Abstract
In the last 100 years, the population of the land of Israel has increased dramatically, accompanied by a very intense and accelerated economic and industrial growth. The objective of the present review is to reveal how these major changes have affected the Mediterranean marine and coastal environment. The present review analyzes the global, regional, and local factors and processes that cause substantial environmental changes affecting a variety of marine habitats and taxa. During the past century these include: (1) seawater warming that enhances the considerable introduction and establishment of non-indigenous tropical, i.e., Lessepsian, species; (2) overfishing of native biota that seems to contribute to this process; (3) sea-level rise, associated with climate change, which may threaten the sensitive intertidal abrasion platforms; (4) chemical, noise, and light pollution and marine debris; (5) massive sand mining from the beaches, which caused severe erosion in many coastal sections and was banned in Israel in 1964; (6) extensive dredging in the sea, mainly related to the construction and development of large ports, which can be detrimental for the benthic biota, especially in rocky substrates; and (7) marine structures (harbors, marinas, detached breakwaters) that interfere with the natural pattern of sand transport along the coast and cause morphological changes (sand erosion or accumulation) on nearby beaches and the seabed. Israel’s coast is presently characterized by intense anthropogenic activity and many stakeholders with considerable conflicts between them and with the marine ecosystem. A few environmental impacts have ceased, and others have been reduced considerably, but the extent of many additional types have increased significantly, and new impacts have appeared in recent years. Some environmental impacts are beyond our control, and others can be reduced by proper management, but it is predicted that certain major environmental impacts, such as Lessepsian migration, will continue in the future at enhanced rates.
1. Introduction
The State of Israel, located in the southeastern Mediterranean (Figure 1), has gone through many geo-political upheavals in the past century. This, together with global changes, has also been reflected in environmental impacts on the coastal zone.
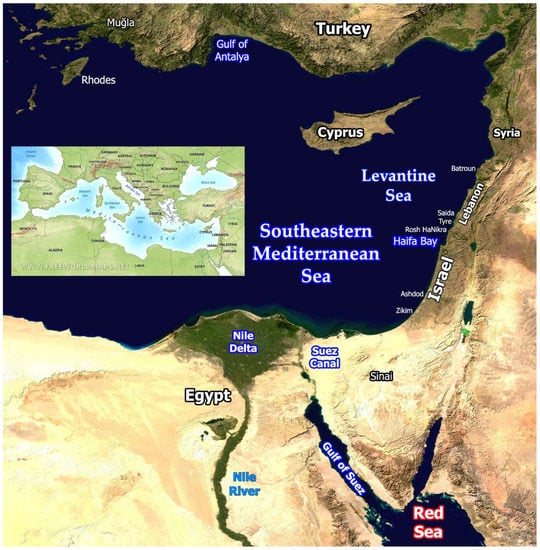
Figure 1.
Eastern Mediterranean. Background: part of “Blue Marble: Land Surface, Shallow Water, and Shaded Topography”. NASA Goddard Space Flight Center Image by Reto Stöckli, Robert Simmon and MODIS Groups; visibleearth.nasa.gov/images/57752/blue-marble-land-surface-shallow-water-and-shaded-topography (accessed on 11 February 2002). Inset: Physical Map of the Mediterranean Sea and its surroundings. www.freeworldmaps.net/europe/mediterranean/physical.html, accessed on 20 December 2002.
During the First World War (1914–1918), the British Empire drove the Turks out of the Levant and put an end to Ottoman rule in this region. The British achieved legitimacy by obtaining a mandate from the League of Nations in June 1922 on part of what had been “Ottoman Syria”. This included Mandatory Palestine (Land of Israel), which preceded the State of Israel, established in May 1948 [1].
In the last 100 years, the population and the economic growth in this region have increased dramatically. Israel’s population, as of July 2022, is 9,567,700 residents [2]. It has almost doubled over the last 30 years due to waves of immigration, natural growth (annual population growth rate for 2021 was 1.6% [2]), and improved life expectancy. This compares with the total population of “Palestine” within the British Mandatory boundaries in 1922 of 752,048 [3]. The total population at the establishment of the State of Israel in 1948 was only 806,000 [4].
Commercial and industrial developments have resulted in enhanced coastal activities that have increased over the years, with considerable impact on the marine environment. Research and development efforts have also expanded over the years, contributing to the improvement of technology and engineering proficiency of projects in the coastal and marine environments and to our scientific knowledge of this area. Coastal and marine technological and scientific progress before and especially after the establishment of Israel have modified not only the dimensions of the effects but also their nature. The first period, which lasted nearly the whole of the 20th century, was characterized by coastal activities, such as sand beach-mining, marine construction, marine transportation, coastal power plants, fishery, security, underwater oil pipelines, water sport, and limited research of marine natural resources and cultural heritage, albeit in a relatively restricted scope. The last 25 years have seen projects associated with modern innovations and technologies, such as seawater desalination, mariculture, submarine natural gas pipelines, sand beach nourishment, and the increasing use of modern materials such as plastics. Even “traditional” activities have taken new directions. For example, the energy economy has been converted from mainly imported crude oil sources to imported coal and natural gas from marine sources in Israel’s exclusive economic zone (EEZ). Shipping has shifted from small, slow vessels to very large, fast, diesel-powered ships and container-based maritime transport (e.g., [5]). Coastal and marine research has benefited from the invention and development of modern tools, such as SCUBA diving, electro-optical and chemical devices, remotely operated environmental sensors, and aerial and satellite technologies.
Today, Israel is considered a highly urbanized nation state. According to the ICBS (Israel Central Bureau of Statistics) report [6], in 2018, a total of 88.9% of the Israeli population, consisting of 8,967,600 inhabitants, lived either in cities (74.2%) or local authorities (14.7%). The highest population density is in the central coastal plain, which is also the economic center of Israel. In the central district, the population density per km2 of land has increased from 122.3 in 1948 to 2268.2 at the end of 2020.
More than five million people (about 60% of the Israeli population) and much of the country’s marine economic and commercial activity (e.g., ports, power plants, desalination plants, coal jetties, and offshore oil terminals) as well as related industries (e.g., refineries, warehouses) are concentrated in the coastal plain. In addition, some areas are military zones closed to public access, leaving only about 130 km (out of 195 km) of coast open to the public for recreational activities (e.g., marinas, authorized bathing beaches, nature reserves), and very few beaches have been left in their natural undeveloped state [7].
The purpose of the present review is to reveal the main environmental impacts on Israel’s Mediterranean coast from the beginning of the British Mandate until today. The review analyzes the processes that drive these impacts, their developments over the last 100 years, and the emergence of new impacts. It examines the effects of environmental impacts on the marine and coastal ecosystems, on the levels of the various habitats and of different taxonomic groups, as well as on people. It also attempts to assess some future trends associated with environmental impacts along the coast.
This article includes a background section examining the abiotic and biotic characteristics of the Israeli Mediterranean coast; a section assessing environmental impacts in the past and present; and a section on nature protection and nature reserves. The discussion section analyzes the state of the Israeli coast and attempts to evaluate the management actions needed to improve it and forecasts possible future developments.
2. Background
2.1. General Environmenntal Conditions
The Mediterranean coast of Israel, as part of the Levantine Sea (Figure 1), is considered to be in a “cul-de-sac” situation, being in contact with the world’s oceans mainly through the temperate Atlantic Ocean [8] and is far from the connection of the Mediterranean to the Atlantic Ocean at the Strait of Gibraltar (Figure 1: inset). Levantine waters have unique physical and chemical and therefore ecological characteristics that are more extreme than the rest of the Mediterranean.
The Mediterranean Sea is characterized by a gradient of environmental conditions from west to east: evaporation increases, precipitation decreases, salinity increases, water temperatures increase, nutrient content in the photic zone decreases, and primary production decreases—reflected in the rest of the food web (including fisheries)—an ultra-oligotrophic Levant basin [9]. Hence, the Mediterranean coast of Israel is characterized by: (1) poverty of the biological production; (2) dynamics—continuous a-biotic changes (e.g., increasing water temperature) and biotic alternations (e.g., invasion of alien species); (3) uniqueness of habitats and ecological processes; and (4) in recent years, intense manmade effects with threats to the biota—with multiple stakeholders having conflicts of interest between them as well as with the marine ecosystem [10,11,12].
The Mediterranean Sea originated in the ancient tropical Tethys Ocean (e.g., [13]). Most of its original tropical biota became extinct during the Messinian salinity crisis (e.g., [14]), when the Mediterranean partially or nearly completely dried up throughout the latter part of the Miocene epoch. It ended when the Atlantic reclaimed the Mediterranean basin with biota of Atlantic origin. From then until the opening of the Suez Canal in 1869, the Mediterranean, including the Levantine basin, was characterized by Atlanto-Mediterranean biota.
2.2. Types of Coasts
The Mediterranean coast of Israel runs about 195 km from Ziqim near the border with Gaza Strip in the south, to Rosh HaNikra near the Lebanese border in the north (Figure 2). Except for Haifa Bay, the Carmel headland, and few small rocky promontories, the coastline is nearly straight, open to the west, and gradually changes its orientation from northeast to approximately north [7].
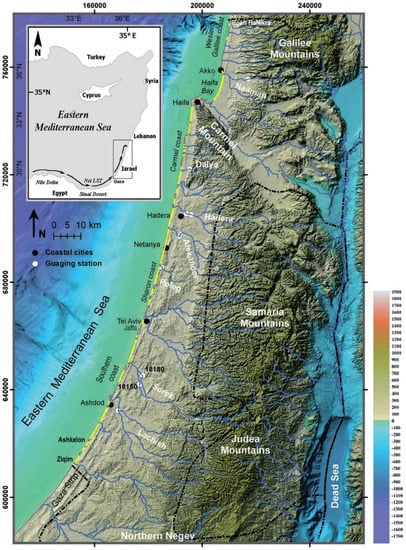
Figure 2.
Locations of Israel’s main coastal cities, sandy beaches (marked yellow), main rivers, and seasonal streams (modified after Lichter et al. 2011 [15]). Background: Shaded relief modified after Hall and Calvo [16]. Inset: The Nile littoral cell longshore sand transport (LST) long-term dominant direction.
The coast of Israel and its adjacent inner shelf, from the shore to maximum 30 m water depth, can be divided into two main sedimentological provinces. The Southern Province stretches 175 km from Ziqim to Akko promontory (northern Haifa Bay) (Figure 2) and is mainly composed of Nile-derived fine quartz sand [7]. This region is considered the northern flank of the Nile littoral cell [17], one of the world’s longest. This coastal compartment runs 650 km along the southeastern Mediterranean from Abu Quir Bay near Alexandria, Egypt, to Haifa Bay [18,19] (Figure 2: inset).
The Northern Province (the Western Galilee coast), however, is a small (20 km long), isolated, rocky littoral cell, partly covered with local coarse carbonate sand [20,21,22]. In general, from the shoreline to about 5 m water depth, the mean grain range is about 150–300 µm and becomes smaller from the south to the north of Israel [23]. However, from about 5 to 30 m water depth, the mean grain size ranges about 100–170 µm uniformly along the coast.
Most beaches in Israel consist mainly of fine quartz sand and have a moderate slope (~1:30) and range from 10 to 30 m wide (Figure 3) [20,23]. Relatively wide sandy beaches (50–100 m) are found mainly along the southern Israel coast (e.g., Figure 3A) and around coastal river estuaries (100–200 m) [24], while beaches less than 20 m and sometimes only a few meters wide are located mainly in the center of the country along the coastal cliff (e.g., Figure 3B).

Figure 3.
Fine quartz sand beach: (1) typical wide beach—for example, in Ziqim (the Southern coast) ((A), photograph by Dov Zviely 16 November 2017). (2) Typical narrow beach—for example, in Netanya (the Sharon coast) ((B), photograph by Dov Zviely 5 January 2017).
Compared to the large number of sandy beaches in Israel, the rocky beaches are less common and are mainly located north of Ashdod Port (~6 km long beachrock); nearby small rocky kurkar (local term for aeolian carbonate-cemented quartz sandstone) promontories (e.g., Jaffa, Akko, Rosh HaNikra); along the Carmel coast (sections of rocky coasts or sandy beaches) (Figure 4A); around the Carmel headland (~5.5 km long mainly rocky coast with small sandy pocket beaches); and along the Western Galilee coast (~20 km long rocky coast with few sandy pocket beaches) (Figure 4) (for locations, see Figure 2).

Figure 4.
Rocky coasts: typical kurkar rocky coast including abrasion platform—for example, Habonim (Carmel coast) ((A), photograph by Dov Zviely 8 August 2020). Massive beachrock outcrop at Shavei Tzion (Western Galilee coast) ((B), photograph by Dov Zviely 18 March 2008).
Israel’s coastal cliff extends between Ashkelon and Hadera (Figure 2) to a total length of about 45 km and rises to 50 m above the beach. Its slope is generally about 75–90° and consists of alternating layers of kurkar and paleosols [25,26,27]. The cliff is poorly consolidated, constantly collapsing and retreating eastwards ([28,29,30,31] and references therein).
The submerged hard substrates along the Israel’s coast include kurkar ridges parallel to the coastline that locally forms a rocky substrate rich with crevices and caves and occurs under water, mainly between the shoreline and ~30 m water depth.
Some of the kurkar substrates appear in the intertidal zone as abrasion platforms, which are more common in the northern Israel coast (e.g., Figure 4A).
Another common rocky formation is beachrock, characterized by the significant presence of marine-associated particles such as shells and coarse sediment, rapidly cemented by calcium carbonate within the intertidal zone. It can be described as tilted stair or tile-like laminated blocks, slightly inclined westward, and known to have the same lamination and a similar declination towards the sea as the hosting beach (e.g., Figure 4B).
The beachrock outcrops can reach hundreds of meters in length, more than 40 m in width, and up to about 1 m in thickness. Its composition is in many cases identical to the detrital components composing the non-consolidated coastal sediment in its close vicinity (waterline).
There are also conglomerate rocks as well as rare limestone along the Carmel headland and Rosh HaNikra coasts.
Finally, 32 small rivers and seasonal streams cut the Israeli coastal zone and flow westward to the Mediterranean Sea (Figure 2). Some of these streams have small drainage basins of only a few square kilometers, while others have drainage basins exceeding 1000 km2 [24].
2.3. The Main Physical Characteristics of the Coastal and Marine Environment
2.3.1. Climate Regime
The eastern Mediterranean Sea can be divided into four sub-seas or basins (from west to east): the Adriatic, Ionian, Aegean, and Levantine Seas [32]. The latter is characterized by hot, dry summers with stable atmospheric conditions; cold, wet winters; and relatively short transitional seasons in spring and autumn [33,34].
The seasonal mean winds are mainly westerly although migratory low-pressure systems moving eastward across the Mediterranean Sea [35] force downwelling-favorable, strong southerly to southwesterly winds along the Israeli coast [36].
During transition seasons, the Red Sea Trough, a tongue of low pressure originating in the Sudanese-Ethiopian “Low”, extends northward from the southern Red Sea towards the eastern Mediterranean at lower atmospheric levels [37].
2.3.2. Wind Regime
During the summer, steady westerly and northwesterly winds dominate the Levantine Basin, strengthened by the Aegean Etesian regime and superimposed by a well-developed coastal sea breeze. The winter winds are predominantly westerlies. In contrast to summer, winter atmospheric conditions are unstable and variable, with occasional cold and dry air outbreaks from the north local cyclogenesis, such as the Cyprus Low [38,39]. Also important are depressions moving eastward across the Mediterranean [35], which generate strong southerly to southwesterly winds along the Israeli coast [40].
Based on wind data recorded in the Ashdod Port area, between 1 April 1993 and 31 March 2011, the Israeli Coastal and Marine Engineering Research Institute (CAMERI) found that approximately 90% of annual winds were light (wind speed below 6 m/s), and about 9% of annual winds were fresh (wind speed between 6 and 10 m/s). Only 0.67–1.2% of annual winds, 1.2–2.7% of winter winds, and 0.34% of summer winds were strong and exceeded 10 m/s [41,42].
The wind direction with speed above 6 m/s was northwest (1.64% occurrence), while the dominant wind direction was south-southeast. Strong southwest winds (above 10 m/s) can generate wave storms and strong currents (0.30% occurrence).
The maximum wind speed (21.8 m/s) was recorded on 12 December 2010 during a winter storm event. The strongest winds were in reasonable agreement with wave storm events in deep water [42].
2.3.3. Wave Regime
The Mediterranean wave climate of Israel can be divided into two seasons: summer (April to October) and winter (November to March). During the summer, the wave climate is characterized by relatively calm sea with a wave climate rarely exceeding 2 m significant wave height (Hs). In the winter, however, the wave climate is characterized by alternating periods of calm sea and storm events, usually of up to 5 m Hs [34,43].
Since 1992, north of Ashdod Port, and since 1993, offshore the Carmel headland (in Haifa), quality wave data have been measured by CAMERI (www.cameri-eng.com, accessed on 24 August 2022) on behalf of the Israel Ports Company (IPC). At these sites (110 km apart; see Figure 2), where water depth is about 24 m, Datawell Waverider directional buoys were deployed to acquire 30 min records of surface elevation and directional spectral information [44].
Other wave measurements are continuously conducted at Hadera and Ashkelon ports by the Israel Oceanographic and Limnological Research (IOLR) (isramar.ocean.org.il/isramar2009/ accessed on 24 August 2022).
The most common wave height in Ashdod and Haifa was measured in the range of 0.5 m ≤ Hs ≤ 1.0 m [45]. Extreme events (Hs > 5.0 m) were rare and measured in Ashdod and Haifa less than 0.1% of the time. From 1993 to 2021, four extreme storms with Hs > 7 m (in February 2001, December 2002, December 2010, and February 2015) were measured in Haifa [46]. These events show that the Israeli coast is affected by relatively very high waves.
A long-term analysis of wave measurements recorded in Ashdod between 1 April 1992 and 31 March 2011 and in Haifa between 11 January 1993–31 March 2011 shows that about 51% of annual waves in Ashdod came from west-northwest [41], while about 70% of the annual waves in Haifa came from west to west-northwest [42].
The most common direction sector for all waves measured in Ashdod and Haifa between 1993 and 2015 was 295–290° and 285–290°, respectively. Extreme waves (Hs > 5 m), however, came from a wider direction sector of 255–315° in Ashdod and 275–310° in Haifa [45].
2.3.4. Longshore Currents and Sand Transport (LST)
Until the construction of the Low Dam at Aswan (1902), and especially after the construction of the High Dam at Aswan (1964), the primary source of sand for the Nile littoral cell was the Nile River. The High Dam’s construction, however, effectively blocked this flow and forced the longshore currents to take sand from the Nile Delta coasts and its seabed, which are continuously eroding ([47] and references therein). The sand is transported by longshore currents eastward to the northern Sinai coast [48,49,50,51] and continues northeastward along the Gaza Strip and Israel’s coasts up to Haifa Bay, which constitutes the northernmost final depositional sink of the Nile littoral cell [17,18,19,44,47,52,53] (Figure 2: inset). LST estimates along the Nile Delta and northern Sinai coasts [54,55] indicate a continuous decrease of sand transport rate as the longshore currents move eastwards and then north-eastwards, up to the Gaza Strip [56,57] and southern Israeli coasts.
Longshore currents are generated along the Israeli coast by radiation stresses of breaking waves in the littoral zone and shearing stresses of local winds acting across the shelf [34,58,59]. Wave-induced currents are generated in the surf zone, generally limited to about 5 m water depth, and during extreme events may extend to about 10 m water depth [60]. Since radiation stresses are generally at least an order of magnitude greater than shear stresses, the former predominate in the surf zone during storms. Beyond this region, however, to about 30 m water depth, shelf currents are generated by local winds. The wave- and wind-induced longshore currents occur in both directions along the Israeli coast. However, the long-term net LST drifts northward along the shallow continental shelf (i.e., 0–30 m water depth), up to Haifa Bay (Figure 2: inset) [17,23,44,47,52,53]. It is estimated that the wave- and wind-induced long-term annual LST rate decreases from about 400,000 m3 net to the northeast at Ziqim [61,62] to ~200,000 m3 at Ashdod, ~100,000 m3 at Tel Aviv, and ~80,000 m3 (±20,000 m3) at the entrance to Haifa Bay, the northern end of the Nile littoral cell.
2.3.5. Tidal Regime
The tidal regime along the Israeli coast exhibits a semi-diurnal and fortnight periodicity and ranges from 15 to 40 cm [63], which is not sufficient to create sediment transport or beach eroding currents but affects the ecology of intertidal biota.
2.4. Marine Habitats
The marine habitats in Israel’s Mediterranean territorial water and EEZ are described in detail following an Environmental Strategic Survey (ESS) produced by the Israel Ministry of Energy and ILOR, www.gov.il/BlobFolder/guide/enviromental_info/he/SEA_G_%20After_Public_comments_102016.pdf (accessed on 24 August 2022), and in [10]. The coastal habitats include soft and hard habitats and open coastal water.
2.4.1. Hard Substrates
In the above-mentioned survey, the hard substrates received a high vulnerability index. This implies that almost all the hard substrata are located on the continental shelf up to 100 m depth, and most of it is concentrated in the northern part of Israel. The hard substrata include abrasion platforms (also called “vermetid reefs” [64]), submerged kurkar ridges, coastal rocks, and artificial hard substrates.
2.4.1.1. Abrasion Platforms
The abrasion platforms (AP) (e.g., Figure 4A) found in the inter-tidal zone are internationally considered a unique habitat. They are inhabited by characteristic biota and are relatively rare, especially along the southern coast. These natural rocky substrates are ecologically imported as well as highly threatened habitats. They are horizontal rocky (kurkar) platforms covered by a biogenic crust. The surface of the platforms is exposed to the air at low tide and are under water at high tide. The organisms that live on the platforms are adapted to survival in environmental conditions of temperature, humidity, salinity, and oxygen saturation that change drastically daily, monthly, and annually. The zonation phenomena associated with the tidal conditions is typical to all coastal marine natural and artificial habitats east of the water line, including AP. Each zone—constantly covered by water, flooded only part of the time, and dry most of the time—has different living conditions. The living organisms are distributed in various zones according to their ability to withstand desiccation and other environmental stressors (such as extreme values of salinity and temperature and strong currents) as well as their need for water cover for feeding and reproduction. The different adaptations of diverse organisms frequently create clear borderlines between the distribution areas of each species, manifested as separate zones. On the surface of the AP, dissolved craters are created: shallow ponds and tidal pools that retain water during low tide. These give rise to various sub-habitats with different conditions and populated by a high diversity of macro algae and animals. The erosion of platforms is prevented by the biological building of the sessile gastropod vermetid worm, Dendropoma anguliferum, which, together with calcareous algae, used to be the main ecosystem builder of the AP. This process enabled the platforms to keep a steady state up to the waterline and thus functioned as a natural breakwater that helped to protect the coastline and its cliff.
2.4.1.2. Submerged Kurkar Ridges
Along the Mediterranean continental shelf of Israel, mainly in the north, there are several submerged kurkar ridges (SKR), which run parallel to the coastline at between 10 and 130 m water depth. They cover about 25% of the area up to 30 m depth, but the coverage decreases in deeper water. On the southern coast, there are a few discontinuous SKR. On the northern coast and down to at least 20–30 m depth, this is the dominant habitat. It is ecologically important because of the high species richness and diversity. Most ridges are submerged, but in certain locations, they protrude above the water surface, creating tiny coastal islets, especially between Akhziv and Rosh HaNikra, that serve as nesting sites for waterfowl. The rocky reef of this habitat supports a very high biological diversity of organisms inhabiting the rocky substrate and around it as well as those dwelling inside the substrate. These diverse biota include algae, invertebrates, and fish—some rare and threatened species.
2.4.1.3. Beachrock
Beachrock (e.g., Figure 4B) is found in the shallowest part of the shore and is considered common and accessible in Israel, but it has been studied less than other coastal marine formations. Zonation is also typical to beachrock. In the driest conditions of the supra-littoral, microorganisms such as Cyanobacteria (“blue-green algae”) live on and inside the hard substrate as well as gastropods of the Littorinidae family and isopods, namely crustaceans of the genus Ligia. Other biotic components of the beachrock are barnacles, crabs, bivalves, and mobile gastropods.
2.4.1.4. Artificial Hard Substrates
The Mediterranean coast of Israel is rich in artificial infrastructures, such as ports, marinas, breakwaters, and power and desalination plants. The numbers, volume and diversity of these structures have increased dramatically over the last 100 years. They serve as artificial hard substrates that function as an additional man-made habitat for marine biota since they supply accessible substrate for settlement of sessile organisms such as algae, coelenterates, bryozoan, bivalves, and barnacles as well as attracting mobile organisms such as crustaceans and fish. In addition, old and modern shipwrecks act as artificial reefs (e.g., Figure 5).

Figure 5.
Part of a shipwreck that is an artificial habitat attracting Lessepsian red squirrelfish (photograph by Hagai Nativ).
2.4.2. Soft Substrates
These habitats are characterized by an unstable and often mobile substrate that is influenced by currents, waves, and mechanical disturbances preventing settlement of many organisms. There is a lack of complex niches, which limits the settlement of sessile species, and thus, the species diversity is relatively low, especially in shallow areas (although biomass may be high). As we move offshore to deeper water, the effect of the waves on the bottom decreases, and the conditions stabilize, enabling a gradual increase in the diversity of invertebrates and fish, including commercial species such as shrimps and soft bottom fish that are the targets of trawl fishing. Even though the biological diversities of soft bottom habitats are lower than those of hard bottoms, one should not minimize the importance of the soft habitat because of lack of knowledge of its species diversity and richness.
2.4.3. The Water Column
The water column (pelagial) contains most of the marine biomass. It has a huge ecological importance to the energy balance as well as the biodiversity of the coast and the bottom since water carries and distributes food particles and propagules of bottom organisms. The biodiversity of the pelagial includes hundreds of species of plants, invertebrates, and fish. Primary producers, such as photosynthetic bacteria and microalgae, are the basis of the food web in this habitat. Primary consumers include species of zooplankton—unicellular animals, copepod crustaceans, shrimps, worms, and jellyfish. The latter can act as a living substrate to attract other pelagic organisms such as fish (e.g., Figure 6) as well as larvae of benthic and pelagic taxa. There is a partial knowledge of the planktonic ecosystem in shallow water down to 10 m water depth but not of that in deeper offshore water. There is insufficient information, especially spatial, of any pelagic taxa, including large organisms such as marine mammals, turtles, sharks, and birds. There is also lack of knowledge on taxa that have considerable effect on the marine ecosystem and human activities, such as jellyfish and the comb jelly.

Figure 6.
The invasive Lessepsian nomadic jellyfish, Rhopilema nomadica, in the open Mediterranean coastal water of Israel with juvenile slender yellowtail kingfish (photograph by Hagai Nativ).
2.4.4. Rare Habitats
There are also rare habitats, such as the Akhziv submarine canyon near Rosh HaNikra [65], meadows of the slender seagrass Cymodocea nodosa, and maërl habitats—coralligenous assemblages of calcareous rhodophytes (e.g., [66]). The ecological knowledge of these habitats is minimal or non-existent.
2.5. Biota
Despite the oligotrophic nature of the Levant and the relatively low biomass, the biodiversity may be locally high due to the presence and prevalence of many species and some unique habitats, such as the abrasion platforms [64]. Some marine species may be termed “flagship species” (e.g., lobsters, sharks, sea turtles, and marine mammals), defined [67] as “popular charismatic species that serve as symbols and rallying points to stimulate conservation awareness and action”.
Most indigenous marine biota are of Atlantic origin, but since the opening of the Suez Canal in 1869, there has been considerable influx of alien marine species, mainly from the Red Sea and the Indian Ocean, in a process called “Lessepsian migration” (e.g., [68,69,70]). Many of these migrant species have established viable reproductive populations on the Mediterranean coast of Israel. Some invasive species are dangerous to humans by being venomous (e.g., stinging jellyfish (Figure 6) and venomous striped eel catfish (Figure 7B)) or poisonous (e.g., pufferfish). Others are also a threat to the ecosystem (e.g., rabbitfish and lionfish (Figure 7A)). The number of multicellular non-indigenous species (NIS) recorded on the Mediterranean coast of Israel is 452, distributed into 245 families. Most of Israeli NIS (87.4%) are considered to have been introduced through the Suez Canal. The most speciose NIS are mollusks, fish, crustaceans, and macro algae, comprising 33%, 21%, 14%, and 12%, respectively, of the total number of recorded NIS (for more details, see Section 3.10.1).

Figure 7.
Lessepsian invasive venomous marine fish on the Mediterranean coast of Israel: (A) lionfish and (B) a school of striped eel catfish near an artificial reef (photograph by Hagai Nativ).
Some additional biological data can be obtained from the Israel Marine Data Center (ISRAMAR) of the ILOR isramar.ocean.org.il/isramarbio/default.aspx (accessed on 24 August 2022).
2.5.1. Plankton
In the oligotrophic environment of the Levant, the coastal phytoplankton depends on nutrient supply, some of which originates from land runoff. This dependence on nutrients also affects the coastal zooplankton community.
Representatives of many taxa of phytoplankton and zooplankton [71] can be found in the pelagic zone of the Israeli Mediterranean coast. Some are hollo-planktonic (spending all their life as pelagic plankton), while others spend only part of their life cycle as plankton. Several benthic taxa have planktonic propagules (eggs, larvae, juvenile stages). Swarms of the Lessepsian jelly Rhopilema nomadica (e.g., Figure 6) were found to feed mainly on micro-zooplankton [72] (see Section 3.10.3.5).
In recent years, repetitive appearances of swarms of ctenophores and siphonophores were also reported from the Mediterranean coast of Israel (e.g., [73]).
2.5.2. Macro-Algae
A total of 307 species of macroalgae were reported on the Israeli Mediterranean shore [74], of which 86 species are regarded as exotic, of which 68% are Lessepsian species, and 20% are of Atlantic origin. The benthic macroalgae comprise an important component in the community of primary producers. They also add to the hard substrate of their habitats by trapping and depositing grains of sand, enhancing the complexity of the habitats, and enabling settlement of other marine biota. There is a deficiency of knowledge of macroalgae in depths of 10–30 m, the distribution of the primary productivity in the marine space, the rates of CO2 fixation (for climate regulation), and the species that make the largest contribution to productivity.
2.5.3. Benthic Invertebrates
Among the sessile benthic invertebrates, there are representatives of diverse phyla, including sponges, coelenterates (early life stages of jellyfish, adult corals (about 10 species in the shallow coast, and deep-water soft corals), and sea anemones), Polychaeta (500–800 species), decapod crustaceans (at least 170 species), barnacles (about 10 species), echinoderms, and Bryozoa (about 50 species), [10,75,76]. Mollusk species were once the most numerous along the Israeli Mediterranean coast (948 species, including 636 gastropods, 257 bivalves, 34 cephalopods, 10 Scaphopoda, 9 Polyplacophora (chitons), and 2 Aplacophora [76]). However, there has been a dramatic change in these taxa in recent years (see Section 3.10.4.1).
2.5.4. Pelagic Invertebrates
Pelagic invertebrates are also divided into those spending their whole lives in the open water and those who spend only certain life stages in the pelagial. Among the best-known pelagic invertebrates are foraminifera, copepods, mollusks, such as octopuses, squid, and nudibranchs, swimming marine worms, jellyfish, and the comb jelly (Ctenophora).
2.5.5. Cartilaginous Fish
There are 58 species of cartilaginous fish (Condrichthys) listed in the Mediterranean water of Israel [77]. The main taxa include rays (Batoidea) (stingrays, guitarfish, skates, and others) and sharks. Most species are predators or scavengers. The population of the whole Mediterranean includes at least 81 species, including 49 sharks, 34 rays, and 1 chimera. Most of the species (35 sharks, 30 rays, and the chimera) were also recorded in the eastern Mediterranean.
2.5.6. Bony Fish
A total of 411 species of bony fish are listed in the Mediterranean waters of Israel [77]. At least 38, mostly newly recorded, species are of Red Sea origin. Most fish species are found only on the shelf, while their richness and prevalence drop sharply in deeper water.
The most common species with considerable biomass are small pelagic planktivorous fish, mainly sardines, and true and jack mackerels. On the substrate of the shelf, they are joined by small fish species that feed mainly on benthic invertebrates. The prominent species, based on biomass, are Indo-Pacific goatfish, breams, and lizardfish. The last three taxa are Lessepsian migrants [77].
2.5.7. Marine Turtles
The two species of marine turtles that are relatively common and reproduce and nest on the Mediterranean coast of Israel are the loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles [78] (Figure 8), with an estimated 100 spawning females of the first species and not more than 10 spawning females of the second.

Figure 8.
Loggerhead (Caretta caretta) (A) and green (Chelonia mydas) (B) sea turtles off the Mediterranean coast of Israel (photograph by Hagai Nativ).
The leatherback sea turtle, Dermochelys coriacea, is a rare visitor to our coasts. Since most of the Israeli coasts are not suitable for nesting due to human activities, the Israel Nature and Parks Authority (INPA) transfers more than 80% of the spawning to six nesting farms in wide sandy beaches. Recently, ten loggerheads and five green turtles were tracked via satellite telemetry tags [78]. They spent their time foraging in a median of 137 km2 core home. The home range size increased to a median of 464 km2 during the inter-nesting season. Migration varied widely, ranging from 87 km from the tagging site in to more than 3000 km. Most turtles migrated short distances within the southeastern Levant Sea, which seems to be a multifunctional habitat for reproduction, migration, and foraging.
2.5.8. Marine Birds
There are 34 species of marine birds known on the Mediterranean coast of Israel. Only ten species can be considered true marine birds (those arriving to shore only for nesting), but there is no quantitative information on their numbers or the number of nesting pairs. Among these are the rare Mediterranean shearwater, Puffinus yelkouan, and other species of shearwater, three species of boobies, several dozen species of seagull, terns, stormy petrels, and skuas. This is a species richness considered relatively high for the Mediterranean [10].
2.5.9. Marine Mammals
Most of the cetacean species known to be present in the Mediterranean Sea also occur in the oligotrophic waters of Israel. Overall, the cetacean fauna of Israel includes 12 species that are either regular or vagrant (for details, see [79]). Only one species, the long-finned pilot whale, has not been recorded in Israel or the whole Levantine Basin. The knowledge of marine mammals on the Mediterranean coast of Israel, especially quantitative information, is partial and restricted in time and space to a narrow strip along the shore and is based on limited observations and stranding records of the investigators and volunteers of IMMRAC (Israeli Marine Mammal Research and Assistance Center). Only one species, the common bottlenose dolphin, Tursiops truncates, (Figure 9), is relatively prevalent, predictable, and accessible enough to enable a comprehensive study of its coastal population (e.g., [80]). There are rare reports of sighting the Mediterranean monk seal, Monachus monachus, on the coast of Israel [81].

Figure 9.
Common bottlenose dolphin, Tursiops truncatus, off the Mediterranean coast of Israel (A) and with a cub (B) (photograph by Aviad Scheinin).
2.6. Fisheries
Hornel [82] supplied a comprehensive (for that time—1935) report of the Mediterranean coastal fisheries in this area. He stated that “There is no dearth of good-quality food-fishes… What is at fault is the inadequate exploitation of these resources”. He claimed that the fishermen used primitive methods. He pointed to the lack of safe harbor accommodation suitable for fish and fishing boats and described the following legal fishing methods: the shore seine, the trammel net, the mullet net, the gill net and throwing nets, hook and line fishing, and traps and trawling (adding that Italian trawlers were supplying a considerable portion of the fish landed). He also mentioned illegal methods, such as the use of explosives and poisonous substances. These illegal methods were used in the first 50 years of the period reviewed, and although their scope has decreased dramatically in later years, they have not completely disappeared. New regulations and prohibitions were recommended for the Mediterranean coastal fisheries in the 1930s [82], including the re-establishment of a Fisheries Service, enforcing the use of nets with larger meshes, and a minimum legal size for fish caught. Many of his recommendations were adopted and modified in later years. He reported that the total landing of all species increased from 1045 tons in 1930–1931 to 1131.6 tons in 1933, and the main species caught were of Atlanto-Mediterranean origin. Estimates of total fisheries removals on the Mediterranean coast of Israel were reconstructed as slightly over 255,400 tons for 1950–2010 [83]. The authors point out that Indo-Pacific organisms are a large and growing component in the multispecies catch of Mediterranean fishes; however, they appear to change species composition and mode of exploitation more than they affect the level of total removals. Total catches of industrial, artisanal, and recreational fisheries (but without discards) increased from about 200 tons in 1950 to about 5000 tons in the 1980s to 1990s but dropped to less than 3000 tons in 2010 [83]. The catch in recent years has been declined to 1500 tons. Basically, some of the fishing methods employed before the establishment of the State of Israel in 1948 have remained, with improvements, in later years. The fishing of some minor targets, such as sponges, and sea turtles [82] has ceased in recent years, while the fishing of shrimps and cephalopods has increased lately due to market demands and high prices. There has always been some recreational fishing in Israel. As the population of Israel has quadrupled since 1950, the recreational fishery has also grown considerably, mostly in the 21st century [84]. It was estimated that more than 70,000 Israelis contribute significantly to fish extraction from the sea through their recreational activities [84]. The recreational catch comprises between 10% and 37% of the total annual fishing yields, which is similar to estimates from other regions of the Mediterranean [85,86].
3. Environmental Impacts
3.1. Changes in Water Temperature and Salinity
Before the completion of the Aswan High Dam in 1964, the Nile discharged into the southern Levantine Sea 50–300 × 106 tons of sediment and 86 × 109 m3 of freshwater annually (between August and November) [17]. Hydrographic measurements of shelf waters have revealed an abrupt decline of salinity of surface water in the late summer months off the southern Israeli coast [87].
Since the ending of the Nile flood in 1965 (and freshwater utilization of other rivers in the Levant), salinity has increased, reaching 39.3% in the fall [88]. Average salinity in the upper mixed layer (0–10 m depth) in the open sea was 39.75% [89].
The eastern Mediterranean and Middle East is warming almost two times faster than the global average and other inhabited parts of the world [90]. This rate is significantly faster than the average rate of warming of the seawater in the world and faster than the rate of warming of other parts of the Mediterranean [91]. Measurements over 40 years reveal a warming trend (0.13 °C/y) far higher than those projected by the Intergovernmental Panel on Climate Change (IPCC) (0.035 °C/y, 2016–2035), possibly due to the longer residence time of water in the Levant [92].
Temperature in the shallow waters of the eastern Mediterranean has risen during the summer by over 3 °C, from a maximum of 28.4 °C in the 1960s to 31.5 °C at present (e.g., [9]). The sea surface temperature in the Levantine Basin increased by about 5.1 °C from 1982 to 2019 and is now the highest in the Mediterranean. The main change is in the summer months. These physical changes make a considerable impact on the biota and ecological processes.
3.2. Sea Level Rise (SLR)
The Survey of Israel (SOI) has been measuring sea level (SL) since 1958. During the past 63 years, the SL has risen by an average increase of about 0.8 mm per year. The rate of SLR has increased through the years (Figure 10).
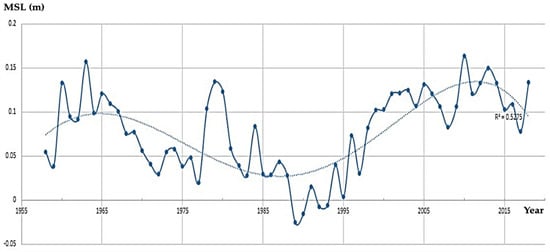
Figure 10.
Mean sea level (MSL) change along the Mediterranean coast of Israel: 1958–2018. (State of Israel, Ministry of Construction and Housing, Survey of Israel).
Although the rate of SLR is considered comparatively slower than the rate of seawater warming, this relatively fast SLR may cause flooding and erosion of the coast and threaten coastal human assets and activities, (e.g., [93]) as well as biota in the intertidal zone [94,95]. In the AR6 report of the IPCC published in August 2021, the SLR in Israel is expected to range between 20 and 30 cm in 2041–2060 (depending on the scenario) and between 40 and 70 cm in 2081–2100, above the baseline of average SL.
3.3. Wind and Wave Storms
Extreme weather events, such as storms, are expected to occur more frequently and with greater intensity due to climate change and are likely to be devastating for ecosystems and humans [95]. For example, an exceptional stranding of the Mediterranean slipper lobster, Scyllarides latus, was reported [96]. A total of 8 and 12 adult lobsters were found in two occasions on a sandy beach of northern Israel close to a winter seasonal shallow rocky complex habitat of this species. Both strandings were recorded immediately following extremely severe storms. The maximum wave heights of these storms were 7.8 and 9 m, and the wave periods were 11.1 and 11.8 s, respectively. This is the first report of stranding for this species and in the whole Scyllaridae family of lobsters.
The predicted increase in the frequency and intensity of wave storms is also expected to increase sediment suspension; enhance water turbidity with negative implications on filtering organisms such as sponges, tunicates, and bivalves; and reduce underwater visibility.
3.4. Ports, Marinas, and Shipping
Several dozen marine structures have been constructed along the Mediterranean coast of Israel since the construction of Haifa Port during the British Mandate, starting in 1929 and completed in 1933 [97] (Figure 11).

Figure 11.
Haifa Port under construction during the British Mandate in 1931 (A) (Library of Congress Prints and Photographs Division Washington, D.C. 20540 USA) (matpc 08935 hdl.loc.gov/loc.pnp/matpc.08935 accessed on 27 September 2022) and the Haifa Port area in 2022 (B) (photograph by Dov Zviely 28 May 2022).
The significant economic growth was accompanied by a considerable growth in commercial shipping. This increase in maritime activities required additional anchorage facilities.
The present structures can be classified into four groups according to their projection into the littoral zone: (1) groins and beach-parallel detached breakwaters, projecting 100 to 200 m offshore, at water depth of 3 to 5 m; (2) marinas, harbors, and power plant cooling basins, projecting 400 to 600 m offshore, at water depth of 5 to 9 m; (3) commercial ports, e.g., Haifa and Ashdod ports, projecting about 2 km offshore from the natural coastline, at water depth of 21 m and 24 m, respectively; and (4) offshore coal jetties at Hadera and Ashkelon ports projecting 2 km offshore, at water depth of 28 m.
3.5. Coastal Erosion and Sand Accumulation
The morphological impact of the marine structures on their adjacent beaches and seabed were studied in detailed by using aerial photographs, coastal geodetic measurements, hydrographic surveys, bathymetric charts, and some ecological surveys. The studies show that sand accumulation has developed at the southern side (upstream), while the northern side (downstream) of marine structures along Israel’s southern coast has eroded. Along Israel’s central and northern coasts, however, this morphological phenomenon is less dominant and even reversed around some small coastal structures [18,19,20,23,47,60,61,98,99,100,101,102,103,104,105] (Table 1). Sand erosion as well as accumulation may increase in magnitude and frequency due to projected escalation in the intensity and occurrence of extreme wave storms (see Section 3.3).

Table 1.
Major marine structures on the Mediterranean coast of Israel 2022.
Sand accumulation and loss may change habitats, with significant implications for their inhabitants. In case of coastal accretion, organisms may be covered by and buried in the sand, and in case of erosion, organisms may be swept away. In both cases, there is a change of the nature of the marine habitats from hard substrate to a soft one and vice versa.
Zifzif is a local term for a medium-to-coarse sand type that includes a certain percentage of sand grains over 0.5 mm in diameter, with or without the remains of shells of marine organisms. Zifzif mining on the Mediterranean beaches for construction purposes was customary before the establishment of the State of Israel. It intensified and continued until 1964. Zifzif was transported, mainly on camels or in carts, to construction sites and brick casting sites. Sand mining was prohibited on Israel’s beaches through the law known as the “Zifzif Law” of 1964. By that time, about 10 million cubic meters of sand had been lost from the country’s beaches.
The continuous mining caused damage to the width of the coast and hence to the coastal habitat and the marine and coastal biodiversity and at the base of the cliffs above the coast.
Following the 1964 legislation, sand mining was concentrated in the dunes east of the coast. From the 1960s until today, about 60 million m3 of dune sand have been mined from the coastal plain, and many dune landscapes have disappeared.
It was estimated that during the 20th century, some 20 million m3 of sand was removed from the coastal zone by mining (legal and illegal) and entrapment of sand around coastal structures [99,105]. This quantity is equivalent to the natural influx of sand to the Israeli coast over 50 years. Possible damages from coastal sand mining are accelerating the retreat of the coastline, including indirect damages such as flooding of the interior of the land during storm events due to the drifting of sand from dunes (and SLR; see Section 3.2); loss of lands of social, economic, or ecological importance; flooding of the coastal area due to the undermining of artificial coastal defenses; and a change in the wave regime. Sand mining can, above and below sea surface, cause extensive damage to the coastal and marine ecosystem—a direct damage due to the removal of the sand, its suspension, and covering of new areas in suspension. It includes also indirect injuries, which result from the suspension of particulate matter in the water, from increasing turbidity and decreasing light penetration, from the release of organic matter and possibly also hazardous chemicals, and from changing the grain size distribution and from topographical changes in the bottom, for example, following the digging of pits.
The Mediterranean coastal zone of Israel and the inner shelf, as mentioned above, is mainly composed of fine quartz sand. This non-renewable resource, which has been used for various purposes, especially in the 20th century; has a high environmental value; and is essential for the Israeli economy and its development.
Over the past 20 years, there has been a dramatic increase in the use of marine sand dredged along the Israeli coast. During this period, the seaports of Ashdod and Haifa (Figure 2) were significantly expanded to include new large-container terminals that could serve large container ships (e.g., the Triple E-class). For the construction, a huge amount of sand of about 20 million m3 dredged from the ports’ vicinity was used to fill the new terminals. Simultaneous with the port expansion, various projects that required several million cubic meters of marine sand were carried out along the Israeli coast, for example: covering pipelines embedded in the seabed, sand bypass operations around the port of Ashdod, and sand beach nourishment activities on several eroded coasts.
The significant increase in demand for marine sand since the beginning of the 21st century and current development plans on the continental shelf and many beaches raises questions about Israel’s future ability to supply marine sand for various uses and serious concern for the marine environment and the fate of the sandy beaches.
Beach nourishment may affect natural ecosystems in the imported site (i.e., borrow area) as well as on the nourished beach. The ecological consequences of the nourishment on coastal biota may be short or long term. The environmental impacts may lead to sedimentation and turbidity that affect light penetration and filtering organisms. It may cause burial of organisms that reside in the nourished area, and the effects of heavy equipment used in the nourishment operation may injure, kill, or affect the behavior and physiology of the native biota. It can change the nature of the local habitat (e.g., altering the grain size and type or change hard substrate to a soft one). Changing the sediment composition may alter the types of organisms that inhabit the nourished beach. Beach nourishment may also displace native biota. It was claimed [106] that beach nourishment in the Haifa Bay enhanced the introduction and dispersal of the non-indigenous, aggressive, omnivorous, invasive Lessepsian moon crab, Matuta victor. However, a recent study [107] proved that the spread and establishment of this invasive species in the Eastern Mediterranean is not associated with beach nourishment.
Maintaining operational depth in the ports and marinas is also important for the coastal sediment balance. Dredging of sand and other sediments from the seabed in the vicinity of ports and marinas located along the Mediterranean coast of Israel is also carried out to maintain the operational depths of these marine structures. These routine activities involve the dredging of large volumes of sand to maintain sailing channels, harbor entrances, maneuvering areas, and the area of the terminals in the Ashdod and Haifa Ports. To a lesser extent, they also involve entrances and other areas of the Ashkelon and the Hadera Ports, the main marinas (Ashkelon, Ashdod, Tel Aviv, and Herzliya), and other smaller anchorages.
Most of the sand dredged in the ports’ channels and anchorage areas was used to build new container terminals. The rest of the sand was dumped in deep water or near the beaches according to the grain size and the polluting material it contained. It is significant that polluted sediment (including sand, silt, clay, and sediment from inside the ports) was removed to maritime waste sites (i.e., Epsilon and Alpha), which are in deep water far beyond Israel’s continental shelf.
In contrast to the dumping of sand dredged in the ports, the sand dredged near the marinas was largely dumped in shallow water (5–10 m water depth) north of the dredging area. It can be estimated from the existing data that the amount of sand dredged in a normal year in all the marinas in Israel is about 100,000 to 150,000 m3. Although this is a large-scale activity, there are no data on the total amount of sand dredged so far around marinas. Moreover, not all the quantity of sand approved for dredging by the authorities has actually been dredged.
From the 1960s until the late 1990s, ports, marinas, detached breakwaters, and other small coastal structures were built along the Mediterranean coast of Israel (see Section 3.4 and Table 1). These marine structures interfered with the LST drift in their surrounding area, and as a result, local morphological changes developed in the nearby seabed and the neighboring sandy beaches [101,102,103,104,105,108,109,110,111,112,113,114,115].
During the 1990s, the planning and environmental authorities in Israel changed their approach to the measures to be adopted to reduce the negative impact of marine structures on the natural LST drift. As a result, and according to National Master Plan 13/B/2 (the “Yovel Port”), the Port and Railways Authority (today IPC—the Israel Ports Development & Assets Company Ltd.) was required to bypass sand accumulated south of Ashdod Port to areas north of the Eshkol power plant. During the period 2000–2004, large amounts of sand were dredged from the seabed (at water depth of 6–10 m) between the Ashdod Marina and Ashdod Port and dumped north of the Eshkol Power Station at a water depth of 6–10 m. The sand bypass was carried out four times, where each time, a sand volume of ~180,000 m3 was transferred (total volume of about 720,000 m3). Between May and August 2011, IPC renewed the sand bypass in the Ashdod Port area. During this activity, sand beach nourishment was implemented for the first time on the Mediterranean coast of Israel. The aims of this activity were: (1) to bypass sand from the huge sandbar stretching south of Ashdod Port main breakwater and (2) to nourish the eroded coast north of the port. For the nourishment, a total volume of sand of about 315,000 m3 was dredged from two sites: between the Ashdod Marina and Ashdod Port at a water depth of 5–8 m (~100,000 m3) and in the Ashdod Port area (~215,000 m3). The sand was deposited between the coastline and water depth of 3 m by rainbowing via a discharge pipe at the bow of the dredging vessel anchored at a water depth of 6 m (Figure 12).

Figure 12.
Nourishment rainbowing operation via discharge pipe. North of Ashdod Port, May–August 2011 (photograph by EDT Marine Construction).
At the end of the operation, a 1 km long coastal section had received nourishment, starting about 2.8 km north of Ashdod Port’s lee breakwater in an area 30 by 80 m (Figure 13A).

Figure 13.
Beachrock coast north of Ashdod Port (A) during nourishment operation (May–August 2011) and (B) in May 2012 (photograph by Dov Zviely).
In spring 2012, a few months after the nourishment was completed, a site visit found no evidence of the massive sand nourishment, while the beach had reverted to its previous rocky state (Figure 13B). A comparative analysis of bathymetric maps showed that in July 2012, half of the nourished sand volume had left the nourished site, and the rest had migrated to deeper water.
Since 2011, beach nourishment has been a preferred method of the Israeli authorities for preserving and expanding eroded beaches and has been carried out (from south to north) in north Ashkelon (2015–2019), north of Ashdod Port (2011), Bat-Yam beach (2020), Netanya beach (2022), and Kiryat Haim beaches (southern Haifa Bay) (2011–2022).
For detailed information on sand nourishment projects carried out in north Ashkelon, the north Ashdod Port area, and in Haifa Bay between 2011 and 2017, see [46] and Figure 14.
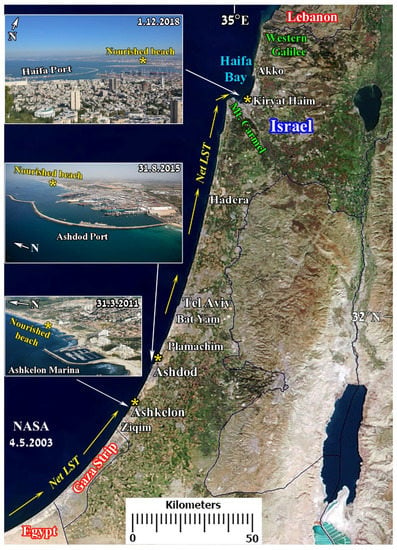
Figure 14.
The Mediterranean coast of Israel and the three sites where beach sand nourishment was carried out between 2011 and 2017: south of Haifa Bay (top inset); north of Ashdod Port (central inset); north Ashkelon (bottom inset). Net longshore sand transport (LST) direction (yellow arrows). Background: part of “Middle East” space image, Jacques Descloitres, MODIS Rapid Response Team, NASA/GSFC, 31 January 2013.
The sand bypass in the Ashdod Port area continued in 2013 (103,000 m3), 2016 (~100,000 m3), and 2019 (203,000 m3). These activities were carried out as part of the Coastal Sand Resource Management Mechanism (the “Manganon”), whose activity and responsibility are specified in the Appendix to National Master Plan 13/B/2/1/A (i.e., the South Port project) [116].
3.6. Coastal Power and Desalination Plants and Fuel Transport
Pinhas Rutenberg founded the Palestine Electric Corporation, currently the Israel Electric Corporation (IEC), in Mandatory Palestine in 1923 and built the first power plant, with a capacity of 300 kW, powered by diesel-fueled engines [117].
The development of the country and the waves of immigration in the 1930s led to a rapid increase in the demand for electricity. Coastal power plants were built in Haifa and Tel Aviv, using fuel oil with steam technology with a capacity of tens of megawatts.
After the establishment of the State of Israel, there was a rapid increase in demand due to the development of industry and the rapid increase in the population. As a result, the Haifa and Tel Aviv coastal stations were expanded with additional steam units. From the 1950s to the 1970s, the Eshkol power station was established in Ashdod, and the expansion of the stations in Haifa and Tel Aviv continued. In the mid-1970s, construction began on the Hadera power plant, which was planned to be powered by coal (instead of the fuel oil that had been used in the steam stations until then). In the mid-1980s, construction began on the Rotenberg power plant in Ashkelon, which was also planned to be powered by coal. These stations were expanded during the 1990s. In the early 21st century, the power plants were converted to operation using natural gas as a substitute for fuel oil and diesel and later also for coal due to the discovery of a lower price natural gas near Israel and to reduce emission of atmospheric pollutants. As at 2019, the IEC capacity was 13,335 MW. About another 30% of the electric energy in Israel was produced by private producers using fossil fuels and renewable energies (IEC Archive: www.iec.co.il/content/archive/pages/iecfoundation, accessed on 24 August 2022).
Although the main environmental effect of coastal electric plants is air pollution, they also affect the marine environment. Already in 1935, it was mentioned [82] that “Contamination by waste oil discharge from oil-vessels is extremely hazardous to fish and marine birds”. In the past, bathing beaches were often polluted by oil spill from tankers transporting fuel for power plants and other users. The use of more modern double-walled tankers and tougher regulations have reduced the risks of oil leaks. However, in February 2021, tar originating from a large offshore crude oil spill from an initially unknown source devastated sea life (including invertebrates, fish, marine turtles, birds, and mammals) in the Mediterranean and spewed tons of tar across more than 180 km of the coastlines of Israel, southern Lebanon, and Gaza (e.g., Figure 15) in what some Israeli officials called “The worst ecological disasters in decades”. This severe pollution event was associated with stormy weather and high waves (e.g., [118] and references therein). Intensive cleaning efforts were conducted along the Israeli Mediterranean coasts until March 2022, at which point 1400 tons of litter and tar had been removed. Investigation of the Israeli Ministry of Protection of the Environment as well as an independent investigation by the NOAA (U.S. National Oceanic and Atmospheric Administration) agency identified the guilty ship in the incident based on strong circumstantial evidence. The ship Emerald, registered under Libyan ownership, left the Persian Gulf, with its Loran ship location transmitters shut down for most of its voyage and in particular when it was opposite Israel, apparently maliciously dumping crude oil.

Figure 15.
Tar pollution on the rocky coast of Habonim (south of Atlit) February 2021 (photograph by Dor Edelist).
Modern coastal power plants pump huge amounts of seawater to cool their systems. This water is released back to the marine environment at a temperature 10 °C higher than the ambient [119]. Despite a variety of filters, marine organisms are trapped during the pumping process. Although the short-term effect of a thermal discharge from a coastal power plant are local and limited to the water surface, it may be detrimental to sessile organisms. It is important to study the effect of this warm discharge on local organisms, such as plankton, in view of the warming trend of the coastal water (see Section 3.1). In coal-operated power plants, some pieces of coal may fall into the water. However, they are found on the bottom only in the vicinity of the unloading dock, and their environmental effect seems to be minor. In the past, fly ash, a product of coal burning, was dumped in maritime waste sites offshore, but at present, there are terrestrial solutions for this material.
Water demand from Israel’s rapidly growing population has outpaced the supply and natural replenishment of potable water. However, this problem has been partially solved by building coastal desalination plants. Presently there are five seawater desalination plants: in Ashkelon (operating from 2005, with production capacity of 121 million cubic m per year (MCM/y)); Palmachim (2007, 90–105 MCM/y), Hadera (2010, 137–159 MCM/y), Sorek (2013, 152–180 MCM/y); and Ashdod (2015, 100 MCM/y) (Israeli Ministry of Environmental Protection) (www.gov.il/he/departments/policies/desalination_facilities_moep_policy, www.iec.co.il/content/archive/pages/iecfoundation, accessed on 24 August 2022)
Three reverse osmosis (SWRO) plants are currently under construction or planned: Sorek-2 will be operated in 2023, Western Galilee in 2025, and Emek Hefer later. Each of these plants will have a production capacity of 200 MCM/y. Even these relatively new installations affect the marine environment. The potential environmental consequences of desalination activities include damage to coastal natural values, damage to the marine environment by the discharge of desalination concentrate water releasing various chemicals into the sea, damage to the food web at the water intakes, and the effects of coastal and underwater piping.
The concentrated brine created in the desalination process is returned to the sea, increasing local seawater salinity. It tends to sink to the bottom due to its high density, creating a hypersaline habitat that is preferred by euryhaline organisms. However, six years’ monitoring of brine discharge from two 240 MCM/y freshwater seawater desalination plants located 0.8 km apart has shown almost no impact on seawater. Israel is planning to more than double its desalination capacity along its 190 km Mediterranean coast by 2050. A long-term adaptable program, in conjunction with specific research and modeling, should be able to assess and predict the impact of large-scale brine discharge on the marine environment [120,121].
Over the years, there has been a significant decrease in the consumption of chemicals in desalination facilities, including the iron consumption in the pre-treatment system. However, following the establishment of desalination facilities along the coast, an increase in phosphorus discharges to the sea began in 2006, with a significant increase in 2009. These sources are 9% of all marine phosphorus pollution in 2014. In addition, new desalination facilities are obliged to treat rinsing fluids containing iron concentrations and dispose of them to a land disposal site instead of to the sea [119,122].
Although the Israeli power plants pump seawater at an order of magnitude greater than the volume pumped by the desalination plants, the latter destroy the entire biota pumped. It is important to study this effect, especially regarding plankton in the vicinity of the intake pipes.
3.7. Chemical Pollution
At the beginning of the 20th century, Israel’s population density was low, and the amount of waste was minimal and consisted mainly of natural materials, so no considerable pollution was created. The waste essentially decomposed by itself or spread over a large area and did not have a significant effect on the marine food web. During the first decades of its existence, the city of Tel Aviv showed rapid urban and demographic development. This growth resulted in a sharp rise of urban by-products, including a vast increase in the volume of sewage that was mainly disposed of in the Mediterranean. The construction of a municipal sewage system, which started in the late 1920s, increased the relative volume of sewage discharged directly into the sea. In 1949, Tel Aviv’s seashore was declared polluted, and bathing was prohibited. This situation lasted intermittently for three decades. The deterioration of the beach area from its previous status as “Tel Aviv’s Riviera” during the early 1940s into a “malodorous urban back yard” only a decade later resulted in a project aimed at sea purification and reuse of the treated sewage for agricultural purposes [123]. Since the beginning of the 1990s, there has been a change in the field of wastewater treatment in Israel (because of environmental considerations as well as the shortage of fresh water). Wastewater treatment facilities were built, significantly reducing the amount discharged into the marine environment. In 1992, only seven wastewater treatment facilities were operating; in 2009, 39 were operating; and as of 2020, there were 89 facilities classified as large (handling over 1000 m3 per day) (Israeli Ministry of Environmental Protection) (www.gov.il/he/departments/guides/sewage_treatment_plants_and_transmission_piping, accessed on 24 August 2022)
One of the main projects is the “Shafdan” (Gush Dan Wastewater Institute). This is a complex, inter-regional system for the collection, treatment, and reuse of municipal wastewater in a densely populated urban area of the central coastal area of Israel, including industrial areas. It serves a population of over two million inhabitants in an urban and industrial area of about 220 km3. It provides about 140,000,000 m3/y of water for agricultural uses [124]. The main by-product of the purification process is about 15,000 m3/day of sludge (with a concentration of 1% dry matter), which consists mostly of bacteria and contains, among other things, heavy metals and toxins. At the end of the process, it produces excess activated sludge (secondary biological sludge only) discharged to the sea through a distribution system at a depth of 38 m. The vicinity of the disposal site was systematically inspected by the ILOR. The findings indicated a non-accumulating site without extensive polluting effect. However, the concentration of organic material enhanced the development of certain marine biota, attracting fish as well as illegal fishermen ([125] and references therein). However, since 2017, there has been a terrestrial solution for the problem of the sludge, and it is no longer disposed of in the sea.
The main sources of marine pollution in Israel in recent years are polluted streams flowing into the Mediterranean, sewage treatment plants, and sewage systems (especially after heavy rain; see also Section 3.8) that cannot cope with the sewage load and discharge waste washings into river channels and/or directly into the sea. Chemical marine pollution originates from marine activity—oil or fuel leaks from ships (see Section 3.6); excess fertilizers washes into the sea; and fishponds sometimes pollute the sea. Untreated sewage from the Gaza Strip is discharged to the sea and, because of the prevailing currents (see Section 2.3.4), affects southern Israel. The Ashkelon desalination plant, which is responsible for desalination of about 20% of the water in Israel (see Section 3.6), has been shut down several times due to fecal coliform bacterial contamination, apparently from untreated wastewater from Gaza discharged into the Mediterranean.
In recent decades, the Israeli Mediterranean coastal environment has been exposed to various pressures due to rapid population growth and urbanization, coastal development, and intensive agriculture and industrial activities. The discharges of these activities released nutrients, heavy metals, and toxic organic compounds to the coastal water. Haifa Bay (Figure 2), in particular, suffered the highest pollution load along the Israeli coast. Since the 1970s and 1980s, high levels of trace metal accumulation originating in effluents of chemical and petrochemical industries in the Haifa Bay area were found in the Bay’s fish and benthic invertebrates (e.g., [126]). There was a considerable reduction in the discharge of toxic materials to the Haifa Bay since 1990. Despite efforts to reduce this pollution, the Bay’s sediments are still polluted with metals (including mercury) as well as concentrations of metals and nutrients in the vicinity of rivers, streams, and outfalls [127,128].
The surface area of the Mediterranean Sea is only 0.7% of the surface of all the seas in the world, but the transport of fuel in the Mediterranean includes 30% of the world’s volume (e.g., Section 3.6). The Mediterranean is a closed basin, and its eastern part is far from the connection to the Atlantic Ocean at the Strait of Gibraltar. Therefore, the rate of exit of pollutants from the Mediterranean and their dilution in the ocean is slow.
In several specific sites along the coasts of Israel, such as in some estuaries, creeks, harbors, and marinas and Haifa Bay, the level of pollutants originating from the land, such as heavy metals, fertilizers, and organic pollutants, is moderate to severe. However, in comparison with other countries and regions in the Mediterranean, the overall environmental condition of the Israeli Mediterranean coastal water is quite good. Firm and effective conservation, legislation, enforcement, and monitoring activities implemented over the past decades have led to a significant improvement in the state of the marine environment regarding chemical pollution. At the same time, the situation is not perfect: there are still discharges of sewage into the sea (all with a permit or in cases of failure), and in the south, there is still an ongoing threat due to the discharge into the sea of the Gaza Strip sewage. The waters of rivers and streams nevertheless carry with them quite high amounts of nutrients derived from agriculture. According to the latest reports of the National Monitoring Program, there are still centers of heavy metal pollution, mainly mercury in the north of Haifa Bay, and there is yet room to improve the water quality in the vicinity of river estuaries.
3.8. Marine Debris
Marine debris (MD) is human-created waste that has deliberately or accidentally been released in the sea, discarded at sea, or reaches the sea through waterways or domestic or industrial outfalls [129]. MD is considered as threat to the marine biodiversity, ecosystems, animal well-being (e.g., [130]) (see, e.g., Section 3.10.4.4 and Section 3.10.4.5), fisheries, maritime transport, recreation and tourism, local societies, and economies.
There is limited information on MD for the first half of the reviewed period. “Ghost nets” (fishing gear abandoned, lost, or otherwise discarded that continues to catch, injure, and kill marine organisms) have a huge impact on the marine environment worldwide (e.g., [131]). This is due to the present scope of fisheries and durable and non-degradable modern material, especially nylon monofilament nets. It was a minor problem in the first half of the 20th century because of the biodegradable nature of fishing nets (e.g., [82]), which, until the early 1960s, were mostly made of natural fibers such as cotton. On the other hand, the percentage of abandoned fishing gear in samplings carried out between 2018 and 2020 at 20–80 m depth of the Mediterranean coast of Israel has increased significantly every year [132]. In 2020, it already constituted a significant portion of the MD in these depths. Shipwrecks and other maritime equipment scattered along the shore may entangle fishing nets and interrupt ship navigation in shallow water. This was a problem in the past and continues to be part of MD. The introduction of plastic material such as high-density polyethylene bottles and the expanding use of plastic in general in the decades following the 1960s exacerbated the MD problem.
Surveys made from 2012 to 2015 along the Mediterranean coast of Israel indicated an average debris density of 0.12 items/m2, and 90% of the items were non-degradable plastic, exceeding the global average of 75%, and this was 20% greater than reported 10 years earlier [133]. The top debris categories were food wrappers and disposables, plastic bags, and cigarette butts [134]. The density of debris on the Israeli coast was much lower than the global average of 1 item/m2 [135]. This low density may be due to the regular cleaning of most of the Israeli coast (i.e., as part of the Israeli Clean Coast Program, as specified in [136]). The MD composition may be a result of widespread use of plastic bags and food wrappers and disposables by the public. Most MD along the Mediterranean coast of Israel, mainly disposable items, derives from the land, mostly probably left by beach users. A recent study [137] revealed that during two years of the COVID-19 pandemic (2020 and 2021), MD concentration along the coasts was considerably reduced. This finding supports the assumption that the reduction in MD reflected the decline in the number beach visitors. Likewise, submerged marine debris along the Israeli Mediterranean coast is mostly plastic, originating from local beach recreation [138]. Submerged marine debris does not seem to accumulate along the Israeli nearshore due to currents and wave regime (see Section 2.3.4). Floating marine debris, including plastic bottles, travels under the influence of winds and sea currents [139]. It can be swept back to shore or travel up to hundreds of kilometers from the release point. Since most of the westward water flow in the eastern Mediterranean is subsurface, it makes the Levant basin a collection area for floating debris. MD may also arrive from neighboring areas such as Gaza and Lebanon. As the municipal garbage dumps of such cities as Beirut and Sidon in Lebanon are located on the shoreline [133], Lebanese debris arrived, e.g., in November 2010, as far south as Tel Aviv, located 140 km south of the Israeli border with Lebanon. A substantial amount of solid waste arrives at Israeli beaches through municipal drainage systems, especially after the first rainstorm at the end of the rainless Israeli summer [140] (e.g., Figure 16). Such rainstorms may also result in organic as well as plastic MD swept into the sea from land and returned to the coast by the action of high storm waves (e.g., Figure 16).

Figure 16.
Solid waste beach outfall of the municipal drainage system of Netanya after the first rainstorm following the rainless Israeli summer ((A), photograph by Galia Pasternak); organic and plastic debris swept to shore after a storm ((B), photograph by Hagai Nativ).
Another invisible type of plastic MD is microplastics. These are microscopic particles of plastic, 0.1 μm–5 mm in diameter, originating from the degradation of larger plastic pieces in the sea and from clothes, paint, tire dust, plastic litter, and personal care products on land (e.g., [141]). Although the real effects of microplastics on marine biota and on human health are still not fully clear, they represent a serious risk for marine ecosystems and human health.
While plastic was first commercially introduced in the late 1940s, initial evidence of microplastics in the sea was first documented during the early 1970s [142]. Exceptionally high levels of microplastics (0.3–5 mm) with a mean abundance of 7.68 ± 2.38 particles/m3 were found on the sea surface along the Israeli Mediterranean coast [143]. These mean values were 1–2 orders of magnitude higher than abundances reported in other parts of the world, including the western Mediterranean. A comparison of the gut contents of rabbitfish collected from the 1960s to the present showed that there was a temporal increase in the proportion of fish with microplastics from about 10% to 92% in 2016 [142]. Microplastic particles were identified in rabbitfish tissues after two weeks’ exposure, suggesting that ingestion of contaminated microplastics by these edible fish might harm them in the long run and perhaps even to those who consume them on a regular basis, e.g., rabbitfish predators and humans [144]. Microplastic sampled on the Israeli coasts was mostly white or colorless (e.g., [134]). These hues have a very similar appearance to planktonic food and thus promote assimilation into the food web.
Dumping of industrial waste and coal ash into the sea, which was practiced in the past, has completely ceased in Israel. However, beach cleaning operations, enforcement, and regulation activities are still trying to cope with the difficult problem of coastal pollution with solid waste and especially with non-biodegradable plastic. The marine environment continues to suffer from MD, mainly plastic floating, in the water column, or sunk to the seabed, in both shallow and deeper waters.
3.9. Acoustic and Light Pollutions
Sound is the sensory cue that travels farthest through the ocean. It travels about 1500 m/s in seawater versus about 340 m/s in air. It is used by marine animals, ranging from invertebrates to cetaceans, to interpret and explore the marine environment and to interact within and among species [145]. Anthropogenic noise is a growing threat to marine life. Strong evidence for such impacts is available for marine mammals (see Section 3.10.4.5), and some studies have found impacts on invertebrates, marine birds, and reptiles. Since sound, carrying information, propagates relatively fast and far under water, many marine animals have evolved a wide range of receptors to detect it. Human interaction with the marine environment entails a continuous growth in the marine ambient noise levels. This effect is particularly noticeable in shallow areas of the Mediterranean, where there is a high level of activity in a wide range of fields such as shipping, construction of marine infrastructure, fishing, resource exploration, and seismic surveys (e.g., [146,147]).
The coastal marine environment was not noiseless under the British Mandate, especially during enhanced maritime activities in World War II and after the establishment of Israel, but there is a dearth of information on this aspect of pollution in this period. This is due to a lack of research and awareness of this problem. However, in the last 50 years, there has been increasing concern on the effects of the acoustic pollution on marine life along our coast [148]. The main sources of man-made transient noise are sonar used by naval vessels, seismic surveys, marine infrastructure works (explosions, pile-driving, sand mining), and drilling, while the total motorized boating and shipping activity has doubled marine ambient noise over the last 50 years. Most research attention in Israel and abroad was and is being directed to the harm caused to marine mammals (see Section 3.10.4.5) although direct negative effects of noise have been observed on more than 50 species of animals of various taxa. The effects of noise worsen with proximity to its source—starting with seemingly harmless behavioral changes, such as curiosity, and ending with serious acoustic injuries [148].
Although more limited than the acoustic sensory modality, due to limitations at night, at depth, and in murky waters, vision is important to most marine organisms. Artificial light pollution is globally widespread in marine environments, altering natural colors, cycles, and intensities of nighttime light, each of which guide a variety of biological processes (e.g., [149]). There are known and potential effects on navigation, reproduction and recruitment, predator–prey interactions, and communication in numerous marine species and ecosystems. In Israel, sources of visual pollution in recent decades include coastal development projects such as seaports, marinas, roads and beach boardwalks, illuminated commercial ships, tourist boats, and natural gas barges. In the Israeli Mediterranean coastal environment, which was mostly dark until about a decade ago, permanent illumination sources were also introduced as part of the development of the natural gas marine infrastructure. Presently, almost all Israeli beaches are exposed to artificial light, and about 78% of them are exposed to intensities of artificial night illumination greater than those of a full moon [95]. This visual pollution has negative effects on marine organisms, such as zooplankton, sea turtles, and migratory birds (see Section 3.10.4.4) ([150] https://mafish.org.il/ accessed on 24 August 2022).
3.10. Ecological Impacts
3.10.1. Lessepsian Migration
F.D. Por [8], the scientist who named the substantial migration of Indo-Pacific species to the Mediterranean through the Suez Canal the “Lessepsian migration” (for Ferdinand Marie de Lesseps, the French diplomat and engineer who built the canal [151]), stated that it is “the most important biogeographic phenomenon witnessed in the contemporary oceans”. The migration is mostly one-way: from the Red Sea to the Mediterranean Sea (there are very few “anti-Lessepsian” migrant species from the Mediterranean to the Red Sea that only reached the southern Gulf of Suez [8]). Today, more than 50 years after this phenomenon was originally recognized, we perceive that the scope of this process and its effects on the Mediterranean marine environment and human beings is much greater that initially assumed. Only 128 “high-probability Lessepsian migrants” (together with 76 “low-probability Lessepsian migrants”) were mentioned in 1978 [8]. More non-indigenous species (NIS) have been recorded along the Israeli coast than anywhere else in the Mediterranean. When calculated per coastline length, the number is of calamitous proportions [70]. These authors report that the overwhelming majority (87.4%) of the 452 Israeli multicellular NIS are considered to have been introduced through the Suez Canal compared to only 147 NIS in 1970. The most speciose taxa are mollusks, fish, crustaceans, and macroalgae, comprising 33%, 21%, 14%, and 12%, respectively, of the total number of recorded NIS. These four major taxa of NIS also comprised most of the NIS in the past but in a slightly different order [8]. A high percentage of species of the four major groups recorded before 1960 has established large populations along the Israeli coast.
The means and route of introduction of NIS are seldom known from direct evidence. They are usually inferred from the biology and ecology of the NIS, their habitats, and pattern of dispersal. It is assumed that Indo-Pacific NIS traverse the Suez Canal and proceed northwards along the Levant coast by active or passive larval or adult movements, aided directly or indirectly by human activity. The Suez Canal is recognized both as a pathway through which NIS may cross naturally and a corridor for shipping-introduced NIS. Fouling organisms can be transferred by slow-moving vessels, mariculture and marine fossil fuel production equipment, and MD. Ballast water is assumed to function as a major pathway for the spread of NIS. Ships pump water, including their biotic content (eggs, larvae, juveniles, and adults of marine organisms), to provide stability and maneuverability during a voyage in one biogeographic region and discharge it in another biogeographic region. The conditions in the ballast water tanks are not ideal for the survival of these marine organisms, especially when the voyage is long. However, modern ships are larger (with larger ballast water tanks) and much faster than 20th century vessels (see Section 3.4), which increases the survivability of potential NIS in the ballast water. In recent years, two-thirds of global marine traffic occurs in the region [152]. Thus, the increase of modern shipping along the Levantine coast has exacerbated Lessepsian migration despite international and national regulations intended to prevent it. It is assumed that active self-propelled movement and drifting of marine organisms through the canal was limited in the first period after its inauguration in 1869; only the hardiest organisms were able to cross this waterway. This was due to salinity barriers of the hypersaline and brackish water lakes, which are part of the canal system [68]. However, these lakes were diluted with time, enlargements of the canal, and the increase in its water circulation. While salinities measured in the Bitter Lakes in 1872 were nearly 70 ppt, as canal water flowed through these lakes, the salinity fell. Salinities of 43–48 ppt measured in the 1970s were not much higher than in the northern Gulf of Suez, the southern opening of the canal. The Nile floods used to coincide with the currents regime in the Levantine basin, creating a relatively low-salinity environment near the northern opening of the canal. This could have been another natural barrier for some of the migrants in their movement into the eastern Mediterranean. This barrier to migration was lessened with the damming of the Nile at Aswan in the 1960s.
Although most Lessepsian NIS are restricted to the upper shelf, possibly due to the relatively shallow depth of the Suez Canal and the higher water temperatures on the shallow Israeli coast, some newly arrived as well as some long-established NIS have been collected on the Israeli deeper shelf and upper slope (e.g., [153]). These include aggressive, carnivorous, and highly fertile NIS, such as the venomous lionfish, Pterois miles (Figure 7), and the swimming crab, Charybdis longicollis, that probably interfere with the native biodiversity of these deeper, sensitive habitats. In addition, marine protected areas (MPAs), such as Israeli nature reserves, are not protected against Lessepsian NIS. Higher or similar biomass of these exotic species was found within MPAs compared to control fished areas [86]. Alien species constitute 23–44% of the fish assemblage and 95–99% of epi-benthic mollusks, including inside the marine reserve [154].
The rate of reporting new records of Lessepsian NIS has increased in recent years. Since the beginning of the 21st century, the number of NIS has increased by 51, 44, 35, and 24 species for mollusks, fish, macroalgae, and crustaceans, respectively [70].
The successive enlargements of the Suez Canal have raised concern over increasing propagule pressure, resulting in continuous introductions of new NIS and associated degradation and loss of native populations, habitats, and ecosystem services (e.g., [155]). It is obvious that a larger and deeper Suez Canal allows many new potentially NIS to gain admittance to the Mediterranean, where increasing temperatures permit their further expansion westwards and northwards [156]. The later authors have demonstrated a correlation between the enlargement of the Suez Canal over the years and the numbers of species probably introduced through the Suez Canal. Factors facilitating Lessepsian migrant colonization and expansion also include the high seawater temperature in the Levantine basin (see Section 3.1). These conditions are advantageous for the thermophilic Lessepsian migrants over indigenous temperate Mediterranean taxa regarding reproduction, growth, and survival.
Overfishing of indigenous biota (see Section 3.10.2) can furthermore contribute to the success of the Lessepsian migrants.
The ecological impacts of Lessepsian migration include out-competition of Atlanto-Mediterranean species (e.g., see Section 3.10.2, Section 3.10.4.1 and Section 3.10.4.3); parasitic invaders (e.g., see Section 3.10.3.4 and Section 3.10.4.1); native species displacement by NIS (e.g., Section 3.10.4.1 and Section 3.10.4.3); and food web phase shift (e.g., Section 3.10.3.2).
Some Lessepsian NIS pose a threat to human health and also have negative effects on coastal and marine industries. These include venomous and poisonous fish (see Section 3.10.2 and Section 3.10.4.3) as well as noxious invertebrates such as the nomadic jellyfish R. nomadica (Figure 6) and the long-spined sea urchin Diadema setosum (see also Section 3.10.3.5 and Section 3.10.4.1). The nomadic jellyfish is the most notorious of the marine health hazards on the Mediterranean coast of Israel. Since its swarms (e.g., Figure 17) occur when the recreational use of beaches is at its highest, the number of affected bathers is in the thousands each year [157].

Figure 17.
A huge swarm of the invasive nomadic jellyfish, Rhopilema nomadica, in the Bay of Haifa, July 2022 (drone photography by Rotem Sade).
Swarms of R. nomadica as well as of other NIS gelatinous organisms plug the filters of coastal electricity and desalination plants (Figure 18). Stinging jellyfish also interrupt trawl and other net fisheries.

Figure 18.
Nomadic jellyfish clogging the sweeping filter of the intake cooling water system (A) in Orot Rabin electric power plant in Hadera (for location see Figure 2) and overflowing the collecting containers (B) (photographs by Avi Algazi).
Rilov et al. [154] stated that “The Levant represents the trailing-edge of distribution of native species where they are exposed to the most extreme temperature and salinity conditions, and the region is also fast-warming and exposed to a great many alien species and strong fishing pressure”. The synergistic effects of Lessepsian migration, seawater warming, and overfishing were and are expected to continue to play a major role in the ecology of the coastal water of Israel.
3.10.2. Fisheries
Fish consumption in Israel is three times higher than local production. Most of the fish supply is imported and from land-based freshwater aquaculture. However, fishing still dominates large marine areas, serves as a source of livelihood and leisure for many Israelis, and has considerable environmental effects [75].
The decline in the fishing catch (see Section 2.6) was caused by the collapse of purse seine fishery, starting in the early 1980s, followed by the collapse of the coastal fisheries in the last two decades. This left trawl fishing as the main supplier of marine fish today. This does not mean that the trawl fishing situation is satisfactory. The declines in the supply of fish occurred despite the advancement of technology and with a considerable rise in operating costs, especially in fuel prices, which have increased significantly compared to fish prices. The cost rises left commercial fishermen with a marginal profit at best. Recreational fisheries were the only section experiencing increases in yields and efforts in recent years [158]. There are new regulations that are limiting recreational fishers (2 fish or 5 kg maximum), and there is a proper licensing today.
Complete stock assessments have not yet been carried out in Israel for any target species. Therefore, we have no idea of the carrying capacity of the system for different species. For this reason, the annual official statistics published by the Israeli Fisheries Department are the best estimate for the food services that the Mediterranean provides. The problem is that these data provide a fragmented time series consisting of estimates that are incomplete based on non-uniform surveys in fishing ports and fishing logs. The Israeli data are a relatively good approximation to reality and have even been significantly upgraded recently. In recent years neighboring countries such as Turkey, Egypt, Lebanon, and Cyprus have surpassed Israel regarding monitoring and stock assessment.
The analysis of the fishery yields supplies only a partial picture. The effort required to make the catch must also be understood. For example, the power of trawler engines has doubled since the 1950s, whereas the coastal fishermen, using mainly trammel nets and long line, gradually upgraded from using oars in the 1940s to the 100 horsepower (HP) engines common today, allowing them to deploy more equipment than before [83].
The collapse of purse seine fishery was expressed in the decrease of the yield of sardines to at least 10% of its level in the peak years. This was due to the damming of the Nile, resulting in decrease in nutrient supply and reduction of marine production as well as socio-economic reasons.
In the short term, fisheries are adversely affected mainly by over-exploitation of the marine ecosystem. Overfishing, i.e., removing fish beyond the natural recovery capabilities of the ecosystem, is currently common in most fishing areas of the world, (e.g., [159]), including Israel. The removal of small fish and sessile organisms from the soft substrate by trawlers has a noticeable effect in the short term. These actions affect the trawl fishery itself and cause considerable damage, especially to the coastal fishery. Dragging the trawl gear across hard substrates (especially by rough-bottom trawl) has a more devastating result since it irreversibly damages the richer and more diverse rocky habitat in the long term. As a rule, removing small fish from the sea is problematic because they are the basis for future generations (e.g., [160]). Care should also be taken in removing the largest fish of any species, as fitness and reproduction tend to increase exponentially with fish size.
After the collapse of the purse seine and coastal fisheries, the trawl fishery has continued to be relatively stable and has remained the main supplier of fresh marine fish in Israel. The marine fishery in Israel is multi-species, including trawl fishery. In the early 1990s, 30–34 trawlers, each with a mean engine power of 214–228 HP, were spending a total of 5152–6214 days at sea each year [161]. The fleet size was frozen at 30 licenses in 1995, but after 1995, only 23 of the 30 vessels were active, possibly due to rising fuel prices and declining catches. This fleet size limit has not proven effective in halting declines in catch per unit effort (Figure 19)—a clear indication of overfishing. A 45-day summer trawling moratorium was established in 1998–1999, but the policy was discontinued the following year despite encouraging interim results, including increasing catches and larger specimens of commercial species [162]. It is interesting that the proportion of NIS in the yield of the trawl fisheries in 2000, just after the moratorium, was lower than before (1990–1994) and after (2008–2010) this moratorium. Since 2017, a 60-day moratorium is enforced on the trawl fishery and on all other methods, including recreational fishing. Only 15 trawlers remain today after a vessel scrapping and buyback program.
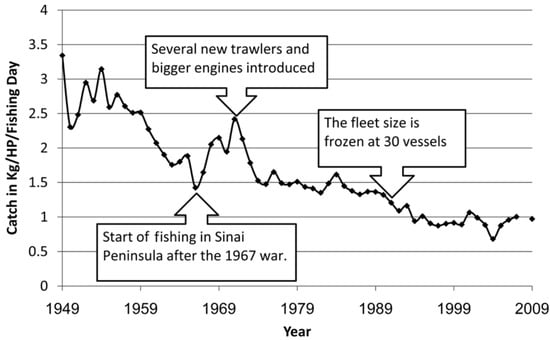
Figure 19.
Declines in CPUE (catch per unit effort) of Israeli trawlers based on data collected by the Department of Fisheries of the Israeli Ministry of Agriculture since 1948 (from [160] with permission).
In the late 1990s, the fleet trawled all year round, spending only about 4000 days/year at sea. The reduction in fishing effort was mitigated by an increase in mean engine power (294 HP), which allows increased effort per sea-day. Before 2005, the fleet trawled for hake in the spring and also for rose shrimps, Parapenaeus longirostris, and red shrimps, Aristeus antennatus and Aristeomorpha foliacea. In the 2000s, stocks dwindled, and the fleet operated almost exclusively on the continental shelf between 15 m (the minimum depth allowed by law) and 150 m [160]. These later authors found that in the Israeli trawl fisheries, discards (organisms smaller than legal size, i.e., juveniles of commercially valuable species or those belonging to species lacking commercial value) increased with the decreasing mesh size of the net. In 1990–1994, 28.3% of the total catch was discarded, and there was a 40.1% discard percentage in shallow hauls. Both the biomass and the number of discarded specimens peaked in summer as well as the percentage of juvenile fish of commercial species. Annual discards for the Israeli trawl fleet for the study period are estimated at 440–700 tons. These findings suggest that a summer moratorium on trawling would reduce discards.
The amazing rate and scale of Lessepsian migration dramatically affects the Mediterranean ecosystem as a whole in both the short and long term (see Section 3.10.1 and Section 3.10.4.3). Lessepsian NIS fish were already sold in the local fish markets in the early 1900s [163], and by the mid-20th century, they constituted a significant part of the Israeli fishery landings (e.g., [164,165]). Some NIS were reported to replace the Mediterranean native species already in 1956. In the late 1960s, Lessepsian fish comprised 20% of the Israeli trawl fishery landings [166]. In the 1990s, their percentage increased to 51% in the upper shelf (to 37 m), 24% at mid-shelf (to 73 m), and 8% in deeper waters [160]. An analysis of the catch of a commercial trawler in 2010–2012 revealed that Lessepsian NIS comprised 54% and 67% of specimens at 20 m and 40 m, respectively [167]. A recent study discovered that the CPUE of alien species increased over time, while for indigenous species, it remained relatively stable between 1996 and 2013 [168]. As NIS are more abundant inshore, the trawler shifted to shallower fishing areas, with a marked change in the revenue structure, with NIS comprising a progressively larger proportion of the revenues. The increase in revenues based on NIS did not fully compensate for the loss of revenues from native species ([70] and references therein). Shifting trawling to shallower water also means more environmental impact at these depths. The most prominent commercial species in the trawl catch in recent years were the following Lessepsian NIS: the brushtooth lizardfish, Saurida undosquamis, and the Randall’s threadfin bream, Nemipterus randalli, which has become the dominant species in the trawl fishery yield. The prevalence of the common pandora, Pagellus erythrinus, a native commercial species similar to N. randalli in its ecological function, has decreased in recent years to about half of its usual values. This is not due to overfishing of the indigenous species but probably because of competition for the same food resources as the invasive fish.
Of the many invertebrate Lessepsian NIS, two contribute significantly to the commercial fisheries: the Japanese tiger prawn, Marsupenaeus japoinus, caught by night trawling in the shallow soft substrate, and the swimming crab of the genus Portunus caught by trammel and gill nets. Unfortunately, the trawl catch also includes a considerable amount of noxious Lessepsian fish such as the venomous striped eel catfish, Plotosus lineatus, which constitutes a nuisance to fishers and bathers, and the highly poisonous pufferfishes of the genus Lagocephalus, which can also cause damage to fishing gear by cutting fishing lines and baits with their strong teeth [158] (Figure 20).

Figure 20.
A catch of the trawl fishery in the Mediterranean coast of Israel in recent years, with many venomous striped eel catfish, rabbitfish, and highly poisonous pufferfishes (photograph by Dor Edelist).
The European hake, Merluccius merluccius, has disappeared from the deep-water trawling due to a combination of sea warming and fishery factors (but reappeared in 2015), and there has also been a steep decline in the catch of indigenous goatfishes of the family Mullidae. Fishing on the continental slope has completely ceased since the 1970s.
In December 2016, the updated fishing regulations entered into force, and their enforcement was transferred from the Fisheries Department to the INPA. Among the new regulations were absolute prohibition of SCUBA fishing and a moratorium on fishing in the recruitment period of indigenous commercial species in the spring and summer. Trawling was forbidden on the northern half of the coast of Israel and in shallow water, the minimum legal size of net mesh was increased, and complete protection of sensitive taxa was established, including cartilaginous fish, sea turtles and marine mammals, and the Mediterranean slipper lobsters, S. latus, all considered “flagship species”. It is not yet clear how the changes in regulations and enforcement will affect the marine biological resources and the fisheries in the long run. Significant numbers of commercial species are caught by several fishing methods. Thus, there is a danger that some fishing “niches” vacated by a few easy-to-control trawlers, due to the new regulations, will be occupied by hundreds of difficult-to-control coastal fishers.
3.10.3. Impacts on Habitats
3.10.3.1. Abrasion Platforms and Beachrocks
These two habitats are the natural hard substrates closest to onshore anthropogenic activities. As such, they are exposed to uprooting, digging, cutting, breaking, sedimentation, freshwater flooding, solid waste (including plastic), chemical pollution, coverage with pipes and other artificial objects, and trampling by recreational line fishers and other visitors.
Before the establishment of the State of Israel, kurkar rock was quarried (e.g., Figure 21) as building material. Since the 1920s, kurkar quarrying has been dramatically reduced due to shifting to alternative concrete, and today, this practice is forbidden.

Figure 21.
Abrasion platforms in the coastal water of Jaffa before they were quarried for building purposes (from [169] Seaward W. H. 1873 Travels Around the World (www.fromoldbooks.org/SewardTravels, accessed on 24 August 2022).
In recent years, the biogenic intertidal ecosystem—the vermetid reefs of the AP—has been threatened by another factor: SLR, driven by anthropogenic climate change, which can be a major threat to this coastal ecosystem [94]. These authors show that biodiversity is much lower on very shallow, permanently submerged, horizontal rocky surfaces compared to that on intertidal reef platforms. The extensive intertidal platforms will permanently drown under even modest SLR scenarios. Therefore, the rich intertidal community will transform, when permanently submerged, either to a very different but still rich community when protected from grazing by highly abundant invasive fish (rabbitfish) or to a much poorer turf community when exposed to such fish grazing. Thus, the reef community net production will drastically drop under permanent submersion.
Since the main ecosystem engineer of the vermetid reefs, the worm shell D. anguliferum (Mollusca), is nearly extinct in the southeast Levantine coasts, it is unlikely that new reefs will be formed higher on the shore in the future, presumably resulting in extensive coastal ecological shifts. Since the vermetid reefs are also assumed to protect the shore from high waves and erosion (see Section 2.4.1.1), their deterioration may also result in morphological decline of the coast. The above authors suggest that in cases where essential/unique intertidal habitats such as vermetid reefs are expected to vanish due to SLR, constructing carefully planned, ecologically friendly, artificial alternatives should be considered.
3.10.3.2. Submerged Kurkar Ridges
The rich and diverse SKR have suffered a multitude of anthropogenic impacts. These include the accumulation of marine debris swept by waves and current that cause physical damage to this sensitive habitat, overfishing by professional (coastal and even trawl fishermen; see Section 3.10.2) and sport fishers alike, and removal of large fish and invertebrates, destruction by fishing equipment, and abandoned gear that acts as a “ghost net” (see Section 3.8). SKR are dug and cut during marine construction, especially of ports. For example, SKR were cut during the construction of the new Bay Port in Haifa Bay. A 2.2 km long and up to 19 m deep entrance channel was dug through SKR to ensure compatibility with the newest giant ships on the market, which are about 400 m long and can carry over 18,000 containers. Underwater sand mining for the construction of ports may create silting that, with the proper wind and currents, might be driven to the nearest SKR, reducing visibility and light penetration there as well as interfering with the vital filtration processes of sponges, bivalves, and other filtering organisms. Recent surveys in this habitat (e.g., [85]) indicate an almost complete dominance of Lessepsian NIS in several main taxa and the complete absence of indigenous species that were very common in the past. Such changes may sometime create a considerable food web phase shift. For example, the marbled spinefoot Siganus rivulatus and the spinefoot S. luridus, both Lessepsian, were first recorded off the coast of Mandate Palestine in 1924. In only a few decades, these schooling herbivorous fish were able to settle in a range of habitats, forming abundant populations and creating marked phase shifts within the food web. Prior to their arrival, herbivores filled a small ecological role within the Levantine ecosystem. With such a high influx of herbivorous species in a short period, this phenomenon has changed the food web, increasing the rate at which algae are consumed and serving as a major prey supply for large predators. They are also affecting fisheries by outcompeting indigenous fish of higher commercial value, such as the seabream of the genus Boops [154,170]. There is a need for more ecological knowledge to estimate the level of conservation required for different components of the SKR. These habitats are today protected from trawl fishing and a considerable proportion is also located in marine protected areas.
3.10.3.3. Artificial Hard Substrates
When constructed in areas where the previous bottom was soft (sand), artificial hard substrates change the nature of the marine environment and interrupt a sequence of soft bottom substrate. Therefore, the coastal infrastructures enable the settlement of organisms typical to hard substrates such as sessile and mobile invertebrates, algae, and concentrate fish. Most of the coastal infrastructures are made from materials foreign to the marine environment, including natural terrestrial materials such as wood and quarry rocks and man-made material such as concrete and steel. Frequently, these materials do not constitute appropriate surfaces for the settlement of typical marine biotic community and are thus populated by invasive alien and pest species (e.g., [171]). In the recent decade, a huge volume (and surface) of man-made hard substrates has been constructed on the Mediterranean coast of Israel, and these are now more common that natural hard substrates.
Artificial reefs (ARs) have been established in Israeli waters mainly to enhance fisheries as a substitute for natural reefs and as an attraction for divers. Several small ARs were also established for research purposes [172,173]. A comparison of the species composition and biomass of bony fish was carried out in a small coastal AR off Haifa (Figure 2) in 1985 and 1995 [174]. Only one species of fish was added in 1995. However, the contribution of Lessepsian fish to the overall fish biomass in the AR increased from 64% to 94%. This was due mainly to a 224% increase in the biomass of the Lessepsian Red Squirrelfish, Sargocentron rubrum, (e.g., Figure 5), and a significant decrease in the biomass of indigenous sparids and dusky groupers.
In addition, old vessels are deployed deliberately as ARs, and shipwrecks also serve this purpose. In Israel, several decommissioned vessels were sunk as ARs [173]. However, inappropriate preparations can cause environmental damage, as happened to a decommissioned navy missile boat sunk off southern Haifa that has disintegrated, with its parts now scattered over a large area of soft bottom at 30 m depth.
AR is defined in the guidelines document of the Convention for the Prevention of Sea Pollution as a result of the dumping of waste materials (London Convention, 1972 [175]) as: “a submerged structure built or placed on the seabed, with the intention of imitating some characteristic of a natural reef…”. The dumping protocol of the Barcelona Convention also considers “placement” of man-made constructions in the sea for the purpose of ARs. To prevent environmental impacts as well as disruption to navigation, an initial document of regulations was prepared and adopted by the Israeli Inter-ministerial Committee for Dumping Waste into the Sea in 2012 [173]. The United Nations has recently updated the guidelines for Placement of AR at Sea [176].
3.10.3.4. Soft Substrates
Alteration, fragmentation, and destruction of this habitat are caused by construction of hard substrates, trawl fisheries, dumping of debris, and other anthropogenic activities. The soft sediment in shallow water is highly impacted by coastal erosion and accretion, especially in the recent decade, due to large-scale shoreline constructions (see Section 3.5). The shallow soft bottom has been continuously and intensively trawled for benthic fish, shrimps, and squids by relatively large fishing equipment. This anthropogenic disturbance has negatively affected the biota in this habitat. Recently introduced fishing regulations may alleviate the fishing pressure on part of this soft bottom coastal environment (see Section 3.10.2). As fishing on the continental slope has been completely terminated since the 1970s due to the distance from ports and rising fuel prices, this deep soft bottom habitat may now be practically considered as a “marine protected area”.
Soft substrates are also affected by Lessepsian migration. Eight species of commercially important NIS shrimps have been recorded in the Levantine Basin. They compose most of the shrimp catch off the Mediterranean coast. However, this high abundance of invasive prawns has led to the decline of a native penaeid prawn, Melicertus kerathurus, which supported commercial Israeli fishery throughout the 1950s. Due to out-competition and its habitat being overrun by these migrants, this native species has since disappeared, with resultant detrimental impacts on commercial fishery [177]. NIS shrimps may be preferred over their local counterparts due to their higher price, e.g., the kuruma shrimp, Penaeus japonicus, which is significantly larger than local prawn species [178].
The Indo-Pacific swimming crab, Charybdis longicollis, was first recorded in the Mediterranean in the mid-1950s and became dominant in silty and sandy substrates off the coast of Israel, making up to 70% of the total biomass in these habitats. Until 1992, none of the specimens collected was infected with the invasive parasite Heterosaccus dollfusi, but in that year, a few infected crabs were collected. The parasite is a barnacle that desexes its host. Within three years, 77% of the crabs collected in Haifa Bay were infected. This rapid increase and high infection rate are attributed to the extremely high population density of the host and the year-round reproduction of the parasite. One effect of this was that the population of the Mediterranean-native shallow-water grey swimming crab, Liocarcinus vernalis, has recovered somewhat [179].
3.10.3.5. The Water Column
There is partial knowledge of the environmental impact of the planktonic ecosystem in shallow water up to 10 m water depth and even less of deeper offshore water. One of the major pelagic organisms is the invasive nomadic jellyfish R. nomadica (see Section 2.4.3, Section 2.5.1, and Section 3.10.2). This Lessepsian invader may critically affect ecosystems and ecosystem services (e.g., [180]). It was first reported in 1977, and from the mid-1980s, it has appeared in gigantic swarms along the Israeli coast mainly in the summer ([69] and references therein) (e.g., Figure 17). It is the largest, most venomous, and most prominent jellyfish species in the eastern Mediterranean. R. nomadica swarms affect tourism by stinging bathers (e.g., [181]), thwarting fishing operations by clogging nets, and interfering with the operation of power and desalination plants (e.g., Figure 18) by blocking seawater intake systems [182]. This species is therefore regarded as one of the worst invasive species in the Mediterranean. Beside physical and economic damage to humans and their industries, the swarms of R. nomadica have an overwhelming impact on the pelagic food web. They feed mainly on micro-zooplankton, and their diet reflects most of the ambient plankton taxa [72,183]. These jellyfish are specialized oligotrophic water feeders, efficiently locating scarce plankton patches and exploiting them to the full [184]. Since they may compete over smaller prey with organisms such as larval fish, they could have considerable impact on the pelagic ecosystem.
Blooms of other gelatinous pelagic organisms, such the invasive ctenophore Mnemiopsis leidyi [185], may also threaten coastal industries and open water ecosystems. This voracious zooplanktivore spread throughout the Black Sea basin after its introduction in the early 1980s throughout northern European coastal waters, and it can now be found off the Mediterranean coast of Israel. Repetitive appearances of swarms of ctenophores and siphonophores reported recently from the Mediterranean coast of Israel (see Section 2.5.1) can also affect coastal and marine devices as well as the ecosystems.
The effects of gelatinous pelagic organisms on coastal installations, such as desalination and electric power plants, are obvious. However, the long-term impacts of the outputs of these industries on the pelagial, such as the discharge of concentrated brine by the first, and of warm cooling water by the second industry still need research.
Another potential environmental hazard in the coastal pelagic zone is microplastic. Since most of the extremely abundant microplastic along our coast is white or colorless (see Section 3.8), it may appear as planktonic food to be consumed by pelagic planktivores with negative consequences to these consumers as well to the subsequent food web. Since microplastic tends to accumulate chemical pollutants from the environment, it may enhance the assimilation of hazardous materials such as heavy metals and organic pollutants into the food web.
3.10.4. Impacts on Specific Taxonomic Group
3.10.4.1. Invertebrates
Artificial light may interfere with vertical migration of zooplankton. Chemical pollution may be accumulated in both phyto- and zooplankton and the subsequent food web, including fish and top predators who consume them, such as marine mammals (see Section 3.10.4.5) and humans. These planktonic organisms are killed during the pumping of seawater in desalination plants (see Section 3.6) although the scope of this problem is not yet clear.
In addition to environmental disruption of gelatinous invertebrates (see Section 3.10.3.5), benthic forms may have also considerable environmental impact. Indigenous and NIS invertebrates constitute the biofouling assemblages on marine vessel surfaces and piping as well as cooling water conduits of coastal power stations (e.g., [186]), causing operational disturbances resulting in high economic investment in coping with this problem. Propagules of invertebrate taxa such as barnacles, mollusks, bryozoans, Coelenterates, sponges, Polychaeta, and ascidians (as well as sessile macroalgae) originate in the water column, settle on the artificial substrates, and develop into heavy biofouling. Before the ban by the International Maritime Organization in 2008, organotin tributyltin (TBT) and triphenyltin were common in anti-fouling paints used in Israel. Exposure to these endocrine disruptors causes imposex, the superimposition of male sexual characters onto females, and impairs reproduction in the whelks Stramonita haemastoma and Hexaplex trunculus, resulting in the decline of these benthic mollusks on the Israeli coast [187].
Mollusks have been the taxa of NIS with the highest number of species in our Mediterranean coast (see Section 3.10.1), and many have established immense populations. For example, the gastropod Conomurex (strombus) persicus is the most prevalent species on the shallow soft substrate. High-density populations of the Indo-Pacific invasive spiny oyster, Spondylus spinosus, occupy vast areas of the rocky habitats along our coast, where they are cemented to the hard substratum [188]. They create reef-like structures and are assumed to have almost completely displaced their indigenous congener, the European thorny oyster, S. gaederopus, endemic to the Mediterranean.
A recent study has indicated that populations of marine mollusks have collapsed in recent decades in parts of the eastern Mediterranean [189]. The authors suggest that the collapse was due to seawater warming that made conditions unsuitable for local populations of marine mollusks as well as some of the arrival of Lessepsian NIS. They recorded only 12% and 5% of historically present native species on shallow subtidal soft and hard substrates, respectively. They state that this is the largest climate-driven regional-scale diversity loss in the oceans documented to date. Importantly, approximately 60% of the recorded shallow subtidal indigenous species did not reach reproductive size, making the shallow shelf a demographic sink. They predict that, as climate warms, this native biodiversity collapse would intensify and expand geographically, counteracted only by Indo-Pacific species entering from the Suez Canal, and that restoration to historical baselines was not achievable.
Crustacean NIS are the second-most abundant invertebrate group in the Mediterranean, with at least 91 species belonging to the order Decapoda [155]. Some make a positive contribution to commercial fisheries, while others out-compete native commercial crustaceans (see Section 3.10.2). The invasion of new Red Sea species of crustacean into the Mediterranean has also facilitated the invasion of their associated parasitic copepods, and some of these parasites have shown an ability to use related native Mediterranean fish species as alternative hosts (e.g., [190]).
The population of the European purple sea urchin, Paracentrotus lividus, once abundant on rocky habitats of the coastline of Israel, has recently collapsed, and today, it is an extremely rare species in the region. A recent study [191] suggests that seawater warming and resource competition that may lead to competitive exclusion by invasive grazers (the two species of Lessepsian rabbitfishes; see Section 3.10.3.1 and Section 3.10.4.3) may be a major cause of the disappearance of this indigenous herbivorous sea urchin. On the other hand, the tropical long-spined sea urchin, Diadema setosum, which may be a health hazard for humans, was recently recorded and identified also on the Mediterranean coast of Israel [192].
3.10.4.2. Cartilaginous Fish
Sharks in the Mediterranean are at extremely high risk, and their populations are rapidly declining [193]. Many are considered “flagship species”. Despite it being banned, fishing of shark and rays still occurs in Israel, and it seems that its level has even increased in recent years by trawlers as by-catch and as a target species in the trammel net fishery. It is possible that the hardness of some trammel net fishermen due to the collapse of the coastal resource is rather expressed in increased capture of these important and protected key top-predator species. Since cartilaginous fish are not consumed by most of the Jewish Israeli population due to religious reasons, they were mostly exported to Gaza Strip [158].
Despite some advance in research in recent years, the scientific knowledge of cartilaginous fish in the coasts of the Levant is limited. Only recently was it found that the shark population includes also Lessepsian species. Similar research is also needed for guitarfish and other taxa. For example, in 2013, about 500 giant devil rays, Mobula mobular, were caught by fishermen off the southern Gaza Strip. These creatures, up to about 5 m across, are famous for their impressive leaps above the water and are listed as endangered species in the IUCN (International Union for Conservation of Nature) list. The fact that they were never recorded off the nearby Israeli coast indicates our poor knowledge of this important group of marine organisms [10].
Winter mixed-species aggregation of two local species of sharks, namely the sandbar, Carcharhinus plumbeus, and the dusky, C. obscurus, was observed in recent years in the discharge of warm cooling water from the large coastal electric power plant in Hadera (e.g., Figure 22). These aggregations have recently created a new type of eco-tourism with the opportunities and challenges of this emerging phenomenon (e.g., [194]). There has been a general increase in shark sightings between 1993 and 2013 compared to the previous two decades. Shark aggregations occur at power plant discharges, most likely due to elevated water temperatures. The aggregations of sharks are a source of interest and attraction for many people, including swimmers, divers, and kayakers. The desire of tourists and therefore of local businesses to take part in this amazing and profitable phenomenon poses certain risks in view of the lack of regulation in the area. Human divers might disturb the sharks and influence behavioral changes. Establishment of proper regulations and enforcement is needed in the area to reduce ecological damage so that the sharks can live with minimum disturbance while allowing a reasonable amount of wildlife tourism.

Figure 22.
Shark aggregation near the discharge of warm cooling water of Orot Rabin Power Station in Hadera (drone photography by Kobi Sror).
3.10.4.3. Bony Fish
The Levant, and especially the Israeli Mediterranean shelf, is the most invaded marine ecosystem in the world, at least regarding bony fish. A staggering 55 Indo-Pacific fish species had established permanent populations in the Mediterranean by 2013, more than any other marine ecosystem in the world [195] (Figure 23). This process is accelerating, with 13 of 27 new arrivals being established in the 21st century alone.
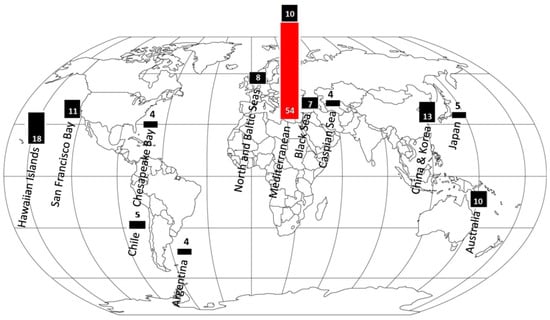
Figure 23.
Number of established invasive marine and brackish fish species reported from invasion hotspots (more than four invasive established species). The red histogram denotes Indo-Pacific migrants (adapted from [195]).
No less than six NIS fish in the second decade of the 21st century have experienced population explosions and are among the most common and widespread species in the eastern Mediterranean. These authors have found significant two-fold increases in NIS fish proportions in shallow waters (15–30 m), where they contributed up to 66% of the ichthyofauna biomass, and 55% in the deeper overall trawling fishing grounds.
This proliferation has resulted in significant declines of certain indigenous species, some to near extinction levels. Despite these profound changes in shelf fish community composition, they have not yet found evidence of declines in species diversity or trophic level. Although these functional properties have remained relatively stable on the shelf, community composition has shifted considerably. They suggest that despite these irreversible alterations, invasions have masked overall changes and diversity declines by replacing native fish with NIS of similar ecological position. Rarer indigenous demersal fish species, such as the greater weever, Trachinus draco, and the Atlantic stargazer, Uranuscopus scaber, have experienced declines of 70–100% and have become extremely rare in the Levant, possibly due to a species-by-species replacement by NIS. This was proposed, for example, for replacement of venomous Trachinus spp. by the venomous invasive striped eel catfish, Plotosus lineatus, (e.g., Figure 7) [196], which shared similar ecological niches. The indigenous meagre, Argyrosomus regius, which was one of the most common commercial fish in the Levant, has since disappeared from local catches [170], while the narrow-barred Spanish mackerel, Scomberomorus commerson, a known Lessepsian migrant, has dramatically increased in population. They suggest that, due to similar life histories and diets, this might be an example of an invasive migrant out-competing a native species and occupying its niche. Decreasing trends were noted in the catch of indigenous red, Mullus barbatus, and striped red, Mullus surmuletus, mullets, while the similar Lessepsian goatfish, golden-banded goatfish, Upeneus moluccensis, and Por’s goatfish, Upeneus pori, displayed oscillating yet overall stable trends in catches over the same period [197]. It was reported [170] that the native mullet had been displaced into deeper, cooler waters, whereas the Lessepsian migrants were caught mainly in shallower, warmer waters. From these data, it was concluded that the Lessepsian migrants apparently have not adapted to the more temperate environment of the deeper areas of the basin but have established dominant populations in the habitats most similar to the tropical sea habitats from where they came. Yet, the invasive Lessepsian lionfish, P. miles (e.g., Figure 7), was recently documented, using a remotely operated vehicle, in five distant mesophotic reefs at 95–120 m depth along the Israeli Mediterranean coast [198], where this successful predator also poses a threat to deep water, colder biota.
Despite their large abundance and biomass, the effect of fish NIS on indigenous Mediterranean populations is still unclear [168,197]. On one hand, the stability of fish size spectra and a lack of clear evidence for the competitive exclusion of indigenous species by NIS suggest limited effects. On the other hand, alien species affected the local food-web structure, thus increasing the competition for local resources. In addition, specific alien species might negatively affect invaded habitats, as is the case for Lessepsian rabbitfish, which negatively affect the local biota due to their intense algal grazing (see Section 3.10.3.1). These authors mention the striking ability of alien Lessepsian fish to rapidly increase in population even when being commercially exploited. They suggest that it might be related to their pre-adaptation to warm climate conditions, such as those occurring in the eastern Mediterranean. Additionally, they argue that tropical fish generally grew faster, which might lead to a higher percentage of individuals reaching maturity under the same fishing regime compared with species from a temperate origin. This argument should also be considered in view of the” Levantine nanism” (dwarfism) phenomenon, [199] characterized by the body size of fish specimens in the Levantine basin being smaller than that of conspecifics in the western Mediterranean. Indigenous Israeli fish also mature sexually at a smaller size than conspecifics from the western Mediterranean. These findings may be explained by lower productivity and perhaps also by the higher water temperature in the Levantine basin than in western regions. It is interesting that “Levantine nanism” was also found in top predators in the Israeli Mediterranean coast, such as the common bottlenose dolphin, T. truncates [200], which prey mainly on bony fish [201].
It is suggested [77] that the recent dramatic increase in the number of Lessepsian fish migrants (average of 2.5 species per year) was most likely due to the increased water influx from the Red Sea to the Mediterranean, following the recent opening of the new parallel 72 km New Canal and the enlargement of other parts of the Suez Canal.
Invasion was placed in the same category as overexploitation, habitat destruction, and pollution [195] processes, which are normally considered as much more critical perturbations to coastal fish communities. In view of these irreversible alterations, the authors anticipate further declines in indigenous fish biomass, abundance, and diversity in the Mediterranean Sea.
3.10.4.4. Marine Turtles and Birds
Marine turtles were targeted by fisheries in the Mediterranean from 1920 to 1970 and have undergone severe exploitation. At least 30,000 to 40,000 turtles were caught along the Palestinian coastline during the 1920s to 1930s ([82,202] and references therein). As a result, the population declined, and by 1970, the trade in turtles in the region had stopped due to lack of profit. Presently populations of sea turtles face major anthropogenic threats worldwide as well as in Israel, both at sea and on their nesting beaches [203]. Following the worldwide reduction of their populations, the IUCN declared all species of sea turtles as endangered, while the International Organization for the Prevention of Trade in Protected Animals (CITES) prohibited trading in their meat or shells. Although intentional capture of marine turtles is now illegal, sea turtles are still incidentally caught by fishing, and according to [202], it is the major cause of their mortality on the Israeli Mediterranean coast. These authors assessed the impact of the Israeli fishery fleet on the green and brown turtle populations, showing that gillnets and trawlers are the main threats to sea turtles in our area. Twenty-one turtles were caught during 1385.5 h of trawling observations—a catch rate of 0.015 turtles per hour. They estimate that 1315 turtles are caught annually by Israeli trawlers, and about 21 turtles are caught each year by each gillnet vessel, yielding an annual estimate of 1672 turtles for the whole gillnet fleet. They found that only a small fraction of the turtles injured by trawlers is represented in the strandings. The mortality rate through trawling and the stranding density is the highest in the region, emphasizing the urgent need to regulate the Israeli fishery (see Section 3.10.2 regarding the updated fishing regulations). These researchers stated that this fishery by-catch posed a major threat to the whole Levantine Sea turtle population, especially during the vulnerable reproduction stage in late spring-summer. Long lines and hooks also injure sea turtles. The fisheries reform has brought about a ~4-fold decline in mortality of turtles. Trawling in the shallow coast, where most turtles were caught, has ended and there is a spring and summer fishing moratorium during the turtles’ reproduction season.
An additional threat at sea is solid waste (see Section 3.8) such as “ghost nets” with which turtles may be entangled and plastic bags that turtles may swallow, causing stomach blockages. Injury by engines of fast-moving boats, especially in shallow water, and by tar originating from oil spills (see Section 3.6) are other threats to turtles. In a recent study [204], it was found that about one-third of 1473 turtles collected by the National Sea Turtle Rescue Center of INPA in 1999–2021 were injured by plastic-related hazards. Fishing-related injuries made up 47%, and polypropylene bag-related injuries made up 32% of the cases. About one-fifth of the treated turtles did not survive. In 2017–2021, a sharp increase was observed in the number of young turtles that were injured or died on the shores due to entanglement (neck and limbs) in polypropylene woven sacks. These bags are now considered as a major life hazard for young sea turtles in the pelagic life stage. These sacks are used in a variety of industries, but the labels on them indicated that their original purpose was to pack food for animals in agriculture, and several ships were suspected as the origin of the sacks at sea. Entanglement in polypropylene bags was more common during the summer months. In the same study, post-mortem autopsies were performed to check the presence of waste in digestive systems of 21 sea turtles of both species collected in 2021. Waste was found in the digestive systems of all the specimens. The concentration of waste was significantly higher in young turtles than in adults, probably resulting in their different habitats. Fishing lines appeared in 8% of the turtle stomachs analyzed, significantly higher than their distribution in MD sampled in this region.
Onshore, the threats are to nests of turtles in sandy beaches and to females in spawning activities. Intense construction has reduced the available spawning habitats (see Section 3.4). Construction and debris also block the access of spawning females to shore. Human activity on bathing beaches, including motorized vehicles, during the breeding season is another threat. Predators such as terrestrial mammals (foxes, jackals), coastal birds, and the tufted ghost crab, Ocypode cursor, may prey on the eggs and hatchlings. To overcome these problems, and since it is estimated that only 150 female brown turtles and 15 female green turtles lay eggs on the Israeli Mediterranean shore, the INPA is working to restore the Mediterranean Sea turtle population. This is done mainly through two long-term projects. One is nest relocation, in which nests of sea turtles are moved to fenced farms for protection. The other is the green project, the breeding nucleus for the green sea turtle in the turtle rescue center that also treats injured turtles.
SLR is a future potential threat that can further reduce the available sandy habitat for spawning.
Artificial light pollution (see Section 3.9) is known to influence the on-beach orientation and nest-site selection of adult female turtles, the on-beach orientation and sea-finding behavior of hatchling turtles, and the dispersal of hatchling turtles at sea [205].
Artificial light may also interfere with the navigation of birds along the coast. Marine birds may be similarly entangled in ghost nets while diving for prey, swallow solid waste and microplastic, be injured by tar from oil spills (see Section 3.6), and be negatively affected by other chemical pollutants. SLR may be a potential threat to coastal birds that feed in the intertidal zone by reducing the area of their feeding grounds.
3.10.4.5. Marine Mammals
Marine mammals (MM) are considered “emblematic species of global conservation concern” [206], similar to some other “flagship species” in Israeli waters, such as lobsters, sea turtles, and sharks. Concern regarding the status of MM along the Israeli Mediterranean coast is legitimate since most of the present numerous anthropogenic factors threatening MM can be expected to increase in the future [207]. Most of the Mediterranean populations of MM have been classified as threatened (critically endangered, endangered, and vulnerable) in the IUCN Red List. Top predators, such as MM, help maintain ecosystem functionality and resilience. They are also considered “umbrella species” in the sense that actions that contribute to their conservation and that of their habitat can benefit marine biodiversity generally and add to the ecological, cultural, and aesthetic value of the marine environment [79]. In their comprehensive essay, the author and his colleague from IMMRAC presented the latest data on MM along the coast, including recommendations to reduce or eliminate existing and potential risks.
Apart from natural causes of mortality of MM (e.g., parasites, harmful algal blooms, predation, infanticide, and violent inter- and intra-species interactions), anthropogenic effects cause the most concern.
In the Fisheries Ordinance of 1937, fish was defined as “any aquatic animal, whether piscine or not”. Animals that could be taken specifically included MM and sea turtles. When trawlers started being deployed in Israel in the 1940s and 1950s, dolphins caused significant damage to the cotton nets and were listed as agricultural pests and, according to verbal reports [79], intentionally killed—including by professional bounty hunters. In the following decades, fishing gear depredation and damage caused by MM in Israel reportedly became insignificant, and the occasional interactions were tolerated, apparently resulting in low levels of direct killings (e.g., shooting or harpooning) [208]. In addition, public awareness of MM and of the risks to which they are exposed have considerably improved.
Bearzi [79] attempted to rank the extant threats on MM in Israel based on their relative importance (at the population level). Important threats included incidental mortality and injury caused by fisheries, anthropogenic noise, and prey depletion. Potentially important threats consisted of chemical contamination, habitat loss and degradation, and climate change. Possibly minor threats included ingestion or entanglement in debris, vessel strikes, oil pollution, intentional and direct takes, and disturbance. However, because of the scarcity of information, such a ranking should be seen only as indicative.
Overfishing has depleted Israel’s fish stocks (see Section 3.10.2), with potentially important consequences for top predators such as MM. Incidental mortality of cetaceans is known to occur in the Mediterranean waters of Israel and is reportedly caused by bottom trawlers and gillnetters According to [209], 20% of all dead-stranded cetaceans show evidence of entanglement in fishing gear. Most bycatch involves trawling gear, with the remaining by-catch being caused by entanglement in trawlers’ lazy lines and in gillnets. All by-catch caused by trawlers involves common bottlenose dolphins, while gillnet victims also include striped dolphin, rough-toothed dolphin, and minke whale [208]. The new fishing regulations, introduced in 2016 (see Section 3.10.2), are expected to decrease the negative interactions between fishing gear, especially trawlers, and MM.
MM use sound for communication, navigation, and foraging and have therefore developed the highest auditory sensitivity among marine organisms. The reported effects of noise on MM include stress and behavioral changes, such as avoidance, auditory masking, changes in hearing threshold, and in extreme cases death [145]. The exploitation of natural gas reserves discovered recently in Israel’s Mediterranean EEZ and the search for additional reserves (and inherent noise-producing activities) are likely to increase pressure on MM. Noisy construction phases including drilling, laying of pipelines, and construction of platforms are also sources of threats to MM as well as repeated and long-lasting high-intensity episodic noise produced during the process leading to platform anchorage. Coastal construction projects involving pile-driving and sand mining (e.g., during the expansion of the ports of Haifa and Ashdod; see Section 3.4), cause noise disturbance to coastal cetaceans near the sites. Another source of potential threats are military acoustic devices. Shipping is an additional and important source of anthropogenic noise considering the high traffic of cargo and other ships along our coast. However, the only specific investigation on the effects of anthropogenic noise on MM in the waters of Israel was a single study in Haifa Bay [210].
Marine traffic can result in collisions and ship strikes. Although collisions between ships and MM are not rare events in the world’s oceans or in the Mediterranean (e.g., [211]), information from Israel is limited to a young fin whale found dying near Ashkelon in February 2008, showing clear evidence of a vessel strike.
Common bottlenose and striped dolphins stranded along our Mediterranean coast between 1994 and 2011 have shown relatively stable concentrations of heavy metals [212,213], and heavy metals were also found in other species of MM. Exceptionally high concentrations of mercury and cadmium were found in stranded MM [214], and high concentrations of DDE (dichloro-diphenyl-dichloro-ethylene), indicative of DDT (dichloro-diphenyl-trichloro-ethane) degradation, were found in common bottlenose dolphins, suggesting high exposure to persistent organo-chlorine pollutants.
The consequences of chemical contamination on MM populations in Israel remain unknown but are potentially important considering that studies in other regions indicate that toxic contamination can affect MM reproduction and health at the individual and population levels and may synergize with other stressors.
Obstruction of the digestive tract by ingested plastic as well as entanglement in plastic and other MD is a known cause of MM death. Plastic debris has become widespread in the marine environment, and the problem is acute on the Israeli Mediterranean coast (see Section 3.8). Several cetacean species stranded along the coast have shown evidence of ingestion or entanglement in debris. For instance, a short-beaked common dolphin was observed for several weeks with a hoop around its neck, a stranded Cuvier’s beaked whale and a Risso’s dolphin had significant amounts of plastics in their stomachs [215], and a mature female common bottlenose dolphin died following ingestion of a piece of fishing net [216].
Specific information on the effect of Lessepsian migration (see Section 3.10.1) on MM off Israel is missing, but at least some dolphin species have been reported to adapt their diet successfully to accommodate such changes [217].
Of all the known anthropogenic threats, only the direct killing of marine mammals seems to have declined, due in part to increased awareness and appreciation of these charismatic animals, but also because MM numbers must have dropped substantially below their historical abundance [79].
Despite multiple anthropogenic threats, such as habitat destruction, overfishing, unintended catch in fishing equipment, and chemical and acoustic pollution, the most abundant MM along our Mediterranean coast, the common bottlenose dolphin, T. truncatus (Figure 9), has been successful in maintaining, so far, a permanent reproductive population of a stable size estimated as 300–400 individuals (e.g., [201]).
4. Nature Protection and Nature Reserve
There are two general approaches to the protection of marine species, especially the endangered ones: protecting certain species and protecting specific habitats. The first approach may be considered less effective because of the typically large scope of the marine space involved and the relatively restricted manpower (rangers) available to enforce this protection. Marine protected areas (MPAs) (also known as marine nature reserve, wildlife refuges, wildlife sanctuaries, nature conservation areas, or no-take zones) are considered to be a major tool for biodiversity conservation at sea (e.g., [218]). MPA can be termed as: “a clearly defined geographical space, recognized, dedicated and managed, through legal or other effective means, to achieve the long-term conservation of nature with associated ecosystem services and cultural values” [219]. MPAs can be protected fully or partially (e.g., only from fishery activities) from anthropogenic threats either throughout the year or in given seasons (e.g., reproductive seasons of selected species). MPA can be designated to protect the entire habitat or selected parts of the habitat, a given species, or certain species assemblages.
Dozens of marine animals in the Mediterranean coast of Israel are protected and must not be harmed in any way, including by fishing. These organisms are protected by virtue of the Declaration of Protected Natural Values, updated in 2019, according to the National Parks and Nature Reserves Law (1998). This list includes all marine mammals, sea turtles, sharks and rays; as well as all mollusks (except some squids and octopuses), echinoderms, and coelenterates; and some marine plants, crustaceans, and fish species.
Until 2012, the marine reserves in Israel covered an area of about 27 km2, i.e., 0.55% of the area of Israel’s territorial waters (up to 12 nautical miles from the coast) [220]. This included two small nature reserves that extend from the coastline westward into the sea (Dor Habonim Sea: ~5.3 km2 between Atlit and Hadera, and Rosh HaNikra: ~9.6 km2; see Figure 2) and several other short and narrow stretches of beach as well as small, protected points. During the British Mandate and in the first years of the State of Israel, there were no declared MPAs apart from marine military zones that were relatively protected, but the anthropogenic pressure on the marine environment was significantly lower than at present.
From the mid-1960s to the early 2000s, seven small marine reserves were declared along Israel’s Mediterranean coast. Two “Mediterranean Reserves” were added to these reserves—the MPA at Rosh HaNikra-Akhziv (RHA) and on the Carmel coast in the southern part of Haifa (Figure 2), where all the natural values are protected. In 2017, a plan was approved for the substantial expansion of the RHA MPA. It is currently the largest marine reserve in Israel, stretching from the Rosh HaNikra cliff in the north to the Nahariya industrial zone to the south and up to 15 km offshore. The reserve has a steep seabed, and it includes deep Mediterranean waters and the deep canyon of Akhziv, which is Israel’s only coastal submarine canyon. It reaches a depth of about 850 m and is known to host a rich benthic community (e.g., [221]).
The State of Israel is a signatory to international treaties in which it pledged to preserve at least 10% of its marine territorial water by 2020. To fulfill this commitment, the INPA promotes the declaration of nature reserves in various areas after collecting and analyzing information and preparing a master plan. Most of the presently declared reserves extend from the coastline to a few hundred meters west of it and do not protect representative parts of all marine habitats or unique habitats. The INPA proposed to declare and operate marine nature reserves to cover 20% of the Israeli Mediterranean territorial waters. The establishment of MPAs is planned to conserve representative areas of the marine habitats that characterize Israel—areas with soft substrates as well as large parts of unique habitats, such as AP, SKR, and other natural hard substrates. Rare habitats such as the underwater canyon and mountain ranges that slope down to the sea and continue below the surface will be fully included in the reserve areas. The total marine area protected in territorial waters included in the Israeli Government Maritime Policy Document for Israel’s Mediterranean Waters stands at 876 km2, which is approximately 21.6% of the area of Israel’s territorial waters (excluding restricted fishing zones, firing ranges, etc.) (for details, see [222]). The new fishery regulations (see Section 3.10.2) have essentially added considerable area of MPAs including sensitive habitats such as SKR.
The suggested factors responsible for the success of a marine reserve [223] include being a “no-take” reserve, the level of enforcement, size of the reserve, age of the reserve, and the detachment from anthropogenic sources of disturbance. The level of enforcement was proven to be a key factor in the efficacy of marine reserves, even for small reserves of up to 30 km2 [224], such as RHA MPA. The original size of this natural reserve before expansion was about 10 km2. It is the oldest MPA in Israel and has been well-protected for over 30 years. Underwater surveys were conducted from 2015 to 2021 by INPA in four of the seven small marine reserves declared along the Israeli Mediterranean coast, including the RHA MPA. These surveys revealed that fish with high commercial value are more abundant, larger, and with higher total biomass inside the reserve than in the control site. This was most pronounced for the dusky grouper, Epinephelus marginatus, which only attained expected reproductive size inside the reserve [86]. Similar results were found for the Mediterranean slipper lobster, S. latus, in the RHA MPA [225].
These results suggest that marine reserves may also serve as a dispersal source of propagules outside the MPA. Additionally, the protected population in a reserve can grow above its carrying capacity, leading to a spillover of adults beyond the reserve’s borders. Thus, marine reserves may increase fishery yields beyond their borders and prevent the collapse of a population due to overfishing (e.g., [226]).
5. Discussion and Conclusions
In the last 100 years, the population of Israel has increased almost 13 times [6]. This dramatic population upsurge was accompanied by very intense and accelerated economic growth. Since most of the population of Israel is concentrated close to the coast and is greatly dependent on the marine space (for import, export, energy resources, freshwater supply from desalination plants, recreation, etc.), this proliferation has been expressed also by a massive increase in the anthropogenic effects on the coastal and marine environments.
While in 1922, the level of man-made activity in our Mediterranean coast was relatively low, today, Israel’s coast is characterized by intense anthropogenic activity and many stakeholders with considerable conflicts between them as well as with the marine ecosystem. Some past environmental impacts, such as killing dolphins, harvesting of sponges, and onshore sand mining, have ceased. Other impacts that have been considerably reduced include the widespread discharge of domestic sewage and dumping of industrial waste (e.g., fly-ash from coal-burning coastal power plants) into the sea. Yet, the extent of many additional types of key environmental impacts (KEI) existing in the first half of the 20th century has increased significantly. In recent years, “modern” effects have been added to them, including dramatic elevations of seawater temperatures and sea level as part of global climate change. Other contemporary impacts are chemical pollution by heavy metals and toxic organic compounds, plastic, and microplastic pollutions and effects of coastal desalination plants and installations of the natural gas industry. In sum, the total of all anthropogenic threats and pressure on Israel’s coast has increased considerably in the 21st century.
The present environmental impacts along the coast should be divided to those that are beyond our control and those that can be reduced, at least to a certain degree.
Israel contributes a relative tiny fraction of the global emission of atmospheric carbon, and even this small portion should be reduced by converting to renewable energy, but the ability to change the effects of global climate on Israel’s marine space is negligible. However, Israel should be prepared for the effects of continued changes in the water temperature and SLR on organisms, habitats, and the human population. An example of this approach is the biogeographic-conservation perspective of the rich Mediterranean shallow rocky reefs. Since both warming and associated bio-invasions continue in the Mediterranean, it is expected not only that the degradation may worsen the conditions of this habitat in our shallow coast, but the degraded reef state will gradually advance westward [154]. These authors suggest that alleviating fishing pressure with marine reserves might make these reefs more resilient to these regional pressures, but invasive species would remain a dominant feature in the system. Therefore, in their opinion, a more realistic conservation target might be the preservation or restoration of ecosystem functions rather than the original native biodiversity. Analyzing the catch of bony fish in the trawl fisheries, Lessepsian species were divided into new invaders and earlier invaders [83]. We should perceive those earlier invaders, which have established long-lasting and permanent reproductive populations, as part of the “new indigenous” biota. It was demonstrated [168] that alien species could become a valuable resource for a local fishing industry, with little effect on indigenous species. However, for the benefit of native commercial species, it is advisable to schedule the fishing season out of recruitment period of the indigenous species, which usually does not overlap with that of the Lessepsian species.
It is probably too early to judge how the new fishing regulations, which came into effect at the beginning of 2017, will affect fisheries and the ecosystem in the long run. They have reduced the areas and the efforts of the fishery and so far, have caused a considerable decline in the fishery yield. What most experts agree, however, is that Lessepsian migration will continue and accelerate. They recognize that most factors that drive the introduction and dispersal of marine NIS along our Mediterranean coast are increasing (e.g., [70]). It is questionable if we will see “Tropicalization of the Mediterranean” (e.g., [227]), but the Levantine basin is the closest to this hypothetical situation. What is obvious, however, is that profound changes in the marine biota and ecosystems, associated with introduction of NIS, will continue, and also affect humans (e.g., new hazardous organisms) in the future.
Infrastructures such as the breakwaters of coastal ports are built to last many years withstanding the destructive forces of waves and currents. However, the processes of coastal erosion and accretion associated with the prevailing LST will continue causing collapse and retreat of coastal cliffs. This will damage buildings on the cliff and bathing beaches as well as natural habitats and their inhabitants. Humans can only try to repair this destruction by protecting the coastal cliffs, routine dredging of sand south of the infrastructures, dumping it north to the man-made structures, and nourishment activities. The sand nourishment projects carried out north of several Israeli coastal infrastructures between 2011 and 2017 were unsuccessful. However, these projects may provide accumulated experience for future durable solutions. For example, pebble nourishment might be the optimal long-lasting solution for protecting eroded beaches to save ancient sites [228]. Faced with the threats of the collapse of coastal cliffs, the Israeli government established the Mediterranean Coastal Cliffs Preservation Company in 2013 with the goal of developing solutions for their protection.
Likewise, there is, at least at present, minimal control on marine debris swept to the Israeli coast from neighboring countries and from the open sea. Moreover, plastic debris dumped along the coast in the past also disintegrates into microplastic, the potentially hazardous nature of which has only recently been revealed. To cope with this problem, activities associated with the debris found on or originating from Israeli coasts and land are limited to beach cleaning, education, legislation, and enforcement (e.g., [229]).
The population of Israel is expected to reach 15 million residents by 2048, and by 2065, it is expected to reach 20 million residents [6]. Due to the narrow width of the State of Israel and the location of its economic centers, it is predicted that this increasing population will be located and/or depend on the Mediterranean coast. The development requirements and the expected density of uses in Israel’s Mediterranean maritime space and especially its territorial waters in the coming years require intelligent planning and management of the space. Considering this, the Planning Administration of the State of Israel established the “Maritime Policy—Israel” project for the Mediterranean space (MPI) in 2012. This national endeavor was led by a team of marine experts in collaboration with officials from various government ministries, a wide range of stakeholders, and with the support of the European Union. A similar program, “Marine Spatial Planning of Israel”, was initiated in academia, led by the Technion, Israel Institute of Technology (e.g., msp-israel.net.technion.ac.il/files/2015/12/MSP_plan.compressed.pdf, accessed on 24 August 2022).
The situation analysis of the MPI has revealed several categories of interactions between users/stakeholders in the maritime space. These are: (1) no-match/ conflict: the two uses cannot coexist in the same space and time; (2) conditional matching/limitations: the two uses can coexist, with limitations on the manner of use, time of use, or location; (3) a match between two examined uses, in which they can coexist without setting conditions or limitations; and (4) opportunity: overlap between the two uses creates potential for one of them. An example of the last category can be enabling some environmentally friendly recreational activity in closed military coastal zones during weekends and holidays in absence of military actions.
Among the basic principles guiding the MPI was that of Ecosystem-Based Management (EBM), with the needs of ecosystems to remain healthy, productive, and offer sustainable services dictating the management of human activity. This approach has been recognized as a leading principle for the management of marine users. Marine spatial planning (MSP) has been recognized globally in recent years as a primary instrument for applying EBM (e.g., [230]).
The MPI deals with the planning of the rules and interactions between the various users at sea to achieve two main objectives: (1) the efficient utilization of Israel’s Mediterranean maritime space resources through the arrangement of the regulations for the activity of the various bodies operating at sea, among others, for the benefit of the national economy and the public and (2) the arrangement of the interactions between the various bodies operating at sea and the required protection of the marine environment and its components as a foundation for sustainable use of marine resources. The MPI proposed several policy principles for management, activity, and development of maritime space as well as for protection of the environment and natural resources. It proposes a comprehensive and detailed list of recommendations in the various aspects of the spatial planning and management of the Mediterranean waters of Israel. Although the output of MPI is a considerable improvement over the past situation, there are still deficiencies in proper legislation (e.g., [70]), enforcement, and the lack of a central authority that will concentrate and coordinate the various activities along the Israeli coast.
Similar measures have been proposed in the past. However, economic and security considerations were too often preferred over environmental considerations in critical decisions regarding the marine ecosystem. The developing bodies often did not recognize/ignore the ecosystem services and did not compensate/reimburse the marine ecosystem. The broad practical implementation of the MPI can be examined and assessed only in the future.
Many of the KEI discussed in the present review probably occur in other countries in the Levantine basin. It is worthwhile to initiate a comparative study of various coasts in the region and particularly to refer to the solutions, if any exist, to different types of KEI in the various countries.
Since the coastal ecosystem in the Levantine basin is very dynamic, and marine and coastal technologies are developing and changing rapidly and frequently, new KEI will emerge. For example, the possible effects of electromagnetic fields on marine life are not fully understood. The sources of such potential effects can be the existing underwater communication cables and the planned electric cable between Israel and Cyprus. Therefore, a continuous and in-depth research and monitoring program of the coastal water is also crucial to maintaining a healthy coastal environment.
Author Contributions
Conceptualization, E.S.; writing, review, and editing, E.S. and D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the assistance of many colleagues for the information contributed for the present review and express gratitude especially to Dan Kerem and Dor Edelist, Department of Maritime Civilizations, University of Haifa; Aviad Scheinin, Apex Marine Predators Lab, Morris Kahn Marine Research Station, Department of Marine Biology; The Leon H. Charney School of Marine Science, University of Haifa; Anat Geffen; and Igal Graiver, the Haifa History Society. Thanks are due also to the following photographers: Aviad Scheinin and Hagai Nativ, Morris Kahn Marine Research Station, University of Haifa; Dor Edelist, Department of Maritime Civilizations, University of Haifa; Galia Pasternak; Rotem Sade, Israel Nature and Parks Authority; Avi Algazi, EMS; Israel Electric Company; and Kobi Sror, Roim Milmala—drone photography.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AP | Abrasion platforms |
| AR | Artificial reefs |
| CAMERI | Coastal and Marine Engineering Research Institute |
| CITES | International Organization for the Prevention of Trade in Protected Animals |
| CPUE | Catch per unit effort |
| DDE | Dichloro-diphenyl-dichloro-ethylene |
| DDT | Dichloro-diphenyl-trichloro-ethane |
| EBM | Ecosystem-Based Management |
| EEZ | Exclusive economic zone |
| ESS | Environmental Strategic Survey |
| HP | Horsepower |
| Hs | Significant wave height |
| IEC | Israel Electric Corporation |
| ICBS | Israel Central Bureau of Statistics |
| IOLR | Israel Oceanographic and Limnological Research |
| IPC | Israel Ports Development & Assets Company Ltd. |
| IPCC | Intergovernmental Panel on Climate Change |
| IMMRAC | Israeli Marine Mammal Research and Assistance Center |
| IMS | Israel Meteorological Service |
| INPA | Israel Nature and Parks Authority |
| ISRAMAR | Israel Marine Data Center |
| IUCN | International Union for Conservation of Nature |
| KEI | Key environmental impacts |
| MCM/y | Million cubic meters per year |
| MD | Marine debris |
| MODIS | Moderate Resolution Imaging Spectroradiometer |
| MPA | Marine Protected Area |
| MPI | Maritime Policy—Israel |
| MM | Marine mammals |
| MSL | Mean sea level |
| MSP | Marine spatial planning |
| NASA/GSFC | National Aeronautics and Space Administration/Goddard Space Flight Center |
| NIS | Non-indigenous species |
| NOAA | National Oceanic and Atmospheric Administration |
| RHA | Rosh HaNikra-Akhziv |
| SWRO | Seawater reverse osmosis |
| SKR | Submerged kurkar ridges |
| SL/SLR | Sea level/sea level rise |
| TBT | Tributyltin |
References
- Garfinkle, A. Origins of the Palestine Mandate. Middle East Prog. 2014, 1–6. Available online: www.glscott.org/uploads/2/1/3/3/21330938/origins_of_the_palestine_mandate.pdf (accessed on 24 August 2022).
- Israel Central Bureau of Statistics. 2022. Available online: www.cbs.gov.il/he/pages/default.aspx# (accessed on 24 August 2022).
- Gavish, D. The Survey of Palestine under the British Mandate, 1920–1948, 1st ed.; Routledge: London, UK, 2005; p. 264. [Google Scholar] [CrossRef]
- Israel Central Bureau of Statistics. State of Israel—70 Years of Statistics, Historical Statistical Atlas 1948–2018. 2021. Available online: www.cbs.gov.il/en/publications/Pages/2021/atlas-2018-e.aspx (accessed on 24 August 2022).
- Yarkoni, H. Seventy Years of Hebrew Shipping in the Eretz Israel, 1927–1997, 1st ed.; Hillel Yarkoni: Haifa, Israel, 2002. (In Hebrew) [Google Scholar]
- Israel Central Bureau of Statistics. Statistical Abstract of Israel (2021) Population Density per Square Kilometer. 2021. Available online: www.cbs.gov.il/en/publications/Pages/2019/Population-Statistical-Abstract-of-Israel-2019-No-70.aspx (accessed on 24 August 2022).
- Bitan, M.; Zviely, D. Lost value assessment of bathing beaches due to sea level rise: A case study of the Mediterranean coast of Israel. J. Coast. Conserv. 2019, 23, 773–783. [Google Scholar] [CrossRef]
- Por, F.D. Lessepsian Migration; Springer: Berlin, Germany, 1978. [Google Scholar]
- Ozer, T.; Gertman, I.; Kress, N.; Silverman, J.; Herut, B. Interannual thermohaline (1979–2014) and nutrient (2002–2014) dynamics in the Levantine surface and intermediate water masses, SE Mediterranean Sea. Glob. Planet. Chang. 2017, 151, 60–67. [Google Scholar] [CrossRef]
- Spanier, E.; Edelist, D.; Perkol-Finkel, S.; Schwartz, I. The natural marine environment. In Policy Document for the Israeli Mediterranean Marine Space. Report A-Survey and Analysis of the Existing State; The Planning Administration, Israel Ministry of Treasury: Jerusalem, Israel, 2015; pp. 69–99, (In Hebrew). Available online: www.gov.il/BlobFolder/guide/current_situation_policy/he/Report_1.pdf (accessed on 24 August 2022).
- Planning Administration. Maritime Policy for Israel’s Mediterranean Waters; Planning Administration, Israel Ministry of Treasury: Jerusalem, Israel, 2020. Available online: https://www.gov.il/BlobFolder/generalpage/policy_maritime/he/Water_Energy_Communication_full_strategy_document_translated_24.1.2021.pdf (accessed on 24 August 2022).
- Spanier, E.; Edelist, D.; Perkol-Finkel, S.; Schwartz, I. The natural marine environment. In Policy Document for the Israeli Mediterranean Marine Space. Report A-Survey and Analysis of the Existing State; The Planning Administration, Israel Ministry of Treasury: Jerusalem, Israel, 2016; Volume I, pp. 165–203. (In Hebrew) [Google Scholar]
- Stampfli, G.; Borel, G. A plate tectonic model for the Paleozoic and Mesozoic constrained by dynamic plate boundaries and restored synthetic oceanic isochrons. Earth Planet. Sci. Lett. 2002, 196, 17–33. [Google Scholar] [CrossRef]
- Gvirtzman, Z.; Heida, H.; Garcia-Castellanos, D.; Bar, O.; Zucker, E.; Enzel, Y. Limited Mediterranean sea-level drop during the Messinian salinity crisis inferred from the buried Nile canyon. Commun. Earth Environ. 2022, 3, 216. [Google Scholar] [CrossRef]
- Lichter, M.; Klein, M.; Zviely, D. Dynamic morphology of small south-eastern Mediterranean river mouths: A conceptual model. Earth Surf. Process. Landf. 2011, 36, 547–562. [Google Scholar] [CrossRef]
- Hall, J.K.; Calvo, R. Digital shaded relief maps of Israel, 1:500,000 scale. In Geological Framework of the Levant; Hall, J.K., Krashenin-nikov, A., Hirsch, F., Benjamini, C., Flexer, A., Eds.; Historical Productions-Hall: Jerusalem, Israel, 2005. [Google Scholar]
- Inman, D.L.; Jenkins, S.A. The Nile littoral cell and man’s impact on the coastal zone of the southeastern Mediterranean. In Proceedings of the 19th International Conference on Coastal Engineering, Houston, TX, USA, 3–7 September 1984; pp. 1600–1617. [Google Scholar] [CrossRef]
- Goldsmith, V.; Golik, A. Sediment transport model of the southeastern Mediterranean coast. Mar. Geol. 1980, 37, 135–147. [Google Scholar] [CrossRef]
- Nir, Y. Offshore Artificial Structures and Their Influence on the Israel and Sinai Mediterranean Beaches. Coast. Eng. 1982, 1982, 1837–1856. [Google Scholar] [CrossRef]
- Emery, K.O.; Neev, D. Mediterranean beaches of Israel. Isr. Geol. Surv. Bull. 1960, 26, 1–24. [Google Scholar]
- Pomerancblum, M. The Distribution of Heavy Minerals and Their Hydraulic Equivalents in Sediments of the Mediterranean Continental Shelf of Israel. J. Sediment. Res. 1966, 36, 162–174. [Google Scholar] [CrossRef]
- Almagor, G.; Gill, D.; Perath, I. Marine Sand Resources Offshore Israel. Mar. Georesour. Geotechnol. 2000, 18, 1–42. [Google Scholar] [CrossRef]
- Golik, A. Pattern of sand transport along the Israeli coastline. Isr. J. Earth Sci. 2002, 51, 191–202. [Google Scholar] [CrossRef]
- Lichter, M.; Zviely, D.; Klein, M. Morphological patterns of southeastern Mediterranean river mouths: The topographic setting of the beach as a forcing factor. Geomorphology 2010, 123, 1–12. [Google Scholar] [CrossRef]
- Gvirtzman, G.; Shachnai, E.; Bakler, N.; Ilani, S. Stratigraphy of the Kurkar Group (Quaternary) of the coastal plain of Israel. Curr. Res. Isr. Geol. Surv. 1984, 1983–1984, 70–82. Available online: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=9141043 (accessed on 24 August 2022).
- Arkin, Y.; Michaeli, L. Short- and long-term erosional processes affecting the stability of the Mediterranean coastal cliffs of Israel. Eng. Geol. 1985, 21, 153–174. [Google Scholar] [CrossRef]
- Porat, N.; Wintle, A.G.; Ritte, M. Mode and timing of kurkar and hamra formation, central coastal plain, Israel. Isr. J. Earth Sci. 2004, 53, 13–25. [Google Scholar] [CrossRef]
- Perath, I.; Almagor, G. The Sharon Escarpment (Mediterranean coast, Israel): Stability, dynamics, risks and environmental management. J. Coast. Res. 2000, 16, 207–224. Available online: www.jstor.org/stable/4300025 (accessed on 24 August 2022).
- Gill, D.; Almagor, G. The Geological Infrastructure of the Coastal Escarpment, Factors Affecting Its Retreat and Methods of Its Preservation; Geological Survey of Israel, Report GSI/21/2002; Geological Survey of Israel: Jerusalem, Israel, 2002. (In Hebrew) [Google Scholar]
- Zviely, D.; Klein, M. Coastal cliff retreat rates at Beit-Yannay, Israel, in the 20th century. Earth Surf. Process. Landf. 2004, 29, 175–184. [Google Scholar] [CrossRef]
- Katz, O.; Mushkin, A. Characteristics of sea-cliff erosion induced by a strong winter storm in the eastern Mediterranean. Quat. Res. 2013, 80, 20–32. [Google Scholar] [CrossRef]
- El-Geziry, T.M.; Bryden, I.G. The circulation pattern in the Mediterranean Sea: Issues for modeller consideration. J. Oper. Oceanogr. 2010, 3, 39–46. [Google Scholar] [CrossRef]
- Reiter, E.R. Handbook for Forecasters in the Mediterranean; Technical Paper No. 5-75; Naval Postgraduate School: Monterey, CA, USA, 1975. [Google Scholar]
- Kit, E.; Kroszynski, U. Marine Policy Plan for Israel: Physical Oceanography, Deep Sea and Coastal Zone Overview; CAMERI-Coastal and Marine Engineering Research Institute: Haifa, Israel, 2014. [Google Scholar]
- Alpert, P.; Neeman, B.U.; Shay-El, Y. Intermonthly Variability of Cyclone Tracks in the Mediterranean. J. Clim. 1990, 3, 1474–1478. [Google Scholar] [CrossRef]
- Rosentraub, Z.; Brenner, S. Circulation over the southeastern continental shelf and slope of the Mediterranean Sea: Direct current measurements, winds, and numerical model simulations. J. Geophys. Res. Earth Surf. 2007, 112, 1–21. [Google Scholar] [CrossRef]
- Krichak, S.O.; Alpert, P.; Krishnamurti, T.N. Red Sea Trough/cyclone development? Numerical investigation. Atmos. Phys. 1997, 63, 159–170. [Google Scholar] [CrossRef]
- Zsoy, E. On the Atmospheric Factors Affecting the Levantine Sea; Technical Report No. 25; European Centre for Medium Range Weather Forecasts: Berkshire, UK, 1981. [Google Scholar]
- Alpert, P.; Reisin, T. An Early Winter Polar Air Mass Penetration to the Eastern Mediterranean. Mon. Weather Rev. 1986, 114, 1411–1418. [Google Scholar] [CrossRef]
- Rosen, D.S. A Concise Physical, Chemical and Biological Characterization of the Eastern Mediterranean with Emphasis on the Israeli Coast; IOLR Report H07/2006; Israel Oceanographic and Limnological Research: Haifa, Israel, 2006. [Google Scholar]
- Levin, A.; Glozman, M.; Keren, Y.; Sladkevich, M.; Kroszynski, U.; Kit, E. Processing of Hydrographic Data at the Ashdod Region; Report P.N. 736; CAMERI: Haifa, Israel, 2012. [Google Scholar]
- Levin, A.; Glozman, M.; Keren, Y.; Sladkevich, M.; Kroszynski, U.; Kit, E. Processing of Hydrographic Data for the Haifa Region; Report P.N. 737; CAMERI: Haifa, Israel, 2012. [Google Scholar]
- Rosen, D.S.; Kaplan, A. Environmental loads design criteria for nearshore structures improved environmental loading design criteria for nearshore structures. In Proceedings of the 30th International Conference on Coastal Engineering, San Diego, CA, USA, 3–8 September 2006; ASCE: Reston, VA, USA; pp. 4456–4468. [Google Scholar]
- Perlin, A.; Kit, E. Longshore Sediment Transport on Mediterranean Coast of Israel. J. Waterw. Port Coast. Ocean Eng. 1999, 125, 80–87. [Google Scholar] [CrossRef]
- Tal, D. Extreme Waves Characteristics and Their Return Period at the Vicinity of Ashdod and Haifa Ports. Master’s Thesis, Department of Geography and Environment Studies, University of Haifa, Haifa, Israel, 2020. (In Hebrew, English Abstract). [Google Scholar]
- Bitan, M.; Zviely, D. Sand Beach Nourishment: Experience from the Mediterranean Coast of Israel. J. Mar. Sci. Eng. 2020, 8, 273. [Google Scholar] [CrossRef]
- Zviely, D.; Kit, E.; Klein, M. Longshore sand transport estimates along the Mediterranean coast of Israel in the Holocene. Mar. Geol. 2007, 238, 61–73. [Google Scholar] [CrossRef]
- Manohar, M. Coastal processes at the Nile Delta coast. Shore Beach 1981, 49, 8–15. Available online: pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCALGEODEBRGM8320129681 (accessed on 24 August 2022).
- Zaghloul, Z.M.; Taha, A.A.; Hamama, H.H. Distribution and drifting of sea bottom sediments off Ras El-Barr to Port Said and their erosion-accretion significance. Egypt. J. Geol. 1982, 1, 25–46. [Google Scholar]
- Stanley, D.J. Sediment transport on the coast and shelf between the Nile Delta and Israeli margin as determined by heavy minerals. J. Coast. Res. 1989, 5, 813–828. Available online: www.jstor.org/stable/4297616 (accessed on 24 August 2022).
- El Din, S.H.S.; Mahar, A.M. Evaluation of sediment transport along the Nile Delta coast. Egypt. J. Coast. Res. 1997, 13, 23–26. Available online: www.jstor.org/stable/4298586 (accessed on 24 August 2022).
- Rohrlicht, V.; Goldsmith, V. Sediment Transport along the Southeast Mediterranean: A geological perspective. Geo-Marine Lett. 1984, 4, 99–103. [Google Scholar] [CrossRef]
- Carmel, Z.; Inman, D.L.; Golik, A. Directional wave measurement at Haifa, Israel, and sediment transport along the Nile littoral cell. Coast. Eng. 1985, 9, 21–36. [Google Scholar] [CrossRef]
- Khalifa, M.A. Adoption of recent formulae for sediment transport calculations applied on the Egyptian Nile delta coastal area. J. Coast. Conserv. 2012, 16, 37–49. [Google Scholar] [CrossRef]
- Frihy, O.E.; Badr, A.A.; Selim, M.A.; El Sayed, W.R. Environmental impacts of El Arish power plant on the Mediterranean coast of Sinai, Egypt. Environ. Earth Sci. 2002, 42, 604–611. [Google Scholar] [CrossRef]
- Hydraulics, D. Port of Gaza, Basic Engineering Study; Final Report Part 11; Coastal Impact Study: Delft, The Netherlands, 1994. [Google Scholar]
- Bosboom, J. Port of Gaza, Morphological Modeling; Report H20-11; Delft Hydraulics: Delft, The Netherlands, 1996. [Google Scholar]
- Kit, E.; Sladkevich, M. Structure of offshore currents on sediment Mediterranean coast of Israel. In 6th Workshop on Physical Processes in Natural Waters; Casamitjana, X., Ed.; University of Girona: Girona, Spain, 2001; pp. 97–100. [Google Scholar]
- Kunitsa, D.; Rosentraub, Z.; Stiassnie, M. Estimates of winter currents on the Israeli continental shelf. Coast. Eng. 2005, 52, 93–102. [Google Scholar] [CrossRef]
- Zviely, D.; Kit, E. S-shape distribution of the longshore sand transport at the Mediterranean coast of Israel. In Proceedings of the Israel Geological Society Annual Meeting, Ashkelon, Israel, 23–27 March 2012; pp. 143–144, (English Abstract). [Google Scholar]
- Zviely, D.; Kit, E. The future of Israel’s marine sand resource supply. In Proceedings of the Israel Geological Society Annual Meeting, Abstracts, Kfar Blum (Upper Galilee), Israel, 26–28 March 2019; p. 141. [Google Scholar]
- Zviely, D. The “Gift of the Nile”—Israel’s marine sand resource: Sources, uses and quantities. In The Maritime Strategic Evaluation for Israel 2018/19; Chorev, S., Gonen, E., Eds.; Maritime Policy & Strategy Research Center, University of Haifa: Haifa, Israel, 2019; pp. 285–301. (In Hebrew) [Google Scholar]
- Rosen, D.S. Charaterization of Meteo-Oceanographic Climate in the Study Sector, Progress Report 4; Report H16/1998; Assessment of marine environmental impacts due to construction of artificial islands on the coast of Israel; Israel Oceanographic and Limnological Research: Haifa, Israel, 1998. [Google Scholar]
- Rilov, G.; Peleg, O.; Guy-Haim, T.; Yeruham, E. Community dynamics and ecological shifts on Mediterranean vermetid reefs. Mar. Environ. Res. 2020, 160, 105045. [Google Scholar] [CrossRef] [PubMed]
- Elasar, M.; Kerem, D.; Angel, D.; Steindler, L.; Herut, B.; Shoham-Frider, E.; Barnea, O.; Almogi, A. Achziv submarine canyon: An oasis in the warming oligotrophic Levantine basin? Rapp. Comm. Int. Mer Médit. 2013, 40, 718. [Google Scholar]
- Martin, C.; Giannoulaki, M.; De Leo, F.; Scardi, M.; Salomidi, M.; Knittweis, L.; Pace, M.L.; Garofalo, G.; Gristina, M.; Ballesteros, E.; et al. Coralligenous and maërl habitats: Predictive modelling to identify their spatial distributions across the Mediterranean Sea. Sci. Rep. 2014, 4, 5073. [Google Scholar] [CrossRef]
- Heywood, V.H.; Watson, R.T. Global Biodiversity Assessment; Cambridge University Press: Cambridge, UK, 1995; Volume 1140. [Google Scholar]
- Spanier, E.; Galil, B.S. Lessepsian migration: A continuous biogeographical process. Endeavour 1991, 15, 102–106. [Google Scholar] [CrossRef]
- Galil, B.S. Alien species in the Mediterranean Sea—Which, when, where, why? In Challenges to Marine Ecosystems; Springer: Dordrecht, Germany, 2008; pp. 105–116. [Google Scholar] [CrossRef]
- Galil, B.S.; Mienis, H.K.; Hoffman, R.; Goren, M. Non-indigenous species along the Israeli Mediterranean coast: Tally, policy, outlook. Hydrobiologia 2021, 848, 2011–2029. [Google Scholar] [CrossRef]
- Belkin, N.; Guy-Haim, T.; Rubin-Blum, M.; Lazar, A.; Sisma-Ventura, G.; Kiko, R.; Morov, A.R.; Ozer, T.; Gertman, I.; Herut, B.; et al. Influence of cyclonic and anti-cyclonic eddies on plankton biomass, activity and diversity in the southeastern Mediterranean Sea. Ocean. Sci. 2022, 18, 693–715. [Google Scholar] [CrossRef]
- Kuplik, Z.; Angel, D.L. Diet composition and some observations on the feeding ecology of the rhizostome Rhopilema nomadica in Israeli coastal waters. J. Mar. Biol. Assoc. United Kingd. 2020, 100, 681–689. [Google Scholar] [CrossRef]
- Gokoglu, M.; Galil, B.S. New records of siphonophores and ctenophores in the Levant Sea. J. Black Sea/Medit. Environ. 2020, 26, 190–202. [Google Scholar]
- Israel, A.; Einav, R. Alien seaweeds from the Levant basin (Eastern Mediterranean Sea), with emphasis to the Israeli shores. Isr. J. Plant Sci. 2017, 64, 99–110. [Google Scholar] [CrossRef]
- Scheinin, A.P. A Report on the State of Nature in the Mediterranean; Hamaarag: Jerusalem, Israel, 2013; p. 127. (In Hebrew) [Google Scholar]
- Stambler, N. The Glory of the Sea: Stability and Change in the Aquatic Systems of Israel; The Israeli Association of Aquatic Sciences: Michmoret, Israel, 2014; p. 584. (In Hebrew) [Google Scholar]
- Golani, D. An updated Checklist of the Mediterranean fishes of Israel, with illustrations of recently recorded species and delineation of Lessepsian migrants. Zootaxa 2021, 4956, 1–108. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Keren, T.; Leader, N.; Weil, G.; Tchernov, D.; Rilov, G. Spatiotemporal hotspots of habitat use by loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles in the Levant basin as tools for conservation. Mar. Ecol. Prog. Ser. 2017, 575, 165–179. [Google Scholar] [CrossRef]
- Bearzi, G. Action Plan for Marine Mammals in Israel, 2017–2022; Israel Marine Mammal Research & Assistance Center (IMMRAC): Haifa, Israel, 2017; p. 101. Available online: www.dolphinbiology.org/_download/IMMAP_2017_web.pdf (accessed on 24 August 2022).
- Kerem, D.; Scheinin, A.; Goffman, O.; Elsar, M.; Hadar, N. Marine mammals in the Mediterranean and northern Red Sea. In The Glory of the Sea: Stability and Change in the Aquatic Systems of Israel; Stambler, N., Ed.; The Israeli Association of Aquatic Sciences: Michmoret, Israel, 2014; pp. 237–254. (In Hebrew) [Google Scholar]
- Roditi-Elasar, M.; Bundone, L.; Goffman, O.; Scheinin, A.P.; Kerem, D.H. Mediterranean monk seal (Monachus monachus) sightings in Israel 2009–2020: Extralimital records or signs of population expansion? Mar. Mammal Sci. 2020, 37, 344–351. [Google Scholar] [CrossRef]
- Hornell, J. Report on the Fisheries of Palestine; Government of Palestine by the Crown Agents for the Colonies: London, UK, 1935.
- Edelist, D.; Scheinin, A.; Sonin, O.; Shapiro, J.; Salameh, P.; Rilov, G.; Benayahu, Y.; Schulz, D.; Zeller, D. Israel: Reconstructed estimates of total fisheries removals in the Mediterranean, 1950–2010. Acta Adriat. 2013, 54, 253–264. [Google Scholar] [CrossRef]
- Schulz, D.; Fleisher, A.; Benayahu, Y. The ecological and economic effects of recreational fishing along the Israeli Mediterranean coast. In Proceedings of the 39th conference of the Israeli Society of Ecology and Environmental Sciences, Megiddo, Israel, 27–28 June 2011; p. 119. (In Hebrew). [Google Scholar]
- Frid, O.; Belmaker, J. Catch dynamics of set net fisheries in Israel. Fish. Res. 2019, 213, 1–11. [Google Scholar] [CrossRef]
- Frid, O.; Lazarus, M.; Malamud, S.; Belmaker, J.; Yahel, R. Effects of marine protected areas on fish communities in a hotspot of climate change and invasion. Mediterr. Mar. Sci. 2022, 23, 157–190. [Google Scholar] [CrossRef]
- Oren, O.H.; Komarovsky, B. The influence of the Nile flood on the shore waters of Israel. Rapp. Réun. Cons. Int. Explor. Mer. Monaco 1961, 16, 655–659. [Google Scholar]
- Oren, O.H.; Hornung, H. Temperatures and salinities off the Israel Mediterranean coast. Bull. Sea Fish. Res. Stn. Haifa 1972, 59, 17–31. [Google Scholar]
- Kress, N.; Gertman, I.; Herut, B. Temporal evolution of physical and chemical characteristics of the water column in the Easternmost Levantine basin (Eastern Mediterranean Sea) from 2002 to 2010. J. Mar. Syst. 2014, 135, 6–13. [Google Scholar] [CrossRef]
- Zittis, G.; Almazroui, M.; Alpert, P.; Ciais, P.; Cramer, W.; Dahdal, Y.; Fnais, M.; Francis, D.; Hadjinicolaou, P.; Howari, F.; et al. Climate Change and Weather Extremes in the Eastern Mediterranean and Middle East. Rev. Geophys. 2022, 60, e2021RG000762. [Google Scholar] [CrossRef]
- Pastor, F.; Valiente, J.; Khodayar, S. A Warming Mediterranean: 38 Years of Increasing Sea Surface Temperature. Remote Sens. 2020, 12, 2687. [Google Scholar] [CrossRef]
- Herut, B.; Rahav, E. The National Monitoring Program of Israel’s Mediterranean Waters—Scientific Report for 2015; IOLR Report H48a/2017, Part I; Israel Oceanographic and Limnological Research: Haifa, Israel, 2017; p. 50. (In Hebrew) [Google Scholar]
- Zviely, D.; Bitan, M.; DiSegni, D. The effect of sea-level rise in the 21st century on marine structures along the Mediterranean coast of Israel: An evaluation of physical damage and adaptation cost. Appl. Geogr. 2015, 57, 154–162. [Google Scholar] [CrossRef]
- Rilov, G.; David, N.; Guy-Haim, T.; Golomb, D.; Arav, R.; Filin, S. Sea level rise can severely reduce biodiversity and community net production on rocky shores. Sci. Total Environ. 2021, 791, 148377. [Google Scholar] [CrossRef]
- Ben-Moshe, N.; Renan, I. State of Nature Report (2022)—Trends and Threats. Hamaarag—Israel’s National Ecosystem; Assessment Program, Steinhardt Museum of Natural History, Tel Aviv University: Tel Aviv, Israel, 2022; p. 121, (In Hebrew with an English Abstract). [Google Scholar]
- Spanier, E.; Miller, E.; Zviely, D. Winter stranding of Mediterranean slipper lobsters, Scyllarides latus. Reg. Stud. Mar. Sci. 2017, 14, 126–131. [Google Scholar] [CrossRef]
- Hilell, K.B.; Allweil, Y. Infrastructure Development and Waterfront Transformations: Physical and Intangible Borders in Haifa Port City. Urban Plan. 2021, 6, 43–57. [Google Scholar] [CrossRef]
- Golik, A. Indirect evidence for sediment transport on the continental shelf off Israel. Geo-Marine Lett. 1993, 13, 159–164. [Google Scholar] [CrossRef]
- Golik, A. Dynamics and management of sand along the Israeli coastline. Bull. Inst. Océanogr. (Monaco) 1997, 18, 97–110. [Google Scholar]
- Shoshany, M.; Golik, A.; Degani, A.; Lavee, H.; Gvirtzman, G. New evidence for sand transport direction along the coastline of Israel. J. Coast. Res. 1996, 12, 311–325. Available online: www.jstor.org/stable/4298483 (accessed on 24 August 2022).
- Klein, M. The environmental impact of marina development on adjacent beaches: A case study of the Herzliya marina, Israel. Appl. Geogr. 2001, 21, 145–156. [Google Scholar] [CrossRef]
- Klein, M.; Lichter, M. Monitoring changes in shoreline position adjacent to the Hadera power station, Israel. Appl. Geogr. 2006, 26, 210–226. [Google Scholar] [CrossRef]
- Klein, M.; Zviely, D.; Kit, E.; Shteinman, B. Experimental study of sediment transport along the central Mediterranean coast of Israel by means of fluorescent sand tracers. J. Coast. Res. 2007, 23, 1462–1470. [Google Scholar] [CrossRef]
- Dror, A. Morphological Changes in Israel’s Mediterranean Coast. Ph.D. Thesis, Department of Geography and Environment Studies, University of Haifa, Haifa, Israel, 2017. (In Hebrew, English Abstract). [Google Scholar]
- Golik, A.; Rosen, D.S. Management of the Israeli Coastal Sand Resources; Report H28/l999; Israel Oceanographic & Limnological Research: Haifa, Israel, 1999. [Google Scholar]
- Innocenti, G.; Stasolla, G.; Mendelson, M.; Galil, B.S. Aggressive, omnivorous, invasive: The Erythraean moon crab Matuta victor (Fabricius, 1781) (Crustacea: Decapoda: Matutidae) in the eastern Mediterranean Sea. Ann. Mag. Nat. Hist. 2017, 51, 2133–2142. [Google Scholar] [CrossRef]
- Zviely, D.; Zurel, D.; Edelist, D.; Bitan, M.; Spanier, E. Does Sand Beach Nourishment Enhance the Dispersion of Non-Indigenous Species? The Case of the Common Moon Crab, Matuta victor (Fabricius, 1781), in the Southeastern Mediterranean. J. Mar. Sci. Eng. 2021, 9, 911. [Google Scholar] [CrossRef]
- Nir, Y. Detached Breakwaters, Groins and Other Marine Constructions and Their Influence on the Israel Mediterranean Beaches; Report MG/2/76; Geological Survey of Israel: Jerusalem, Israel, 1976; p. 33. (In Hebrew) [Google Scholar]
- Golik, A.; Rosen, D.S.; Golan, A.; Shoshany, M.; DiCastro, D.; Harari, P. Ashdod Port’s effect on the shoreline, seabed and sediments. In Proceedings of the 19th International Conference on Coastal Engineering, Orlando, FL, USA, 30 September–3 October 1996; Chapter 339. pp. 4376–4389. [Google Scholar]
- Zviely, D. The Impact of the Herzliya Marina on the Width of Its Neighboring Beaches. Master’s Thesis, Department of Geography, University of Haifa, Haifa, Israel, 2000; p. 101, (In Hebrew, English Summary). [Google Scholar]
- Zviely, D. Sedimentological Processes in Haifa Bay in Context of the Nile Littoral Cell. Ph.D. Thesis, Department of Geography and Environment Studies, University of Haifa, Haifa, Israel, 2006. (In Hebrew, English Abstract). [Google Scholar]
- Zviely, D.; Klein, M.; Rosen, D.S. The impact of the Herzliya marina, Israel, on the width of its neighboring beaches. In Proceedings of the 27th International Conference on Coastal Engineering, Sydney, Australia, 16–21 July 2000; Volume 2. Poster 62. [Google Scholar]
- Zviely, D.; Kit, E.; Rosen, B.; Galili, E.; Klein, M. Shoreline migration and beach-nearshore sand balance over the last 200 years in Haifa Bay (SE Mediterranean). Geo-Mar. Lett. 2008, 29, 93–110. [Google Scholar] [CrossRef]
- Rosen, D.S. Study of 50 years coastal changes at Hadera, Israel. In Proceedings of the 23rd International Conference on Coastal Engineering, Venice, Italy, 4–9 October 1992; Chapter 259. pp. 3399–4409. [Google Scholar]
- Almagor, G.; Perath, l. The Mediterranean Coast of Israel; Report GS1/28/2016; Geological Survey of Israel, Ministry of Energy: Jerusalem, Israel, 2020. (In Hebrew)
- Zviely, D. Morphological Changes along Ashdod Coasts at the Years 1946–2013 and the Potential Sand along the Ashdod Port Main Breakwater; DZ-2017-10-02; The Ministry of Environmental Protection, Marine and Coasts Division: Zichron Yaakov, Israel, 2017. (In Hebrew)
- Shamir, R. Current Flow: The Electrification of Palestine; Stanford University Press: Stanford, CA, USA, 2013. [Google Scholar]
- García-Sánchez, G.; Mancho, A.M.; Ramos, A.G.; Coca, J.; Wiggins, S. Structured pathways in the turbulence organizing recent oil spill events in the Eastern Mediterranean. Sci. Rep. 2022, 12, 3662. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y. Monitoring the Marine Environment, the Site of the Orot Rabin Power Plants, the Israel Electric Company, the Hadera Desalination Plant, the H2ID Company; A Report for 2020; The Ministry of Environmental Protection: Zichron Yaakov, Israel, 2021; p. 81, (In Hebrew). Available online: ieccontent.iec.co.il/media/fduldhx2 (accessed on 24 August 2022).
- Kress, N.; Galil, B. Impact of seawater desalination by reverse osmosis on the marine environment. In Efficient Desalination by Reverse Osmosis: A Guide to RO Practice; IWA: London, UK, 2015; pp. 177–202. [Google Scholar]
- Kress, N.; Gertner, Y.; Shoham-Frider, E. Seawater quality at the brine discharge site from two mega size seawater reverse osmosis desalination plants in Israel (Eastern Mediterranean). Water Res. 2019, 171, 115402. [Google Scholar] [CrossRef] [PubMed]
- Netanyahu, S. Seawater desalination-resilience, challenges and risks. Ecol. Environ. 2017, 8, 11. (In Hebrew). Available online: magazine.isees.org.il/?p=16359 (accessed on 24 August 2022).
- Balslev, Y. The Pollution and Purification of Tel Aviv Sea Shore, 1909–1982. Horiz. Geogr. 2012, 78, 103–121. (In Hebrew) [Google Scholar]
- Haarstrick, A.; Bahadir, M. Water and its Global Meaning. In Water and Wastewater Management; Bahadir, M., Haarstrick, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 3–14. [Google Scholar]
- Sapiro, T. Environmental and International Aspects of Sludge Discharge from the Shafdan to the Mediterranean; The Information Center of the Knesset: Jerusalem, Israel, 2010. (In Hebrew) [Google Scholar]
- Shefer, E.; Silverman, J.; Herut, B. Trace metal bioaccumulation in Israeli Mediterranean coastal marine mollusks. Quat. Int. 2015, 390, 44–55. [Google Scholar] [CrossRef]
- Herut, B.; Rahav, E. The National Monitoring Program of Israel’s Mediterranean Waters—Scientific Report for 2015; IOLR Report H48a/2017, Part III; Israel Oceanographic and Limnological Research: Haifa, Israel, 2017. (In Hebrew) [Google Scholar]
- Shoham-Frider, E.; Gertner, Y.; Guy-Haim, T.; Herut, B.; Kress, N.; Shefer, E.; Silverman, J. Legacy groundwater pollution as a source of mercury enrichment in marine food web, Haifa Bay, Israel. Sci. Total Environ. 2020, 714, 136711. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Sciences. Marine litter. In Assessing Potential Ocean Pollutants; A Report of the Study Panel on Commission on Natural Resources; Natural Research Council, National Academy of Sciences: Washington, DC, USA, 1975.
- Gall, S.; Thompson, R. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Gajanur, A.R.; Jaafar, Z. Abandoned, lost, or discarded fishing gear at urban coastlines. Mar. Pollut. Bull. 2022, 175, 113341. [Google Scholar] [CrossRef] [PubMed]
- Segal, Y. The National Monitoring Program of Israel’s Mediterranean Waters—Scientific Report on Marine Litter for 2020; IOLR Report H21/2021; Israel Oceanographic and Limnological Research: Haifa, Israel, 2021. [Google Scholar]
- Golik, A.; Gertner, Y. Litter on the israeli coastline. Mar. Environ. Res. 1992, 33, 1–15. [Google Scholar] [CrossRef]
- Pasternak, G.; Zviely, D.; Ribic, C.A.; Ariel, A.; Spanier, E. Sources, composition and spatial distribution of marine debris along the Mediterranean coast of Israel. Mar. Pollut. Bull. 2017, 114, 1036–1045. [Google Scholar] [CrossRef]
- Galgani, F.; Hanke, G.; Maes, T. Global distribution, composition and abundance of marine litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin, Germany, 2015; pp. 29–56. [Google Scholar] [CrossRef]
- Alkalay, R.; Pasternak, G.; Zask, A. Clean-coast index—A new approach for beach cleanliness assessment. Ocean Coast. Manag. 2007, 50, 352–362. [Google Scholar] [CrossRef]
- Segal, Y.; Gertner, Y.; Sisma-Ventura, G.; Zurel, D.; Herut, B. The State of Beach Litter Pollution during the COVID-19 Pandemic: A Case Study of the Israeli Coasts. Coast. Manag. 2022, 50, 372–384. [Google Scholar] [CrossRef]
- Pasternak, G.; Ribic, C.A.; Spanier, E.; Ariel, A.; Mayzel, B.; Ohayon, S.; Zviely, D. Nearshore survey and cleanup of benthic marine debris using citizen science divers along the Mediterranean coast of Israel. Ocean Coast. Manag. 2019, 175, 17–32. [Google Scholar] [CrossRef]
- Pasternak, G.; Zviely, D.; Ariel, A.; Spanier, E.; Ribic, C.A. Message in a bottle—The story of floating plastic in the eastern Mediterranean Sea. Waste Manag. 2018, 77, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.; Ribic, C.A.; Spanier, E.; Zviely, D. Stormwater systems as a source of marine debris: A case study from the Mediterranean coast of Israel. J. Coast. Conserv. 2021, 25, 27. [Google Scholar] [CrossRef]
- Cantasano, N. Marine Pollution by Microplastics in the Mediterranean Sea. J. Mar. Sci. Eng. 2022, 10, 858. [Google Scholar] [CrossRef]
- Van der Hal, N.V.D.; Yeruham, E.; Angel, D.L. Dynamics in microplastic ingestion during the past six decades in herbivorous fish on the Mediterranean Israeli coast. In Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea; Cocca, M., Di Pace, E., Errico, M., Gentile, G., Montarsolo, A., Mossotti, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 159–165. [Google Scholar] [CrossRef]
- van der Hal, N.; Ariel, A.; Angel, D.L. Exceptionally high abundances of microplastics in the oligotrophic Israeli Mediterranean coastal waters. Mar. Pollut. Bull. 2017, 116, 151–155. [Google Scholar] [CrossRef]
- van der Hal, N.; Yeruham, E.; Shukis, D.; Rilov, G.; Astrahan, P.; Angel, D.L. Uptake and incorporation of PCBs by eastern Mediterranean rabbitfish that consumed microplastics. Mar. Pollut. Bull. 2019, 150, 110697. [Google Scholar] [CrossRef]
- Duarte, C.M.; Chapuis, L.; Collin, S.P.; Costa, D.P.; Devassy, R.P.; Eguiluz, V.M.; Erbe, C.; Gordon, T.A.C.; Halpern, B.S.; Harding, H.R.; et al. The soundscape of the Anthropocene ocean. Science 2021, 371, eaba4658. [Google Scholar] [CrossRef]
- van Geel, N.C.; Risch, D.; Wittich, A. A brief overview of current approaches for underwater sound analysis and reporting. Mar. Pollut. Bull. 2022, 178, 113610. [Google Scholar] [CrossRef]
- Rodrigo-Saura, F.J.; Poveda, P.; Carbajo, J.; Ramis, J. Monitoring long-term underwater acoustic pollution in Mediterranean Sea waters. In Proceedings of INTER-NOISE and NOISE-CON Congress and Conference, Madrid, Spain, 16–19 June 2019; Institute of Noise Control Engineering: Reston, VA, USA, 2019; Volume 259, pp. 5877–5888. [Google Scholar]
- Kerem, D. The world of silence? The need to limit noise at sea. Ecol. Environ. 2014, 5, 61–62. (In Hebrew) [Google Scholar]
- Davies, T.W.; Duffy, J.P.; Bennie, J.; Gaston, K.J. The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ. 2014, 12, 347–355. [Google Scholar] [CrossRef]
- The Blue Half; Israeli Mediterranean Conservation Reform; the Society for the Protection of Nature in Israel. The Dark Side of Light: Light Pollution in the Foreground of the Sea; The Blue Half: Tel Aviv-Yafo, Israel, 2022; (In Hebrew). Available online: mafish.org.il/marine-planning/light-pollution/ (accessed on 24 August 2022).
- Por, F.D. The Canuellidae (Copepoda, Harpacticoida) in the waters around the Sinai Peninsula and the problem of “Lessepsian” migration of this family. Isr. J. Zool. 1969, 18, 169–178. [Google Scholar] [CrossRef]
- Ulman, A.; Ferrario, J.; Occhpinti-Ambrogi, A.; Arvanitidis, C.; Bandi, A.; Bertolino, M.; Bogi, C.; Chatzigeorgiou, G.; Çiçek, B.A.; Deidun, A.; et al. A massive update of non-indigenous species records in Mediterranean marinas. PeerJ 2017, 5, e3954. [Google Scholar] [CrossRef]
- Galil, B.S.; Danovaro, R.; Rothman, S.B.S.; Gevili, R.; Goren, M. Invasive biota in the deep-sea Mediterranean: An emerging issue in marine conservation and management. Biol. Invasions 2019, 21, 281–288. [Google Scholar] [CrossRef]
- Rilov, G.; Peleg, O.; Yeruham, E.; Garval, T.V.; Vichik, A.; Raveh, O. Alien turf: Overfishing, overgrazing and invader domination in south-eastern Levant reef ecosystems. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 28, 351–369. [Google Scholar] [CrossRef]
- Galil, B.S.; Boero, F.; Campbell, M.L.; Carlton, J.T.; Cook, E.; Fraschetti, S.; Gollasch, S.; Hewitt, C.L.; Jelmert, A.; MacPherson, E.; et al. ‘Double trouble’: The expansion of the Suez Canal and marine bioinvasions in the Mediterranean Sea. Biol. Invasions 2015, 17, 973–976. [Google Scholar] [CrossRef]
- Galil, B.; Marchini, A.; Occhipinti-Ambrogi, A.; Ojaveer, H. The enlargement of the Suez Canal—Erythraean introductions and management challenges. Manag. Biol. Invasions 2017, 8, 141–152. [Google Scholar] [CrossRef]
- Galil, B. Poisonous and venomous: Marine alien species in the Mediterranean Sea and human health. In Invasive Species and Human Health; Mazza, G., Tricarico, E., Eds.; CABI Invasive Series 10; CABI: Wallingford, UK, 2018; pp. 1–15. [Google Scholar]
- Edelist, D.; Rilov, G. Trends in Israeli fishing in the Mediterranean Sea. Ecol. Environ. 2014, 5, 90–97. (In Hebrew) [Google Scholar]
- Wiesmeth, H. Overfishing. In Environmental Economics; Springer: Cham, Switzerland, 2022; pp. 269–305. [Google Scholar]
- Edelist, D.; Sonin, O.; Golani, D.; Rilov, G.; Spanier, E. Spatiotemporal patterns of catch and discards of the Israeli Mediterranean trawl fishery in the early 1990s: Ecological and conservation perspectives. Sci. Mar. 2011, 75, 641–652. [Google Scholar] [CrossRef]
- Snovsky, G.; Shapiro, J. The Fishery and Aquaculture of Israel 1996 in Figures; The State of Israel, Ministry of Agriculture, Department of Fisheries: Rishon LeTsiyon, Israel, 1997.
- Pisanty, S.; Sonin, O.; Alperovic, A. Assessment of the Influence of a Summer Trawling Ban on the Catch of the Fishery; Technical Paper; State of Israel, Ministry of Agriculture and Rural Development, Department of Fisheries: Rishon LeTsiyon, Israel, 2000; p. 17. (In Hebrew)
- Bodenheimer, F.S. Animal Life in Palestine. An Introduction to the Problems of Animal Ecology and Zoogeography; L. Mayer: Jerusalem, Israel, 1935. [Google Scholar]
- Wirszubski, A. On the biology and biotope of the red mullet Mullus barbatus L. Bull. Sea. Fish. Res. Stn. Caesarea 1953, 7, 1–20. [Google Scholar]
- Oren, O.H. Changes in the temperature of the eastern Mediterranean Sea in relation to the catch of the Israel trawl fishery during the years 1954–1955 and 1955–1956. Bull. Inst. Océanogr. (Monaco) 1957, 1102, 1–13. [Google Scholar]
- Ben-Tuvia, A. Man-made changes in the eastern Mediterranean Sea and their effect on the fishery resources. Mar. Biol. 1973, 19, 197–203. [Google Scholar] [CrossRef]
- Goren, M.; Galil, B.S.; Diamant, A.; Stern, N.; Levitt-Barmats, Y. Invading up the food web? Invasive fish in the southeastern Mediterranean Sea. Mar. Biol. 2016, 163, 180. [Google Scholar] [CrossRef]
- Van Rijn, I.; Kiflawi, M.; Belmaker, J. Alien species stabilize local fisheries catch in a highly invaded ecosystem. Can. J. Fish. Aquat. Sci. 2020, 77, 752–761. [Google Scholar] [CrossRef]
- Seward, W.H.; Seward, O.R.; William, H. Seward’s Travels around the World; No. 34186; Appleton: New York, NY, USA, 1873. [Google Scholar]
- Galil, B.S. Loss or gain? Invasive aliens and biodiversity in the Mediterranean Sea. Mar. Pollut. Bull. 2007, 55, 314–322. [Google Scholar] [CrossRef]
- Castro, N.; Gestoso, I.; Marques, C.S.; Ramalhosa, P.; Monteiro, J.G.; Costa, J.L.; Canning-Clode, J. Anthropogenic pressure leads to more introductions: Marine traffic and artificial structures in offshore islands increases non-indigenous species. Mar. Pollut. Bull. 2022, 181, 113898. [Google Scholar] [CrossRef]
- Edelist, D.; Spanier, E. Influence of Levantine Artificial Reefs on the fish assemblage of the surrounding seabed. Mediterr. Mar. Sci. 2009, 10, 35–54. [Google Scholar] [CrossRef]
- Bettelheim, G.; Amir, R. Placement of artificial reefs in Israel. Ecol. Environ. 2014, 5, 58–59. (In Hebrew) [Google Scholar]
- Spanier, E. Changes in the ichthyofauna of an artificial reef in the southeastern Mediterranean in one decade. Sci. Mar. 2000, 64, 279–284. [Google Scholar] [CrossRef]
- London Convention. Convention on the Prevention of Marine Pollution by Dumping of Wastes and Other Matter. 1972. Available online: www.imo.org/en/OurWork/Environment/Pages/London-Convention-Protocol.aspx (accessed on 24 August 2022).
- UNEP/MED 5.5. Updated Guidelines for Regulating the Placement of Artificial Reefs at Sea; UNEP/MED WG.461/13; UNEP: Tunis, Tunisia, 2019; Available online: www.rac-spa.org/nfp14/documents/01_working_documents/wg_461_13_en.pdf (accessed on 24 August 2022).
- Galil, B.S. Seeing Red: Alien species along the Mediterranean coast of Israel. Aquat. Invasions 2007, 2, 281–312. [Google Scholar] [CrossRef]
- Tosunoğlu, Z.; Soykan, O.; Duruer, E.; Kinacigil, T. Contribution to Some Biological and Fishery Aspects of Commercial Penaeid Prawns in Mersin Bay (Northeastern Mediterranean, Turkey). Crustaceana 2008, 81, 577–585. [Google Scholar] [CrossRef]
- Galil, B.S. Lessepsian immigration: Human impact on Levantine Biogeography. In The Biodiversity Crisis and Crustacea—Proceedings of the Fourth International Crustacean Congress Crustacean Issues 12; von Vaupel Klein, J., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 50–51. [Google Scholar]
- Edelist, D.; Knutsen, Ø.; Ellingsen, I.; Majaneva, S.; Aberle, N.; Dror, H.; Angel, D.L. Tracking Jellyfish Swarm Origins Using a Combined Oceanographic-Genetic-Citizen Science Approach. Front. Mar. Sci. 2022, 9, 869619. [Google Scholar] [CrossRef]
- Ghermandi, A.; Galil, B.; Gowdy, J.; Nunes, P.A. Jellyfish outbreak impacts on recreation in the Mediterranean Sea: Welfare estimates from a socioeconomic pilot survey in Israel. Ecosyst. Serv. 2015, 11, 140–147. [Google Scholar] [CrossRef]
- Edelist, D.; Guy-Haim, T.; Kuplik, Z.; Zuckerman, N.; Nemoy, P.; Angel, D.L. Phenological shift in swarming patterns of Rhopilema nomadica in the Eastern Mediterranean Sea. J. Plankton Res. 2020, 42, 211–219. [Google Scholar] [CrossRef]
- Gengel, E.; Kuplik, Z.; Angel, D.; Heifetz, E. A physics-based model of swarming jellyfish. arXiv 2022, arXiv:2211.07800. [Google Scholar] [CrossRef]
- Kuplik, Z.; Kerem, D.; Angel, D. Respiration Rates, Metabolic Demands and Feeding of Ephyrae and Young Medusae of the Rhizostome Rhopilema nomadica. Diversity 2021, 13, 320. [Google Scholar] [CrossRef]
- Fuentes, V.L.; Angel, D.L.; Bayha, K.M.; Atienza, D.; Edelist, D.; Bordehore, C.; Gili, J.-M.; Purcell, J.E. Blooms of the invasive ctenophore, Mnemiopsis leidyi, span the Mediterranean Sea in 2009. In Jellyfish Blooms: New Problems and Solutions 12; Springer: Dordrecht, The Netherlands, 2010; pp. 23–37. [Google Scholar] [CrossRef]
- Glazer, A. Recruitment and Settlement of Sessile Marine Organisms on Different Artificial Substrates in a Power Plant in the South-Eastern Mediterranean. Ph.D. Dissertation, University of Haifa, Haifa, Israel, 2002. [Google Scholar]
- Rilov, G.; Gasith, A.; Evans, S.; Benayahu, Y. Unregulated use of TBT-based antifouling paints in Israel (eastern Mediterranean): High contamination and imposex levels in two species of marine gastropods. Mar. Ecol. Prog. Ser. 2000, 192, 229–238. [Google Scholar] [CrossRef]
- Shabtay, A.; Rilov, G.; Benayahu, Y. The Indo-Pacific oyster Spondylus spinosus Schreibers, 1793 in the Eastern Mediterranean Sea: Reproductive features. Molluscan Res. 2015, 35, 206–212. [Google Scholar] [CrossRef]
- Albano, P.G.; Steger, J.; Bošnjak, M.; Dunne, B.; Guifarro, Z.; Turapova, E.; Hua, Q.; Kaufman, D.S.; Rilov, G.; Zuschin, M. Native biodiversity collapse in the eastern Mediterranean. Proc. R. Soc. B Boil. Sci. 2021, 288, 20202469. [Google Scholar] [CrossRef]
- Maillard, C.; Raibaut, A.; Castri, F. Human activities and modifications of ichtyofauna of the Mediterranean Sea: Effect on parasitosis. In Biological Invasions in Europe and the Mediterranean Basin; Springer: Dordrecht, The Netherlands, 1990; pp. 297–305. [Google Scholar] [CrossRef]
- Yeruham, E.; Shpigel, M.; Abelson, A.; Rilov, G. Ocean warming and tropical invaders erode the performance of a key herbivore. Ecology 2020, 101, e02925. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, O.; Kroh, A. Needle in a haystack—Genetic evidence confirms the expansion of the alien echinoid Diadema setosum (Echinoidea: Diadematidae) to the Mediterranean coast of Israel. Zootaxa 2018, 4497, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Barash, A.; Pickholtz, R.; Blaustein, L.; Rilov, G. Seasonal aggregations of sharks near coastal power plants in Israel: An emerging phenomenon. Mar. Ecol. Prog. Ser. 2018, 590, 145–154. [Google Scholar] [CrossRef]
- Shamir, Z.Z.; Shamir, S.Z.; Becker, N.; Scheinin, A.; Tchernov, D. Evidence of the impacts of emerging shark tourism in the Mediterranean. Ocean Coast. Manag. 2019, 178, 104847. [Google Scholar] [CrossRef]
- Edelist, D.; Rilov, G.; Golani, D.; Carlton, J.T.; Spanier, E. Restructuring the Sea: Profound shifts in the world’s most invaded marine ecosystem. Divers. Distrib. 2013, 19, 69–77. [Google Scholar] [CrossRef]
- Edelist, D.; Golani, D.; Rilov, G.; Spanier, E. The invasive venomous striped eel catfish Plotosus lineatus in the Levant: Possible mechanisms facilitating its rapid invasional success. Mar. Biol. 2011, 159, 283–290. [Google Scholar] [CrossRef]
- Maureaud, A.; Frelat, R.; Pécuchet, L.; Shackell, N.; Mérigot, B.; Pinsky, M.L.; Amador, K.; Anderson, S.C.; Arkhipkin, A.; Auber, A.; et al. Are we ready to track climate-driven shifts in marine species across international boundaries? -A global survey of scientific bottom trawl data. Glob. Chang. Biol. 2021, 27, 220–236. [Google Scholar] [CrossRef]
- Stern, N.; Rothman, S.B.; Hüseyinoglu, M.F.; Öztürk, B. Iron Lion Zion: The successful, albeit lingered, invasion of the lionfish in the Israeli Mediterranean Sea. In Lionfish Invasion and Its Management in the Mediterranean Sea; Turkish Marine Research Foundation (TUDAV) Publication Istanbul: Istanbul, Turkey, 2018; Volume 49, pp. 51–56. [Google Scholar]
- Sonin, O.; Spanier, E.; Levi, D.; Patti, B.; Rizzo, P.; Andreoli, M.G. Nanism (dwarfism) in fish: A comparison between red mullet Mullus barbatus from the southeastern and the central Mediterranean. Mar. Ecol. Prog. Ser. 2007, 343, 221–228. [Google Scholar] [CrossRef]
- Sharir, Y.; Kerem, D.; Gol’Din, P.; Spanier, E. Small size in the common bottlenose dolphin Tursiops truncatus in the eastern Mediterranean: A possible case of Levantine nanism. Mar. Ecol. Prog. Ser. 2011, 438, 241–251. [Google Scholar] [CrossRef]
- Scheinin, A.; Kerem, D.; Lojen, S.; Liberzon, J.; Spanier, E. Resource partitioning between common bottlenose dolphin (Tursiops truncatus) and the Israeli bottom trawl fishery? Assessment by stomach contents and tissue stable isotopes analysis. J. Mar. Biol. Assoc. United Kingd. 2014, 94, 1203–1220. [Google Scholar] [CrossRef]
- Levy, Y.; Frid, O.; Weinberger, A.; Sade, R.; Adam, Y.; Kandanyan, U.; Berkun, V.; Perry, N.; Edelist, D.; Goren, M.; et al. A small fishery with a high impact on sea turtle populations in the eastern Mediterranean. Zool. Middle East 2015, 61, 300–317. [Google Scholar] [CrossRef]
- Levi, Y. Eastern Mediterranean Sea Turtle Ecology and Conservation: Spatiotemporal Patterns, Fishing Impacts, Health Diagnostics and Husbandry. Ph.D. Dissertation, University of Haifa, Haifa, Israel, 2017. [Google Scholar]
- Sassoon, S.; Levy, Y.; Segal, Y. The Effects of Marine Litter on Sea Turtles in Israel; Report to The Israeli Ministry for the Protection of the Environment; Ministry of Environmental Protection: Zichron Yaakov, Israel, 2022. [Google Scholar]
- Marangoni, L.F.B.; Davies, T.; Smyth, T.; Rodríguez, A.; Hamann, M.; Duarte, C.; Pendoley, K.; Berge, J.; Maggi, E.; Levy, O. Impacts of artificial light at night in marine ecosystems—A review. Glob. Chang. Biol. 2022, 8, 5346–5367. [Google Scholar] [CrossRef] [PubMed]
- Piante, C.; Ody, D. Blue Growth in the Mediterranean Sea: The Challenge of Good Environmental Status. MedTrends Project. WWF-France. 2015. Available online: https://d2ouvy59p0dg6k.cloudfront.net/downloads/medtrends_regional_report.pdf (accessed on 24 August 2022).
- Reynolds, J.E., III; Marsh, H.; Ragen, T.J. Marine mammal conservation. Endanger. Species Res. 2009, 7, 23–28. [Google Scholar] [CrossRef]
- Kerem, D.; Edelist, D. National Overview on the Current Status of Cetacean-Fisheries Conflicts in Israel, 1993–2008. In Proceedings of the International Workshop on Bycatch within the ACCOBAMS Area, Rome, Italy, 17–18 September 2008; p. 5. [Google Scholar]
- Goffman, O. Incidental cetacean bycatch along the Mediterranean coast of Israel. Israel Cetaceans—Risks and chances. In Proceedings of the IMMRAC 1st International Conference, Ashdod, Israel, October 2011. (In Hebrew). [Google Scholar]
- Zuriel, Y.; Kerem, D.; Scheinin, A. Long-Term Passive Acoustic Monitoring of Bottlenose Dolphins (Tursiops truncatus) in Haifa Bay. Environmental Report on the Influence of Sub-Marine Noise from Haifa Port Expanding on Marine Mammals; IMMRAC: Michmoret, Israel, 2016. (In Hebrew) [Google Scholar]
- Pirotta, V.; Grech, A.; Jonsen, I.D.; Laurance, W.F.; Harcourt, R.G. Consequences of global shipping traffic for marine giants. Front. Ecol. Environ. 2019, 17, 39–47. [Google Scholar] [CrossRef]
- Shoham-Frider, E.; Kress, N.; Wynne, D.; Scheinin, A.; Roditi-Elsar, M.; Kerem, D. Persistent organochlorine pollutants and heavy metals in tissues of common bottlenose dolphin (Tursiops truncatus) from the Levantine Basin of the Eastern Mediterranean. Chemosphere 2009, 77, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Shoham-Frider, E.; Goffman, O.; Harlavan, Y.; Kress, N.; Morick, D.; Roditi-Elasar, M.; Shefer, E.; Kerem, D. Trace elements in striped dolphins (Stenella coeruleoalba) from the Eastern Mediterranean: A 10-years perspective. Mar. Pollut. Bull. 2016, 109, 624–632. [Google Scholar] [CrossRef]
- Shoham-Frider, E.; Kerem, D.; Roditi-Elasar, M.; Goffman, O.; Morick, D.; Yoffe, O.; Kress, N. Trace elements in tissues of cetacean species rarely stranded along the Israeli Mediterranean coast. Mar. Pollut. Bull. 2014, 83, 376–382. [Google Scholar] [CrossRef]
- Shoham-Frider, E.; Amiel, S.; Roditi-Elasar, M.; Kress, N. Risso’s dolphin (Grampus griseus) stranding on the coast of Israel (eastern Mediterranean). Autopsy results and trace metal concentrations. Sci. Total Environ. 2002, 295, 157–166. [Google Scholar] [CrossRef]
- Levy, A.M.; Brenner, O.; Scheinin, A.; Morick, D.; Ratner, E.; Goffman, O.; Kerem, D. Laryngeal Snaring by Ingested Fishing Net in a Common Bottlenose Dolphin (Tursiops truncatus) off the Israeli Shoreline. J. Wildl. Dis. 2009, 45, 834–838. [Google Scholar] [CrossRef]
- Brand, D. The Effect of Lessepsian Migration on the Diets of the Common Bottlenose Dolphin (Tursiops truncatus) and the Short-Beaked Common Dolphin (Delphinus delphis) along the Israeli Coastline. Master’s Thesis, University of Haifa, Haifa, Israel, 2013; p. 92, (In Hebrew, with an English Summary). [Google Scholar]
- Bianchi, C.N.; Azzola, A.; Cocito, S.; Morri, C.; Oprandi, A.; Peirano, A.; Sgorbini, S.; Montefalcone, M. Biodiversity Monitoring in Mediterranean Marine Protected Areas: Scientific and Methodological Challenges. Diversity 2022, 14, 43. [Google Scholar] [CrossRef]
- Dudley, N. Guidelines for Applying Protected Area Management Categories; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- Gabrié, C.; Lagabrielle, E.; Bissery, C.; Crochele, E.; Meola, B.; Webster, C.; Claudet, J.; Chassanite, A.; Marinesque, S.; Robert, P.; et al. The Status of Marine Protected Areas in the Mediterranean Sea; MedPAN & RAC/SPA: Marseille, France, 2012. [Google Scholar]
- Roditi-Elasar, M.; Kerem, D.; Lazar, M.; Barneah, O.; Almogi-Labin, A.; Angel, D.L. Benthic macro-faunal abundance and diversity and sediment distribution in Akhziv submarine canyon and the adjacent slope (eastern Levant Basin, Mediterranean Sea). Mediterr. Mar. Sci. 2019, 20, 521–531. [Google Scholar] [CrossRef]
- Israel Planning Administration. Maritime Policy-Policy Document for Israel’s Mediterranean Waters, A Platform for Policy Formulation—Background and Work Processes; Israel Planning Administration: Jerusalem, Israel, 2020.
- Edgar, G.J.; Stuart-Smith, R.D.; Willis, T.J.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.F.; Berkhout, J.; et al. Global conservation outcomes depend on marine protected areas with five key features. Nature 2014, 506, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Giakoumi, S.; Scianna, C.; Plass-Johnson, J.; Micheli, F.; Grorud-Colvert, K.; Thiriet, P.; Claudet, J.; Di Carlo, G.; Di Franco, A.; Gaines, S.D.; et al. Ecological effects of full and partial protection in the crowded Mediterranean Sea: A regional meta-analysis. Sci. Rep. 2017, 7, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Miller, E. Do Marine Nature Reserves Enhance the Conservation of the Mediterranean Slipper Lobster (Scyllarides latus)? Master’s Thesis, University of Haifa, Haifa, Israel, 2020. [Google Scholar]
- Lester, S.E.; Halpern, B.S.; Grorud-Colvert, K.; Lubchenco, J.; Ruttenberg, B.I.; Gaines, S.D.; Airamé, S.; Warner, R.R. Biological effects within no-take marine reserves: A global synthesis. Mar. Ecol. Prog. Ser. 2009, 384, 33–46. [Google Scholar] [CrossRef]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar] [CrossRef]
- Bitan, M.; Galili, E.; Spanier, E.; Zviely, D. Beach Nourishment Alternatives for Mitigating Erosion of Ancient Coastal Sites on the Mediterranean Coast of Israel. J. Mar. Sci. Eng. 2020, 8, 509. [Google Scholar] [CrossRef]
- Portman, M.E.; Pasternak, G.; Yotam, Y.; Nusbaum, R.; Behar, D. Beachgoer participation in prevention of marine litter: Using design for behavior change. Mar. Pollut. Bull. 2019, 144, 1–10. [Google Scholar] [CrossRef]
- Ansong, J.; Gissi, E.; Calado, H. An approach to ecosystem-based management in maritime spatial planning process. Ocean Coast. Manag. 2017, 141, 65–81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).