Abstract
Rice brown spot (BS) disease, caused by Bipolaris oryzae, is a significant threat to rice production worldwide. In this study, a major quantitative trait locus (QTL), qBS11, associated with resistance to BS in rice, was identified and fine-mapped. A recombinant inbred line (RIL) population from a cross between the susceptible variety Zhenshan97 and the resistant variety C309 was used for QTL mapping. Using composite interval mapping (CIM) and bulked segregant analysis sequencing (BSA-seq), qBS11 was narrowed to a 244.6 kb interval on chromosome 11, explaining up to 47.7% of the phenotypic variance. Fine mapping identified several potential candidate genes, including LOC_Os11g41170 and LOC_Os11g41210, encoding disease resistance proteins. The resistance exhibited by qBS11 was found to be partially dominant, with heterozygotes showing medium resistance. High broad-sense heritability (89.2%) confirmed the dominance of genetic factors in BS resistance. Additionally, regulatory region variations in the candidate genes suggest a gene dosage effect, which may explain the partial dominance observed for qBS11. This study provides valuable insights into the genetic basis of BS resistance and offers a foundation for breeding BS-resistant rice varieties through molecular marker-assisted selection (MAS). The findings also pave the way for future functional studies of the identified genes.
1. Introduction
Rice (Oryza sativa L.) is one of the world’s most important staple crops, and ensuring stable productivity is crucial for both national and global food security. Among the numerous fungal diseases affecting rice, brown spot (BS), is recognized as one of the major impediments to maximizing yield and quality []. Susceptible plants exhibit reduced chlorophyll and lignin content, decreased antioxidant enzyme activity, and damaged cellular structures, ultimately leading to leaf wilting [,]. Critically, grain infection can result in fragile, unfilled kernels, commonly known as shriveled grains, significantly affecting harvest quality [].
Traditionally, BS has been regarded as a secondary issue, primarily reflecting the increased susceptibility of rice plants under physiological stresses such as drought, nutrient imbalance, and silicon deficiency. As a result, BS was not considered a primary disease [,]. However, numerous field and laboratory studies have confirmed that BS is caused by the specific pathogenic fungus Bipolaris oryzae (anamorph of Cochliobolus miyabeanus), which exhibits clear infectious and epidemiological characteristics []. Multiple segregation and pathogenicity tests have shown that Bipolaris oryzae induces typical brown spot symptoms on various rice cultivars and wild rice species [], and it can be transmitted through multiple pathways, including seeds and leaves [,]. The pathogen exhibits high genetic diversity and pathogenic variability across different regions and rice varieties and undergoes both sexual and asexual reproduction, which enhances its adaptability and spread [,].
BS is exacerbated by environmental conditions such as high temperature, high humidity, water stress, and nutrient deficiencies, especially nitrogen (N) and potassium (K) []. These stresses mainly weaken plant resistance and facilitate pathogen infection. Current control methods are often integrated, involving chemical fungicides (frequently combined with blast disease control), seed disinfection, cultural practices, and the application of silicon, which enhances resistance [,,]. However, these methods are often insufficient or costly, underscoring the necessity of developing genetically resistant varieties.
Genetic research into BS resistance has focused on the identification of quantitative trait loci (QTLs). Resistance QTLs have been mapped using various genetic populations, including doubled haploid (DH) lines, recombinant inbred lines (RILs), and backcross inbred lines (BILs), with resistance loci primarily located on Chromosomes 1, 4, and 11 [,,,]. Additionally, genome-wide association studies (GWAS) using natural populations have also been an effective approach for mapping BS resistance loci []. Despite progress in mapping QTLs, the functional characterization and cloning of major resistance genes remain limited. Several genes, including OsSnRK1, OsFBN6, OsExo70F3, and OsBSR820, have been shown to enhance resistance to BS through overexpression [,,], but natural variation for these genes has not been fully exploited for breeding purposes. Additionally, loss-of-function lines for the Osmed30 gene displayed enhanced resistance to BS compared to their wild-type counterparts []. However, despite these efforts, only a few BS-associated genes have been mapped, and even fewer cloned genes are available for use in breeding.
Compared to the cloning and functional analysis of disease resistance genes for other pathogens, the depth and breadth of BS research remain limited. This study utilized a RIL population derived from the cross between a highly susceptible indica rice variety, Zhenshan97 (ZS97), and a resistant variety, C309. Preliminary QTL mapping using this RIL population in a disease-susceptible field over three years identified three QTLs, including a major QTL, qBS11, which explained up to 51.4% of the phenotypic variation. A BSA-mapping approach was employed to further analyze qBS11 using a backcross population (BC4F2), followed by fine mapping, ultimately localizing qBS11 to a 250 kb region. This study provides candidate genes and genetic resources for the cloning and breeding of BS resistance.
2. Materials and Methods
2.1. Plant Materials and Population Construction
The parental lines used in this study were the susceptible variety ZS97 and the resistant variety C309. A stable RIL population was developed by crossing ZS97 and C309 to produce the F1 generation, followed by continuous self-pollination to obtain the F2 generation. Three hundred individuals were randomly selected and advanced through the single-seed descent method up to the F8 generation, resulting in 209 genetically stable RIL families [].
For bulked segregant analysis sequencing (BSA-seq), 37 extreme-resistant and 37 extreme-susceptible plants were selected from 209 RIL lines to form two DNA pools for next-generation sequencing: the high-resistant pool (HR-pool) and the highly susceptible pool (HS-pool).
A whole-genome chromosome segment substitution lines (CSSLs) population was constructed with C309 as the donor parent and ZS97 as the recurrent parent. After backcrossing to the BC4F1 generation, a CSSL harboring the fragment of qBS11, identified through QTL mapping, was selected for self-pollination, resulting in the BC4F2 population consisting of 2000 individuals. This population was subsequently used for fine mapping of qBS11.
2.2. Pathogen Isolation and Verification
Rice leaves exhibiting typical brown spot symptoms were collected from naturally infected plants in the field. Approximately 0.2 cm2 tissue pieces were excised from the lesion margins, including both symptomatic and adjacent healthy tissues. The leaf segments were surface sterilized sequentially in 70% ethanol for 30 s, 1% mercuric chloride for 2 min, and rinsed five times with sterile distilled water. Sterilized tissues were dried on sterile filter paper and placed on potato dextrose agar (PDA) medium supplemented with 50 μg/mL streptomycin and 50 μg/mL penicillin to inhibit bacterial contamination. The plates were incubated at 28 °C for 3–5 days. Emerging fungal hyphae from the tissue segments were transferred onto fresh PDA plates and subcultured two to three times until pure cultures were obtained. Colonies exhibiting morphological characteristics typical of Bipolaris spp.—including dark olive to brown mycelia and elliptical, multi-septate conidia—were selected for further analysis. Pure fungal isolates were maintained on PDA slants at 4 °C for short-term storage and in sterile glycerol solution (25%, v/v) at –80 °C for long-term preservation.
2.3. Pathogenicity Verification
To confirm the pathogenicity of the isolated fungi, Koch’s postulates were applied. Fungal isolates identified as Bipolaris spp. were used for pathogenicity testing. The confirmed isolates were cultured on PDA plates for 3 days and then transferred to PDA liquid medium for incubation at 28 °C with shaking at 120 rpm for 2 days. After incubation, the fungal culture was homogenized for 5 min. The resulting spore suspension was uniformly sprayed onto the leaves of ZS97 and C309 plants at the tiller stage using a spray applicator. To maintain humidity, the ZS97 and C309 plants were covered with plastic wrap and incubated at 28 °C for 48 h. After the incubation period, the plants were further cultured at 28 °C for 3 days. Disease symptoms on the rice leaves were observed and recorded.
2.4. Molecular Identification and Sequencing
Genomic DNA was extracted from the mycelial isolates using the fungal genomic DNA extraction kit (DP317, TIANGEN, Beijing, China). The extracted DNA was used as a template for PCR amplification of the internal transcribed spacer (ITS) regions using the primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The PCR reaction mixture contained approximately 40–60 ng of DNA template, 1.5 μL of 10× buffer (Mg2+ plus), 0.75 μL of dNTPs (2 mM), 2.5 nM of each primer, and 0.5 U of rTaq polymerase (Takara Bio, Beijing, China), with deionized water added to a final reaction volume of 20 μL. The PCR program was as follows: initial denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s; with a final extension at 72 °C for 1 min. The PCR products were recovered for Sanger sequencing using the primers of ITS1 and ITS4. The resulting sequences were compared against the NCBI nucleotide database using BLASTn (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 9 November 2025) to confirm the taxonomic identity of the fungal isolates.
2.5. Field Phenotyping of RIL Population and Backcross Population
In the summers of 2022, 2023, and 2024, 209 RIL lines and their parental lines were planted in Danzhou, China (109.51° E, 19.51° N, altitude 130.6 m). The meteorological data for the entire growing period (from February to July) including monthly average temperature, monthly average humidity, and monthly average precipitation are listed in Table S1. Meteorological data are based on historical reanalysis datasets from the European Centre for Medium-Range Weather Forecasts (ECMWF)/National Aeronautics and Space Administration (NASA), provided by www.xihe-energy.com (accessed on 9 November 2025). Three different field plots were selected each year to serve as the three replicates, with the replicates assigned using a completely randomized block design. In each replicate, 5 plants from each line were planted, and 3 plants with consistent growth were randomly selected for phenotypic analysis. The average phenotype score of these selected plants was used as the final phenotypic value. Fungal inoculation was performed according to the method outlined in the Fungal Pathogenicity Validation section. During the tillering stage, a spore suspension of Bipolaris oryzae was uniformly sprayed onto the rice plant leaves. Artificial inoculation and natural infection were conducted in 2022, 2023, and 2024, and disease symptoms were subsequently observed and recorded. Disease severity was assessed using a scale, where Grade 1 indicates high resistance (HR), Grade 3 indicates medium resistance (MR), Grade 5 indicates medium susceptibility (MS), Grade 7 indicates susceptibility (S), and Grade 9 indicates high susceptibility (HS) (Table S2). The phenotyping and fungal inoculation of the backcross population (BC4F2) followed the same methods as used for the RILs, with the experiment conducted in the summer of 2025.
2.6. Heritability Calculation
The heritability was calculated using an REML (Restricted Maximum Likelihood) approach with a mixed-effects model (phenotype~(1|Genotype)) to estimate the variance components for Genotype () and Residual (). Broad-sense heritability () was calculated as , where is the median number of observations per genotype. Narrow-sense heritability () was roughly estimated as . Genotype × Environment (G × E) interactions were not considered in this model.
2.7. The DNA Library Construction, Sequencing, Genotyping and QTL Mapping
The next-generation sequencing and genotype analysis of the RIL population, including the construction of the bin map, have been previously reported in our earlier studies [].
The QTL mapping was carried out using the QTL.gCIMapping.GUI software (version 2.1.1) [], setting a genome scan step size of 1 cM and a significance threshold of logarithm of odds (LOD) ≥ 2.5. Data visualization was partially conducted using the ggplot2 package [].
2.8. BSA-Seq Analysis
To independently validate the location of the major QTL qBS11, DNA samples from two pools (HR-pool and HS-pool) were collected from the RILs. Equal amounts of genomic DNA from each individual within the pools were pooled for sequencing, with the goal of generating approximately 12 Gb of clean data (>30× coverage) per pool. Sequencing was performed by Biomarker Technologies Co., Ltd. (Beijing, China).
Fastp software (version 0.23.4) [] was used for adapter removal and quality filtering, with low-quality bases removed by calculating the average quality score using a 4-bp sliding window. Bases following any window with an average score below 15 were discarded and only reads longer than 50 bp were retained. The cleaned data were then aligned to a rice Nipponbare (IRGSP-1.0) [] using BWA (version 0.7.19) []. SAMtools (version 1.9) [] was used to convert the data and remove PCR duplicates. Variant calling, including the identification of SNPs and InDels, was performed with the Genome Analysis Toolkit (GATK) (version 4.2) []. A rigorous set of filtering criteria was applied to the raw VCF file to ensure that only high-quality, informative, and reliably phased SNPs were used for subsequent Bulk Segregant Analysis. Initially, a basic quality check was performed, retaining only bi-allelic SNPs with a quality score (QUAL) and mapping quality (MQ) exceeding 30. Furthermore, given the approximate 30× mean sequencing depth, stringent depth filtering was crucial to mitigate stochastic sampling error: SNPs were retained only if their read depth (DP) in both the HR-pool and the HS- pool was within the optimal range of 15× to 75×, which eliminates both extremely low-coverage sites and potential paralogs or repeat regions. Specifically, SNPs were discarded if they were nearly fixed in both pools simultaneously—defined as an SNP-index less than 0.1 in both pools or greater than 0.9 in both pools. The VCF variants from the two pools were analyzed using the DeepBSA method (version 1.4) []. Three algorithms, including △SNP-index, G′, and SmoothLOD, were employed for BSA-seq analysis. The threshold for identifying peaks of the ΔSNP-index, G′, and SmoothLOD was set using the “auto” parameter in the software.
2.9. Fine Mapping
Based on the results of QTL mapping and BSA-seq, the candidate region for qBS11 was initially defined between the InDel markers InD11-119 and InD11-161. Within this interval, 20 InDel markers, selected from a previous study [], were screened for polymorphisms using the parental lines. After polymorphism screening, 9 pairs of markers with clear polymorphisms were chosen for further analysis. For fine mapping, disease evaluation of the BC4F2 population was conducted using both artificial inoculation and natural infection. Genotyping of 192 BC4F2 progenies was performed using the markers InD11-119, IJ-InD11-320, InD11-148, InD11-156, and InD11-161. A genetic linkage map was subsequently constructed using MapMaker [], and by integrating phenotypic data, the location of qBS11 was confirmed using QTL IciMapping (version 3.1) []. These markers were subsequently used for genotyping the highly resistant and highly susceptible progenies of BC4F2 populations. Through recombination analysis in individual plants, the candidate interval for qBS11 was progressively narrowed down. Concurrently, phenotypic re-measured was conducted on the recombinant individuals. The primers used in this study are listed in Table S3. The complete experimental strategy used to identify and fine mapping of the target locus is illustrated in Figure S1.
3. Results
3.1. Pathogen Confirmation and Phenotypic Distribution
Through natural field infection, it was found that ZS97 and C309 exhibited different levels of resistance to brown spot disease. When grown in nutrient-deficient sandy soil, ZS97 showed a high susceptibility to brown spot disease, with more than 50% of the leaf area affected, which was classified as high susceptibility (HS, Grade 9). In contrast, C309 exhibited a disease-resistant phenotype, classified as high resistance (HR, Grade 1) (Figure 1A). The disease resistance grades of the two parental lines showed significant differences based on Student’s t-tests (p < 0.01). Additionally, the hull of ZS97 was also observed to display a susceptible phenotype (Figure 1B).
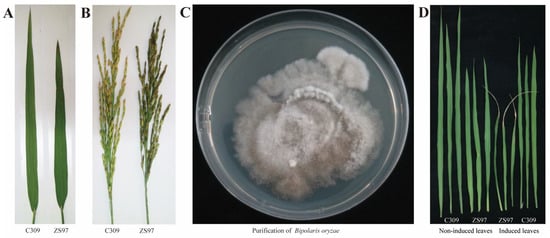
Figure 1.
Phenotypic characterization of the parental lines. (A) Comparison of susceptibility to BS disease in parental leaf samples. (B) Comparison of susceptibility to BS disease in parental panicle samples. (C) Morphological characteristics of Bipolaris oryzae. (D) Phenotypic analysis of BS disease after fungal inoculation in parental lines.
The isolated Bipolaris spp. colonies were cultured on PDA plates. Initially, the fungal mycelium appeared white and fluffy. After 4 days of incubation, the colony color gradually changed to dark gray, and the reverse side of the agar turned from pale yellow to black, with the colony growing closely and spreading over the medium. Continued incubation led to the formation of brown droplets on the surface of the colony, accompanied by the growth of numerous sclerotia (Figure 1C). The fungal mycelium was then cultured in liquid medium, and pathogenicity was tested according to Koch’s postulates. The confirmed isolates were inoculated onto the leaves of ZS97 and C309 rice plants at the tillering stage. After 7 days, rice leaves exhibited disease symptoms similar to those observed in naturally infected plants in the field, with brown lesions and yellow halos surrounding the lesions (Figure 1D). The severity of the disease was more pronounced, with leaf margins showing signs of necrosis, indicating that the inoculum may have been applied at a higher concentration than in natural field infections.
The PCR-amplified ITS sequence was subsequently subjected to Sanger sequencing, and the resulting sequences were compared against the NCBI database using BLASTn. The highest sequence similarity was observed with Bipolaris oryzae, a pathogen known to infect rice, with a sequence identity of 99%. These results confirm that the isolated fungal strain is responsible for brown spot disease in rice.
3.2. Phenotypic Analysis of the RIL Population
Following artificial inoculation with Bipolaris oryzae, the disease phenotype of the RIL population was evaluated. The phenotypic distribution of the BS resistance in the RIL population over three years exhibited a skewed normal distribution (Figure S2), suggesting the involvement of both genetic and environmental factors in shaping the observed phenotypic variation.
The broad-sense heritability () of BS was calculated based on three replicates across three years (2022, 2023, and 2024). The heritability estimates for BS were consistently high across all three years, with values of 89.8% in 2022, 82.4% in 2023, and 89.2% in 2024. These high heritability values suggest that genetic factors play a dominant role in the phenotypic variation of BS, indicating that the trait is largely controlled by genetic components. The narrow-sense heritability () of BS was calculated across three years (2022, 2023, and 2024), with the following estimates: 44.9% in 2022, 41.2% in 2023, and 44.6% in 2024. These moderate heritability values suggest that while genetic factors do play a significant role in the expression of BS, there is also a considerable influence from environmental factors and non-additive genetic effects, such as dominance and epistasis.
3.3. Initial QTL Mapping of BS via GCIM
The phenotypic frequency distribution histograms over the three years indicate that brown BS behaves as a continuous trait (Figure S2), which is controlled by QTLs. Therefore, based on the phenotypic and genotypic data, QTL mapping was conducted to identify loci associated with BS resistance.
Through QTL mapping across three years, three loci responsible for controlling BS were identified. These loci, designated as qBS3, qBS7, and qBS11 (Figure 2 and Table 1), were mapped as follows: qBS3 was positioned on chromosome 3 at 1.69 cM, with an additive effect of −0.46, explaining 5.1% of the phenotypic variance (PVE). qBS7, located on chromosome 7 at 9.74 cM, exhibited an additive effect of −0.38, explaining 3.6% of the variance. The qBS11 locus, identified on chromosome 11 (23.7–23.8 Mb), showed the most substantial effect, accounting for between 36.0% and 47.7% of the variance. Importantly, qBS11 demonstrated the highest stability, being detected in all three years under varying environmental conditions. These findings indicate that resistance to BS in the RIL population is primarily governed by a major-effect locus, qBS11. Although several additional QTLs were detected, their individual effects were relatively minor, suggesting that qBS11 plays a predominant role in conferring BS resistance, while the minor QTLs may contribute small additive effects under specific environmental conditions.
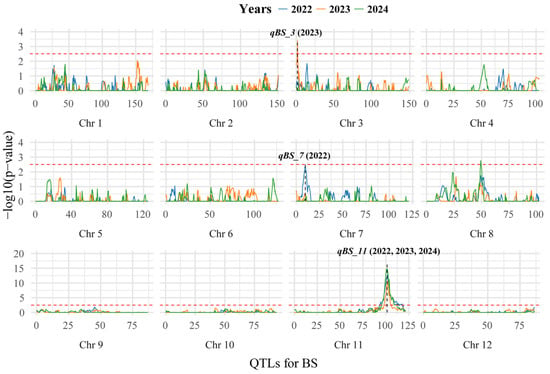
Figure 2.
Mapping QTLs for BS by genome-wide CIM using the RILs. The dashed red line represents the threshold for significance (−log10 (p-value) = 2.5). The labels indicate the positions of significant QTLs.

Table 1.
QTL for BS resistance.
3.4. BSA-Seq for BS
The average alignment rate to the reference genome across both the H-pools and L-pools was 96.5% and 97.4%, respectively, with an average sequencing depth of 33.0-fold and 31.4-fold, respectively. The VCF variants obtained from GATK processing of the HR-pool and HS-pool were analyzed using the DeepBSA method [], employing three algorithms: ∆SNP-index, G′, and SmoothLOD. All three algorithms consistently revealed a distinct and prominent major peak on chromosome 11 (Figure 3), indicating the presence of a novel major QTL associated with resistance to BS.
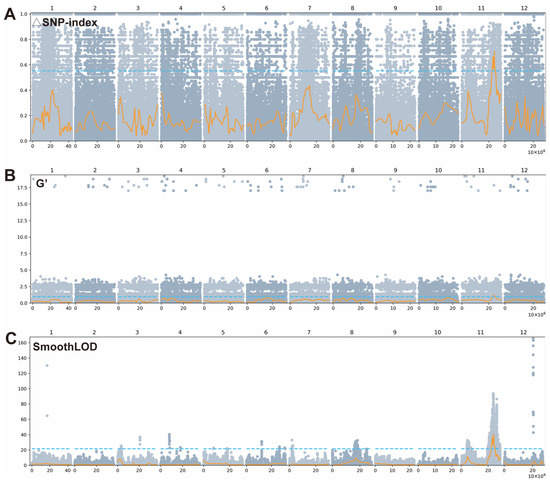
Figure 3.
The BSA results were analyzed using △SNP-index (A), G′ (B), and SmoothLOD (C) algorithms from DeepBSA. Each gray dot represents a SNP, the yellow line indicates the LOESS fit of all points, and the blue line represents the default threshold.
According to the △SNP-index algorithms, the candidate interval was located between 22.6 Mb and 24.8 Mb (Figure 3A and Table S4), with a peak at 24.2 Mb and a maximum ΔSNP-index value of 0.7. The G′ algorithms analysis refined this region to 22.9–24.4 Mb (Figure 3B and Table S4), with a peak position at 23.7 Mb and a G′ value of 1.1. Similarly, the SmoothLOD algorithms detected a consistent signal within 22.3–25.3 Mb (Figure 3C and Table S4), peaking at 24.2 Mb with a maximum LOD score of 40.5.
All three algorithms consistently identified a candidate gene region on chromosome 11 (22.3–25.3 Mb), corresponding to the major QTL qBS11 that controls brown spot disease resistance in this population. Notably, this genomic interval overlaps precisely with the QTL qBS11 previously identified through QTL mapping, confirming that the locus detected by BSA-seq represents the same qBS11. This convergence between linkage analysis and bulked segregant analysis provides strong support that qBS11 is the major and stable QTL responsible for conferring resistance to BS in rice.
In addition, consistent results were obtained through other BSA methods [], which further confirmed the identification of the major QTL qBS11 on chromosome 11 within the 22.7–24.8 Mb region (Figure S3). These findings reinforce the robustness of our analysis and highlight the pivotal role of qBS11 in conferring resistance to brown spot disease in this population.
3.5. Fine Mapping of qBS11
Based on previous QTL mapping and BSA-seq results, the qBS11 interval is located between the InDel markers InD11-119 and InD11-161. Therefore, using these two InDel markers, a heterozygous CSSL (BC4F1) from this region was selected for further fine mapping to minimize the influence of other QTLs on the results. For the fungal inoculation test in the BC4F2 population, the plant of heterozygous genotype (ZS97qBS11qbs11) exhibited medium BS resistance (MR, Grade 5) between the two homozygous genotypes (ZS97qbs11qbs11 and ZS97qBS11qBS11), indicating that qBS11 may be a partial dominant gene. For the disease resistance survey of 2000 plants, 455 plants exhibited high resistance (Grade 1), 1020 plants showed medium resistance (Grades 3, 5, and 7), and 525 plants displayed high susceptibility (Grade 9) ( = 5.70 < = 5.99, p = 0.58), thus confirming the partial dominant nature of qBS11.
A genetic linkage map was constructed for 192 BC4F2 progenies using five InDel markers. By integrating the phenotypic data, the major QTL qBS11 was mapped between InD11-148 and InD11-156, with a genetic distance of 5.5 cm (Figure 4A).
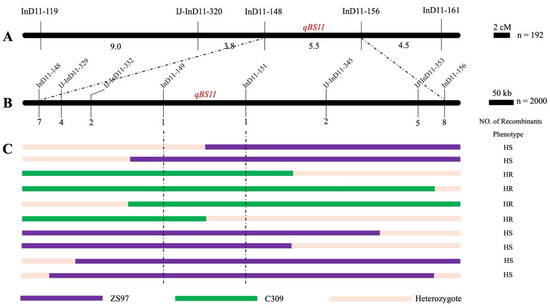
Figure 4.
Identification of candidate genomic region. (A) The genetic linkage map constructed by 5 InDel markers. The numbers below the map indicate the genetic distance (cM) between two markers. (B) Fine mapping of the qBS11. The numbers below the fine mapping diagram indicate the number of recombinant individuals. (C) Several representative recombinant individuals. The small black box represents the scale bar.
Since qBS11 is a partial dominant gene, identifying extreme resistance (HR) and extreme susceptibility (HS) phenotypic individuals’ genotypes were crucial for completing the fine mapping analysis. Subsequently, genotyping of 980 extreme individuals was performed using the markers InD11-148, InD11-156, and their flanking markers IJ-InD11-329, IJ-InD11-332, InD11-149, InD11-151, IJ-InD11-345, and IJ-InD11-353. A total of 30 recombinant individuals (defined by a crossover event between the flanking markers) were identified (Figure 4B). Based on the genotype and phenotype, qBS11 was finally mapped to a 244.6 kb interval between InD11-149 and InD11-151 (Figure 4C).
3.6. Candidate Gene Analysis Within the qBS11 Fine-Mapped Interval
Within the fine-mapped interval of qBS11, spanning approximately 244.6 kb, a total of 26 predicted genes were identified (Table S5). Among them, several genes encode proteins with putative functions related to disease resistance, signal transduction, and stress response. Notably, LOC_Os11g41170 and LOC_Os11g41210, both annotated as disease resistance protein RPM1, putative, belong to the nucleotide-binding site–leucine-rich repeat (NBS-LRR) family, which is commonly involved in pathogen recognition and defense signal activation. In addition, LOC_Os11g41140, encoding a C3HC4-type zinc finger domain-containing protein, and LOC_Os11g41150, annotated as a nitrilase-associated protein, may participate in transcriptional regulation and secondary metabolism associated with stress responses.
Genes such as LOC_Os11g41130 (vacuolar protein sorting-associated protein 26) and LOC_Os11g41160 (phosphoserine phosphatase, chloroplast precursor) may be involved in intracellular transport or primary metabolic pathways that indirectly affect defense-related signaling. Furthermore, multiple transposon and retrotransposon proteins (e.g., LOC_Os11g41050, LOC_Os11g41110, LOC_Os11g41190, LOC_Os11g41270, and LOC_Os11g41280) were detected in this region.
Based on the results from BSA-seq analysis, the variations in the parental lines ZS97 and C309 were further examined (Table S6). It was found that the genes LOC_Os11g41100, LOC_Os11g41130, LOC_Os11g41140, LOC_Os11g41160, LOC_Os11g41170, LOC_Os11g41180, LOC_Os11g41190, LOC_Os11g41210, LOC_Os11g41260, and LOC_Os11g41290 exhibited variations, including synonymous_variant, missense_variant, downstream_gene_variant, upstream_gene_variant, 5_prime_UTR_variant, 3_prime_UTR_variant, frameshift_variant, and disruptive_inframe_deletion. Upon further functional annotation and analysis, it was found that LOC_Os11g41170 and LOC_Os11g41210, both encoding disease resistance proteins RPM1, were strongly associated with BS resistance. Therefore, LOC_Os11g41170 and LOC_Os11g41210 are the most likely candidate genes for further validation and functional analysis.
4. Discussion
4.1. The Critical Role of Genetic Resistance in Combating BS
This study successfully identified and fine-mapped qBS11, a major QTL conferring resistance to rice BS, utilizing a RIL population derived from a cross between the highly resistant variety C309 and the susceptible variety ZS97. This discovery provides a valuable genetic resource for rice resistance breeding while offering profound insight into the complex quantitative genetic basis of this trait.
BS, caused by the phytopathogenic fungus Bipolaris oryzae has traditionally been viewed as a secondary symptom primarily reflecting physiological stress [,], such as nutrient deficiencies (e.g., nitrogen, potassium, silicon) or environmental stress []. However, by rigorously fulfilling Koch’s postulates and confirming the isolated strain’s identity through ITS sequencing and BLASTn comparison (99% sequence identity), this study unequivocally establishes BS as a primary fungal disease with clear infectious and epidemiological characteristics.
The parental line ZS97 exhibited extreme susceptibility (HS, Grade 9), with over 50% of the leaf area affected, and hull susceptibility, when grown under the sandy, nutrient-poor, tropical conditions typical of the Hainan region. This phenomenon highlights the synergistic relationship between environmental stress and pathogen virulence, where adverse conditions (nutrient depletion, high temperature, and humidity) significantly compromise the plant’s innate defenses, thus facilitating Bipolaris oryzae invasion and propagation. Given the relatively high optimal growth temperature of Bipolaris oryzae [], the frequency and severity of BS outbreaks are projected to increase globally under climate change scenarios. Consequently, the development and deployment of stable, highly effective genetic resistance genes, such as qBS11, represent a critical strategy to ensure stable rice yields and global food security, offering a superior alternative to costly and often non-durable chemical or agronomic interventions.
Phenotypic analysis of the RIL population revealed a skewed normal, continuous distribution of BS resistance, characteristic of a polygenic trait governed by multiple QTLs. The trait exhibited consistently high broad-sense heritability ), ranging from 82.4% to 89.8% across three years of replicated trials. Such high values strongly affirm the dominant role of genetic factors in controlling phenotypic variation for BS resistance, providing a solid foundation for successful marker-assisted selection (MAS).
Conversely, the narrow-sense heritability () estimates were moderate, ranging from 41.2% to 44.9%. The significant disparity between and implies that non-additive genetic effects (such as dominance or epistasis) and genotype-by-environment (G × E) interactions contribute substantially to the observed phenotypic variation. This complex genetic architecture necessitates breeding efforts to prioritize major-effect QTLs with robust stability, ensuring reliable resistance expression across diverse environmental conditions. Minor QTLs, such as the transiently detected qBS3 and qBS7, while contributing small additive effects (PVE of 5.1% and 3.6%, respectively), lack the stability required for effective resistance under complex G × E scenarios. Thus, focusing research resources on qBS11, which consistently explains 36.0% to 47.7% of the phenotypic variance, is a logical and necessary choice for maximizing breeding efficiency from a quantitative genetic perspective.
4.2. Fine Mapping, Inheritance, and Molecular Identification of the Major Locus qBS11
In this study, a major QTL, qBS11 was successfully identified and fine-mapped, associated with resistance to BS in rice. Through a combination of QTL mapping and BSA-seq, the candidate region for qBS11 was narrowed on chromosome 11 to a 244.6 kb interval, located between the markers InD11-149 and InD11-151. This QTL exhibited consistent resistance across three years of field testing, further confirmed by phenotypic data, and validated using molecular markers. Our results are consistent with the previous findings, and we have further narrowed the interval, which is beneficial for gene cloning.
Adair [] suggested that resistance was recessive, involving several genes, while later studies, such as those by Balal et al. [], identified two dominant genes associated with resistance and one with susceptibility. Goel et al. [] further hypothesized that resistance involved additive, dominant, and gene interactions. In our study, the resistance conferred by qBS11 appears to be partial dominant, as evidenced by the intermediate resistance phenotype (Medium Resistance, Grade 5) in the heterozygous genotype (ZS97qBS11qbs11), compared to the two homozygous genotypes (ZS97qbs11qbs11 and ZS97qBS11qBS11), aligning with these earlier findings. The broad-sense heritability estimates for BS resistance were high (89.2% in 2024), suggesting that the phenotypic variation is largely controlled by genetic factors, which supports the notion that qBS11 plays a major role in BS resistance. In fact, many genes associated with rice blast disease resistance, as well as bacterial blight resistance genes, exhibit partial dominance [,].
Through a fine mapping approach involving the identification of extreme resistant (HR) and extreme susceptible (HS) phenotypes, we were able to pinpoint the qBS11 locus to a 244.6 kb region on chromosome 11, which contained 26 predicted genes. Notably, genes such as LOC_Os11g41170 and LOC_Os11g41210, both encoding disease resistance protein RPM1-like proteins, were located within this interval. These genes are part of the nucleotide-binding site–leucine-rich repeat (NBS-LRR) family, known for their role in pathogen recognition and activation of defense signaling [,,]. RPM1 is a resistance protein that belongs to the CC-NB-LRR class, distinguished by its coiled-coil (CC) domain instead of the typical TIR domain found in other NBS-LRR proteins []. The transfer of the Arabidopsis NLR gene RPM1 (D505V) into rice enhanced broad-spectrum resistance to various pathogens, including fungal pathogen Magnaporthe oryzae, bacterial pathogen Xanthomonas oryzae pv. oryzae (Xoo), and pest brown planthopper (BPH, Nilaparvata lugens Stål) []. The identification of multiple missense mutations in these genes, as well as variations in their UTR regions, suggests that these genes are potential candidates for conferring BS resistance.
These regulatory region variations, particularly the multiple UTR mutations in the candidate genes LOC_Os11g41170 and LOC_Os11g41210 (Table S6), likely contribute to the transcriptional changes in the C309 allele. These changes could lead to higher basal or induced transcription levels, which in turn could increase the protein accumulation. This gene dosage effect suggests that in the heterozygote, the single functional C309 resistance allele produces a concentration of the R-protein below the threshold required for maximum defense activation, resulting in medium resistance (Grade 5). Conversely, only in the homozygous state (qBS11qBS11), when the R-protein reaches the necessary threshold concentration, can full defense activation and maximal resistance (Grade 1) be achieved. This dose-dependent R-gene activation mechanism offers a plausible explanation for qBS11’s partial dominance, differentiating its mode of action from that of recessive resistance genes, which often involve the loss of a host susceptibility factor [].
4.3. Physical Co-Localization and Allelic Hypotheses
qBS11’s precise location falls within the 22.3–25.3 Mb region of Chromosome 11, which is a known hotspot for BS resistance QTLs [,,], including loci previously reported from diverse geographical origins. For instance, the celebrated BS resistance gene bsr1 (or qBSfR11), derived from the variety ‘Tadukan,’ has been fine-mapped to a highly overlapping interval []. Furthermore, the QTL qBSR11-kn, originating from ‘CH45,’ covers a similar range of 19.1–25.1 Mb [].
Despite this physical co-localization, a fundamental difference exists in the mode of inheritance: qBS11 exhibits partial dominance, whereas bsr1 is reported to be a recessive gene [], additionally, the authors hypothesize that qBS11-kn and bsr1 may be the same gene []. This contradiction strongly suggests that qBS11 and bsr1/qBS11-kn are not the same gene.
4.4. Molecular Breeding Prospects and Functional Analysis Outlook
The successful fine-mapping and molecular hypotheses proposed herein hold significant basic scientific value and provide immediately deployable tools and clear future directions for BS resistance breeding.
The fine-mapping effort yielded high-quality InDel molecular markers (e.g., InD11-149 and InD11-151) that are tightly linked to qBS11. The extremely short physical distance (<244.6 kb) between these markers and the target locus significantly minimizes the probability of recombination. The linkage fidelity is a key determinant of marker reliability in MAS. These highly reliable InDel markers, characterized by high polymorphism and co-dominance, are ideally suited for implementation in high-throughput screening platforms. They allow breeders to accurately and efficiently screen for the desired homozygous resistant plants (qBS11qBS11) in early generations (e.g., F2 or BC generations), thereby accelerating the introgression of the superior C309 resistance allele into elite, high-yielding susceptible backgrounds. The findings from this work not only contribute to the molecular understanding of rice-pathogen interactions but also offer valuable genetic resources for breeding BS-resistant rice varieties through MAS.
Given the high genetic diversity, rapid variability, and capacity for sexual reproduction in Bipolaris oryzae, reliance on a single resistance gene often leads to a lack of durability. To ensure long-lasting and broad-spectrum resistance, the strategy of gene pyramiding, which involves co-selecting and assembling multiple R-genes with distinct mechanisms or recognition specificities, is essential [].
The partial dominance of qBS11 offers significant advantages in breeding, particularly in hybrid breeding and gene pyramiding for durable disease resistance. Unlike recessive genes, which require both parents to carry the recessive allele to express resistance, qBS11 allows disease resistance to be expressed in heterozygous hybrids, thereby reducing breeding time and costs. This makes it a more efficient option for hybrid breeding, as it eliminates the need to introduce the gene into both parents. Furthermore, its partial dominance results in stronger resistance in the homozygous state, providing breeders with the flexibility to create varieties with multiple disease resistance traits through gene pyramiding, thus enhancing the durability of resistance. Overall, qBS11 offers higher breeding value, benefiting both conventional and hybrid breeding strategies.
qBS11, as a major-effect locus, should be strategically pyramided with the minor QTLs identified in this study (qBS3 and qBS7) and other known resistance loci (e.g., qBSR6-kt on Chromosome 6 []). This layered defense strategy ensures that even if the pathogen overcomes the major recognition conferred by qBS11, the complementary, quantitative resistance provided by the minor QTLs will continue to suppress disease severity, thereby extending the effective lifespan of the resistant cultivar.
The critical next step is to leverage the 244.6 kb fine-mapped interval to ultimately isolate the causal gene for qBS11 through map-based cloning. Our in silico analysis of the two primary candidates in this region has provided a powerful lead. While LOC_Os11g41210 lacks obvious functional lesions, all three missense mutations in the susceptible allele of LOC_Os11g41170 were found to be clustered within a highly conserved N-terminal Winged Helix (WH) domain, which is a classic DNA-binding motif []. The WH is a DNA-binding motif characterized by an H1-S1-H2-H3-S2-W1-S3-W2 arrangement [], which employs a DNA-recognition helix for sequence-specific contacts in the major groove and small β-sheet “wings” (W1, W2) to interact with the minor groove or backbone. This versatile domain, which may also mediate protein–protein interactions, is found in a wide array of proteins with diverse biological functions, including transcriptional repressors [], transcription factors [,], helicases [,], endonucleases [], histones, and transposases [].
This finding strongly prioritizes LOC_Os11g41170 for rigorous functional validation. This process requires functional complementation experiments (introducing the C309 resistant allele into the susceptible ZS97 background to restore resistance) and targeted loss-of-function studies using overexpression and gene editing technologies (CRISPR/Cas9) to definitively confirm it as the actual qBS11. Additionally, gene expression analysis will be an essential step to further validate these candidates. Although we have not yet conducted such analysis, differential gene expression studies using RNA-Seq or reverse-transcription quantitative PCR (RT-qPCR) will help confirm the functional roles of the candidate gene.
Furthermore, the discovery of the WH domain provides a specific, testable hypothesis for its mechanism. The WH domain is a classic DNA-binding motif, suggesting LOC_Os11g41170 may function as a transcriptional regulator. Therefore, structural biology and interaction analyses are urgently needed to validate how the missense mutations within this WH domain impact its function—whether by disrupting its ability to bind target DNA or by impeding its recognition of Bipolaris oryzae effectors. Finally, the separate dosage effect hypothesis, derived from the regulatory region variants, must also be quantitatively verified as a potential molecular basis for qBS11’s partial dominance.
5. Conclusions
In this study, the major QTL qBS11 associated with BS resistance in rice was successfully identified and fine-mapped. Through QTL mapping and BSA-seq, qBS11 was localized to a 244.6 kb region on chromosome 11, which accounts for a substantial portion of the phenotypic variation in resistance. Several candidate genes within this interval were identified, including disease resistance proteins, which are strongly associated with brown spot resistance. A robust genetic target for further map-based cloning and the development of molecular markers for MAS in rice breeding programs aimed at improving resistance to BS was provided. The understanding of the genetic mechanisms underlying disease resistance was enhanced, and the groundwork for breeding disease-resistant rice varieties was laid.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture15232417/s1: Figure S1: Flowchart illustrating the complete experimental pipeline.; Figure S2: Histogram plots of BS resistance traits in three years.; Figure S3: Identification of the major QTL qBS11 associated with BS through another BSA-seq analysis method; Table S1: Meteorological data for the growing period (February to July); Table S2: Standardized evaluation system for rice BS resistance; Table S3: InDel markers used in this study. Table S4: Results of three BSA algorithms. Table S5: Genes within the qBS11 Interval. Table S6: Gene variations identified within the candidate interval of qBS11.
Author Contributions
Conceptualization, W.H. and Z.X.; methodology, Q.L.; software, W.H.; validation, Q.L., Y.Z. and W.H.; formal analysis, W.H.; investigation, Y.Z. and Y.L.; resources, Q.L.; data curation, W.H.; writing—original draft preparation, Q.L., Y.Z. and W.H.; writing—review and editing, Q.L., Y.Z., W.H. and Z.X.; visualization, W.H.; supervision, Z.X.; project administration, W.H.; funding acquisition, W.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No. 1630032021015), and Natural Scientific Foundation of Hainan (Nos. 323RC532 and NHXXRCXM202326).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the main text and Supplementary Materials. The other data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BS | brown spot |
| QTL | quantitative trait locus |
| RIL | recombinant inbred line |
| CIM | composite interval mapping |
| BSA-seq | bulked segregant analysis sequencing |
| MAS | marker-assisted selection |
| N | nitrogen |
| K | potassium |
| DH | doubled haploid |
| BILs | backcross inbred lines |
| GWAS | genome-wide association studies |
| ZS97 | Zhenshan 97 |
| HR-pool | high-resistant pool |
| HS-pool | highly susceptible pool |
| CSSLs | chromosome segment substitution lines |
| PDA | potato dextrose agar |
| ITS | internal transcribed spacer |
| HR | high resistance |
| MR | medium resistance |
| MS | medium susceptibility |
| S | susceptibility |
| HS | high susceptibility |
| LOD | logarithm of odds |
| H2 | broad-sense heritability |
| h2 | narrow-sense heritability |
| PVE | phenotypic variance |
| NBS-LRR | nucleotide-binding site–leucine-rich repeat |
| G × E | genotype-by-environment |
| CC | coiled-coil |
References
- Barnwal, M.K.; Kotasthane, A.; Magculia, N.; Mukherjee, P.K.; Savary, S.; Sharma, A.K.; Singh, H.B.; Singh, U.; Sparks, A.; Variar, M. A review on crop losses, epidemiology and disease management of rice brown spot to identify research priorities and knowledge gaps. Eur. J. Plant Pathol. 2013, 136, 443–457. [Google Scholar] [CrossRef]
- Chhabra, R.; Sharma, R.; Hunjan, M.S.; Sharma, V.K.; Sharma, P.; Chauhan, S.K. Microstructural and metabolic variations induced by Bipolaris oryzae inciting brown spot disease of rice. Cereal Res. Commun. 2023, 51, 953–968. [Google Scholar] [CrossRef]
- Ashfaq, B.; Arshad, H.M.I.; Atiq, M.; Yousaf, S.; Saleem, K.; Arshad, A. Biochemical profiling of resistant phenotypes against bipolaris oryzae causing brown spot disease in rice. Front. Agron. 2021, 3, 675895. [Google Scholar] [CrossRef]
- Sunder, S.; Singh, R.; Agarwal, R. Brown spot of rice: An overview. Indian Phytopathol. 2014, 67, 201–215. [Google Scholar]
- Kaboré, K.H.; Kassankogno, A.I.; Tharreau, D. Brown spot of rice: Worldwide disease impact, phenotypic and genetic diversity of the causal pathogen Bipolaris oryzae, and management of the disease. Plant Pathol. 2025, 74, 908–922. [Google Scholar] [CrossRef]
- Liu, Y.L.; Tang, J.R.; Li, Y.; Zhou, H.K. First report of Bipolaris oryzae causing leaf spot on cultivated wild rice (Oryza rufipogon) in China. Plant Dis. 2021, 105, 1857. [Google Scholar] [CrossRef]
- M Bekheet, S.M.; El-Bebany, A.F.; Aboshosha, S.S.; Saleh, M.M.; M Shams, A.H. Pathogenicity evaluation of Bipolaris oryzae isolates on Egyptian rice cultivars. Alex. Sci. Exch. J. 2021, 42, 609–617. [Google Scholar] [CrossRef]
- Kabore, K.H.; Kassankogno, A.I.; Adreit, H.; Milazzo, J.; Guillou, S.; Blondin, L.; Chopin, L.; Ravel, S.; Charriat, F.; Barro, M. Genetic diversity and structure of Bipolaris oryzae and Exserohilum rostratum populations causing brown spot of rice in Burkina Faso based on genotyping-by-sequencing. Front. Plant Sci. 2022, 13, 1022348. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Castell-Miller, C.; Javan-Nikkhah, M.; Naghavi, M.R.; Dehkaei, F.P.; Leng, Y.; Puri, K.; Zhong, S. Population structure, genetic diversity, and sexual state of the rice brown spot pathogen Bipolaris oryzae from three Asian countries. Plant Pathol. 2018, 67, 181–192. [Google Scholar] [CrossRef]
- Carvalho, M.P.; Rodrigues, F.A.; Silveira, P.R.; Andrade, C.C.L.; Baroni, J.C.P.; Paye, H.S.; Loureiro Junior, J.E. Rice resistance to brown spot mediated by nitrogen and potassium. J. Phytopathol. 2010, 158, 160–166. [Google Scholar] [CrossRef]
- Nargave, S.; Gehlot, J.; Buri, R.; Jakhar, M.; Damor, J.S.; Jain, S. Biological and chemical management strategy to control brown spot disease in rice caused by Bipolaris oryzae. Int. J. Plant Soil Sci. 2023, 35, 531–537. [Google Scholar] [CrossRef]
- Balgude, Y.S.; Gaikwad, A.P.; Tirmali, A.M. Integrated management of sheath rot and brown spot of paddy. IJCS 2020, 8, 1354–1359. [Google Scholar] [CrossRef]
- Fortunato, A.A.; Rodrigues, F.A.; Datnoff, L.E. Silicon control of soil-borne and seed-borne diseases. In Silicon and Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2015; pp. 53–66. [Google Scholar]
- Matsumoto, K.; Ota, Y.; Seta, S.; Nakayama, Y.; Ohno, T.; Mizobuchi, R.; Sato, H. Identification of QTLs for rice brown spot resistance in backcross inbred lines derived from a cross between Koshihikari and CH45. Breed. Sci. 2017, 67, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Katara, J.; Sonah, H.; Deshmukh, R.; Chaurasia, R.; Kotasthane, A. Molecular analysis of QTLs associated with resistance to brown spot in rice (Oryza sativa L.). Indian J. Genet. Plant Breed. 2010, 70, 17–21. [Google Scholar]
- Ota, Y.; Matsumoto, K.; Honda, Y.; Ohashi, S.; Nakamura, D.; Mizobuchi, R.; Sato, H. Identification of QTLs for brown spot resistance in rice. Breed. Sci. 2025, 75, 325–333. [Google Scholar] [CrossRef]
- Cao, F.-Y.; Lee, G.-H.; Zeng, Y.; Lee, A.-R.; Park, S.-Y.; Jang, S.-G.; Cho, L.-H.; Kim, S.T.; Lee, J.; Kwon, S.-W. Genome-wide identification and functional characterization of brown spot resistance genes in rice (Oryza sativa L.). J. Agric. Food Chem. 2025, 73, 14089–14098. [Google Scholar] [CrossRef]
- Filipe, O.; De Vleesschauwer, D.; Haeck, A.; Demeestere, K.; Höfte, M. The energy sensor OsSnRK1a confers broad-spectrum disease resistance in rice. Sci. Rep. 2018, 8, 3864. [Google Scholar] [CrossRef]
- Cao, F.-Y.; Zeng, Y.; Lee, A.-R.; Kim, B.; Lee, D.; Kim, S.-T.; Kwon, S.-W. OsFBN6 enhances brown spot disease resistance in rice. Plants 2024, 13, 3302. [Google Scholar] [CrossRef]
- Lin, Q.; Gan, P.; Zhou, Y.; Lin, Y.; Xie, Z.; Hu, W. Identification of QTLs for internode length and diameter associated with lodging resistance in rice. bioRxiv 2025. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, Y.; Dunwell, J.M.; Zhang, Y. QTL. gCIMapping. GUI v2. 0: An R software for detecting small-effect and linked QTLs for quantitative traits in bi-parental segregation populations. Comput. Struct. Biotechnol. J. 2020, 18, 59–65. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. WIREs Comp. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Subgroup, G.P.D.P. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Shi, S.; Zhang, H.; Wang, X.; Chen, H.; Li, W.; Li, L. DeepBSA: A deep-learning algorithm improves bulked segregant analysis for dissecting complex traits. Mol. Plant 2022, 15, 1418–1427. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, T.; Wang, P.; Wang, B.; Song, J.; Han, Z.; Chen, L.; Liu, K.; Xing, Y. Development of whole-genome agarose-resolvable LInDel markers in rice. Rice 2020, 13, 1. [Google Scholar] [CrossRef]
- Lincoln, S.E.; Daly, M.J.; Lander, E.S. Constructing genetic linkage maps with MAPMAKER/EXP Version 3.0: A tutorial and reference manual. In A Whitehead Institute for Biomedical Research Technical Report; Whitehead Institute for Biomedical Research: Cambridge, MA, USA, 1993; Volume 3, pp. 1–47. [Google Scholar]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Wachsman, G.; Modliszewski, J.L.; Valdes, M.; Benfey, P.N. A SIMPLE pipeline for mapping point mutations. Plant Physiol. 2017, 174, 1307–1313. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ota, Y.; Yamakawa, T.; Ohno, T.; Seta, S.; Honda, Y.; Mizobuchi, R.; Sato, H. Breeding and characterization of the world’s first practical rice variety with resistance to brown spot (Bipolaris oryzae) bred using marker-assisted selection. Breed. Sci. 2021, 71, 474–483. [Google Scholar] [CrossRef]
- Adair, C.R. Inheritance in Rice of Reaction to Helminthosporium oryzae and Cercospora oryzae; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 1941. [Google Scholar]
- Balal, M.; Omar, R.; El-Khadem, M.; Aidy, I. Inheritance of resistance to the brown spot disease of rice, Cochliobolus miyabeanus. Agric. Res. Rev. 1979, 57, 119–133. [Google Scholar]
- Goel, R.; Ritu Bala, R.B.; Kuldeep Singh, K.S. Genetic characterization of resistance to brown leaf spot caused by Drechslera oryzae in some wild rice (Oryza sativa) lines. Indian J. Agric. Sci. 2006, 76, 705–707. [Google Scholar]
- Zhang, J.; Li, X.; Jiang, G.; Xu, Y.; He, Y. Pyramiding of Xa7 and Xa21 for the improvement of disease resistance to bacterial blight in hybrid rice. Plant Breed. 2006, 125, 600–605. [Google Scholar] [CrossRef]
- Mulbah, Q.S.; Shimelis, H.A.; Laing, M.D. Combining ability and gene action of three components of horizontal resistance against rice blast. Euphytica 2015, 206, 805–814. [Google Scholar] [CrossRef]
- Torres, M.d.; Sanchez, P.; Fernandez-Delmond, I.; Grant, M. Expression profiling of the host response to bacterial infection: The transition from basal to induced defence responses in RPM1-mediated resistance. Plant J. 2003, 33, 665–676. [Google Scholar] [CrossRef]
- Gao, Z.; Chung, E.-H.; Eitas, T.K.; Dangl, J.L. Plant intracellular innate immune receptor Resistance to Pseudomonas syringae pv. maculicola 1 (RPM1) is activated at, and functions on, the plasma membrane. Proc. Natl. Acad. Sci. USA 2011, 108, 7619–7624. [Google Scholar] [CrossRef]
- Zhao, G.; Guo, D.; Wang, L.; Li, H.; Wang, C.; Guo, X. Functions of RPM1-interacting protein 4 in plant immunity. Planta 2021, 253, 11. [Google Scholar] [CrossRef]
- Mackey, D.; Holt, B.F.; Wiig, A.; Dangl, J.L. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 2002, 108, 743–754. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Wang, Z.; Meng, F.; Zhang, S.; Wu, X.; Zhang, Z.; Gao, Z. Overexpression of Arabidopsis nucleotide-binding and leucine-rich repeat genes RPS2 and RPM1 (D505V) confers broad-spectrum disease resistance in rice. Front. Plant Sci. 2019, 10, 417. [Google Scholar] [CrossRef]
- Sato, H.; Ando, I.; Hirabayashi, H.; Takeuchi, Y.; Arase, S.; Kihara, J.; Kato, H.; Imbe, T.; Nemoto, H. QTL analysis of brown spot resistance in rice (Oryza sativa L.). Breed. Sci. 2008, 58, 93–96. [Google Scholar] [CrossRef]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Oladosu, Y.; Okporie, E.; Onyishi, G.; Utobo, E.; Ekwu, L.; Swaray, S. Marker-assisted selection and gene pyramiding for resistance to bacterial leaf blight disease of rice (Oryza sativa L.). Biotechnol. Biotechnol. Equip. 2019, 33, 440–455. [Google Scholar] [CrossRef]
- Gajiwala, K.S.; Burley, S.K. Winged helix proteins. Curr. Opin. Struct. Biol. 2000, 10, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Clark, K.L.; Burley, S.K.; Darnell, J.E., Jr. Hepatocyte nuclear factor 3/fork head or “winged helix” proteins: A family of transcription factors of diverse biologic function. Proc. Natl. Acad. Sci. USA 1993, 90, 10421–10423. [Google Scholar] [CrossRef]
- Cicero, M.P.; Hubl, S.T.; Harrison, C.J.; Littlefield, O.; Hardy, J.A.; Nelson, H.C. The wing in yeast heat shock transcription factor (HSF) DNA-binding domain is required for full activity. Nucleic Acids Res. 2001, 29, 1715–1723. [Google Scholar] [CrossRef]
- Liu, J.; Smith, C.L.; DeRyckere, D.; DeAngelis, K.; Martin, G.S.; Berger, J.M. Structure and function of Cdc6/Cdc18: Implications for origin recognition and checkpoint control. Mol. Cell 2000, 6, 637–648. [Google Scholar] [CrossRef]
- Yamada, K.; Miyata, T.; Tsuchiya, D.; Oyama, T.; Fujiwara, Y.; Ohnishi, T.; Iwasaki, H.; Shinagawa, H.; Ariyoshi, M.; Mayanagi, K. Crystal structure of the RuvA-RuvB complex: A structural basis for the Holliday junction migrating motor machinery. Mol. Cell 2002, 10, 671–681. [Google Scholar] [CrossRef]
- Wah, D.A.; Hirsch, J.A.; Dorner, L.F.; Schildkraut, I.; Aggarwal, A.K. Structure of the multimodular endonuclease Fok I bound to DNA. Nature 1997, 388, 97–100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).