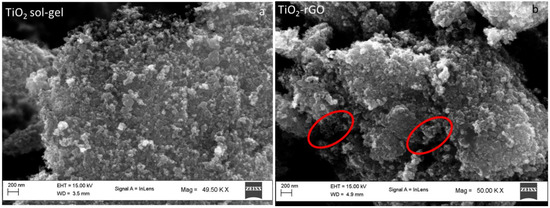
Figure 3.
SEM images of (a) TiO2 sol-gel, and (b) TiO2-rGO, with the rGO sheets highlighted in red.
The TiO2 sol-gel showed a high surface area (59 m2/g) compared to the TiO2-rGO (53 m2/g) and consequently a low mean pore size diameter (Table 1). The decrease in surface area and the increase in the pore size in the TiO2-rGO was ascribed to the inclusion of TiO2 on rGO and to the further thermal treatments necessary for the synthesis of this sample.

Table 1.
BET surface area and mean pore size diameter of the examined samples.
3.2. Spermiotoxicity Test with TiO2 Sol-Gel
Through the eosin test, the respective percentages of mortality in the performed replicas were calculated. The images below (Figure 4a–d) are examples of fields examined under the optical microscope which show how after 30 min of exposure to concentrations of 10, 20, and 40 μg/mL of TiO2 sol-gel it is possible to observe a higher percentage of mortality in the spermatozoa as the exposure concentration of the nanoparticle increases.
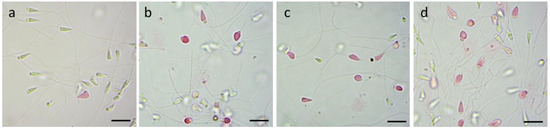
Figure 4.
Vitality evaluation with eosin test on P. lividus spermatozoa exposed to TiO2 sol-gel for 30 min: (a) CTRL group; (b) spermatozoa exposed to 10 μg/mL; (c) spermatozoa exposed to 20 μg/mL; (d) spermatozoa exposed to 40 μg/mL. Scale bar a,b,c,d = 5 µm.
The figure below (Figure 5a) shows the percentages of vitality of P. lividus spermatozoa exposed to the TiO2 sol-gel nanoparticle, at 30 and 60 min of exposure. Through the two-way ANOVA test, it is possible to state that there is a highly significant statistical difference (p < 0.001) between the control group and the exposed groups for all the concentrations used while there is a statistical significance (p < 0.05) for exposure times.
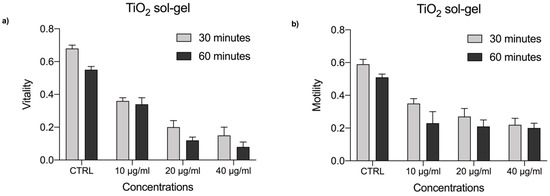
Figure 5.
(a) Vitality rate of P. lividus sperm exposed to TiO2 sol-gel at concentrations of 10, 20, and 40 μg/mL for 30 and 60 min. (b) Motiliy rate of P. lividus sperm exposed to TiO2 sol-gel at concentrations of 10, 20, and 40 μg/mL for 30 and 60 min. CTRL = control.
These results show that sperm vitality after 30 min of exposure to concentrations of 10, 20, and 40 μg/mL drastically decreases and the percentages are, respectively, 36%, 20%, and 15%, compared to the control (CTRL) which presents a percentage of vitality equal to 68%. From the 60 min exposure at the concentration of 10 μg/mL, the vitality is 34%. At the concentration of 20 μg/mL it decreases to 12%, and is 8% at the concentration of 40 μg/mL of TiO2 sol-gel, while the percentage of vitality of the control sample spermatozoa (CTRL) is 55%.
Concerning motility (Figure 5b) of the spermatozoa exposed to the TiO2 sol-gel at 30 min of exposure, the percentage is 35% at the lowest exposure concentration (10 μg/mL), while that of the control group (CTRL) has a value equal to 59%. Concentrations of 20 μg/mL and 40 μg/mL follow, which present a motility percentage of 27% and 22%, respectively. At 60 min of exposure, as the concentrations increase, the percentage of motile spermatozoa decreases and the percentage of motility is, respectively, 23%, 21%, and 20%, while for the control sample a percentage equal to 51% is recorded.
3.3. Spermiotoxicity Test with TiO2-rGO
Mortality rates in all replicates were obtained from non-viable sperm counts in the five reading fields for each slide. The images below (Figure 6a–d) show examples of fields examined under the optical microscope after 30 min of exposure to concentrations of 10 μg/mL, 20 μg/mL, and 40 μg/mL of TiO2-rGO. The percentage of mortality of the spermatozoa presents higher values as the exposure concentration of the nanocompound increases.
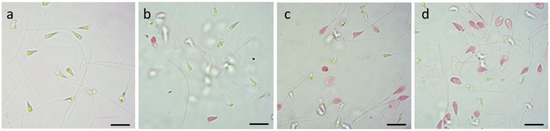
Figure 6.
Vitality evaluation with eosin test on P. lividus spermatozoa exposed to TiO2-rGO for 30 min: (a) CTRL group; (b) spermatozoa exposed to 10 μg/mL; (c) spermatozoa exposed to 20 μg/mL; (d) spermatozoa exposed to 40 μg/mL. Scale bar a,b,c,d = 5 µm.
The following figure (Figure 7a) shows the percentages of vitality of P. lividus spermatozoa exposed to the TiO2-rGO nanocompound both at 30 and at 60 min of exposure. Through the two-way ANOVA test, it was possible to state that there is a highly significant statistical difference (** p < 0.01) between the control group and the exposed groups for all concentrations and exposure times.
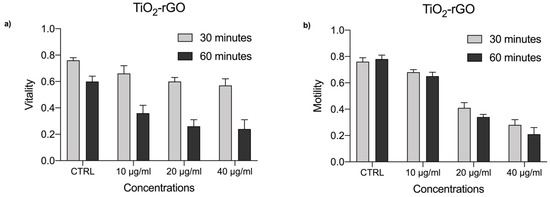
Figure 7.
(a) Vitality rate of P. lividus sperm exposed to TiO2-rGO at concentrations of 10, 20, and 40 μg/mL for 30 and 60 min. (b) Motility rate of P. lividus sperm exposed to TiO2-rGO at concentrations of 10, 20, and 40 μg/mL for 30 and 60 min. CTRL = control.
The vitality of male gametes at concentrations of 10, 20, and 40 μg/mL after 30 min was reduced, showing the respective values of 66%, 60%, and 57%. After 60 min of exposure, the vitality drastically reduced showing the respective values of 36%, 26%, and 24% compared with the control group (CTRL), which showed a percentage of vitality equal to 76% after 30 min and 60% after 60 min of exposure.
Furthermore, at the respective exposure concentrations (10, 20, and 40 μg/mL) motility was reduced to 68%, 41%, and 28%, while after 60 min of exposure the motility decreases, presenting the respective values of 65%, 34%, and 21%, compared with the control group (CTRL) which showed a percentage of motility equal to 76% after 30 min and 78% after 60 min of exposure (Figure 7b).
4. Discussion and Conclusions
The presence of emerging contaminants in marine waters is a worrying and alarming problem. Indeed, the detection in traces of nanoparticles used for both industrial and domestic purposes is becoming more frequent. Furthermore, nanoparticles have been recently recognized as excellent photocatalysts in the purification of wastewater, particularly when they are combined with other compounds, such as graphene oxide (GO). However, the uncontrolled release of NPs could affect the marine ecosystem as they can interfere with physiological and biological processes of marine organisms. The fact that nanoparticles, such as TiO2 NPs, are used in a great variety of products, such as pharmaceuticals and cosmetics, and in the production of paints, paper, and plastic materials raises a series of questions on their impact in ecosystems [31].
This study evaluated the effects of the exposure of spermatozoa of P. lividus to 10, 20, 40 μg/mL of TiO2 sol-gel and TiO2-rGO at 30 and 60 min (Figure 8). The results obtained showed a significant reduction in the vitality and motility of the spermatozoa at all concentrations and exposure times tested for both nanomaterials. This study is the first preliminary work concerning these newly synthesized materials and confirms their toxic effects on spermatozoa of the sea urchin P. lividus.
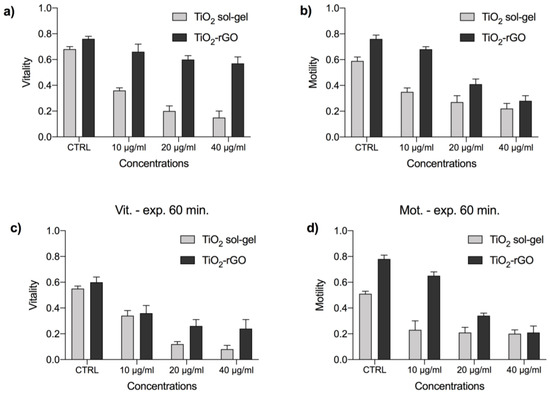
Figure 8.
Comparison of the vitality (a,c) and motility (b,d) rates of P. lividus sperm exposed to TiO2 sol-gel and TiO2-rGO and at concentrations of 10, 20, and 40 μg/mL for 30 (a,b) and 60 min (c,d). CTRL = control groups.
Observing vitality and motility values, it is possible to state that the toxicity of TiO2-rGO increases with exposure time and concentrations, although the prolonged exposure damages the spermatozoa the most.
On the other hand, exposure to TiO2 sol-gel determined a higher mortality already at 30 min of exposure, showing a higher toxicity for the tested concentrations than for the exposure time. As can be seen from the data, the TiO2 sol-gel caused a considerable immobility and mortality already at the concentration of 10 μg/mL compared with the percentage obtained for TiO2-rGO. In fact, when the exposure of the latter was prolonged to 60 min, the mortality values were similar to those of TiO2 sol-gel at 30 min.
Hence, the results of our research provide new insights on the deleterious effects of the newly synthesized nanocompounds TiO2-rGO and TiO2 sol-gel on marine life, revealing the vulnerability of P. lividus spermatozoa to such compounds. This also highlights the potential negative effects for aquatic organisms and ecosystems in general. Our findings suggest caution when applying these and similar compounds to water remediation when they will be available on a large scale. The marine environment is today subject to a high quantity of pollutants of anthropic origin. Hence, it is important to understand the effects and mechanisms of interactions between synthetic products and marine life to prevent disastrous consequences on marine ecosystems and aquatic systems in general. In this regard, some studies have recently demonstrated the deleterious effect of commonly used substances, such as sunscreens and other NPs [32,33]. Results from these studies clearly showed a deleterious effect on the development of the sea urchins P. lividus and Arbacia lixula (Linnaeus, 1758). Other research investigated metal nanoparticle toxicity on different developmental stages of P. lividus. These studies showed a high sensitivity to ZnO particles that induced the development of anomalies in larvae, although no compromission of fertilization processes were recorded [22,23]. Other authors showed that TiO2 NPs used in a sunscreen products against ultraviolet radiations produced abnormalities related to the skeletal growth in P. lividus [34]. The results obtained agree with the data present in the literature, in which it has been observed that uncomplexed TiO2, at the same concentrations, influenced the fertilization and development of exposed P. lividus larvae. Indeed, some authors highlighted interferences of TiO2 NPs with the bio-mineralization processes of larvae when spermatozoa of P. lividus were exposed to these contaminants [24]. Moreover, even individually, GO have been shown to have negative effects on the development of P. lividus, causing anomalies during early-stage phases [35].
In conclusion, it can be deduced that the application of TiO2 in the context of water remediation could represent a good solution; however, it would be advisable to carry out further studies to evaluate the concentration and effects of nanoparticles dispersed in the marine environment which could negatively affect survival, reproduction, and development of marine organisms. In this context, the utilization of sea urchin spermatozoa as a model for ecotoxicological tests can be considered a good tool for the evaluation and estimation of the deleterious effect of nanoparticles used in water remediation [36]. Therefore, it will be necessary to deepen the studies on the possible effects of NPs on fertilization and the first stages of development of P. lividus that is already threatened by various xenobiotics and contaminants present in the marine environment, through overfishing and habitat destruction [37,38,39]. Lastly, ecotoxicological tests should be performed on each newly synthetized compound before its introduction into marine environments to evaluate the associated risks for marine life.
Author Contributions
Conceptualization, S.I. (Sara Ignoto); methodology, S.I. (Sara Ignoto), R.F., S.A.B. and M.V.B.; software, S.I. (Sara Ignoto) and F.T.; validation, F.T., A.S. and M.V.B.; formal analysis, S.I. (Sara Ignoto); investigation, S.I. (Sara Ignoto) and R.P.; data curation, S.I. (Sara Ignoto); writing—original draft preparation, S.I. (Sara Ignoto); writing—review and editing, S.I. (Sara Ignoto), R.P., E.M.S., M.C., G.F., S.I. (Stefania Indelicato), R.F., S.A.B., G.I., F.T., A.S., M.V.B.; visualization, S.I. (Sara Ignoto); supervision, M.V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are available on request.
Acknowledgments
S.I. thanks the Industrial PhD program (PON RICERCA ED INNOVAZIONE 2014−2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naidu, R.; Espana, V.A.A.; Liu, Y.; Jit, J. Emerging contaminants in the environment: Risk-based analysis for better management. Chemosphere 2016, 154, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Piwowarska, D.; Kiedrzyńska, E. Xenobiotics as a contemporary threat to surface waters. Ecohydrol. Hydrobiol. 2021, 22, 337–354. [Google Scholar] [CrossRef]
- Rego, R.M.; Kuriya, G.; Kurkuri, M.D.; Kigga, M. MOF based engineered materials in water remediation: Recent trends. J. Hazard. Mater. 2021, 403, 123605. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- De Oliveira, M.; Frihling, B.E.F.; Velasques, J.; Magalhães Filho, F.J.C.; Cavalheri, P.S.; Migliolo, L. Pharmaceuticals residues and xenobiotics contaminants: Occurrence, analytical techniques and sustainable alternatives for wastewater treatment. Sci. Total Environ. 2020, 705, 135568. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Abbas, G.; Qurashi, A.; Hussain, M. Efficient catalyst development for deep aerobic photocatalytic oxidative desulfurization: Recent advances, confines, and outlooks. Catal. Rev. 2022, 64, 789–834. [Google Scholar] [CrossRef]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Enhancing the photocatalytic oxidation of dibenzothiophene using visible light responsive Fe and N co-doped TiO2 nanoparticles. Ceram. Int. 2017, 43, 973–981. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Contado, C.; Pagnoni, A. TiO2 in commercial sunscreen lotion: Flow field-flow fractionation and ICP-AES together for size analysis. Anal. Chem. 2008, 80, 7594–7608. [Google Scholar] [CrossRef]
- Hund-Rinke, K.; Simon, M. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids (8 pp). Environ. Sci. Pollut. Res. 2006, 13, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.K.; Kim, E.J.; Lee, J.; Lee, S. Potential risks of TiO2 and ZnO nanoparticles released from sunscreens into outdoor swimming pools. J. Hazard. Mater. 2016, 317, 312–318. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2010, 56, 1639–1657. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Balsamo, S.A.; Fiorenza, R.; Condorelli, M.; Pecoraro, R.; Brundo, M.V.; Lo Presti, F.; Sciré, S. One-pot synthesis of TiO2-rGO photocatalysts for the degradation of groundwater pollutants. Materials 2021, 14, 5938. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.X. Significance of physicochemical and uptake kinetics in controlling the toxicity of metallic nanomaterials to aquatic organisms. J. Zhejiang Univ. Sci. A 2014, 15, 573–592. [Google Scholar] [CrossRef]
- Fairbairn, E.A.; Keller, A.A.; Mädler, L.; Zhou, D.; Pokhrel, S.; Cherr, G.N. Metal oxide nanomaterials in seawater: Linking physicochemical characteristics with biological response in sea urchin development. J. Hazard. Mater. 2011, 192, 1565–1571. [Google Scholar] [CrossRef]
- Timerbaev, A.R.; Kuznetsova, O.V.; Keppler, B.K. Current trends and challenges in analysis and characterization of engineered nanoparticles in seawater. Talanta 2021, 226, 122201. [Google Scholar] [CrossRef]
- Minetto, D.; Libralato, G.; Ghirardini, A.V. Ecotoxicity of engineered TiO2 nanoparticles to saltwater organisms: An overview. Environ. Int. 2014, 66, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Manzo, S.; Miglietta, M.L.; Rametta, G.; Buono, S.; Di Francia, G. Embryotoxicity and spermiotoxicity of nanosized ZnO for Mediterranean Sea urchin Paracentrotus lividus. J. Hazard. Mater. 2013, 254, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Manzo, S.; Schiavo, S.; Oliviero, M.; Toscano, A.; Ciaravolo, M.; Cirino, P. Immune and reproductive system impairment in adult sea urchin exposed to nanosized ZnO via food. Sci. Total Environ. 2017, 599, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Aluigi, M.G.; Ferrando, S.; Gallus, L.; Ramoino, P.; Gatti, A.M.; Falugi, C. Developmental abnormalities and changes in cholinesterase activity in sea urchin embryos and larvae from sperm exposed to engineered nanoparticles. Aquat. Toxicol. 2013, 130, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Sugni, M.; Ciappellano, S.G.; Biressi, A.C.M.; Fernandes, D.; Porte, C.; Candia, M.D. Sexual dimorphism in the sea urchin Paracentrotus lividus: Morphological and biochemical analysis. In International Workshop Status and Management of the Edible Sea Urchin Paracentrotus lividus in Mediterranean Sea: Book of Abstracts; Mazzola, A., Chemello, R., Gianguzza, P., Eds.; Università di Palermo: Palermo, Italy, 2010; p. 44. [Google Scholar]
- Brundu, G.; Cannavacciuolo, A.; Nannini, M.; Somma, E.; Munari, M.; Zupo, V.; Farina, S. Development of an efficient, non-invasive method for identifying gender year-round in the sea urchin Paracentrotus lividus. Aquaculture 2023, 564, 739082. [Google Scholar] [CrossRef]
- Fabbrocini, A.; D’Adamo, R.; Del Prete, F.; Maurizio, D.; Specchiulli, A.; Oliveira, L.F.; Sansone, G. The sperm motility pattern in ecotoxicological tests. The CRYO-Ecotest as a case study. Ecotoxicol. Environ. Saf. 2016, 123, 53–59. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Dattilo, S.; et al. Preferential removal of pesticides from water by molecular imprinting on TiO2 photocatalysts. Chem. Eng. J. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Lin, M.H.; Morshedi, M.; Srisombut, C.; Nassar, A.; Oehninger, S.M.D. Plasma membrane integrity of cryopreserved human sperm: An investigation of the results of the hypoosmotic swelling test, the water test, and eosin-Y staining. Fertil. Steril. 1998, 70, 1148–1155. [Google Scholar] [CrossRef]
- Sajjad, A.K.L.; Shamaila, S.; Tian, B.; Chen, F.; Zhang, J. One step activation of WOx/TiO2 nanocomposites with enhanced photocatalytic activity. Appl. Catal. B Environ. 2009, 91, 397–405. [Google Scholar] [CrossRef]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol–gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Manno, D.; Serra, A.; Buccolieri, A.; Panzarini, E.; Carata, E.; Tenuzzo, B.; Izzo, D.; Vergallo, C.; Rossi, M.; Dini, L. Silver and carbon nanoparticles toxicity in sea urchin Paracentrotus lividus embryos. BioNanoMaterials 2013, 14, 229–238. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Damiani, E.; Marcellini, F.; Falugi, C.; Tiano, L.; Brugè, F.; Danovaro, R. Sunscreen products impair the early developmental stages of the sea urchin Paracentrotus lividus. Sci. Rep. 2017, 7, 7815. [Google Scholar] [CrossRef] [PubMed]
- Catalano, R.; Labille, J.; Gaglio, D.; Alijagic, A.; Napodano, E.; Slomberg, D.; Campos, A.; Pinsino, A. Safety evaluation of TiO2 nanoparticle-based sunscreen UV filters on the development and the immunological state of the sea urchin Paracentrotus lividus. Nanomaterials 2020, 10, 2102. [Google Scholar] [CrossRef] [PubMed]
- Mesarič, T.; Sepčić, K.; Drobne, D.; Makovec, D.; Faimali, M.; Morgana, S.; Falugi, C.; Gambardella, C. Sperm exposure to carbon-based nanomaterials causes abnormalities in early development of purple sea urchin (Paracentrotus lividus). Aquat. Toxicol. 2015, 163, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Volpi Ghirardini, A.; Arizzi Novelli, A. A sperm cell toxicity test procedure for the Mediterranean species Paracentrotus lividus (Echinodermata: Echinoidea). Environ. Technol. 2001, 22, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Scanu, S.; Soetebier, S.; Piazzolla, D.; Tiralongo, F.; Mancini, E.; Romano, N.; Marcelli, M. Concentrations of As, Cd, Cr, Ni, and Pb in the echinoid Paracentrotus lividus on the coast of Civitavecchia, northern Tyrrhenian Sea, Italy. Reg. Stud. Mar. Sci. 2015, 1, 7–17. [Google Scholar] [CrossRef]
- Scalisi, E.M.; Pecoraro, R.; Salvaggio, A.; Corsaro, A.; Messina, G.; Ignoto, S.; Lombardo, B.M.; Brundo, M.V. Evaluation of dimethoate toxicity on fertilization and on embryonic development of Paracentrotus lividus (Lamarck, 1816). Toxicol. Res. 2020, 9, 537–543. [Google Scholar] [CrossRef]
- Pais, A.; Serra, S.; Meloni, G.; Saba, S.; Ceccherelli, G. Harvesting effects on Paracentrotus lividus population structure: A case study from northwestern Sardinia, Italy, before and after the fishing season. J. Coast. Res. 2012, 28, 570–575. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).