Safety and Toxicological Risk Assessment of Northern Algerian Honeys
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents and Materials
2.3. Sample Preparation and Analysis
- Linearity: Stock multi-element standard solutions were prepared and suitably diluted to construct seven-point calibration curves. For each point of the curve, six replicates (n = 6) were analyzed. Correlation coefficients (R2) were used to check the linearity of the calibration curves. The linear ranges were 0.5–200.0 μg/L for As, Be, Cd, Cr, Li, Mo, Ni, Pb, Sb, Co, Cu, Fe, Mn, Se, Sn, Ti, and Zn; 0.1–5.0 mg/L for K, Ca, Na, and Mg; and 1–100 μg/L for Hg.
- Sensitivity: The limit of detection (LOD) and limit of quantification (LOQ) were determined for each target analyte. They were determined as 3.3 σ/S and 10 σ/S, respectively, where σ is the standard deviation of the mean value obtained by the analysis of ten blanks and S is the slope of the relative calibration curve.
- Trueness: An internal honey sample was first prepared and analyzed through the procedure described above and then spiked at three known concentration levels (i.e., 0.1, 0.2, and 0.5 mg/kg for Pb, Ni, Cr, Sb, As, Cd, Sn and Hg; 0.2, 1.0, and 2.0 mg/kg for Fe, Li, Be, Zn, Se, Ti, Mn, Mo, Cu, and Co; and 0.2, 0.5, and 1.0 g/kg for Ca, Na, Mg, and K) and re-analyzed. Hence, the trueness was calculated by considering both the experimental concentrations of the element derived from the sample analysis and the known amount of the standard spiked to the sample. Results are reported as the mean %value, averaged across the three different concentration levels, each replicated three times.
- Precision: Intraday and interday precision were evaluated for each element by analyzing six replicates of the honey sample spiked at the lowest concentration levels on the same day and over a longer period (i.e., one week). Results are reported as RSD%, averaged across the replicate measurements
2.4. Statistical Analysis
2.5. Toxicological Risk Assessment
3. Results
3.1. Profile of Inorganic Elements in Algerian Honeys
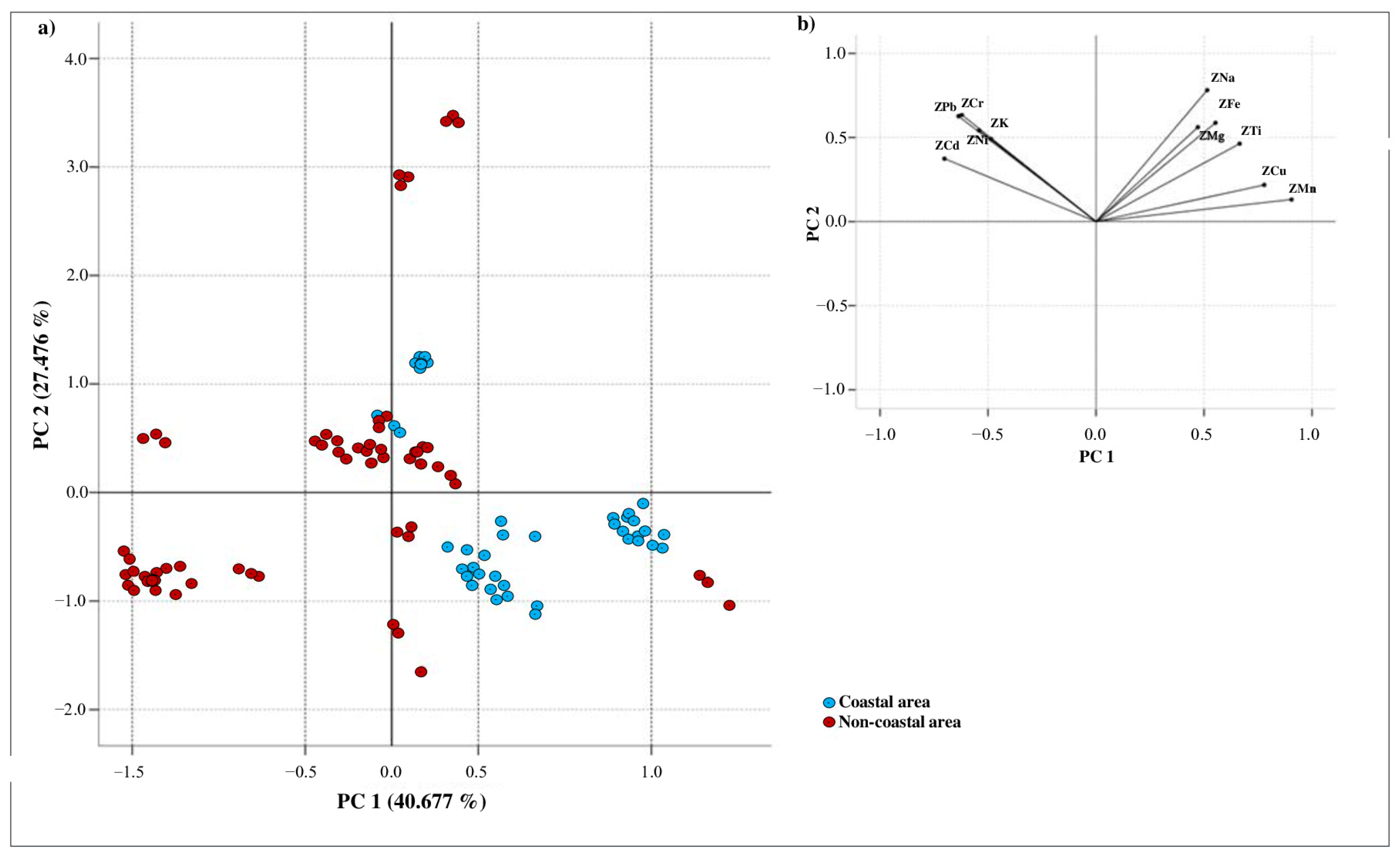
3.2. Comparison of Inorganic Elements in Honeys from Coastal and Non-Coastal Areas
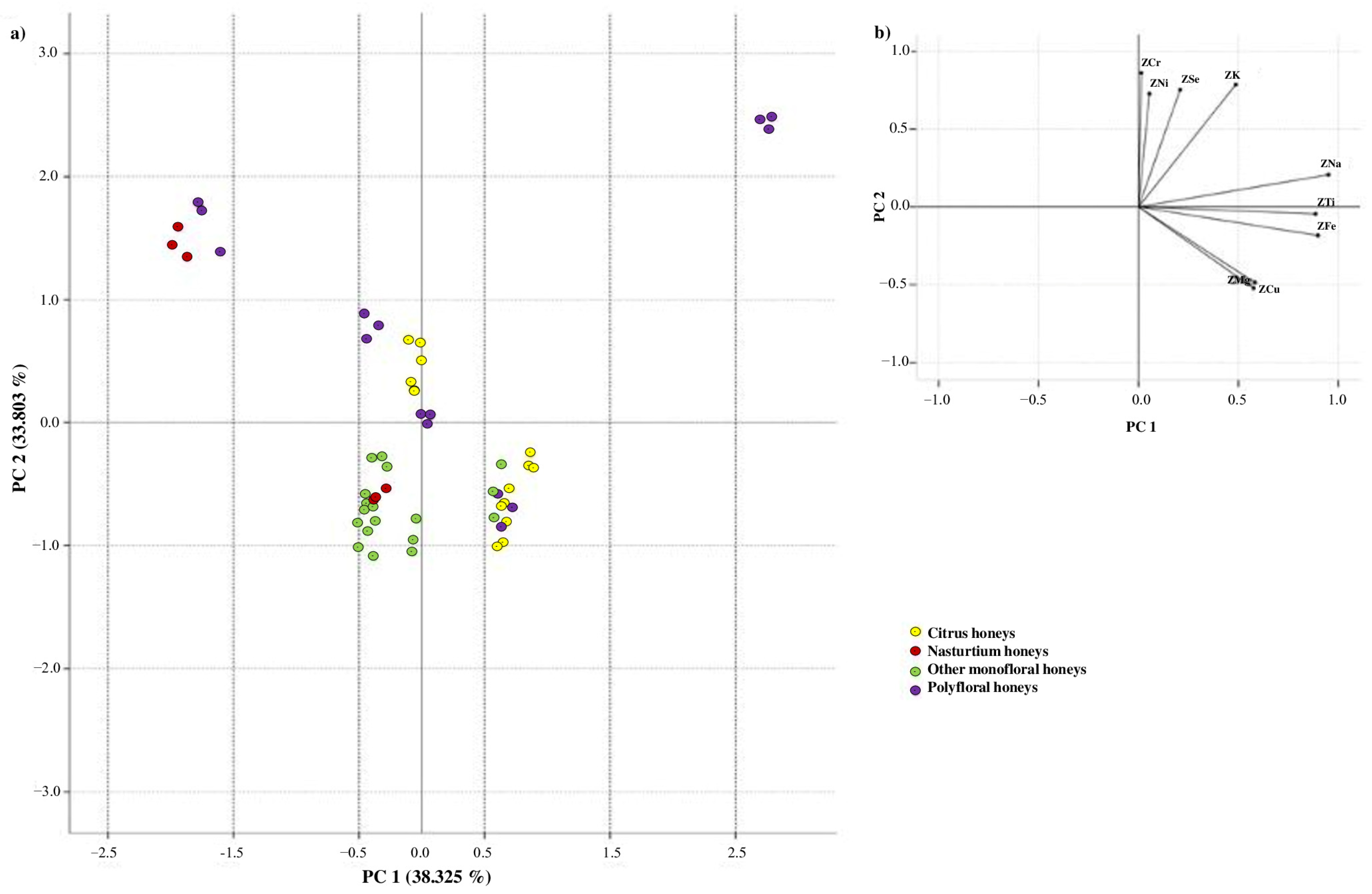
3.3. Comparison of Inorganic Elements in Honeys in Relation to the Botanical Origin
3.4. Statistical Analysis
3.4.1. Geographical Origin
3.4.2. Botanical Origin
3.5. Toxicological Risk Assessment
4. Discussions
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Codex Alimentarius Commission. Revised Codex Standard for Honey. Codex Stan 12-1981, Rev.1 (1987), Rev.2 (2001). Available online: https://www.ihc-platform.net/codex2001.pdf (accessed on 20 April 2025).
- Council Directive of the European Union. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities 2002, 10, 47–52. [Google Scholar]
- Derrar, S.; Nava, V.; Ayad, M.A.; Saim, M.S.; Aggad, H.; Spanò, I.M.; Litrenta, F.; Leonardi, M.; Albergamo, A.; Lo Turco, V.; et al. Safety assessment of honeys from northern and southern Algerian regions. Agriculture 2024, 14, 1503. [Google Scholar] [CrossRef]
- Chefrour, C.; Draiaia, R.; Tahar, A.; Ait Kaki, Y.; Bennadja, S.; Battesti, M.J. Physicochemical characteristics and pollen spectrum of some north-east Algerian honeys. Afr. J. Food Agric. Nutr. Dev. 2009, 9, 1276–1293. [Google Scholar] [CrossRef]
- Di Bella, G.; Potortì, A.G.; Beltifa, A.; Ben Mansour, H.; Nava, V.; Lo Turco, V. Discrimination of Tunisian honey by mineral and trace element chemometrics profiling. Foods 2021, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Kedzierska-Matysek, M.; Teter, A.; Skałecki, P.; Topyła, B.; Domaradzki, P.; Poleszak, E.; Florek, M. Residues of pesticides and heavy metals in Polish varietal honey. Foods 2022, 11, 2362. [Google Scholar] [CrossRef] [PubMed]
- Hernández, O.M.; Fraga, J.M.G.; Jiménez, A.I.; Jimenez, F.; Arias, J.J. Characterization of honey from the Canary Islands: Determination of the mineral content by atomic absorption spectrophotometry. Food Chem. 2005, 93, 449–458. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: A comprehensive review. Compr. Rev. Food. Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef]
- WHO/JEFCA. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives on Arsenic. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/1863 (accessed on 13 October 2025).
- WHO/JEFCA. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives on Cadmium. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/1376 (accessed on 13 October 2025).
- EFSA CONTAM Panel. Scientific Opinion on the update of the risk assesment of nickel in food and drinking water. EFSA J. 2020, 18, 6268. [Google Scholar]
- EFSA CONTAM Panel. Risk assessment of complex organoarsenic species in food. EFSA J. 2024, 22, e9112. [Google Scholar]
- Pohl, P. Determination of metal content in honey by atomic absorption and emission spectrometries. Trends Anal. Chem. 2009, 28, 117–128. [Google Scholar] [CrossRef]
- Terrab, A.; González, A.G.; Díez, M.J.; Heredia, F.J. Mineral content and electrical conductivity of the honeys produced in Northwest Morocco and their contribution to the characterisation of unifloral honeys. J. Sci. Food Agric. 2003, 83, 637–643. [Google Scholar] [CrossRef]
- Fernández-Torres, R.; Perez-Bernal, J.L.; Bello-Lopez, M.A.; Callejon-Mochon, M.; Jimenez-Sanchez, J.C.; Guiraúm-Pérez, A. Mineral content and botanical origin of Spanish honeys. Talanta 2005, 65, 686–691. [Google Scholar] [CrossRef]
- Çobanoğlu, D.N.; Temizer, İ.K.; Felek, İ.; Şimşek, A.; Dündar, O. Botanical origin of honey: Implications for mineral composition and potential toxic element safety. Chem. Biodivers. 2025, 22, e00318. [Google Scholar] [CrossRef]
- Pavlin, A.; Kocar, D.; Imperl, J.; Kolar, M.; Marolt, G.; Petrova, P. Honey origin authentication via mineral profiling combined with chemometric approaches. Foods 2023, 12, 2826. [Google Scholar] [CrossRef]
- Vlad, I.A.; Bartha, S.; Goji, G.; Tăut, I.; Rebrean, F.A.; Burescu, L.I.N.; Pășcuț, C.G.; Moțiu, P.T.; Tunduc, A.; Bunea, C.I.; et al. Comprehensive assessment of potentially toxic element (PTE) contamination in honey from a historically polluted agro-industrial landscape: Implications for agricultural sustainability and food safety. Agriculture 2025, 15, 1176. [Google Scholar] [CrossRef]
- Glevitzky, M.; Corcheş, M.T.; Popa, M.; Vică, M.L. Honey as a bioindicator: Pollution’s effects on its quality in mining vs. protected sites. Appl. Sci. 2025, 15, 7297. [Google Scholar] [CrossRef]
- Chafik, B.M.; Adnène, B.I. Determination of heavy metals in honey samples from different regions of the northeast of Algeria: According to an urban gradient. Pollution 2022, 8, 820–829. [Google Scholar] [CrossRef]
- Yayinie, M.; Atlabachew, M. Multi-element analysis of honey from Amhara Region-Ethiopia for quality, bioindicator of environmental pollution, and geographical origin discrimination. Biol. Trace Elem. Res. 2022, 200, 5283–5297. [Google Scholar] [CrossRef]
- Haider, Y.; Adjlane, N.; Martin-Hernandez, R.; Haddad, N. Beekeeping in Algeria: Evaluation of beekeeping practices, trends of management, and challenges. Afr. J. Food Agric. Nutr. Dev. 2024, 25, 25542–25564. [Google Scholar] [CrossRef]
- Homrani, M.; Escuredo, O.; Rodriguez-Flores, M.S.; Fatiha, D.; Mohammed, B.; Homrani, A.; Seijo, C. Botanical origin, pollen profile, and physicochemical properties of Algerian honey from different bioclimatic areas. Foods 2020, 9, 938. [Google Scholar] [CrossRef]
- Tamali, H.S.; Ozkirim, A. Beekeeping activities in Turkey and Algeria. Mellifera 2019, 19, 30–40. [Google Scholar]
- Commission Regulation (EU). 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Foodstuffs and Repealing Regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/?uri=CELEX:32023R0915 (accessed on 20 April 2025).
- Zerrouk, S.; Bahloul, R. Palynological and physicochemical properties of multifloral honey produced in some regions of Algeria. J. Apic. Res. 2023, 62, 345–354. [Google Scholar] [CrossRef]
- Massous, A.; Ouchbani, T.; Lo Turco, V.; Litrenta, F.; Nava, V.; Albergamo, A.; Potortì, A.G.; Di Bella, G. Monitoring Moroccan Honeys: Physicochemical Properties and Contamination Pattern. Foods 2023, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Principles of environmental impact assessment review. In Appendix A: Environmental Impact Assessment Checklist; U.S. EPA: Washington, DC, USA, 1998. [Google Scholar]
- Bertil, M.; Örnemark, U. The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics; LGC: Teddington, UK, 2014. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization Statistic Database. 2013. Available online: https://www.fao.org/faostat/en/#home (accessed on 20 April 2025).
- European Communities Commission. Regulation (EU). No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA Relevance. Available online: http://data.europa.eu/eli/reg/2011/1169/2018-01-01 (accessed on 20 April 2025).
- EFSA (European Food Safety Authority). Dietary reference values for nutrients summary report. EFSA Support. Publ. 2017, 14, e15121E. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific opinion on arsenic in food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific opinion on safety and efficacy of cobalt compounds (E3) as feed additives for all animal species: Cobaltous acetate tetrahydrate, basic cobaltous carbonate monohydrate and cobaltous sulphate heptahydrate, based on a dossier submitted by TREACEEIG. EFSA J. 2012, 10, 2971. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). Summary and Conclusions—Fifty-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- EFSA (European Food Safety Authority). Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. 2015, 13, 4002. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Cadmium. In Safety Evaluation of Certain Food Additives and Contaminants/Prepared by the Sixtyfirst Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series No. 52; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- WHO (World Health Organization). Arsenic. In Joint Expert WHO/FAO Expert Committee on Food Additives and Contaminants; Food Additives Series No. 24; WHO: Geneva, Switzerland, 1988. [Google Scholar]
- WHO (World Health Organization). Nickel in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality. 2005. Available online: https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/nickel-background-document.pdf?sfvrsn=90644b9f_9 (accessed on 21 April 2025).
- WHO (World Health Organization). Lead in safety evaluation of certain food additives and contaminants. In Prepared by the Fiftythird Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series No. 44; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Duru, C.E.; Duru, I.A. Phytochemical evaluation and health risk assessment of honey from an Apiary in Amizi, Ikuano local government area, Abia State, Nigeria. Sci. Afr. 2021, 13, e00885. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Risk-Based Concentration Table. 2010. Available online: https://archive.epa.gov/region9/superfund/web/html/index-23.html (accessed on 21 April 2025).
- Albergamo, A.; Bartolomeo, G.; Messina, L.; Rando, R.; Di Bella, G. Traceability of Opuntia spp. In Opuntia spp.: Chemistry, Bioactivity and Industrial Applications; Springer International Publishing: Cham, Switzerland, 2021; pp. 457–482. [Google Scholar]
- Barreiros, J.; Cepeda, A.; Franco, C.; Nebot, C.; Vázquez, B. Analysis of minerals in honey and their nutritional implications. J. Food Compos. Anal. 2024, 136, 106733. [Google Scholar] [CrossRef]
- Haouam, L.; Tahar, A.; Dailly, H.; Lahrichi, A.; Chaqroune, A.; Abdennour, C. Physicochemical properties and major elements contents of Algerian honeys from semi-arid regions. Emir. J. Food Agric. 2016, 28, 107–115. [Google Scholar] [CrossRef]
- Habati, M.; Gherib, A.; Bakchiche, B.; Benmebarek, A. A Study on the physicochemical, antioxidant properties and mineral content of five honeys produced in the central region of Algeria. Sci. Study Res-Chem. C 2017, 18, 121. [Google Scholar]
- Elamine, Y.; Inácio, P.M.; da Graça Miguel, M.; Carlier, J.D.; Costa, M.C.; Estevinho, L.M.; Gomes, H.L. Electrical impedance spectroscopy for potassium content analysis and botanical origin identification of honey. Food Chem. 2024, 453, 139605. [Google Scholar] [CrossRef]
- Zerrouk, S.; Seijo, M.C.; Escuredo, O.; Rodriguez-Flores, M.S. Characterization of Ziziphus lotus (jujube) honey produced in Algeria. J. Apic. Res. 2017, 57, 166–174. [Google Scholar] [CrossRef]
- Oliveira, S.S.; Alves, C.N.; Boa Morte, E.S.; de Freitas Santos Junior, A.; Oliveira Araujo, G.; Muniz Batista Santos, D.C. Determination of essential and potentially toxic elements and their estimation of bioaccessibility in honeys. Microchem. J. 2019, 151, 104221. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Tan, S.W.; Yusof, Y.A.; Chua, L.S. Classification of honey from its bee origin via chemical profiles and mineral content. Food Anal. Methods 2017, 10, 19–30. [Google Scholar] [CrossRef]
- Kocsis, M.; Bodó, A.; Koszegi, T.; Csepregi, R.; Filep, R.; Hoffmann, G.; Farkas, Á. Quality Assessment of Goldenrod, Milkweed and Multifloral Honeys Based on Botanical Origin, Antioxidant Capacity and Mineral Content. Int. J. Mol. Sci. 2022, 23, 769. [Google Scholar] [CrossRef]
- Nakib, R.; Ghorab, A.; Harbane, S.; Saker, Y.; Ouelhadj, A.; Rodriguez-Flores, M.S.; Seijo, M.C.; Escudero, O. Sensory attributes and chemical composition: The case of three monofloral honey types from Algeria. Foods 2024, 13, 2421. [Google Scholar] [CrossRef]
- Bereksi-Reguig, D.; Bouchentouf, S.; Allali, H.; Adamczuk, A.; Kowalska, G.; Kowalski, R. Trace elements and heavy metal contents in west Algerian natural honey. J. Anal. Methods Chem. 2022, 2022, 7890856. [Google Scholar] [CrossRef]
- Tariba Lovakovic, B.; Lazarus, M.; Brcic Karaconji, I.; Jurica, K.; Zivkovic Semren, T.; Lusic, D.; Brajenovic, N.; Pelaic, Z.; Pizent, A. Multi-elemental composition and antioxidant properties of strawberry tree (Arbutus unedo L.) honey from the coastal region of Croatia: Risk-benefit analysis. J. Trace Elem. Med. Biol. 2018, 45, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Licata, P.; Potortì, A.G.; Crupi, R.; Nava, V.; Qada, B.; Rando, R.; Bartolomeo, G.; Dugo, G.; Lo Turco, V. Mineral content and physico-chemical parameters of honey from North regions of Algeria. Nat. Prod. Res. 2022, 36, 636–643. [Google Scholar] [CrossRef]
- Bora, F.D.; Andrecan, A.F.; Călugăr, A.; Bunea, C.I.; Popescu, M.; Petrescu-Mag, I.V.; Bunea, A. Comprehensive elemental profiling of Romanian honey: Exploring regional variance, honey types, and analyzed metals for sustainable apicultural and environmental practices. Foods 2024, 13, 1253. [Google Scholar] [CrossRef] [PubMed]
- Ben Amar, Y.M.; Nava, V.; Mouad, L.B.; Brigui, J.; Chouaibi, N.; Potortì, A.G.; Litrenta, F.; Albergamo, A.; Di Bella, G. Proximate composition and mineral profile of Moroccan and Italian carobs. J. Food Compos. Anal. 2025, 143, 107628. [Google Scholar] [CrossRef]
- Mehdi, Y.; Mutlaq, A.; Al-Balas, Q.; Azzi, E.; Bouadjela, L.; Taïbi, N.; Bachari, K. Physicochemical characterization and determination of chloramphenicol residues and heavy metals in Algerian honeys. Environ. Sci. Poll. Res. 2018, 25, 33322–33333. [Google Scholar] [CrossRef]
- Bereksi-Reguig, D.; Allali, H.; Bouchentouf, S.; Adamczuk, A.; Kowalska, G.; Kowalski, R. Analysis of trace-elements and toxic heavy metals in honeys from Tlemcen Province, north-western Algeria. Agric. Conspec. Sci. 2020, 85, 367–374. [Google Scholar]
- Messai, A.; Idres, A.; Benselhoub, A. Mineralogical characterization of limonitic iron ore from the Rouina mine, Ain Defla (Algeria). J. Geol. Geograph. Geoecol. 2018, 27, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Abederahmane, N.; Khochemane, L.; Gadri, L.; Rais, K.; Bennis, O. Impact of air pollution with dust in the Ouenza iron mine-NE Algeria. Min. Sci. 2018, 25, 19–31. [Google Scholar] [CrossRef]
- Flamminii, F.; Consalvo, A.; Cichelli, A.; Chiaudani, A. Assessing Mineral Content and Heavy Metal Exposure in Abruzzo Honey and Bee Pollen from Different Anthropic Areas. Foods 2024, 13, 1930. [Google Scholar] [CrossRef]
- Sedik, P.; Horska, E.; Adam, S.; Miskeje, M. Mineral content as an aspect of nutrition marketing: Case study of honey market in Slovakia. J. Food Nutr. Res. 2020, 59, 185. [Google Scholar]
- Ysbaa, S.; Haddouche, O.; Boutaleb, A.; Sami, L.; Kolli, O. Mineralization and fluid inclusion characteristics of Pb-Zn-Fe-Ba (Cu, F, Sr) ore-deposits in northern east of Algeria. Arab. J. Geosci. 2021, 14, 957. [Google Scholar] [CrossRef]
- Sager, M.; Maleviti, E. Elemental composition of honeys from Greece-possible use as environmental indicators. J. Nutr. Food Sci. 2011, 8, 2. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Bammou, M.; Sellam, K.; El Midaoui, A.; Bourkhis, B.; Ennassir, J.; Alem, C.; Filali-Zegzouti, Y. Physicochemical properties of eleven monofloral honey samples produced in Morocco. Arab. J. Basic Appl. Sci. 2019, 26, 476–487. [Google Scholar] [CrossRef]
- Abeslami, A.; El Farissi, H.; Cacciola, F.; El Bachiri, A.; Sindic, M.; Fauconnier, M.-L.; Bruneau, E.; Talhaoui, A. Unveiling the Mineral and Sugar Richness of Moroccan Honeys: A Study of Botanical Origins and Quality Indicators. Molecules 2025, 30, 150. [Google Scholar] [CrossRef]
- Bilandzic, N.; Sedak, M.; Dokic, M.; Boskovic, A.G.; Florijancic, T.; Boskovic, I.; Kovacic, M.; Puskadija, Z.; Hruskar, M. Assessment of Toxic and Trace Elements in Multifloral Honeys from Two Regions of Continental Croatia. Bull. Environ. Contam. Toxicol. 2019, 104, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, G.; Sopaj, F.; Tasev, K.; Stafilov, T.; Sajn, R.; Pacarizi, M. Analysis of chemical elements in honey samples in the territory of Kosovo. J. Food Comp. Anal. 2023, 124, 105505. [Google Scholar] [CrossRef]
- Alemu, M.; Gaudie, A.; Tefera, M. Physicochemical properties and levels of selected trace metals in honey from North Gondar, Ethiopia. Ethiop. J. Nat. Comp. Sci. 2021, 1, 90–102. Available online: http://journal.uog.edu.et/index.php/EJNCS (accessed on 20 April 2025).
- Adugna, E.; Hymete, A.; Birhanu, G.; Ashenef, A. Determination of some heavy metals in honey from different regions of Ethiopia. Cogent Food Agric. 2020, 6, 1764182. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Pavlicek, D.; Orešcanin, V.; Varenina, I.; Sedak, M.; Bilandžic, N. Mineral concentrations in different types of honey originating from three regions of continental Croatia. Foods 2024, 13, 2754. [Google Scholar] [CrossRef]
- Chebli, A.I.; Zergui, A.; Amziane, A.; Zebbiche, Y.; Abdennour, S. Metals in honey, cow’s milk and eggs in North-East Algeria and health risk. Food Addit. Contam. Part B 2024, 18, 55–64. [Google Scholar] [CrossRef]
- Albu, A.; Simeanu, C.; Pop, I.M.; Pui, A.; Tarcau, D.; Cucu-Man, S.-M. selected characteristics of multifloral Honeys from North-Eastern Romania. Agriculture 2024, 14, 26. [Google Scholar] [CrossRef]
- Zenunovic, A.; Keran, H.; Srabovic, E. Content of heavy metals in different types of honey. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7, 277–280. [Google Scholar] [CrossRef]
- Llico-Saénz, C.E.; Villanueva, J.J.; Vargas-Rocha, L. Cadmium and lead contamination in highland honey from Peru: A potential health risk. Innovaciencia 2025, 13, e4801. [Google Scholar] [CrossRef]
- Zergui, A.; Boudalia, S.; Joseph, M.L. Heavy metals in honey and poultry eggs as indicators of environmental pollution and potential risks to human health. J. Food Comp. Anal. 2023, 119, 105255. [Google Scholar] [CrossRef]
- Gajek, M.; Wysocki, P.; Mordaka, K.; Szunkowska Jozwik, M.I. Elemental composition of bee products from diverse botanical sources: Implications for consumer safety. J. Food Compos. Anal. 2025, 147, 108059. [Google Scholar] [CrossRef]
- Yaiche Achour, H.; Khali, M. Composition physicochimique des miels algériens. Determination des éléments traces et des éléments potentiellement toxiques. Afr. Sci. 2014, 10, 127–136. [Google Scholar]
- Sana; Ahmad, W.; Anwar, F.; Ismail, H.; Farid, M.; Ayub, M.A.; Sumrra, S.H.; Emenike, C.; Starowicz, M.; Zubair, M. Multifactorial evaluation of honey from pakistan: Essential minerals, antioxidant potential, and toxic metal contamination with relevance to human health risk. Foods 2025, 14, 2493. [Google Scholar] [CrossRef]
- Inaudi, P.; Garzino, M.; Abollino, O.; Malandrino, M.; Giacomino, A. Honey: Inorganic composition as possible marker for botanical and geological assignment. Molecules 2025, 30, 1466. [Google Scholar] [CrossRef]
- Junior, M.M.S.; Novais, J.S.; Felix, C.S.A.; Silveira, L.R.; Costa, G.P.; de Andrade, J.B. Human health risk assessment of mercury in Apis mellifera honey samples from the Atlantic Forest in Bahia, Brazil. J. Food Compos. Anal. 2025, 146, 107935. [Google Scholar] [CrossRef]
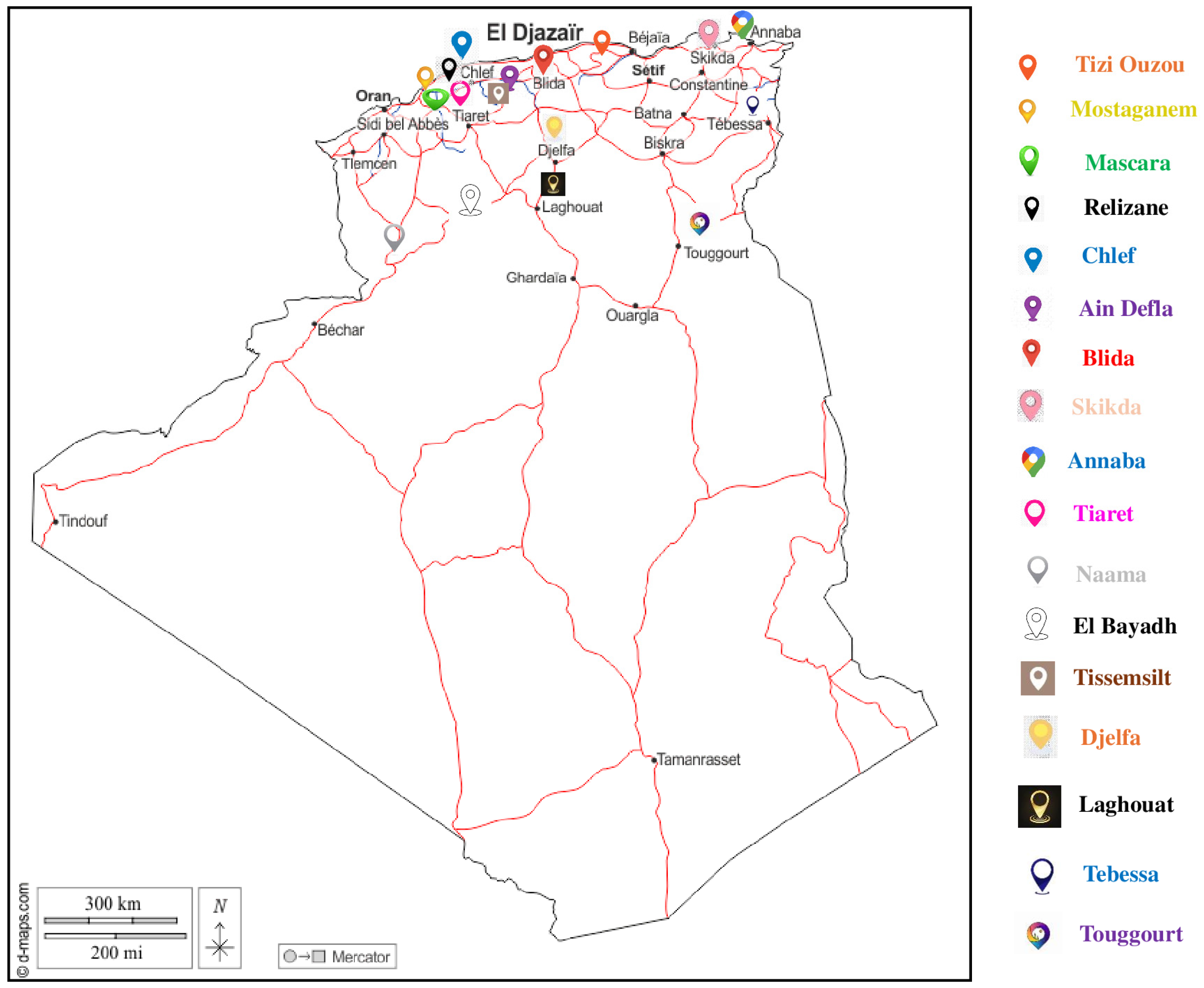
| Apiary Code | N. Honey Sample | Geographical Origin | Botanical Origin |
|---|---|---|---|
| HS-1 | 1 | Tizi Ouzou | Pinus silvestris |
| 2 | |||
| 3 | |||
| HS-2 | 4 | Laghouat | Ziziphus lotus |
| 5 | |||
| 6 | |||
| HS-3 | 7 | Djelfa | Ziziphus lotus–Silybum marianum |
| 8 | |||
| 9 | |||
| HS-4 | 10 | Tissemsilt | Tamarix africana |
| 11 | |||
| 12 | |||
| HS-5 | 13 | Blida | Citrus sinensis |
| 14 | |||
| 15 | |||
| HS-6 | 16 | Laghouat | Euphorbia officinarum |
| 17 | |||
| 18 | |||
| HS-7 | 19 | Tizi Ouzou | Quercus ilex–Eucalyptus globulus |
| 20 | |||
| 21 | |||
| HS-8 | 22 | Djelfa | Nasturtium officinalis |
| 23 | |||
| 24 | |||
| HS-9 | 25 | Touggourt | Helianthus annuus |
| 26 | |||
| 27 | |||
| HS-10 | 28 | Annaba | Eucalyptus globulus |
| 29 | |||
| 30 | |||
| HS-11 | 31 | Tizi Ouzou | Quercus ilex |
| 32 | |||
| 33 | |||
| HS-12 | 34 | Tizi Ouzou | Nasturtium officinalis |
| 35 | |||
| 36 | |||
| HS-13 | 37 | Skikda | Eucalyptus globulus |
| 38 | |||
| 39 | |||
| HS-14 | 40 | Tebessa | Rosmarinus officinalis |
| 41 | |||
| 42 | |||
| HS-15 | 43 | Tizi Ouzou | Ceratonia siliqua |
| 44 | |||
| 45 | |||
| HS-16 | 46 | Djelfa | Multifloral |
| 47 | |||
| 48 | |||
| HS-17 | 49 | Chlef | Ziziphus lotus–Thymus vulgaris |
| 50 | |||
| 51 | |||
| HS-18 | 52 | Blida | Citrus sinensis |
| 53 | |||
| 54 | |||
| HS-19 | 55 | El Bayadh | Euphorbia officinarum |
| 56 | |||
| 57 | |||
| HS-20 | 58 | Djelfa | Silybum marianum |
| 59 | |||
| 60 | |||
| HS-21 | 61 | Djelfa | Eruca sativa |
| 62 | |||
| 63 | |||
| HS-22 | 64 | Ain Defla | Pinus halepensis–Quercus ilex |
| 65 | |||
| 66 | |||
| HS-23 | 67 | Mostaganem | Multifloral |
| 68 | |||
| 69 | |||
| HS-24 | 70 | Tiaret | Eruca sativa |
| 71 | |||
| 72 | |||
| HS-25 | 73 | Relizane | Ziziphus lotus |
| 74 | |||
| 75 | |||
| HS-26 | 76 | Naâma | Multifloral |
| 77 | |||
| 78 | |||
| HS-27 | 79 | Skikda | Citrus sinensis |
| 80 | |||
| 81 | |||
| HS-28 | 82 | Tiaret | Multifloral |
| 83 | |||
| 84 | |||
| HS-29 | 85 | Mostaganem | Citrus sinensis |
| 86 | |||
| 87 | |||
| HS-30 | 88 | Tebessa | Ziziphus lotus |
| 89 | |||
| 90 | |||
| HS-31 | 91 | Mascara | Tamarix africana |
| 92 | |||
| 93 | |||
| HS-32 | 94 | Tizi Ouzou | Erica arborea–Lavandula stoechas |
| 95 | |||
| 96 | |||
| HS-33 | 97 | Mostaganem | Eucalyptus gomphocephala |
| 98 | |||
| 99 | |||
| HS-34 | 100 | Mostaganem | Pinus halepensis–Rosmarinus officinalis |
| 101 | |||
| 102 | |||
| HS-35 | 103 | Mostaganem | Citrus sinensis |
| 104 | |||
| 105 | |||
| HS-36 | 106 | Chlef | Multifloral |
| 107 | |||
| 108 |
| Parameter | Setting |
|---|---|
| Nebulizer | Concentric PFA |
| RF-generator | 1550 W |
| Sample depth | 5 mm |
| Interface | Sample and skimmer cones in Ni |
| Interface pressure | 1.89 × 100 Pa |
| Argon flow (plasma/auxiliary/carrier) | 14/0.8/1.1 L/min |
| Sample introduction flow | 0.93 L/min |
| Scanning condition | Number of replicates: 3, dwell time: 1 s |
| CCT gas flow (He) | 4.7 mL/min |
| Vacuum | <7.5 × 10−7 Pa |
| Extract Lens 1 voltage | 1.5 V |
| Spray chamber temperature | 2.7 °C |
| Integration times | 0.5 s/point for As, Se, and Fe; 0.1 s/point for other elements |
| Element | R2 | LOD (mg/kg) | LOQ (mg/kg) | Trueness (%) | Precision (RSD%) | |
|---|---|---|---|---|---|---|
| Intraday | Interday | |||||
| As | 0.9996 | 0.001 | 0.003 | 98.00 | 1.4 | 1.5 |
| Be | 0.9996 | 0.003 | 0.010 | 96.50 | 1.1 | 1.2 |
| Ca | 0.9989 | 0.095 | 0.314 | 91.50 | 1.1 | 1.3 |
| Cd | 0.9998 | 0.001 | 0.003 | 100.50 | 1.0 | 1.2 |
| Co | 0.9995 | 0.003 | 0.010 | 96.25 | 0.9 | 1.1 |
| Cr | 0.9998 | 0.003 | 0.010 | 95.50 | 0.7 | 0.8 |
| Cu | 0.9997 | 0.005 | 0.017 | 97.75 | 0.9 | 1.0 |
| Fe | 0.9995 | 0.010 | 0.033 | 96.10 | 0.6 | 0.7 |
| K | 0.9988 | 0.105 | 0.347 | 90.25 | 1.1 | 1.2 |
| Li | 0.9996 | 0.003 | 0.010 | 96.80 | 1.0 | 1.2 |
| Mg | 0.9994 | 0.070 | 0.231 | 97.50 | 1.1 | 1.3 |
| Mn | 0.9995 | 0.004 | 0.013 | 97.35 | 1.2 | 1.4 |
| Mo | 0.9996 | 0.003 | 0.010 | 97.15 | 1.0 | 1.1 |
| Na | 0.9989 | 0.085 | 0.281 | 92.00 | 0.8 | 0.9 |
| Ni | 0.9994 | 0.003 | 0.010 | 98.85 | 1.0 | 1.3 |
| Pb | 0.9999 | 0.001 | 0.003 | 102.50 | 0.7 | 0.8 |
| Sb | 0.9993 | 0.003 | 0.010 | 97.00 | 1.0 | 1.1 |
| Se | 0.9995 | 0.004 | 0.013 | 94.50 | 1.1 | 1.3 |
| Sn | 0.9993 | 0.003 | 0.010 | 97.25 | 1.2 | 1.3 |
| Ti | 0.9995 | 0.003 | 0.010 | 96.00 | 0.9 | 1.0 |
| Zn | 0.9997 | 0.010 | 0.033 | 97.15 | 1.2 | 1.3 |
| Hg | 0.9998 | 0.003 | 0.010 | 98.00 | 0.9 | 1.0 |
| Element | RfD (μg/Kgbw/Day) |
|---|---|
| As | 0.3 |
| Cd | 1 |
| Cr | 3 |
| Cu | 40 |
| Fe | 9 |
| Mn | 140 |
| Ni | 20 |
| Pb | 3.5 |
| Zn | 300 |
| Samples | Macro-Elements | Trace Elements | Potentially Toxic Elements | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Ca | Mg | K | Fe | Co | Cr | Cu | Mn | Se | Ti | Zn | Sn | As | Cd | Ni | Pb | Hg | |
| HS-1 | 82.90 ± 2.63 | 273.31 ± 3.49 | 107.37 ± 2.18 | 614.59 ± 3.36 | 14.83 ± 0.23 | 0.03 ± 0.01 | 0.06 ± 0.01 | 1.25 ± 0.09 | 3.64 ± 0.41 | 0.23 ± 0.03 | 0.19 ± 0.03 | 3.50 ± 0.23 | <LOQ | <LOQ | 0.02 ± 0.01 | 0.11 ± 0.03 | 0.02 ± 0.01 | <LOQ |
| HS-2 | 54.84 ± 3.88 | 95.31 ± 3.23 | 71.05 ± 4.08 | 11,504.46 ± 241.40 | 18.27 ± 0.23 | <LOQ | 0.13 ± 0.02 | 0.60 ± 0.04 | 0.10 ± 0.01 | 0.38 ± 0.04 | <LOQ | 9.28 ± 0.23 | 0.14 ± 0.01 | <LOQ | 1.02 ± 0.05 | 0.31 ± 0.05 | 0.64 ± 0.10 | <LOQ |
| HS-3 | 45.37 ± 3.82 | 158.33 ± 8.24 | 31.31 ± 2.42 | 1606.19 ± 41.83 | 6.52 ± 0.33 | 0.50 ± 0.05 | 0.26 ± 0.03 | 0.72 ± 0.05 | 0.23 ± 0.02 | 0.37 ± 0.03 | 0.04 ± 0.01 | 1.77 ± 0.12 | 0.03 ± 0.01 | <LOQ | 0.05 ± 0.01 | 0.41 ± 0.03 | 0.19 ± 0.02 | <LOQ |
| HS-4 | 89.12 ± 1.89 | 278.40 ± 4.81 | 34.81 ± 3.17 | 820.01 ± 4.28 | 10.02 ± 0.17 | <LOQ | 0.09 ± 0.01 | 3.67 ± 0.10 | 4.27 ± 0.14 | 0.12 ± 0.02 | 0.28 ± 0.04 | 1.05 ± 0.08 | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.09 ± 0.01 | 0.34 ± 0.03 | 0.12 ± 0.02 | <LOQ |
| HS-5 | 118.51 ± 4.55 | 314.40 ± 8.40 | 82.65 ± 1.92 | 924.10 ± 9.57 | 9.17 ± 0.08 | 0.20 ± 0.03 | 0.21 ± 0.05 | 2.77 ± 0.21 | 1.23 ± 0.11 | 0.35 ± 0.03 | 0.49 ± 0.05 | 14.65 ± 0.63 | <LOQ | 0.06 ± 0.02 | 0.28 ± 0.04 | 0.26 ± 0.05 | 0.71 ± 0.05 | <LOQ |
| HS-6 | 46.31 ± 2.65 | 97.74 ± 2.54 | 79.15 ± 2.88 | 12,606.41 ± 323.81 | 19.07 ± 1.85 | <LOQ | 0.15 ± 0.01 | 0.51 ± 0.05 | 0.16 ± 0.02 | 0.34 ± 0.04 | <LOQ | 8.59 ± 0.46 | 0.08 ± 0.02 | <LOQ | 0.81 ± 0.03 | 0.32 ± 0.05 | 0.52 ± 0.04 | <LOQ |
| HS-7 | 64.58 ± 3.02 | 286.79 ± 4.12 | 108.15 ± 1.36 | 643.41 ± 4.68 | 14.34 ± 0.30 | 0.03 ± 0.01 | 0.09 ± 0.02 | 1.35 ± 0.07 | 3.41 ± 0.18 | 0.21 ± 0.02 | 0.22 ± 0.03 | 3.88 ± 0.21 | <LOQ | <LOQ | 0.02 ± 0.01 | 0.14 ± 0.02 | 0.04 ± 0.01 | <LOQ |
| HS-8 | 50.50 ± 2.78 | 195.70 ± 4.50 | 35.36 ± 2.27 | 1526.11 ± 26.15 | 5.84 ± 0.16 | 0.47 ± 0.02 | 0.18 ± 0.03 | 0.80 ± 0.06 | 0.27 ± 0.02 | 0.34 ± 0.02 | 0.02 ± 0.01 | 1.58 ± 0.10 | 0.04 ± 0.01 | <LOQ | 0.04 ± 0.00 | 0.41 ± 0.04 | 0.20 ± 0.03 | <LOQ |
| HS-9 | 64.03 ± 3.17 | 192.13 ± 4.92 | 142.67 ± 4.96 | 1054.08 ± 24.93 | 5.15 ± 0.14 | 0.38 ± 0.05 | 0.13 ± 0.02 | 0.53 ± 0.09 | 0.49 ± 0.06 | 0.28 ± 0.03 | 0.04 ± 0.01 | 1.27 ± 0.05 | 0.02 ± 0.00 | <LOQ | 0.03 ± 0.01 | 0.23 ± 0.03 | 0.24 ± 0.02 | <LOQ |
| HS-10 | 161.80 ± 3.20 | 380.28 ± 7.99 | 106.79 ± 6.84 | 659.92 ± 14.67 | 16.74 ± 1.02 | 0.32 ± 0.04 | 0.15 ± 0.03 | 1.74 ± 0.17 | 2.23 ± 0.07 | 0.17 ± 0.03 | 0.33 ± 0.02 | 9.94 ± 0.17 | <LOQ | 0.03 ± 0.01 | 0.43 ± 0.04 | 0.19 ± 0.03 | 0.86 ± 0.04 | 0.04 ± 0.01 |
| HS-11 | 58.45 ± 2.22 | 270.62 ± 1.71 | 128.88 ± 4.48 | 591.31 ± 3.96 | 14.10 ± 0.14 | 0.02 ± 0.01 | 0.06 ± 0.01 | 1.22 ± 0.05 | 3.55 ± 0.23 | 0.23 ± 0.02 | 0.20 ± 0.03 | 3.43 ± 0.25 | <LOQ | <LOQ | 0.04 ± 0.01 | 0.13 ± 0.03 | 0.05 ± 0.01 | <LOQ |
| HS-12 | 72.17 ± 1.90 | 283.32 ± 2.13 | 122.55 ± 14.90 | 610.92 ± 5.34 | 13.16 ± 0.31 | 0.02 ± 0.01 | 0.09 ± 0.01 | 1.38 ± 0.06 | 3.96 ± 0.06 | 0.25 ± 0.04 | 0.24 ± 0.02 | 4.15 ± 0.11 | <LOQ | <LOQ | 0.01 ± 0.01 | 0.15 ± 0.03 | 0.07 ± 0.02 | <LOQ |
| HS-13 | 173.68 ± 3.71 | 334.11 ± 4.40 | 191.08 ± 4.48 | 718.46 ± 6.38 | 19.61 ± 0.59 | 0.43 ± 0.02 | 0.18 ± 0.02 | 3.20 ± 0.15 | 2.65 ± 0.11 | 0.23 ± 0.02 | 0.41 ± 0.02 | 14.19 ± 0.20 | <LOQ | 0.05 ± 0.01 | 0.47 ± 0.05 | 0.31 ± 0.02 | 0.91 ± 0.03 | 0.05 ± 0.01 |
| HS-14 | 98.58 ± 7.45 | 412.99 ± 8.27 | 93.21 ± 8.23 | 601.78 ± 10.36 | 10.75 ± 0.12 | 0.16 ± 0.03 | 0.13 ± 0.02 | 2.94 ± 0.22 | 4.16 ± 0.16 | 0.16 ± 0.01 | 0.23 ± 0.02 | 16.80 ± 0.51 | <LOQ | <LOQ | 0.22 ± 0.02 | 0.35 ± 0.04 | 0.81 ± 0.03 | <LOQ |
| HS-15 | 70.96 ± 4.90 | 295.49 ± 1.83 | 156.47 ± 7.25 | 675.74 ± 4.16 | 15.90 ± 0.30 | 0.02 ± 0.01 | 0.11 ± 0.02 | 2.61 ± 0.06 | 6.25 ± 0.31 | 0.41 ± 0.04 | 0.19 ± 0.03 | 6.24 ± 0.55 | <LOQ | <LOQ | 0.03 ± 0.01 | 0.19 ± 0.04 | 0.04 ± 0.01 | <LOQ |
| HS-16 | 51.37 ± 3.37 | 178.34 ± 7.86 | 44.00 ± 3.37 | 1620.35 ± 26.52 | 6.13 ± 0.12 | 0.56 ± 0.03 | 0.25 ± 0.05 | 0.63 ± 0.06 | 0.24 ± 0.05 | 0.39 ± 0.04 | 0.04 ± 0.01 | 1.47 ± 0.07 | 0.02 ± 0.01 | <LOQ | 0.05 ± 0.01 | 0.42 ± 0.04 | 0.24 ± 0.03 | <LOQ |
| HS-17 | 107.11 ± 4.01 | 341.11 ± 5.29 | 62.63 ± 2.23 | 777.28 ± 5.62 | 13.83 ± 0.72 | <LOQ | 0.13 ± 0.02 | 4.30 ± 0.22 | 5.19 ± 0.06 | 0.19 ± 0.01 | 0.32 ± 0.03 | 1.75 ± 0.11 | 0.09 ± 0.01 | 0.03 ± 0.01 | 0.12 ± 0.03 | 0.43 ± 0.02 | 0.25 ± 0.04 | <LOQ |
| HS-18 | 118.91 ± 8.16 | 315.34 ± 3.92 | 72.50 ± 2.53 | 924.89 ± 4.68 | 8.93 ± 0.16 | 0.21 ± 0.04 | 0.23 ± 0.03 | 2.68 ± 0.12 | 1.20 ± 0.06 | 0.33 ± 0.04 | 0.47 ± 0.03 | 14.15 ± 0.23 | <LOQ | 0.08 ± 0.02 | 0.33 ± 0.05 | 0.28 ± 0.06 | 0.61 ± 0.04 | <LOQ |
| HS-19 | 775.48 ± 7.28 | 404.90 ± 7.44 | 675.71 ± 4.45 | 9625.49 ± 29.87 | 42.04 ± 1.67 | 1.40 ± 0.05 | 0.95 ± 0.03 | 1.65 ± 0.05 | 4.84 ± 0.11 | 0.45 ± 0.03 | 1.55 ± 0.04 | 2.24 ± 0.09 | <LOQ | 0.01 ± 0.00 | 0.05 ± 0.01 | 0.53 ± 0.04 | 0.69 ± 0.06 | <LOQ |
| HS-20 | 49.75 ± 3.24 | 154.77 ± 5.81 | 24.25 ± 1.87 | 1691.98 ± 30.10 | 5.39 ± 0.14 | 0.67 ± 0.06 | 0.28 ± 0.03 | 0.84 ± 0.06 | 0.22 ± 0.03 | 0.31 ± 0.02 | 0.04 ± 0.01 | 1.87 ± 0.09 | 0.04 ± 0.01 | <LOQ | 0.07 ± 0.02 | 0.40 ± 0.04 | 0.18 ± 0.03 | <LOQ |
| HS-21 | 57.94 ± 2.70 | 173.45 ± 4.09 | 32.90 ± 3.18 | 1640.29 ± 10.57 | 6.59 ± 0.17 | 0.63 ± 0.04 | 0.18 ± 0.03 | 0.81 ± 0.07 | 0.28 ± 0.05 | 0.38 ± 0.05 | 0.03 ± 0.00 | 1.80 ± 0.09 | 0.04 ± 0.01 | <LOQ | 0.05 ± 0.01 | 0.44 ± 0.04 | 0.15 ± 0.02 | <LOQ |
| HS-22 | 97.30 ± 1.79 | 311.91 ± 6.69 | 165.74 ± 6.09 | 807.77 ± 4.42 | 11.16 ± 0.39 | <LOQ | 0.08 ± 0.01 | 3.49 ± 0.06 | 4.38 ± 0.06 | 0.11 ± 0.02 | 0.22 ± 0.03 | 1.35 ± 0.11 | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.10 ± 0.03 | 0.34 ± 0.02 | 0.18 ± 0.03 | <LOQ |
| HS-23 | 176.29 ± 5.24 | 206.49 ± 4.17 | 99.28 ± 7.50 | 929.50 ± 4.47 | 16.80 ± 0.96 | 0.03 ± 0.01 | 0.05 ± 0.02 | 5.10 ± 0.06 | 6.33 ± 0.21 | 0.25 ± 0.03 | 0.46 ± 0.03 | 2.77 ± 0.09 | 0.12 ± 0.02 | <LOQ | <LOQ | 0.19 ± 0.03 | 0.04 ± 0.01 | <LOQ |
| HS-24 | 80.14 ± 3.19 | 114.52 ± 3.62 | 48.06 ± 1.36 | 773.56 ± 14.57 | 14.49 ± 0.97 | 0.04 ± 0.01 | 0.36 ± 0.03 | 5.37 ± 0.17 | 6.17 ± 0.17 | 0.16 ± 0.01 | 0.86 ± 0.06 | 8.06 ± 0.19 | 0.13 ± 0.02 | 0.06 ± 0.01 | 0.35 ± 0.03 | 0.31 ± 0.03 | 0.70 ± 0.05 | <LOQ |
| HS-25 | 60.22 ± 3.41 | 285.57 ± 7.54 | 47.18 ± 3.60 | 462.10 ± 25.89 | 8.70 ± 0.46 | 0.31 ± 0.04 | 0.09 ± 0.01 | 0.99 ± 0.08 | 3.04 ± 0.23 | 0.21 ± 0.02 | 0.12 ± 0.03 | 4.38 ± 0.27 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.02 ± 0.01 |
| HS-26 | 861.90 ± 35.31 | 429.31 ± 7.31 | 124.02 ± 3.93 | 13,337.56 ± 233.94 | 35.78 ± 2.18 | 0.80 ± 0.05 | 0.57 ± 0.04 | 1.79 ± 0.07 | 3.02 ± 0.12 | 0.62 ± 0.04 | 2.35 ± 0.09 | 5.22 ± 0.19 | <LOQ | 0.02 ± 0.01 | 0.07 ± 0.01 | 0.51 ± 0.03 | 0.73 ± 0.03 | <LOQ |
| HS-27 | 175.58 ± 3.60 | 354.96 ± 7.07 | 201.20 ± 5.24 | 741.64 ± 8.43 | 19.84 ± 0.33 | 0.45 ± 0.04 | 0.17 ± 0.02 | 3.27 ± 0.15 | 2.73 ± 0.06 | 0.23 ± 0.02 | 0.41 ± 0.03 | 14.04 ± 0.18 | <LOQ | 0.05 ± 0.00 | 0.46 ± 0.04 | 0.31 ± 0.01 | 0.98 ± 0.03 | 0.04 ± 0.01 |
| HS-28 | 69.34 ± 1.85 | 97.41 ± 2.81 | 56.41 ± 2.54 | 771.53 ± 7.35 | 14.34 ± 1.18 | 0.06 ± 0.01 | 0.40 ± 0.03 | 0.79 ± 0.03 | 0.05 ± 0.01 | 0.21 ± 0.02 | 0.74 ± 0.04 | 12.86 ± 0.55 | 0.19 ± 0.04 | 0.13 ± 0.01 | 1.18 ± 0.10 | 0.38 ± 0.03 | 0.75 ± 0.03 | <LOQ |
| HS-29 | 190.06 ± 4.07 | 272.13 ± 20.48 | 82.87 ± 1.93 | 896.60 ± 6.40 | 19.34 ± 0.21 | 0.03 ± 0.01 | 0.04 ± 0.01 | 5.07 ± 0.08 | 7.13 ± 0.10 | 0.23 ± 0.03 | 0.45 ± 0.04 | 2.97 ± 0.08 | 0.13 ± 0.02 | <LOQ | <LOQ | 0.21 ± 0.03 | 0.03 ± 0.01 | <LOQ |
| HS-30 | 93.94 ± 2.27 | 435.32 ± 10.76 | 100.78 ± 4.49 | 614.44 ± 8.12 | 10.77 ± 0.30 | 0.20 ± 0.02 | 0.13 ± 0.02 | 2.83 ± 0.15 | 4.17 ± 0.07 | 0.16 ± 0.02 | 0.24 ± 0.02 | 17.02 ± 0.12 | <LOQ | <LOQ | 0.23 ± 0.03 | 0.39 ± 0.02 | 0.84 ± 0.05 | <LOQ |
| HS-31 | 125.96 ± 5.73 | 83.46 ± 1.59 | 104.16 ± 1.26 | 380.34 ± 2.75 | 28.31 ± 0.73 | <LOQ | 0.03 ± 0.01 | 5.30 ± 0.17 | 8.94 ± 0.15 | 0.14 ± 0.02 | 0.26 ± 0.05 | 3.21 ± 0.10 | 0.08 ± 0.02 | <LOQ | <LOQ | 0.11 ± 0.03 | 0.01 ± 0.01 | <LOQ |
| HS-32 | 77.13 ± 1.09 | 257.00 ± 2.23 | 97.34 ± 6.47 | 650.52 ± 4.37 | 15.15 ± 0.47 | 0.04 ± 0.01 | 0.11 ± 0.02 | 1.20 ± 0.07 | 4.13 ± 0.13 | 0.25 ± 0.03 | 0.17 ± 0.02 | 3.61 ± 0.16 | <LOQ | <LOQ | 0.03 ± 0.01 | 0.09 ± 0.03 | 0.10 ± 0.03 | <LOQ |
| HS-33 | 175.59 ± 4.47 | 193.90 ± 5.95 | 107.31 ± 11.56 | 962.06 ± 10.85 | 19.63 ± 0.77 | 0.01 ± 0.01 | 0.04 ± 0.01 | 6.06 ± 0.41 | 5.94 ± 0.17 | 0.25 ± 0.04 | 0.48 ± 0.06 | 4.01 ± 0.10 | 0.11 ± 0.03 | <LOQ | <LOQ | 0.19 ± 0.03 | 0.03 ± 0.01 | <LOQ |
| HS-34 | 163.66 ± 3.72 | 216.95 ± 5.22 | 83.25 ± 2.52 | 910.41 ± 7.81 | 18.22 ± 0.30 | 0.02 ± 0.01 | 0.05 ± 0.01 | 4.92 ± 0.05 | 6.74 ± 0.10 | 0.26 ± 0.03 | 0.45 ± 0.04 | 3.44 ± 0.12 | 0.12 ± 0.03 | <LOQ | <LOQ | 0.21 ± 0.04 | 0.03 ± 0.01 | <LOQ |
| HS-35 | 172.57 ± 12.38 | 231.01 ± 7.16 | 92.66 ± 4.10 | 830.81 ± 17.88 | 18.57 ± 0.18 | 0.01 ± 0.01 | 0.04 ± 0.01 | 5.42 ± 0.22 | 6.61 ± 0.14 | 0.23 ± 0.04 | 0.42 ± 0.04 | 2.52 ± 0.12 | 0.14 ± 0.03 | <LOQ | <LOQ | 0.18 ± 0.02 | 0.02 ± 0.01 | <LOQ |
| HS-36 | 114.40 ± 3.86 | 377.39 ± 6.11 | 67.97 ± 2.78 | 788.17 ± 4.05 | 13.86 ± 0.29 | <LOQ | 0.13 ± 0.02 | 4.56 ± 0.13 | 5.20 ± 0.06 | 0.20 ± 0.01 | 0.31 ± 0.03 | 1.80 ± 0.05 | 0.09 ± 0.01 | 0.03 ± 0.01 | 0.15 ± 0.03 | 0.44 ± 0.04 | 0.24 ± 0.04 | <LOQ |
| Regulatory limits | - | - | 25.00 a | - | 15.00 a | - | - | 5.00 a | - | - | - | 5.00 a | - | 0.01–0.5 a | 0.05 a | - | 0.10 b | - |
| Element | Honeys from the Coastal Area (n = 42) | Honeys from the Non-Coastal Area (n = 66) | p-Value |
|---|---|---|---|
| Ca | 275.45 ± 5.20 a | 247.63 ± 5.72 a | 0.205 |
| Cd | 0.17 ± 0.02 a | 0.26 ± 0.02 b | 0.000 |
| Co | 0.10 ± 0.01 a | 0.44 ± 0.04 a | 0.070 |
| Cr | 0.09 ± 0.01 a | 0.23 ± 0.02 b | 0.000 |
| Cu | 3.13 ± 0.11 a | 2.21 ± 0.10 b | 0.002 |
| Fe | 16.87 ± 0.41 a | 13.87 ± 0.57 b | 0.000 |
| K | 745.42 ± 6.85 a | 2948.13 ± 49.58 b | 0.000 |
| Mg | 120.37 ± 5.39 a | 99.84 ± 3.34 b | 0.000 |
| Mn | 4.67 ± 0.15 a | 2.63 ± 0.08 b | 0.000 |
| Na | 129.67 ± 3.74 a | 146.86 ± 5.29 b | 0.000 |
| Ni | 0.18 ± 0.02 a | 0.35 ± 0.03 b | 0.000 |
| Pb | 0.23 ± 0.01 a | 0.41 ± 0.03 b | 0.000 |
| Se | 0.25 ± 0.03 a | 0.28 ± 0.02 a | 0.496 |
| Sn | 0.12 ± 0.02 a | 0.07 ± 0.01 a | 0.079 |
| Ti | 0.33 ± 0.03 a | 0.43 ± 0.03 b | 0.041 |
| Zn | 5.62 ± 0.17 a | 6.01 ± 0.20 a | 0.076 |
| Element | Monofloral Honeys | Polyfloral Honeys (n = 33) | p-Value | ||
|---|---|---|---|---|---|
| Citrus (n = 15) | Nasturtium (n = 6) | Others (n = 54) | |||
| Ca | 297.57 ± 9.41 a | 239.51 ± 3.32 b | 295.43 ± 4.92 a | 257.79 ± 5.65 a,b | 0.184 |
| Cd | 0.36 ± 0.04 a | 0.03 ± 0.00 c,b | 0.08 ± 0.01 b,c | 0.36 ± 0.04 a | 0.271 |
| Co | 0.18 ± 0.02 a | 0.24 ± 0.01 a | 0.05 ± 0.01 b | 0.36 ± 0.04 a | 0.132 |
| Cr | 0.14 ± 0.02 a | 0.14 ± 0.02 a | 0.08 ± 0.01 c | 0.28 ± 0.03 b | 0.007 |
| Cu | 3.84 ± 0.15 a | 1.09 ± 0.06 d | 2.53 ± 0.09 c,b | 2.57 ± 0.07 b,c | 0.004 |
| Fe | 15.17 ± 0.19 a | 9.50 ± 0.23 b | 13.90 ± 0.25 a | 17.38 ± 0.94 a | 0.046 |
| K | 863.60 ± 9.39 a | 1068.52 ± 15.74 a,b | 694.88 ± 5.76 c | 3489.42 ± 55.27 b,a | 0.000 |
| Mg | 106.38 ± 3.14 a,b | 78.96 ± 8.58 a | 114.43 ± 4.14 a,b | 78.33 ± 4.02 a | 0.019 |
| Mn | 3.78 ± 0.09 a | 2.12 ± 0.04 a | 4.31 ± 0.19 a | 2.97 ± 0.09 a | 0.117 |
| Na | 155.13 ± 6.55 a | 61.33 ± 2.34 c | 94.25 ± 3.47 b | 254.66 ± 9.93 a | 0.000 |
| Ni | 0.25 ± 0.03 a | 0.28 ± 0.03 a | 0.21 ± 0.03 a | 0.39 ± 0.03 b | 0.001 |
| Pb | 0.47 ± 0.02 a | 0.14 ± 0.02 a | 0.19 ± 0.02 a | 0.40 ± 0.03 a | 0.083 |
| Se | 0.27 ± 0.03 a | 0.29 ± 0.03 a | 0.20 ± 0.02 b | 0.33 ± 0.02 a | 0.007 |
| Sn | 0.14 ± 0.02 a | 0.04 ± 0.01 a | 0.08 ± 0.02 a | 0.11 ± 0.02 a | 0.051 |
| Ti | 0.45 ± 0.03 a | 0.13 ± 0.01 c | 0.25 ± 0.03 b | 0.78 ± 0.04 a | 0.000 |
| Zn | 9.67 ± 0.25 a | 2.86 ± 0.10 d,b,c | 5.40 ± 0.24 c,b,d | 4.82 ± 0.19 b,c,d | 0.111 |
| Element | Coastal Honeys | Non-Coastal Honeys | ||||||
|---|---|---|---|---|---|---|---|---|
| North African Consumer | European Consumer | North African Consumer | European Consumer | |||||
| EDI (mg/d) | % RDA or AI | EDI (mg/d) | % RDA or AI | EDI (mg/d) | % RDA or AI | EDI (mg/d) | % RDA or AI | |
| Ca | 8.3 × 10−2 | 0.01 | 5.0 × 10−1 | 0.06 | 7.5 × 10−2 | 0.01 | 4.5 × 10−1 | 0.06 |
| Cr | 2.7 × 10−5 | 0.07 | 1.6 × 10−4 | 0.40 | 7.0 × 10−5 | 0.18 | 4.2 × 10−4 | 1.05 |
| Cu | 9.4 × 10−4 | 0.09 | 5.6 × 10−3 | 0.56 | 6.6 × 10−4 | 0.07 | 4.0 × 10−3 | 0.40 |
| Fe | 5.4 × 10−3 | 0.04 | 3.2 × 10−2 | 0.23 | 4.5 × 10−3 | 0.03 | 2.7 × 10−2 | 0.19 |
| K | 2.2 × 10−1 | 0.01 | 1.3 × 100 | 0.07 | 8.9 × 10−1 | 0.05 | 5.3 × 100 | 0.27 |
| Mg | 3.6 × 10−2 | 0.01 | 2.2 × 10−1 | 0.06 | 3.0 × 10−2 | 0.01 | 1.8 × 10−1 | 0.05 |
| Mn | 1.4 × 10−3 | 0.07 | 9.5 × 10−3 | 0.48 | 0.8 × 10−3 | 0.04 | 3.0 × 10−3 | 0.15 |
| Na | 3.9 × 10−2 | <0.01 | 2.3 × 10−1 | 0.01 | 4.8 × 10−2 | <0.01 | 2.6 × 10−1 | 0.01 |
| Se | 7.4 × 10−5 | 0.14 | 4.4 × 10−4 | 0.80 | 8.5 × 10−5 | 0.16 | 5.1 × 10−4 | 0.93 |
| Zn | 1.7 × 10−3 | 0.02 | 1.0 × 10−2 | 0.10 | 1.8 × 10−3 | 0.02 | 1.0 × 10−2 | 0.10 |
| Element | Coastal Honeys | Non-Coastal Honeys | ||||||
|---|---|---|---|---|---|---|---|---|
| North African Consumer | European Consumer | North African Consumer | European Consumer | |||||
| EDI (μg/kgb.w./d) | % TDI or TWI or BMDL01 or PTWI or UI | EDI (μg/kgb.w./d) | % TDI or TWI or BMDL01 or PTWI or UI | EDI (μg/kgb.w./d) | % TDI or TWI or BMDL01 or PTWI or UI | EDI (μg/kgb.w./d) | % TDI or TWI or BMDL01 or PTWI or UI | |
| As | 1.9 × 10−4 | 0.06 | 1.1 × 10−3 | 0.37 | 1.9 × 10−4 | 0.06 | 1.1 × 10−3 | 0.37 |
| Cd | 7.2 × 10−4 | 0.20 | 4.3 × 10−3 | 1.20 | 1.1 × 10−3 | 0.31 | 6.6 × 10−3 | 1.85 |
| Co | 4.5 × 10−4 | 0.20 | 2.7 × 10−3 | 1.18 | 1.9 × 10−3 | 0.83 | 1.1 × 10−2 | 4.81 |
| Cu | 1.3 × 10−2 | <0.01 | 8.0 × 10−2 | 0.02 | 9.5 × 10−3 | <0.01 | 5.7 × 10−2 | 0.01 |
| Hg | 1.9 × 10−4 | 0.03 | 1.1 × 10−3 | 0.19 | 8.6 × 10−5 | 0.02 | 5.1 × 10−4 | 0.09 |
| Mn | 2.0 × 10−2 | 0.01 | 1.2 × 10−1 | 0.03 | 1.1 × 10−2 | 0.01 | 6.8 × 10−2 | 0.02 |
| Ni | 8.0 × 10−4 | <0.01 | 4.8 × 10−3 | 0.02 | 1.5 × 10−3 | 0.01 | 9.0 × 10−3 | 0.04 |
| Pb | 9.6 × 10−4 | 0.19 | 5.8 × 10−3 | 1.16 | 1.8 × 10−3 | 0.36 | 1.5 × 10−3 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava, V.; Rechidi-Sidhoum, N.; Lo Turco, V.; Spanò, I.M.; Albergamo, A.; Benklaouz, M.B.; Benameur, Q.; Litrenta, F.; Potortì, A.G.; Di Bella, G. Safety and Toxicological Risk Assessment of Northern Algerian Honeys. Agriculture 2025, 15, 2421. https://doi.org/10.3390/agriculture15232421
Nava V, Rechidi-Sidhoum N, Lo Turco V, Spanò IM, Albergamo A, Benklaouz MB, Benameur Q, Litrenta F, Potortì AG, Di Bella G. Safety and Toxicological Risk Assessment of Northern Algerian Honeys. Agriculture. 2025; 15(23):2421. https://doi.org/10.3390/agriculture15232421
Chicago/Turabian StyleNava, Vincenzo, Nadra Rechidi-Sidhoum, Vincenzo Lo Turco, Irene Maria Spanò, Ambrogina Albergamo, Meki Boutaiba Benklaouz, Qada Benameur, Federica Litrenta, Angela Giorgia Potortì, and Giuseppa Di Bella. 2025. "Safety and Toxicological Risk Assessment of Northern Algerian Honeys" Agriculture 15, no. 23: 2421. https://doi.org/10.3390/agriculture15232421
APA StyleNava, V., Rechidi-Sidhoum, N., Lo Turco, V., Spanò, I. M., Albergamo, A., Benklaouz, M. B., Benameur, Q., Litrenta, F., Potortì, A. G., & Di Bella, G. (2025). Safety and Toxicological Risk Assessment of Northern Algerian Honeys. Agriculture, 15(23), 2421. https://doi.org/10.3390/agriculture15232421










