Abstract
Rice (Oryza sativa L.) is a vital staple crop, and the environmental risk assessment of transgenic varieties is crucial for formulating biosafety policies. Rice pollen grains are spherical, with an average diameter of 40.03 ± 2.75 μm. This study established a standardized protocol for in vitro pollen germination by first optimizing key culture conditions. A single-factor experimental design identified the optimal medium composition as 150 g/L sucrose, 40 mg/L boric acid, 20 mg/L calcium chloride, 10 mg/L monopotassium phosphate, and 10 mg/L magnesium sulfate. The ideal germination temperature was determined to be 31 ± 1 °C, with no germination observed below 16 °C or above 40 °C. Pollen germination rates declined significantly within 5 min post-isolation and ceased completely after 30 min. Building on this optimized protocol, a standardized evaluation method was developed, defining key assessment conditions at temperatures of 25/31/37 °C and post-isolation times of 0/5/15 min. Under these defined conditions, the pollen viability of glyphosate-resistant transgenic rice G2-6 was compared to its non-transgenic recipient ZH11. No significant differences were found at any tested time–temperature combination (p > 0.05). This work establishes a practical and reproducible standard for transgenic rice pollen assessment, offering a scientific basis for evidence-based biosafety regulation and policy-making.
1. Introduction
With the global population projected to exceed 9.5 billion by 2050, ensuring sustainable agricultural productivity has emerged as a critical challenge [,]. Concurrently, biosynthetic technologies and gene-editing techniques have witnessed expanding applications in agricultural innovation. Biotechnology-driven breeding, particularly represented by transgenic technology, provides an effective solution to enhance crop yields and stress resilience [,,]. According to ISAAA statistics, the global cultivation area of genetically modified (GM) crops reached approximately 109.4 million hectares by 2019, demonstrating significant contributions to food security and economic growth []. However, rigorous environmental safety assessments must precede the commercialization of these biotechnological products to evaluate their potential adverse impacts on ecosystems []. Biosafety concerns have now become a pivotal constraint limiting both the advancement of transgenic biotechnology and the widespread agricultural adoption of genetically engineered products []. Over-reliance on empirically derived risk models could foster a misleading sense of security among regulators, which could in turn compromise their capacity to comprehensively address risks that resist straightforward scientific quantification []. Thus, the establishment of a sound environmental risk assessment system constitutes an urgent necessity.
Within the biosafety evaluation framework, environmental risk assessment (ERA) plays a central role in predicting unintended ecological consequences, with transgene escape mediated through pollen-driven gene flow being a primary concern [,]. This process may alter the genetic composition of wild relatives, and even low-frequency hybridization events could lead to transgene persistence in natural ecosystems, particularly if the transgenes confer a selective advantage, potentially disrupting ecological equilibrium [,]. The scale of this risk is not solely a function of distance but is intrinsically linked to pollen viability under realistic environmental conditions—a key determinant of fertilization success and effective dispersal range []. Pollen encountering suboptimal temperatures or prolonged dispersal times exhibits rapidly declining viability, meaning a meaningful ERA must assess performance across a spectrum of environmentally relevant scenarios, not just under idealized laboratory conditions. While multiple pollen viability assessment methods exist (e.g., TTC, MTT, and I2-KI staining), their limitations in simulating natural conditions raise substantial concerns. For instance, in rice, aniline blue and TTC staining are known to yield false-positive results, overestimating environmental risks [,]. Consequently, in vitro pollen germination remains the most direct, effective, and accurate method for assessing functional pollen viability, as it can be adapted to model these critical ecological variables.
Rice, the world’s third-largest cereal crop after maize and wheat, serves as the primary food source for nearly half of the global population. Given its paramount importance in agriculture, promoting the development of genetically modified rice is imperative. Although rice pollen exhibits relatively weak in vitro viability, it retains the capacity for natural hybridization at low frequencies []. This biological characteristic, coupled with its known hybridization potential with wild Oryza species, elevates the priority of transgenic ERA for rice []. However, a parallel, standardized protocol for the environmental release assessment of transgenic rice remains absent, creating a significant methodological gap in the biosafety evaluation pipeline for one of the world’s most important staple crops.
This study was designed specifically to bridge this gap. Our primary objective was not to define the universal “optimal” conditions for rice pollen germination, but rather to establish a standardized and reproducible system for the comparative assessment of pollen viability between transgenic and non-transgenic rice lines under a range of ecologically realistic stress conditions. To this end, we defined a set of key evaluation parameters—including a standardized culture medium and, crucially, a series of temperature regimes and post-isolation timescales. These parameters were explicitly selected to simulate realistic field scenarios: optimal growing temperatures, high-temperature stress, mild cold stress, and the temporal decay of viability corresponding to increasing dispersal distance. Within this standardized and ecologically contextualized framework, we then conducted a focused case study to compare the pollen viability of a glyphosate-resistant transgenic rice line (G2-6) with its non-transgenic recipient (ZH11). The established system provides regulatory bodies with a critical tool to ensure that any potential differences in pollen performance between GM and non-GM lines are detected consistently and under conditions that are relevant to environmental risk assessment.
2. Materials and Methods
2.1. Plant Material and Pollen Collection
The perennial wild rice ZH11 and herbicide-resistant transgenic rice line G2-6 were collected from the Sanya population (18°21′ N, 109°10′ E) of Hainan Province, China, and used as the pollen donor. The planting conditions were set as follows: row spacing of 30 cm and plant spacing of 12 cm. Seeds of the non-transgenic wild-type rice ZH11 were provided by Cropedit Biotechnology Company Limited, Beijing, China, while seeds of the transgenic rice line G2-6 were supplied by the Biotechnology Research Institute, Chinese Academy of Agricultural Sciences (CAAS), Beijing, China []. The optimization of in vitro culture temperature, desiccation duration, and culture medium was conducted using the wild-type ZH11. Three days prior to pollen collection, rice plants at the pre-germination stage were carefully dug up from the field with roots intact. The entire plants, along with adhering soil and field water, were placed in 60 cm diameter buckets and transported to the laboratory, where they were maintained outdoors under natural conditions. Pollen collection was consistently conducted between 11:00 a.m. and 2:00 p.m. local time. Ambient temperature during pollen collection ranged from 28 to 32 °C with 65–80% relative humidity, and three biological replicates were performed for each treatment group, with in vitro germination assays immediately conducted following pollen collection.
2.2. Experimental Materials
All reagents used in the experiments were of analytical grade. The chemical reagents used in the culture medium preparation, including sucrose (CAS:57-50-1), H3BO3 (CAS:10043-35-3), CaCl2 (CAS:10043-52-4), KH2PO4 (CAS:7778-77-0), and MgSO4 (CAS:10034-99-8) were procured from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.3. Preparation and Optimization of Culture Media
Stock solutions were filter-sterilized (0.22 μm PVDF membrane) and stored at 4 °C for ≤30 days. Final medium was prepared by diluting stocks with sterile deionized water (18.2 MΩ·cm) to achieve target concentrations. The stock solutions were diluted to working concentrations prior to use. The optimization process commenced with the baseline medium M4 containing 175 g/L sucrose, which had been previously established as the optimal carbohydrate concentration. Building upon this formulation, we systematically tested combinatorial gradients of the following components: H3BO3 (20, 40 and 60 mg/L), CaCl2 (20, 40 and 60 mg/L), KH2PO4 (0, 10 and 20 mg/L), and MgSO4 (0, 10 and 20 mg/L).
2.4. Temperature Treatment of Pollen
To simulate the diverse temperature conditions during natural anther dehiscence and pollen dispersal, in vitro pollen germination was assessed across a gradient of temperatures. For each assay, a 10 mL aliquot of liquid culture medium was dispensed into a 35 mm Petri dish. Anthers were gently crushed with fine-tipped forceps and immersed in the medium, followed by gentle agitation to ensure homogeneous pollen distribution. Each pollen sample was tested with three biological replicates. The plates were then incubated in precision-controlled chambers at specified temperatures ranging from 16 °C to 40 °C (specifically, 16, 22, 25, 28, 31, 34, 37, and 40 °C) for a 15 min period at each temperature. Following incubation, the germination rates were quantified through microscopic examination.
2.5. Pollen In Vitro Treatment
For the in vitro treatment of pollen, 20 mL of distilled water was added to a 90 mm Petri dish, and a dry filter paper was placed in a 35 mm dish positioned at the center of the 90 mm Petri dish. Pollen grains were evenly distributed on the dry filter paper, and the assembly was incubated at 28 °C in a precision incubator for specified durations. Following hydration, the pollen was transferred to a fresh 35 mm Petri dish containing liquid medium and gently agitated to ensure uniform distribution. Finally, the cultures were maintained at 31 °C for 15 min in a controlled-humidity environment.
2.6. Pollen Germination and Data Analysis
All medium components (all reagents were analytical grade in this study) were dissolved in distilled water. To prevent component degradation from heat sterilization, the medium was filter-sterilized using 0.22 μm membrane filters. 10 mL of the medium was dispensed into 35 mm Petri dishes. Anthers were carefully excised using forceps, placed at the center of the medium, and gently crushed to release pollen grains. The dishes were slightly agitated to ensure even pollen distribution. The plates were incubated at 31 °C in a precision incubator for 15 min. After incubation, samples were examined under an Olympus microscope (Model SZX2-ILLTQ Olympus Corporation, Tokyo, Japan). For each replicate, >20 pollen grains were imaged. Pollen was considered germinated when the tube length exceeded half of the grain diameter. Germination rates were quantified using Image J (version 1.8.0). Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test in GraphPad Prism (version 8.0.2). The comparison of different pollen viability data was performed using Student’s t-test in Excel software (version 2509). The raw data for all analyses are provided in Supplementary Materials. The pollen germination rate was calculated using the following formula:
2.7. Microscopic Examination of Pollen Morphology
Observations were conducted using an Olympus stereo microscope (Model SZX2-ILLTQ Olympus Corporation, Tokyo, Japan) with the following imaging parameters: Exposure time: 60 ms, Exposure compensation: +1/3 EV, Resolution: 1920 × 1200 pixels, Aspect ratio: 16:10. The Petri dish was positioned under the microscope stage, and the focusing knobs were adjusted to bring pollen grains into sharp focus. Digital images were subsequently captured using the integrated camera system (Model DP74 Olympus Corporation, Tokyo, Japan) and archived in JPG format via cellSens Standard v2.3 imaging software.
3. Results
3.1. Microscopic Observation of Pollen Morphology
Rice, as a self-pollinating crop, exhibits relatively weaker pollen viability compared to cross-pollinating crops such as maize. Consequently, the initial pollen status significantly influences pollen germination rates. To maintain experimental consistency, we conducted a series of preliminary trials and identified that anthers reaching the upper half of the glume just prior to pollen release demonstrated optimal germination potential (Figure 1b). These selected anthers were subsequently employed for experimental procedures.

Figure 1.
Screening of tested rice (Cultivar ZH11) pollen and morphological observation. (a) Anthers positioned within the lower portion of the lemma-palea enclosure. (b) Anthers ascending to the upper lemma-palea junction prior to spikelet opening. (c) Anthers partially exserted through the recently split lemma margins. (d) Fully exserted anthers having completed pollen shedding. (e) The apical portions of the lemma and palea were surgically excised. (f) Microscopic morphological observation of In Vitro pollen in rice (n = 57), bar = 200 μm.
Fresh pollen grains were immediately immersed in distilled water for morphological characterization under microscopy. The pollen grains displayed subspherical morphology with uniform dimensions ranging from 38 to 42 μm in diameter (n = 57) (Figure 1f). The observed structural integrity and size consistency confirmed the suitability of these pollen samples for subsequent experimental phases.
3.2. Screening of the Initial Medium
Through systematic optimization based on existing pollen culture media research, we first identified sucrose (as carbon source), boric acid (H3BO3) and calcium chloride (CaCl2) (as micronutrients) as essential components [,,,].
A series of preliminary experiments identified sucrose, CaCl2, and H3BO3 as essential components of the culture medium. To identify the optimal concentrations of medium components, an orthogonal experimental design was implemented to systematically evaluate the effects of varying concentrations of sucrose (50, 100, 150, 175, 200, and 400 g/L), H3BO3 (20, 40, and 60 mg/L), and CaCl2 (20, 40, and 60 mg/L) on in vitro pollen germination rates in rice. The results demonstrated that medium M4, containing 175 g/L sucrose, achieved the highest pollen germination rate, establishing this as the optimal carbohydrate concentration for in vitro rice pollen germination (Table 1). Further analysis indicated that both M10 and M11 media produced the most effective micronutrient synergy, significantly outperforming other formulations. Among them, the M10 medium, containing 40 mg/L H3BO3 and 20 mg/L CaCl2, demonstrated a superior performance, achieving an in vitro pollen germination rate of 85.03 ± 3.7% (Table 2). This systematic approach ensures reproducible quantification of rice pollen viability under controlled conditions.

Table 1.
Germination percentage of pollen cultured on media containing various concentrations of sucrose, different letters indicate statistically significant differences (p < 0.05).

Table 2.
Germination percentage of pollen cultured on media containing various concentrations of boric acid and calcium chloride, different letters indicate statistically significant differences (p < 0.05).
3.3. Further Optimization of Pollen Germination Medium
To further optimize the culture medium, systematic testing of additional micronutrients revealed that varying concentrations of monopotassium phosphate (KH2PO4) (0, 10, 20 mg/L) and magnesium sulfate (MgSO4) (0, 10, 20 mg/L) enhanced the germination rate of isolated rice pollen (Table 3). Both KH2PO4 and MgSO4 exhibited optimal efficacy at 10 mg/L. Based on these findings, the refined culture medium formulation (containing 175 g/L sucrose, 40 mg/L H3BO3, 20 mg/L calcium chloride, 10 mg/L KH2PO4, and 10 mg/L MgSO4) was formulated and adopted for subsequent experimental phases (Figure 2). With reference to existing standards for transgenic pollen viability culture, after the culture medium was determined, we also needed to determine the incubation temperature and the post-excision time [].

Table 3.
Germination percentage of pollen cultured on media containing various concentrations of monopotassium phosphate and magnesium sulfate, different letters indicate statistically significant differences (p < 0.05).
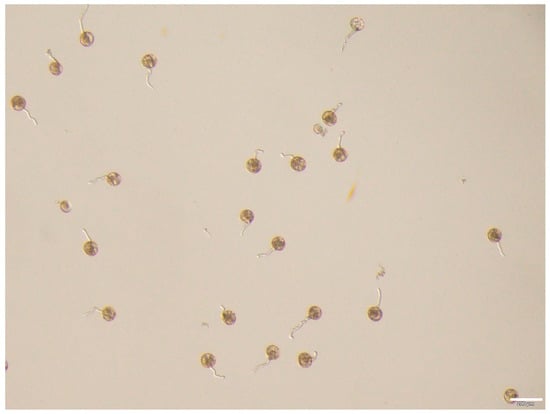
Figure 2.
The germination performance of rice (Cultivar ZH11) pollen in the optimized M17 medium, bar = 200 μm.
3.4. Determination of Pollen Culture Temperature
The data revealed a triphasic response pattern: Complete germination inhibition occurred at 16 °C. A positive thermal correlation was observed from 16 to 31 °C, with maximum germination efficiency achieved at 31 °C. Subsequently, a significant negative correlation emerged from 31 to 40 °C, culminating in complete germination cessation at 40 °C (Figure 3). This thermal optimum (31 °C) was therefore designated as the control condition for subsequent assays.
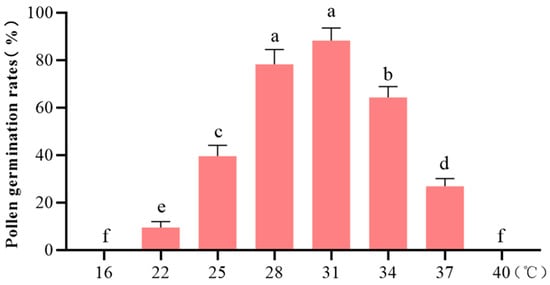
Figure 3.
Pollen germination rate in response to different temperature. Different letters indicate statistically significant differences (p < 0.05), and bars represent standard deviations.
Further analysis revealed marginal germination at 22 °C. While 28 °C maintained comparable viability to 31 °C (p > 0.05), statistically significant reductions (p < 0.05) were recorded at 25 °C, 34 °C, and 37 °C. Notably, the more pronounced reductions at 25 °C and 37 °C relative to 31 °C established these thresholds as representative hypothermic and hyperthermic stress conditions, respectively. Consequently, a three-tiered temperature framework (25/31/37 °C) was implemented to evaluate pollen environmental resilience under controlled in vitro stress simulations.
3.5. Determination of Post-Excision Time for Pollen
Pollen-mediated gene flow via wind dispersal facilitates cross-pollination, with the effective distance of transgene escape being critically dependent on pollen viability under in vitro conditions []. To evaluate the temporal effects of post-excision duration on pollen viability, germination rates were systematically analyzed at varying time intervals (0, 1, 2, 3, 4, 5, 6, 9, 12, 15, 20, and 30 min) post-isolation (Figure 4). The results demonstrated a time-dependent decline in germination efficiency. Compared to freshly collected pollen (0 min), no significant differences (p > 0.05) in germination rates or viability were observed within the first 4 min post-excision. However, a statistically significant reduction (p < 0.05) in germination rate became apparent after 5 min, with progressive deterioration observed over time. A sharp decline in viability occurred from 12 to 15 min post-isolation, culminating in complete loss of germination capacity (0% germination) by 30 min. Based on these findings, critical time points of 0, 5 and 15 min post-excision were established for subsequent evaluation of pollen environmental stress tolerance (Figure A2).
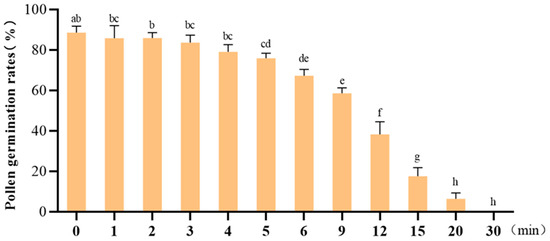
Figure 4.
Pollen germination rates following varying post-excision durations. Different letters indicate statistically significant differences (p < 0.05), and bars represent standard deviations.
3.6. The Transgenic Event in G2-6 Did Not Affect Pollen Viability Relative to ZH11 Controls
To assess the potential impact of genetic modification on pollen viability, we conducted comparative experiments using herbicide-tolerant transgenic rice (G2-6) and its non-transgenic parental cultivar (ZH11) []. Pollen grains from both genotypes were collected and subjected to in vitro storage for 0, 5, and 15 min, followed by incubation at 25 °C, 31 °C, and 37 °C for 15 min.
A two-tailed t-test was performed to analyze pollen germination rates under different storage durations periods and temperature conditions. The results demonstrated no statistically significant differences (p > 0.05) in in vitro pollen germination rates between transgenic G2-6 and non-transgenic ZH11 across all nine treatment combinations (Figure 5). Collectively, these data indicate that the genetic modification process did not exert significant adverse effects on pollen viability in the transgenic rice line G2-6 compared to its non-transgenic counterpart ZH11.
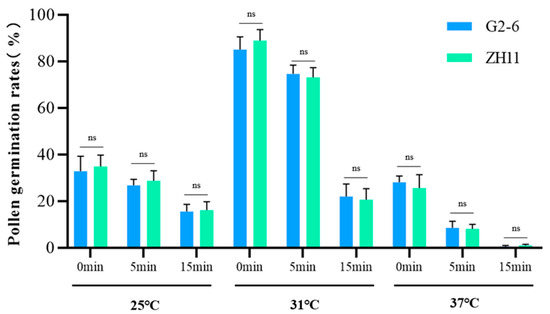
Figure 5.
Pollen viability of transgenic rice G2-6 versus non-transgenic ZH11 under varying post-excision durations and temperature regimes. ns: not significance, determined using a t-test (p > 0.05), and bars represent standard deviations.
4. Discussion
During our experimental investigations, we observed that rice pollen viability is highly susceptible to environmental and operational factors due to its inherently weak in vitro stability. Consequently, the selection of developmentally synchronized pollen grains proved critical for experimental reproducibility. We systematically compared pollen germination rates across four distinct anther developmental stages: Pre-emergence anthers localized in the lower lemma–palea region; emergent anthers positioned at the upper lemma–palea junction; initial dehiscence-stage anthers during spikelet opening; post-dehisced anthers fully exposed post-pollination. Quantitative analysis revealed highly significant differences in germination efficiency among these stages (Figure 1a–d). The highest germination rate was achieved using pollen from emergent anthers at the upper lemma–palea junction (Figure A1b). The optimal pollen selection criterion was defined by anthers that exhibited immediate dehiscence upon contact with the central air–liquid interface of the culture medium following gentle extraction with fine-tipped forceps.
Successful in vitro pollen germination depends on a precisely optimized culture medium, generally composed of carbohydrates, trace elements, and supplementary components. Boron and calcium play essential roles in pollen germination [,]. Specifically, boron is required for pollen tube growth by forming borate esters through binding with pectin in the cell wall, thereby enhancing wall elasticity and plasticity []. Meanwhile, calcium ions (Ca2+) serve as crucial secondary messengers during this process. During pollen germination, the stigma releases Ca2+ to establish a concentration gradient, which directs the oriented growth of pollen tubes through chemotropism [,]. Additionally, KH2PO4 provides essential phosphate groups for ATP synthesis and potassium ions (K+) to regulate osmotic balance, while MgSO4 supplies Mg2+ as a cofactor for energy metabolism-related enzymes and stabilizes cell wall architecture through pectin crosslinking [,,]. Together, these components support efficient pollen tube elongation by sustaining redox homeostasis and cytoskeletal dynamics. In our preliminary screening of various formulations—including additives such as polyethylene glycol 4000 (PEG 4000), thiamine (VB1), and manganese sulfate—these compounds, though reported to enhance pollen germination rates in prior studies, exhibited no statistically significant effects in our experimental system []. This discrepancy may arise from species-specific micronutrient requirements or divergent physical parameters governing pollen viability across taxa.
This study establishes a comprehensive methodology for assessing pollen viability in GM crops, providing critical technical support for environmental risk evaluation of biotechnological agricultural products. Pollen viability is influenced not only by plant species, but also by climatic conditions, and even further modulated by geographical distribution or genotypic variations [,,]. Against this complex background, China has developed national standards for pollen viability assessment in several major crops such as maize, soybean, and cotton []. Although the culture media used in these protocols are distinct for each species, the temperature treatments and post-isolation durations are consistently applied. However, our experimental findings indicate that these standardized criteria are not directly applicable to rice, primarily due to its inherently weaker pollen viability. This observation underscores the substantial interspecific variation in pollen survival capacity and highlights the necessity of developing species-specific evaluation protocols—with tailored temperature regimes, isolation durations, and culture medium formulations. Consequently, this methodology not only provides critical insights for environmental biosafety assessment but also serves as a valuable reference for establishing future species-specific standards—offering practical guidance on how to efficiently screen culture media, and adjust isolation times and temperatures based on pollen vitality. Ultimately, this supports the development of science-based biosafety regulations, including isolation distance requirements, coexistence strategies, and long-term ecological impact projections for GM crops across diverse agroecosystems.
5. Conclusions
This study pioneers the systematic optimization of in vitro pollen germination medium for rice, establishing for the first time the quantitative relationships between pollen germination efficiency and critical environmental parameters. Initial investigations confirmed the critical influence of pollen viability status at harvest on germination success rates, establishing a methodological recommendation to prioritize anthers positioned in the upper lemma–palea enclosure for germination assays. Results demonstrate that rice pollen viability is significantly influenced by medium composition (optimized at 175 g/L sucrose, 40 mg/L H3BO3, 20 mg/L CaCl2, 10 mg/L KH2PO4, and 10 mg/L MgSO4), post-excision duration (showing time-dependent decline), and incubation temperature (optimal at 31 °C). Guided by the principle of substantial equivalence, we used the same germination medium for both transgenic and non-transgenic rice. We developed a novel transgenic pollen assessment protocol employing three post-excision intervals (0/5/15 min) and temperature regimes (25/31/37 °C), enabling comparative viability analysis between GM and non-GM varieties.
This study establishes a comprehensive framework for transgenic crop environmental risk assessment by pioneering the development of a species-optimized in vitro pollen germination medium—one that not only achieves unprecedented initial germination efficiency under controlled conditions but also features a significantly simplified formulation compared to conventional media. More importantly, this work describes a standardized in vitro protocol for pollen viability assessment that directly supports environmental risk assessment by improving the evaluation of transgene flow potential in genetically modified rice. The methodology delivers robust datasets crucial for biosafety verification (particularly for gene flow-related risk evaluation) and precision breeding initiatives, with transformative potential in advancing agricultural biotechnology innovation and informing evidence-based biosafety governance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15232420/s1.
Author Contributions
Conceptualization, Y.C.; Methodology, Y.C. and C.L.; Software, Y.C. and J.Y.; Validation, X.Z., J.Y. and Y.D.; Formal analysis, J.Y. and D.W.; Investigation, J.Y.; Resources, Y.D.; Data curation, Y.C.; Writing—original draft, Y.C. and C.L.; Writing—review & editing, Y.C. and X.Z.; Visualization, Y.C.; Supervision, X.W.; Project ad-ministration, X.W. and Z.W.; Funding acquisition, X.W. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Major Project of China on Agricultural Biotechnology Breeding, grant number 2023ZD04062.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
Authors Yufeng Dong and Dongmei Wang were employed by the Cropedit Biotechnology Company Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| H3BO3 | Boric acid |
| CaCl2 | Directory of open access journals |
| KH2PO4 | Monopotassium phosphate |
| MgSO4 | Magnesium sulfate |
| GM | Genetically modified |
| VB1 | Vitamin B1 |
Appendix A
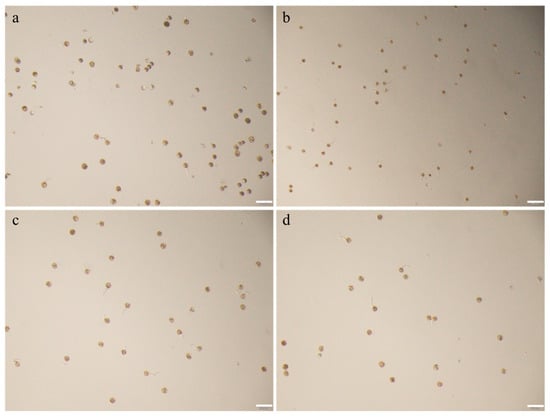
Figure A1.
Pollen Germination in Relation to Anther Position in Rice (Cultivar ZH11). (a) Pollen germination status in anthers positioned at the lower lemma-palea enclosure, bar = 200 μm. (b) Pollen germination status in anthers ascending at the upper lemma-palea junction prior to spikelet opening, bar = 200 μm. (c) Pollen germination status in anthers partially exserted through the recently split lemma margins, bar = 200 μm. (d) Pollen germination dynamics in fully exserted anthers beyond the lemma-palea enclosure, bar = 200 μm.
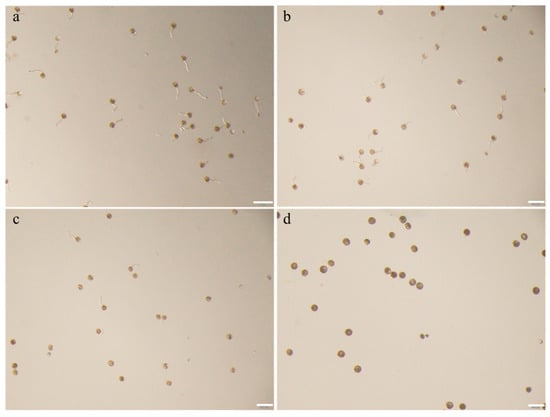
Figure A2.
Pollen germination rate in response to different temperature. (a) Pollen germination of post-excision, bar = 200 μm. (b) Pollen germination after 5 min of post-excision. bar = 200 μm. (c) Pollen germination after 15 min of post-excision, bar = 200 μm. (d) Pollen germination after 30 min of post-excision, bar = 200 μm.
References
- Vollset, S.E.; Goren, E.; Yuan, C.W.; Cao, J.; Smith, A.E.; Hsiao, T.; Bisignano, C.; Azhar, G.S.; Castro, E.; Chalek, J.; et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef]
- Grafton, R.Q.; Williams, J.; Jiang, Q. Food and water gaps to 2050: Preliminary results from the global food and water system (GFWS) platform. Food Secur. 2015, 7, 209–220. [Google Scholar] [CrossRef]
- Hu, H.H.; Dai, M.Q.; Yao, J.L.; Xiao, B.Z.; Li, X.H.; Zhang, Q.F.; Xiong, L.Z. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 12987–12992. [Google Scholar] [CrossRef]
- Shepherd, D.N.; Mangwende, T.; Martin, D.P.; Bezuidenhout, M.; Kloppers, F.J.; Carolissen, C.H.; Monjane, A.L.; Rybicki, E.P.; Thomson, J.A. Maize streak virus-resistant transgenic maize: A first for Africa. Plant Biotechnol. J. 2007, 5, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Li, P.C.; Li, X.Y.; Wen, N.; Wang, Y.X.; Lu, W.; Lin, M.; Lang, Z.H. In maize, co-expression of GAT and GR79-EPSPS provides high glyphosate resistance, along with low glyphosate residues. Abiotech 2023, 4, 277–290. [Google Scholar] [CrossRef] [PubMed]
- ISAAA. Global Status of Commercialized Biotech/GM Crops: 2019—ISAAA Brief 55-2019; ISAAA: Ithaca, NY, USA, 2019. [Google Scholar]
- Lubieniechi, S.A.; Van Eenennaam, A.L.; Smyth, S.J. Regulation of animal and plant agricultural biotechnology. Trends Biotechnol. 2025, 43, 511–521. [Google Scholar] [CrossRef]
- Ludlow, K.; Falck-Zepeda, J.; Smyth, S.J. Risk-appropriate, science-based innovation regulations are important. Trends Biotechnol. 2025, 43, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, K.L.; Yao, Y. China’s regulatory change toward genome-edited crops. Trends Biotechnol. 2024, 42, 801–806. [Google Scholar] [CrossRef]
- Jank, B.; Gaugitsch, H. Assessing the environmental impacts of transgenic plants. Trends Biotechnol. 2001, 19, 371–372. [Google Scholar] [CrossRef]
- Lu, B.R.; Yang, C. Gene flow from genetically modified rice to its wild relatives: Assessing potential ecological consequences. Biotechnol. Adv. 2009, 27, 1083–1091. [Google Scholar] [CrossRef]
- Chandler, S.; Dunwell, J.M. Gene flow, risk assessment and the environmental release of transgenic plants. Crit. Rev. Plant Sci. 2008, 27, 25–49. [Google Scholar] [CrossRef]
- Noack, F.; Engist, D.; Gantois, J.; Gaur, V.; Hyjazie, B.F.; Larsen, A.; M’Gonigle, L.K.; Missirian, A.; Qaim, M.; Sargent, R.D.; et al. Environmental impacts of genetically modified crops. Science 2024, 385, eado9340. [Google Scholar] [CrossRef]
- Pasquet, R.S.; Peltier, A.; Hufford, M.B.; Oudin, E.; Saulnier, J.; Paul, L.; Knudsen, J.T.; Herren, H.R.; Gepts, P. Long-distance pollen flow assessment through evaluation of pollinator foraging range suggests transgene escape distances. Proc. Natl. Acad. Sci. USA 2008, 105, 13456–13461. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.K.; Deng, Y.T.; Tang, F.; Zhao, L.K.; Zhao, L.X.; Wang, Y.; Dai, X.B.; Zhou, Z.L.; Cao, Q.H. Screening and optimisation of in vitro pollen germination medium for sweetpotato (Ipomoea batatas). Plant Methods 2023, 19, 93. [Google Scholar] [CrossRef]
- Khatun, S.; Flowers, T.J. The estimation of pollen viability in rice. J. Exp. Bot. 1995, 46, 151–154. [Google Scholar] [CrossRef]
- Gealy, D.R.; Mitten, D.H.; Rutger, J.N. Gene flow between red rice (Oryza sativa) and herbicide-resistant rice (O-sativa): Implications for weed management. Weed Technol. 2003, 17, 627–645. [Google Scholar] [CrossRef]
- Dong, Y.F.; Jin, X.; Tang, Q.L.; Zhang, X.; Yang, J.T.; Liu, X.J.; Cai, J.F.; Zhang, X.B.; Wang, X.J.; Wang, Z.X. Development and Event-specific Detection of Transgenic Glyphosate-resistant Rice Expressing the G2-EPSPS Gene. Front. Plant Sci. 2017, 8, 885. [Google Scholar] [CrossRef]
- Jia, W.Q.; Wang, Y.L.; Mi, Z.R.; Wang, Z.; He, S.L.; Kong, D.Z. Optimization of culture medium for in vitro germination and storage conditions of Exochorda racemosa pollen. Front. Plant Sci. 2022, 13, 994214. [Google Scholar] [CrossRef]
- Castillo, S.E.; Tovar, J.C.; Shamin, A.; Gutirerrez, J.; Pearson, P.; Gehan, M.A. A protocol for Chenopodium quinoa pollen germination. Plant Methods 2022, 18, 65. [Google Scholar] [CrossRef]
- Wu, Y.L.; Gao, W.Y.; Zhou, Y.Z.; Guo, H.Y. Optimization of In Vitro Germination and storage of Armeniaca sibirica Pollen. Sci. Hortic. 2022, 304, 111309. [Google Scholar] [CrossRef]
- Announcement No. 423 of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Available online: https://www.moa.gov.cn/govpublic/ncpzlaq/202107/t20210714_6371842.htm (accessed on 26 October 2025).
- Song, Z.P.; Lu, B.R.; Zhu, Y.G.; Chen, J.K. Gene flow from cultivated rice to the wild species Oryza rufipogon under experimental field conditions. N. Phytol. 2003, 157, 657–665. [Google Scholar] [CrossRef]
- Wang, S.H.; Chen, F.; Zhou, K.D. In vitro germination of rice pollen. Crop Sci. 2000, 5, 609–612. (In Chinese) [Google Scholar]
- Tian, C.; Lu, H.; Wang, F.; Zhang, C.Y.; Lin, J.X. Effects of medium components on in vitro pollen germination and pollen tube growth of Picea wilsonii Mast. J. Beijing For. Univ. 2007, 29, 47–52. [Google Scholar]
- Kwack, B.H. Studies on cellular site of calcium action in promoting pollen growth. Physiol. Plant. 1967, 20, 825–833. [Google Scholar] [CrossRef]
- Wang, Q.L.; Lu, L.D.; Wu, X.Q.; Li, Y.Q.; Lin, J.X. Boron influences pollen germination and pollen tube growth in Picea meyeri. Tree Physiol. 2003, 23, 345–351. [Google Scholar] [CrossRef]
- Pierson, E.S.; Miller, D.D.; Callaham, D.A.; Shipley, A.M.; Rivers, B.A.; Cresti, M.; Hepler, P.K. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell 1994, 6, 1815–1828. [Google Scholar] [CrossRef]
- Chen, S.Q.; Wang, Z.; Liu, M.X.; Xie, Z.W.; Wang, H.H. Pollen Grain Germination and Pollen Tube Growth in Pistil of Rice. Rice Sci. 2008, 15, 125–130. [Google Scholar] [CrossRef]
- Ye, L.; Lv, D.; Jian, M.; Tian, H. Isolation of sperm cells of Allium tuberosum Roxb. J. Mol. Cell Biol. 2008, 41, 323–328. [Google Scholar]
- Zhao, R.; Hu, X.; Yuan, D.Y.; Masabni, J.; Xiong, H.; Zou, F. Orthogonal test design for optimizing culture medium for in vitro pollen germination of interspecific oil tea hybrids. An. Acad. Bras. Cienc. 2021, 93, e20190431. [Google Scholar] [CrossRef]
- Moor, A. The effect of magnesium sulphate (MgSO4) on capsicum pollen quality: A magneziumszulfat (MgSO4) hatasa a paprikapollen ninosegere. Zoldsegtermesztesi Kut. Intez. Bull. 1993, 25, 71–74. [Google Scholar]
- Brunet, J.; Ziobro, R.; Osvatic, J.; Clayton, M.K. The effects of time, temperature and plant variety on pollen viability and its implications for gene flow risk. Plant Biol. 2019, 21, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Boavida, L.C.; McCormick, S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 2007, 52, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Kakani, V.G.; Reddy, K.R.; Koti, S.; Wallace, T.P.; Prasad, P.V.V.; Reddy, V.R.; Zhao, D. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann. Bot. 2005, 96, 59–67. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).