Abstract
The electrokinetic remediation (EKR) method has been extensively considered for the removal of inorganic pollutants from contaminated dredged sediment. In addition, the use of chelating agents as electrolyte solutions has been beneficial in increasing the mobility of metals. This study investigated the metals’ (Cd, Cr, Cu, Pb, and Zn) mobilities by assessing the effect of two environmentally friendly chelating agents, ethylenediaminedisuccinic acid (EDDS) and citric acid (CA), in enhancing the EKR efficiency under a periodic voltage gradient. The results showed that, for the same concentration (0.1 mol L−1), CA is more suitable for enhancing the removal of Cr (67.83%), Cu (59.77%), and Pb (32.05%) by chelating and desorbing them from the sediment matrix and concentrating them in the electrode compartments. EDDS provided efficiency to improve the Cd extraction percentage (45.87%), whereas CA and EDDS had comparable improvement removal impacts on Zn EKR (39.32% and 41.37%, respectively). From the comparison with previous results obtained with a continuous voltage, applying a periodic voltage gradient associated with a low concentration of chelating agents led to a promising result.
1. Introduction
Dumping contaminated sediments into the marine environment could result in significant environmental impacts on the fauna and flora [1]. This operation is thus rigorously restricted in accordance with French legislation and standards. As such, contaminated sediment from harbors and inland waterways should be managed and treated separately on land as waste if necessary. Sediment treatment can be divided into physical, biological, and chemical technologies including thermal degradation/extraction, biological decontamination, chemical oxidation/reduction/separation, electrokinetics, stabilization/solidification, and washing [2,3]. Of these, EKR is a reliable method for separating pollutants from different polluted materials. EKR was initially developed as a mitigation method for polluted soil, and, during the last 30 years, it has been recognized as a practical in situ and ex situ remediation technique for clay-rich soils and fine-grained, low-permeability harbor sediments. When electric potential is applied via electrodes integrated into the porous medium, it induces various complex mechanisms, such as electrolysis, electromigration, electro-osmosis, and electrophoresis [4]. The voltage gradient generates the electro-osmotic flow of water [5,6], and solubilized neutral pollutants can be also transported with the pore fluid [7]. Enhanced EKR is highly dependent on the type of processing solution used. The main reaction, inherent to the EKR, is water electrolysis. It generates H+ at the anode side and OH- at the cathode side, and thus causes metals to precipitate close to the cathode side because of the alkaline pH rise [8]. Over the last few years, chelating agents have been extensively used to enhance metals’ solubilization for EKR [9,10,11,12,13]. The needed amount of chelating agent depends on the concentration of metals to be chelated and of the kind of chelating agent used. Most of the complexes are negatively charged when the pH values range between 4 and 10, and should, therefore, migrate towards the anode. Each metal complex has an optimum pH and an active pH range in which the metal complex is stable [11,12]. Chelating agents, such as ethylenediaminetetraacetic acid (EDTA) and citrate, are ligands capable of coordinating with central metal cations in at least two sites to form chelate complexes. Due to the particular molecular structure of chelating agents, they may form multiple bonds to a single metal ion, even from sorbed species and solid precipitates. In EKR, chelated metal cations (Mn+) can take place in the form of more soluble anionic complexes that can be transported, such as M-EDTA- and M-citrate- [14,15,16].
In previous studies on EKR [12,17], it was found to be interesting to use chelating agents, such as citric acid (CA), EDDS, nitrilotriacetic acid (NTA), or EDTA, for the removal of many metals when an electric field was continuously applied. However, less investigation has been carried out on the advantages of the application of a periodic electric potential associated with chelating agents [12,18]. For this purpose, a series of EKR tests, improved by various chelating agents, was carried out in this study. This periodicity generally generates an electrical current that follows an up-and-down pattern. When the voltage was not applied, it allowed time for the mass transfer of charged solutes from the soil to the aqueous medium, causing a higher current when the voltage was applied again [18]. Moreover, the use of a periodic voltage gradient can contribute to generating a significantly higher overall EOF than when a continuous voltage is applied. Cameselle et al. described that a longer contact time between sediment particles and CA (when voltage was not applied or during the preconditioning step) could favor its sorption onto particles, which modifies the zeta-potential, leading to a more negatively charged surface and to a better EOF [7]. Therefore, applying a periodic voltage gradient can enhance the decontamination of the overall sediment column: the highest pulsed current intensity can be favorable for metal removal [19]. The present study aims to compare and evaluate the enhancement effect of CA and EDDS in EKR with the application of a periodic voltage gradient for metals’ (Cd, Cr, Cu, Pb, and Zn) removal from dredged, naturally contaminated sediment. The originality of our research is that it covers a wide range of metallic elements, since a few reported experiments are dealing with so many simultaneous elements in a single run. Another advantage is the use of environmentally friendly EDDS chelating agent that is scarcely used, despite its interest. The novelty of this investigation is the application of a periodic voltage gradient associated with a low concentration of chelating agents (CA and EDDS) to extend the previous study [11] using a continuous voltage.
2. Materials and Methods
2.1. Sediment Sampling
A dredged sediment sample was collected at the disposal site (Tancarville, Normandy, France) and stored at a temperature of 4 °C. Its properties are presented in Table 1.

Table 1.
Properties of the dredged sediment.
2.2. Experimental Setup and EKR Test
The experimental EKR setup, described in previous papers [17,19], was composed of Teflon material, including a sediment cell (containing 0.4 kg of material) and two electrode compartments. Graphite electrode plates were placed in each electrode compartment, separated from the sediment by a porous filter paper (0.45 µm) and a grid. Two pumps filled the electrodes reservoirs with aqueous processing fluids. After each EKR test, the sediment was removed from the cell and cut into four slices (S1 to S4 from the anode to the cathode) for analysis.
Different processing electrolytes containing EDDS-Na3 or citric acid (CA) were prepared at a concentration of 0.1 M and used to supply the two electrode compartments. A voltage gradient (1 Vcm−1) was applied periodically (5 days on/2 days off cycles) for a duration of 24 days (Table 2). During the EKR experiment, the outlet effluent’s volumes were measured continuously and the cumulative electroosmotic flow (EOF) was evaluated as the difference between the input and output volume of the additive solution in each electrode compartment.

Table 2.
Experimental conditions.
2.3. Analytical Methods
For the metals’ extraction, three aliquots of treated sediment were collected from each sliced section, dried for 48 h at 35 °C, and ground. Each 0.5 g sub-sample was introduced into PTFE tube vessels, sealed, and heated for 10 min in a microwave unit (170 °C, 1200 W output) in order to mineralize the sediment using 10 mL of concentrated nitric acid: hydrochloric acid 3:1 (v:v) (MarsX, CEM Corporation, Matthews, IL, USA). After cooling, the liquid extracts were diluted to 50 mL and filtered through 0.45 µm PTFE filters. The metal concentrations (Cd, Cr, Cu, Pb, and Zn) were analyzed in triplicate using an ICP-AES (ICAP 6300, Thermo Fisher Scientific, Waltham, MA, USA).
3. Results
3.1. Current Variation and Electro-Osmotic Flow (EOF)
As shown in Figure 1, the general trend of electrical current showed a decrease at the beginning, and then stabilized at a low value [17]. The initial higher values were due to the solubilization of inorganic species, which lead to the rise of the electrical conductivity [20]. After a while, the ionic species were depleted as they moved by electromigration, causing the current to decrease with time until achieving a stable value [21].
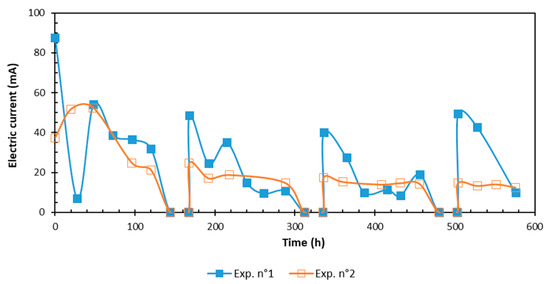
Figure 1.
Time evolution of the electric current.
The electric current generated by the applied periodic voltage followed an up-and-down pattern. When the voltage was not applied, it allowed time for the mass transfer of charged solutes from the soil to the aqueous medium, causing a higher current when the voltage was applied again [18,19]. The most important value of electric current was observed in the EDDS experience, while the undermost value was noted for the CA because high amounts of Na+ counterions were introduced with EDDS. Using chelating agents to solubilize the metal elements contained in the porous medium increased the current intensity and conductivity.
The cumulative EOF in the cathode compartment for each experiment is presented in Figure 2. The highest cumulative EOF (1966 mL) was noted for the Exp.1-test (EDDS). The cumulative EOF can be affected by variations in the zeta potential [22]. Moreover, it is reported in the literature that the zeta potential is impacted by the surface charge of solid particles, the pH, and the ions’ concentrations in the pore solution [23]. These factors are able to not only affect the EOF level, but also its direction, which can be inversed from the cathode to the anode in particular cases. The chelating agents selected for these experiments do not only enhance the removal rate by the formation of chelates/complexes and the increase of the metals’ solubility, but they also modify the interstitial fluid chemistry and therefore directly affect the zeta potential of the sediment particle surfaces [24,25]. The result provided by the EDDS test shows an increase in the cumulative EOF, which can be correlated with the highest value of electrical current.
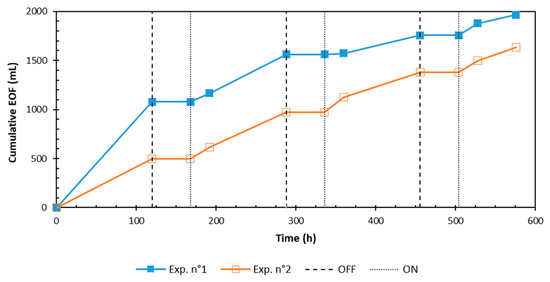
Figure 2.
Time evolution of cumulative EOF in the cathode.
3.2. Sediment pH and Electrical Conductivity (EC)
Figure 3 displays the pH values that were monitored in the sediment samples after the EKR test. It shows that the sediment underwent an overall pH modification compared to the initial value, and the pH value at the anode was lower than that at the cathode. This trend was more remarkable for CA than EDDS. Using CA as an acid buffer helped to maintain a low acidification process when compared to the initial pH value, particularly near the anode where H+ was produced by water electrolysis. However, the high amount of carbonates in the natural sediment, responsible for its buffer capacity, prevented the advance of the acidic front from the anode to the cathode [26].
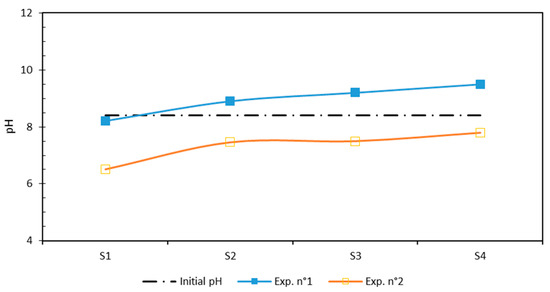
Figure 3.
Distribution of pH after EKR test.
As can be seen in Figure 3, the addition of EDDS led to a notable rise in the pH value, particularly near the cathode where OH- was produced by water electrolysis, causing alkaline pH values of 8.2 and 9.5 close to the anode and the cathode, respectively. Unlike CA, EDDS is not an acid buffer and could not contribute to acidifying the sediment matrix, even at the anode side.
The measured EC values in each section of the sediment sample from the anode to cathode are shown in Figure 4. The global tendency of the sediment EC after EKR treatment was at a lower level than the initial value, indicating a significant decrease in ionic species. When using CA as a processing fluid, the EC stayed higher close to the anode area under acidic conditions (allowing the solubilization of metal ions), but it decreased near the cathode because of the significant migration of mobile ions near the cathode, which migrated efficiently. In contrast, in the EDDS test, the value of EC of the sediment samples increased as it approached the cathode. This behavior was the effect of ion precipitation due to the alkaline conditions (8.9 to 9.5), which led the ions to not be depleted as they were not soluble in alkaline pore water [12].
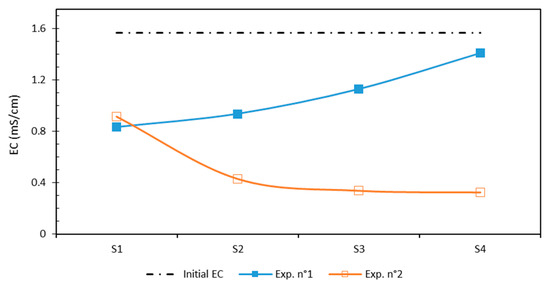
Figure 4.
Distribution of EC after EKR test.
3.3. Metal Removal
The metals removal efficiency after each test, defined as the ratio of the removed concentration and initial one, is shown in Figure 5. Solubilized cationic metals migrate through electromigration from the anode to the cathode side, but, as mentioned above, the buffering capacity of the natural sediment counteracts the acidification of the sediment and prevents metal solubilization and electromigration. However, the chelating agents EDDS and CA helped to desorb metals and form anionic complexes that migrated from the cathode to the anode through the matrix [11,14,27,28]. Considering the EDDS solution, the results indicated that the metal with the better removal efficiency was Cr (nearly 54 %) and Pb was the least removed metal (about 5.5 %). After EKR treatment, the target metals’ removal efficiencies were achieved in the order of Cr > Cu > Cd > Zn > Pb. When using EDDS, the stability constant values with Ca, Mg, and Fe were shown much lower than those with metals such as Cd, Cu, Pb, and Zn [27]. This led to a reduction in the competition between major cations and metals for complex formation and induced great metal removal for Cd, Cu, Pb, and Zn [27]. Using CA as a chelating agent, the metal recoveries were in the order of Cr > Cu > Zn ≈ Cd > Pb. The best-removed metal was Cr (about 67.8 %) and Pb was the least eliminated from the sediment matrix (about 32.1%). With the exception of Cd and Zn, CA was more efficient than EDDS for eliminating Cr, Cu, and Pb, as the electromigration of solubilized species near the anode side could occur in addition to the migration of the complexes. Pb was particularly difficult to remove from the sediment when using EDDS as a processing fluid at 0.1 mol L−1, and in a pH range of 8.2–9.5. The Pb removal efficiency for the CA test was six times higher, but Pb remained more difficult to remove. Indeed, Pb has a significantly higher ionic radius than the other studied metals and the chelation with EDDS or CA was probably more difficult. Additionally, it seemed that the elimination of Pb was favored by the acidic environment induced by the CA buffer. With EDDS enhancement, the result shows that the mobility of Pb was not effective, in contrast to reported results in the literature [28]. Additionally, in a previous study on the same sediment type and using EDDS with a continuous voltage [11], the best metal removals were obtained in the order of Cu > Cr > Cd ≈ Pb > Zn, which was different from that obtained in the present study. In comparison, better removals were obtained when using a periodic voltage as in this setup for all target metals, except for Pb. Applying a periodic voltage enhanced the metals’ removal, as reported previously, because the pulsed current intensity was favorable for metal removal [19].
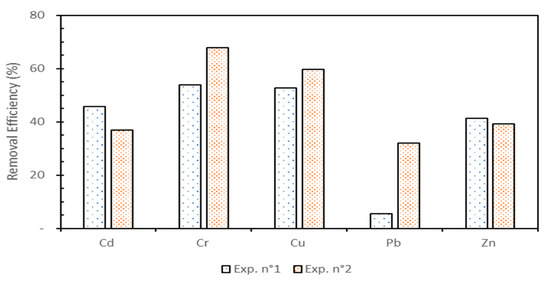
Figure 5.
Metal removal efficiencies after each test.
The enhancement of Cd removal by the EDDS chelate formation was higher than that obtained in the CA test. Regarding Cd, the migration as a free cation was probably low, even at a pH of around 6.5, in the case of CA application. Moreover, the stability of Cd complexes with citrate is low; therefore, its migration to the anode was low [29]. It can be seen from Figure 5 that the percentage removal efficiency of Zn was quite high for the two tests. Using EDDS or CA led to similar results (respectively 41.4% and 39.3% removals). The Zn was disposed to precipitate as a hydroxide when the pH was >7; its removal could mainly be attributed to the formation of chelates with EDDS or CA, which, in preference, move toward the anode compartment. The effectiveness of the chelating agent in the removal of metals is affected by several parameters, such as the metal ions, the electrolyte conditions, and the most important parameter, pH [10,30]. However, the ratio of chelate/metal must also be considered. Here, using a low concentration (0.1 M) of EDDS showed significant removals of the targeted metals, except Pb, and these results are similar to those obtained with a concentration twice higher [27].
4. Conclusions
The EKR experiments demonstrate that significantly enhanced recovery of metals from dredged sediment was obtained by using an intermittent gradient voltage combined with a weak concentration of chelating agents. The results provided for CA, an environmentally friendly compound, indicated effective removals of all of the target metals. However, EDDS, which is also a green chelating agent, was also very efficient for metal removal, except for Pb. CA and EDDS had equal effectiveness in eliminating Zn, but EDDS was more effective for Cd removal. This investigation established that the metals’ removal was not solely correlated with the stability constants of metal–chelate complexes, but was also influenced by the pH of the sediment matrix and the metal speciation, as mentioned in a previous study [11]. Applying a periodic voltage has positive effects on enhancing the mobility of metals and reducing energy and additive consumption.
Author Contributions
M.-T.A., Supervision, conceptualization, methodology, and writing—original draft preparation. A.B. Conceived the study and was in charge of overall direction and planning, validation, and writing—reviewing and editing. F.P.-K. contributed to the interpretation of the results, verified the analytical method, and the reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
This study did not report any data.
Acknowledgments
This work was supported by Region Normandie (France) in the framework of SEDEVAR Research project (SCALE Network).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Svensson, N.; Norén, A.; Modin, O.; Karlfeldt Fedje, K.; Rauch, S.; Strömvall, A.-M.; Andersson-Sköld, Y. Integrated Cost and Environmental Impact Assessment of Management Options for Dredged Sediment. Waste Manag. 2022, 138, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Song, Y.; Yuan, P.; Cui, X.; Qiu, G. The Remediation of Heavy Metals Contaminated Sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Perelo, L.W. Review: In Situ and Bioremediation of Organic Pollutants in Aquatic Sediments. J. Hazard. Mater. 2010, 177, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.R.; Urbanek, A.; Khodadoust, A.P. Electroosmotic Dewatering of Dredged Sediments: Bench-Scale Investigation. J. Environ. Manag. 2006, 78, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Olivier, J.; Vaxelaire, J.; Hoadley, A.F.A. Electrical Field: A Historical Review of Its Application and Contributions in Wastewater Sludge Dewatering. Water Res. 2010, 44, 2381–2407. [Google Scholar] [CrossRef]
- Ammami, M.T.; Song, Y.; Benamar, A.; Portet-Koltalo, F.; Wang, H. Electro-Dewatering of Dredged Sediments by Combined Effects of Mechanical and Electrical Processes: Influence of Operating Conditions. Electrochim. Acta 2020, 353, 136462. [Google Scholar] [CrossRef]
- Cameselle, C.; Reddy, K.R. Development and Enhancement of Electro-Osmotic Flow for the Removal of Contaminants from Soils. Electrochim. Acta 2012, 86, 10–22. [Google Scholar] [CrossRef]
- Nogueira, M.G.; Pazos, M.; Sanromán, M.A.; Cameselle, C. Improving on Electrokinetic Remediation in Spiked Mn Kaolinite by Addition of Complexing Agents. Electrochim. Acta 2007, 52, 3349–3354. [Google Scholar] [CrossRef]
- Amrate, S.; Akretche, D.E.; Innocent, C.; Seta, P. Removal of Pb from a Calcareous Soil during EDTA-Enhanced Electrokinetic Extraction. Sci. Total Environ. 2005, 349, 56–66. [Google Scholar] [CrossRef]
- Giannis, A.; Nikolaou, A.; Pentari, D.; Gidarakos, E. Chelating Agent-Assisted Electrokinetic Removal of Cadmium, Lead and Copper from Contaminated Soils. Environ. Pollut. 2009, 157, 3379–3386. [Google Scholar] [CrossRef]
- Song, Y.; Ammami, M.-T.; Benamar, A.; Mezazigh, S.; Wang, H. Effect of EDTA, EDDS, NTA and Citric Acid on Electrokinetic Remediation of As, Cd, Cr, Cu, Ni, Pb and Zn Contaminated Dredged Marine Sediment. Environ. Sci. Pollut. Res. 2016, 23, 10577–10586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Qiu, Z.; Tang, H.; Wang, H.; Sima, W.; Liang, C.; Liao, Y.; Li, Z.; Wan, S.; Dong, J. Coupled with EDDS and Approaching Anode Technique Enhanced Electrokinetic Remediation Removal Heavy Metal from Sludge. Environ. Pollut. 2021, 272, 115975. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, Z.; Li, A.; Cui, C. Enhanced Electrokinetic Remediation of Heavy Metals Contaminated Soil by Biodegradable Complexing Agents. Environ. Pollut. 2021, 283, 117111. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-C.; Yang, J.-S.; Jeon, E.-K.; Baek, K. Enhanced-Electrokinetic Extraction of Heavy Metals from Dredged Harbor Sediment. Environ. Sci. Pollut. Res. 2015, 22, 9912–9921. [Google Scholar] [CrossRef]
- Song, Y.; Cang, L.; Xu, H.; Wu, S.; Zhou, D. Migration and Decomplexation of Metal-Chelate Complexes Causing Metal Accumulation Phenomenon after Chelate-Enhanced Electrokinetic Remediation. J. Hazard. Mater. 2019, 377, 106–112. [Google Scholar] [CrossRef]
- Song, Y.; Cang, L.; Zuo, Y.; Yang, J.; Zhou, D.; Duan, T.; Wang, R. EDTA-Enhanced Electrokinetic Remediation of Aged Electroplating Contaminated Soil Assisted by Combining Dual Cation-Exchange Membranes and Circulation Methods. Chemosphere 2020, 243, 125439. [Google Scholar] [CrossRef]
- Ammami, M.T.; Benamar, A.; Wang, H.; Bailleul, C.; Legras, M.; Le Derf, F.; Portet-Koltalo, F. Simultaneous Electrokinetic Removal of Polycyclic Aromatic Hydrocarbons and Metals from a Sediment Using Mixed Enhancing Agents. Int. J. Environ. Sci. Technol. 2014, 11, 1801–1816. [Google Scholar] [CrossRef] [Green Version]
- Maturi, K.; Reddy, K.R.; Cameselle, C. Surfactant-Enhanced Electrokinetic Remediation of Mixed Contamination in Low Permeability Soil. Null 2009, 44, 2385–2409. [Google Scholar] [CrossRef]
- Ammami, M.T.; Portet-Koltalo, F.; Benamar, A.; Duclairoir-Poc, C.; Wang, H.; Le Derf, F. Application of Biosurfactants and Periodic Voltage Gradient for Enhanced Electrokinetic Remediation of Metals and PAHs in Dredged Marine Sediments. Chemosphere 2015, 125, 1–8. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior; John Wiley & Sons: New York, NY, USA, 2005; Volume 3. [Google Scholar]
- Maturi, K.; Reddy, K.R. Simultaneous Removal of Organic Compounds and Heavy Metals from Soils by Electrokinetic Remediation with a Modified Cyclodextrin. Chemosphere 2006, 63, 1022–1031. [Google Scholar] [CrossRef]
- Acar, Y.B.; Alshawabkeh, A.N. Principles of Electrokinetic Remediation. Environ. Sci. Technol. 1993, 27, 2638–2647. [Google Scholar] [CrossRef]
- Kaya, A.; Yukselen, Y. Zeta Potential of Clay Minerals and Quartz Contaminated by Heavy Metals. Can. Geotech. J. 2005, 42, 1280–1289. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Yeung, A.T.; Koenig, A.; Li, H.-J. Effects of Chelating Agents on Zeta Potential of Cadmium-Contaminated Natural Clay. Sep. Sci. Technol. 2009, 44, 2203–2222. [Google Scholar] [CrossRef]
- Popov, K.; Glazkova, I.; Myagkov, S.; Petrov, A.; Sedykh, E.; Bannykh, L.; Yachmenev, V. Zeta-Potential of Concrete in Presence of Chelating Agents. Colloids Surf. A: Physicochem. Eng. Asp. 2007, 299, 198–202. [Google Scholar] [CrossRef]
- Ouhadi, V.R.; Yong, R.N.; Shariatmadari, N.; Saeidijam, S.; Goodarzi, A.R.; Safari-Zanjani, M. Impact of Carbonate on the Efficiency of Heavy Metal Removal from Kaolinite Soil by the Electrokinetic Soil Remediation Method. J. Hazard. Mater. 2010, 173, 87–94. [Google Scholar] [CrossRef]
- Suzuki, T.; Niinae, M.; Koga, T.; Akita, T.; Ohta, M.; Choso, T. EDDS-Enhanced Electrokinetic Remediation of Heavy Metal-Contaminated Clay Soils under Neutral PH Conditions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 440, 145–150. [Google Scholar] [CrossRef]
- Zhang, T.; Zou, H.; Ji, M.; Li, X.; Li, L.; Tang, T. Enhanced Electrokinetic Remediation of Lead-Contaminated Soil by Complexing Agents and Approaching Anodes. Environ. Sci. Pollut. Res. 2014, 21, 3126–3133. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Rolle, E.; Ceremigna, D.; De Propris, L.; Gabellini, M.; Tornato, A. A Kinetic Study of Chelant-Assisted Remediation of Contaminated Dredged Sediment. J. Hazard. Mater. 2006, 137, 1458–1465. [Google Scholar] [CrossRef]
- Gidarakos, E.; Giannis, A. Chelate Agents Enhanced Electrokinetic Remediation for Removal Cadmium and Zinc by Conditioning Catholyte PH. Water Air Soil Pollut. 2006, 172, 295–312. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).