Blue Light Enhances Photosynthetic Efficiency and Antioxidant Capacity in Mullein (Verbascum phlomoides L.) Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Light Treatments
2.2. Sample Preparation
2.3. Determination of Chlorophyll and Carotenoid Content
2.4. Determination of Antioxidant Capacity with FRAP Method
2.5. Determination of Total Phenols and Flavonoids
2.6. Efficiency of the Photosynthetic Activity in Young Verbascum Plants
2.7. Data Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphological Parameters of Verbascum phlomoides L.
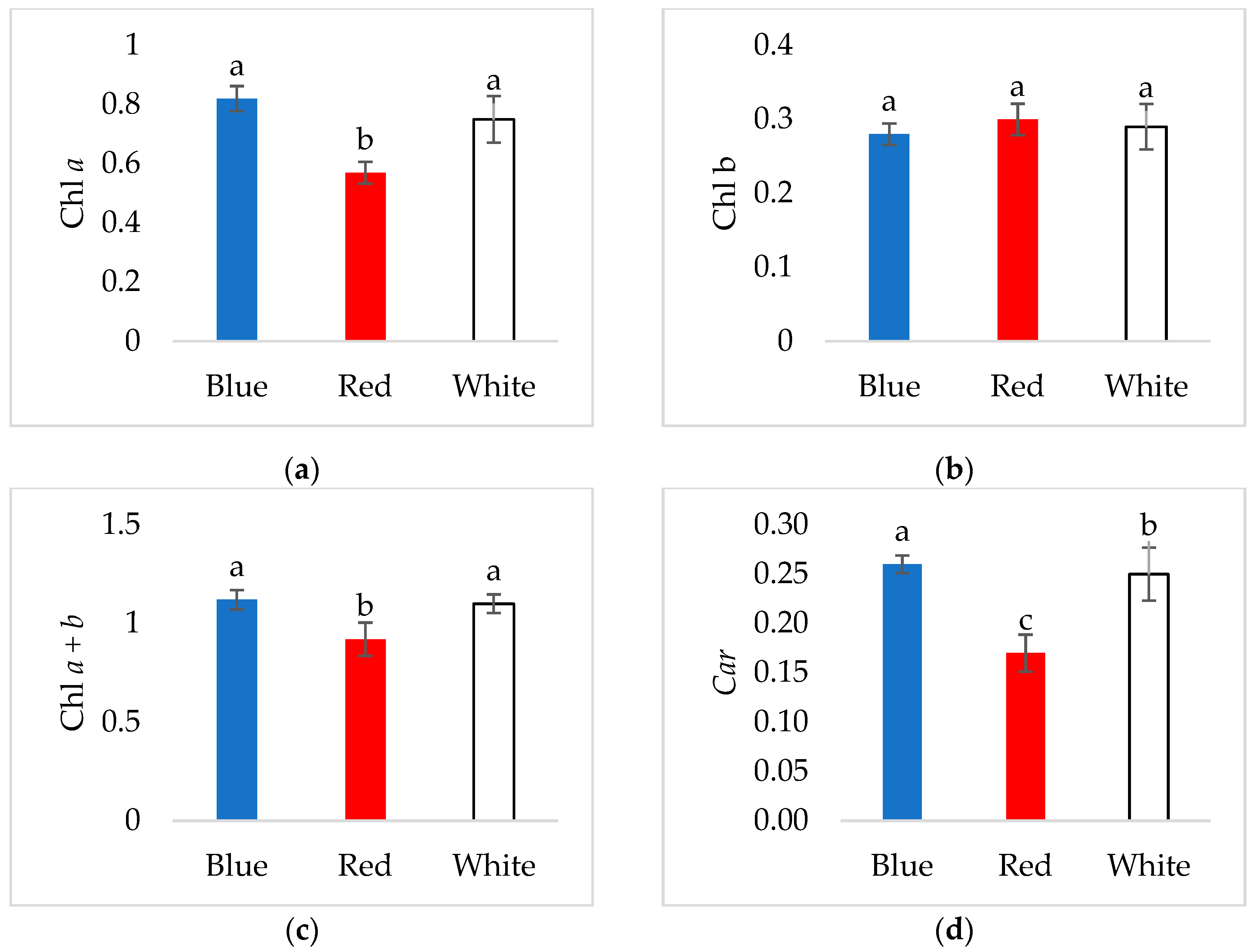
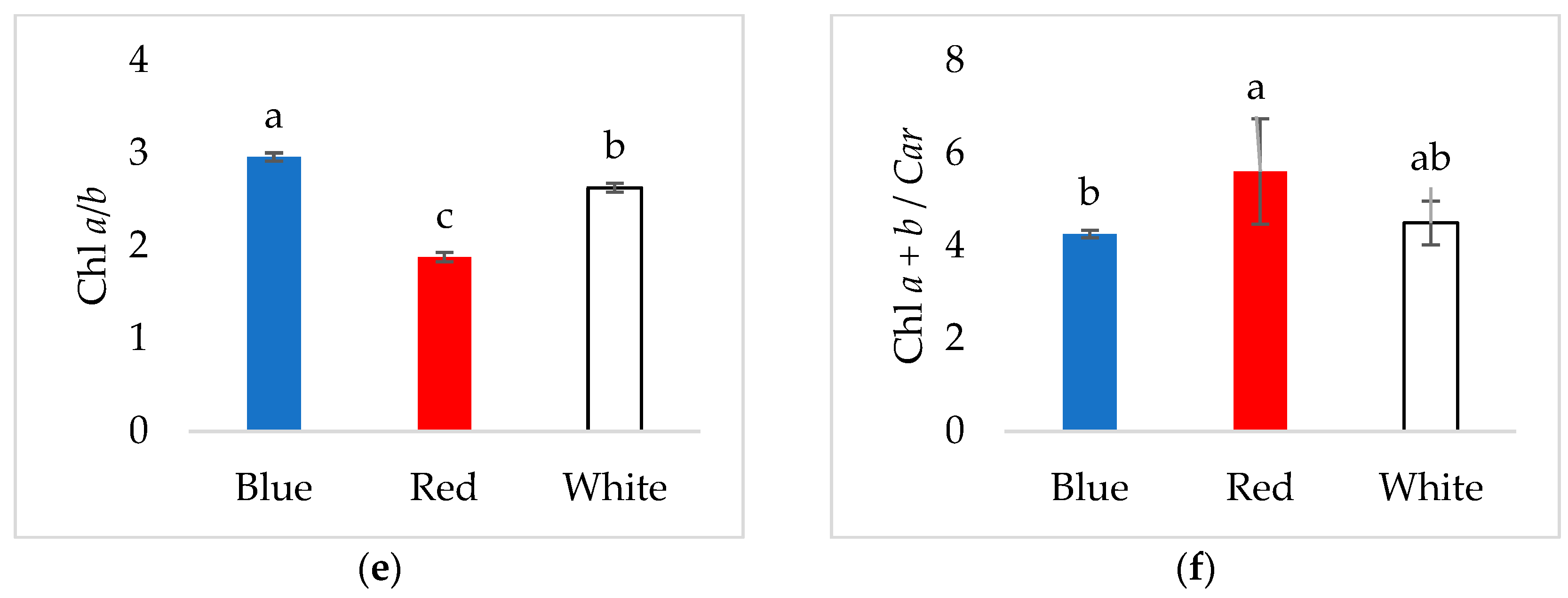
3.2. Pigment Content in Young Verbascum Leaves
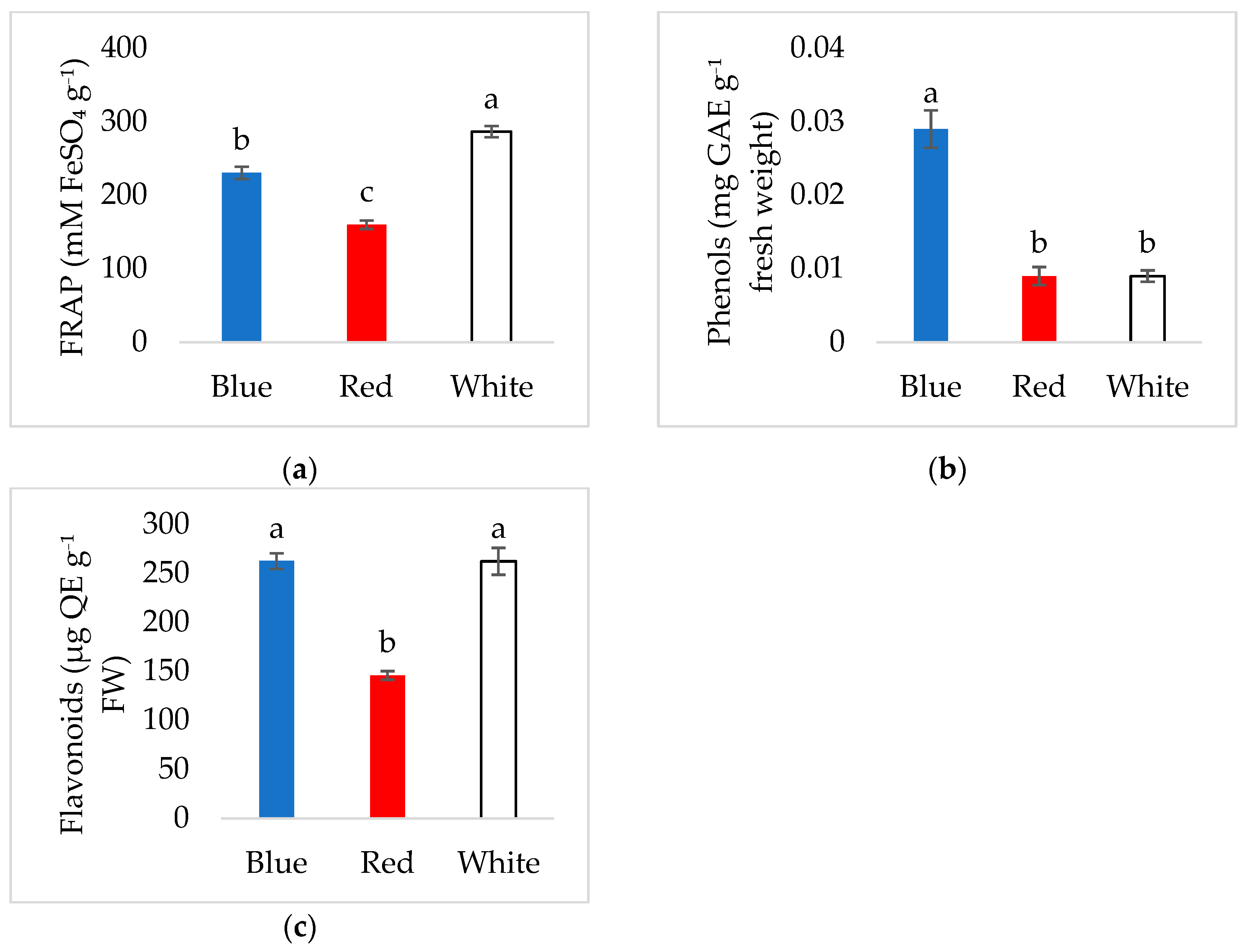
3.3. Antioxidant Activity and Phenolic Content of Young Verbascum Leaves
3.4. Leaf Area and Specific Leaf Area of Young Verbascum Leaves
3.5. Chlorophyll Fluorescence Parameters
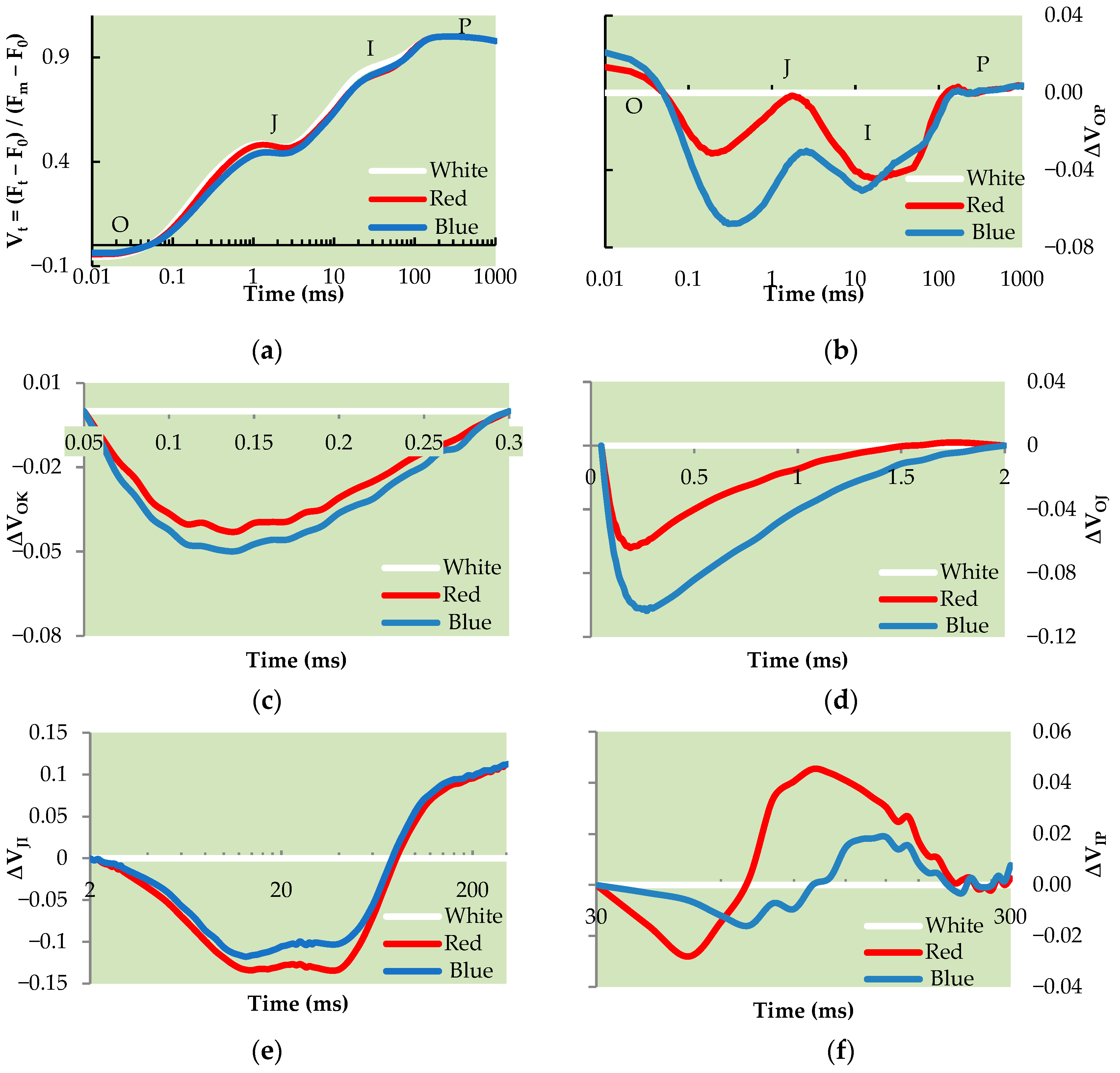
3.6. OJIP Transient Curve
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nikolić, T. (Ed.) Verbascum phlomoides L. Flora Croatica Database; Faculty of Science, University of Zagreb: Zagreb, Croatia, 2015; Available online: http://hirc.botanic.hr/fcd (accessed on 6 October 2025).
- Ghahremani, A.; Pirbalouti, A.G.; Mozafari, H.; Habibi, D.; Sani, B. Phytochemical and morpho-physiological traits of mullein as a new medicinal crop under different planting pattern and soil moisture conditions. Ind. Crops Prod. 2020, 145, 111976. [Google Scholar] [CrossRef]
- Marian, E.; Gratiela Vicas, L.; Jurca, T.; Muresan, M.; Pallag, A.; Stan, R.L.; Sevastre, B.; Diaconeasa, Z.; Ionescu, C.M.L.; Hangan, A.C. Salvia officinalis L. and Verbascum phlomoides L.: Chemical, antimicrobial, antioxidant and antitumor investigations. Rev. Chim. 2018, 69, 365–370. [Google Scholar] [CrossRef]
- Garg, R.; Dobhal, K.; Singh, A. Utilization of Medicinal Herbal Plants in the Management of Respiratory Conditions. In Immunopathology of Chronic Respiratory Diseases; Athari, S.S., Nasab, E.M., Eds.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Blanco-Salas, J.; Hortigón-Vinagre, M.P.; Morales-Jadán, D.; Ruiz-Téllez, T. Searching for scientific explanations for the uses of Spanish folk medicine: A review on the case of mullein (verbascum, Scrophulariaceae). Biology 2021, 10, 618. [Google Scholar] [CrossRef]
- Mihaylova, R.; Elincheva, V.; Gevrenova, R.; Zheleva-Dimitrova, D.; Momekov, G.; Simeonova, R. Targeting inflammation with natural products: A mechanistic review of iridoids from Bulgarian medicinal plants. Molecules 2025, 30, 3456. [Google Scholar] [CrossRef]
- Pongkitwitoon, B.; Putalun, W.; Triwitayakorn, K.; Kitisripanya, T.; Kanchanapoom, T.; Boonsnongcheep, P. Anti-inflammatory activity of verbascoside- and isoverbascoside-rich Lamiales medicinal plants. Heliyon 2024, 10, e23644. [Google Scholar] [CrossRef] [PubMed]
- Grigorov, M.; Pavlović, D.; Zlatković, B.; Dragićević, A.; Tadić, V.; Matejić, J.; Mladenović Antić, S.; Ilić, D.; Nešić, I. Comparative study of three Verbascum species (V. phlomoides, V. niveum and V. speciosum)—Chemical characterisation and biological activities. Nat. Prod. Commun. 2025, 20, 1–15. [Google Scholar] [CrossRef]
- Angeloni, S.; Zengin, G.; Sinan, K.I.; Ak, G.; Maggi, F.; Caprioli, G.; Kaplan, A.; Bahşi, M.; Çakılcıoğlu, U.; Bouyahya, A.; et al. An insight into Verbascum bombyciferum extracts: Different extraction methodologies, biological abilities and chemical profiles. Ind. Crops Prod. 2021, 161, 113201. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Albu, C.; Radu, G.L. Verbascum phlomoides and Solidago virgaureae herbs as natural source for preventing neurodegenerative diseases. J. Herb. Med. 2016, 6, 180–186. [Google Scholar] [CrossRef]
- Armatu, A.; Bodîrlău, R.; Nechita, C.B.; Niculaua, M.; Teacă, C.A.; Ichim, M.; Spiridon, I. Characterization of biologically active compounds from Verbascum phlomoides by chromatography techniques. I. Gas chromatography. Rom. Biotechnol. Lett. 2011, 16, 6297–6304. [Google Scholar]
- Yılmaz, M.; Genç, G.E. Overview of ethnobotanical, phytochemical and biological activity relations of Verbascum species in worldwide. Turk. J. Biod. 2024, 7, 131–154. [Google Scholar] [CrossRef]
- Lisjak, M.; Ocvirk, D.; Špoljarević, M.; Teklić, T.; Liović, I.; Špoljarić Marković, S.; Volenik, M.; Mijić, A. The effect of seed priming with hydrogen sulfide on germination and biochemical indicators of drought stress in sunflower seedlings. Poljoprivreda 2025, 31, 1–12. [Google Scholar] [CrossRef]
- Negi, S.; Kumar, P.; Kumar, A.; Kumar, V.; Irfan, M. Environmental variables affecting apple fruit development and bioactive compounds: Biochemical insights and biotechnological advances. J. Plant Growth Regul. 2025, 1–20. [Google Scholar] [CrossRef]
- Rapčan, I.; Radočaj, D.; Jurišić, M. A length-of-season analysis for maise cultivation from the land-surface phenology metrics using the Sentinel-2 images. Poljoprivreda 2025, 31, 92–98. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, J.; Dayananda, B.; Li, J. Effect of light intensities on the photosynthesis, growth and physiological performances of two maple species. Front. Plant Sci. 2022, 13, 999026. [Google Scholar] [CrossRef]
- Varga, I.; Kristić, M.; Lisjak, M.; Tkalec Kojić, M.; Iljkić, D.; Jović, J.; Kristek, S.; Markulj Kulundžić, A.; Antunović, M. Antioxidative Response and Phenolic Content of Young Industrial Hemp Leaves at Different Light and Mycorrhiza. Plants 2024, 13, 840. [Google Scholar] [CrossRef]
- Süntar, İ.; Tatlı, I.I.; Akkol, E.K.; Keleş, H.; Kahraman, Ç.; Akdemir, Z. An ethnopharmacological study on Verbascum species: From conventional wound healing use to scientific verification. J. Ethnopharmacol. 2010, 132, 408–413. [Google Scholar] [CrossRef]
- Gupta, A.; Atkinson, A.N.; Pandey, A.K.; Bishayee, A. Health--promoting and disease--mitigating potential of Verbascum thapsus L. (common mullein): A review. Phytother. Res. 2022, 36, 1507–1522. [Google Scholar] [CrossRef] [PubMed]
- Grigorov, M.; Pavlović, D. Quality control, phenolic content and in vitro antioxidant potential of orange mullein flower and leaf. Biol. Nyssana 2021, 12, 123–129. [Google Scholar] [CrossRef]
- Idris, A.; Linatoc, A.C.; Bakar, M.F.A.; Ibrahim, Z.T.; Audu, Y. Effect of light quality and quantity on the accumulation of flavonoid in plant species. J. Sci. Technol. 2018, 10, 32–45. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants Response to Light Stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Song, Y.; Liu, W.; Wang, Z.; He, S.; Jia, W.; Shen, Y.; Sun, Y.; Xu, Y.; Wang, H.; Shang, W. Effect of different monochromatic LEDs on the environmental adaptability of Spathiphyllum floribundum and Chrysanthemum morifolium. Plants 2023, 12, 2964. [Google Scholar] [CrossRef]
- Lin, X.; Liu, S.; He, S.; Liu, J.; Liu, Y.; Ye, C.; Li, H. Stomatal opening and transcriptomic events in cut carnation leaves in response to blue light. Hortic. Environ. Biotechnol. 2025, 66, 1357–1376. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Q.; Xin, Y.; Mei, Z.; Gao, A.; Liu, W.; Yu, L.; Chen, X.; Wang, N. Analyses of the photosynthetic characteristics, chloroplast ultrastructure, and transcriptome of apple (Malus domestica) grown under red and blue lights. BMC Plant Biol. 2021, 21, 483. [Google Scholar] [CrossRef]
- Spaninks, K.; Lamers, G.; van Lieshout, J.; Offringa, R. Light quality regulates apical and primary radial growth of Arabidopsis thaliana and Solanum lycopersicum. Sci. Hortic. 2023, 317, 112082. [Google Scholar] [CrossRef]
- Jishi, T.; Kimura, K.; Matsuda, R.; Fujiwara, K. Effects of temporally shifted irradiation of blue and red LED light on cos lettuce growth and morphology. Sci. Hortic. 2016, 198, 227–232. [Google Scholar] [CrossRef]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. The role of blue and red light in the orchestration of secondary metabolites, nutrient transport and plant quality. Plants 2023, 12, 2026. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Vidaković-Cifrek, Ž.; Tkalec, M. Chlorophyll a Fluorescence in Evaluation of Plant Responses to Environmental Signals. In Chlorophyll a Fluorescence Measurements in Croatia-First Twenty Years; Lepeduš, H., Viljevac Vuletić, M., Zdunić, Z., Eds.; Monograph of Agricultural Institute Osijek: Osijek, Croatia, 2023; pp. 19–27. Available online: https://www.poljinos.hr/wp-content/uploads/2023/10/Chlorophyll-a-Fluorescence-Measurements-in-Croatia-First-Twenty-Years.pdf (accessed on 6 October 2025).
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Brestič, M.; Živčák, M.; Hauptvogel, P.; Misheva, S.; Kocheva, K.; Yang, X.; Li, X.; Allakhverdiev, S.I. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth. Res. 2018, 136, 245–255. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterise and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Barboričová, M.; Filaček, A.; Mlynáriková Vysoká, D.; Gašparovič, K.; Živčák, M.; Brestič, M. Sensitivity of fast chlorophyll fluorescence parameters to combined heat and drought stress in wheat genotypes. Plant Soil. Environ. 2022, 68, 309–316. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction), Photosystem II: Basics applications of the OJIPfluorescence transient. J. Photochem. Photobiol. B 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Gao, Q.; Liao, Q.; Li, Q.; Yang, Q.; Wang, F.; Li, J. Effects of LED red and blue light component on growth and photosynthetic characteristics of coriander in plant factory. Horticulturae 2022, 8, 1165. [Google Scholar] [CrossRef]
- Jang, I.T.; Lee, J.H.; Shin, E.J.; Nam, S.Y. Evaluation of growth, flowering, and chlorophyll fluorescence responses of Viola cornuta cv. Penny Red Wing according to spectral power distributions. J. People Plants Environ. 2023, 26, 335–349. [Google Scholar] [CrossRef]
- Park, B.G.; Lee, J.H.; Shin, E.J.; Kim, E.A.; Nam, S.Y. Light quality influence on growth performance and physiological activity of Coleus cultivars. Int. J. Plant Biol. 2024, 15, 807–826. [Google Scholar] [CrossRef]
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–461. [Google Scholar] [CrossRef]
- Wettstein, D. Chlorophyll–letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–487. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Quality and nutritional value of strawberry fruit under long term salt stress. Food Chem. 2008, 107, 1413–1420. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.M.; Brown, C.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, W.; Yang, S.; Ding, X.; Zou, J. Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Y.; Chu, H.; Chen, X.; Guo, H.; Yuan, H.; Yan, D.; Zheng, B. Effects of different light qualities on seedling growth and chlorophyll fluorescence parameters of Dendrobium officinale. Biologia 2017, 72, 735–744. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Zhang, M.; Ju, J.; Hu, Y.; He, R.; Song, J.; Liu, H. Meta-Analysis of the Impact of Far-Red Light on Vegetable Crop Growth and Quality. Plants 2024, 13, 2508. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, S.P.; Mahapatra, S.K.; Swain, D.; Rout, G.R. Deeper insights into the physiological and metabolic functions of the pigments in plants and their applications: Beyond natural colorants. Physiol. Plant. 2025, 177, e70168. [Google Scholar] [CrossRef]
- Kolarov, R.; Prvulović, D.; Gvozdenac, S. Antioxidant capacity of wild-growing orange mullein (Verbascum phlomoides L.). Agro-Knowl. J. 2021, 22, 127–135. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, J.; Liu, M.; Li, Y.; Zheng, J. Effects of different LED spectra on the antioxidant capacity and nitrogen metabolism of Chinese cabbage (Brassica rapa L. ssp. Pekinensis). Plants 2024, 13, 2958. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of ascorbate accumulation and metabolism in lettuce by the red: Blue ratio of continuous light using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef]
- Thongtip, A.; Mosaleeyanon, K.; Janta, S.; Wanichananan, P.; Chutimanukul, P.; Thepsilvisut, O.; Chutimanukul, P. Assessing light spectrum impact on growth and antioxidant properties of basil family microgreens. Sci. Rep. 2024, 14, 27875. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Xie, L.; Ye, J.; Zhou, L.; Yu, D.; Wang, Q.W. Light quality regulates growth and flavonoid content in a widespread forest understorey medicinal species Scutellaria Baicalensis Georgi. Front. Plant Sci. 2024, 15, 1488649. [Google Scholar] [CrossRef]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Moosavi-Nezhad, M.; Pedersen, C.; Gruda, N.S.; Salami, S.A. Monochromatic blue light enhances crocin and picrocrocin content by upregulating the expression of underlying biosynthetic pathway genes in saffron (Crocus sativus L.). Front. Hortic. 2022, 1, 960423. [Google Scholar] [CrossRef]
- Takemiya, A.; Inoue, S.I.; Doi, M.; Kinoshita, T.; Shimazaki, K.I. Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 2005, 17, 1120–1127. [Google Scholar] [CrossRef]
- Ke, X.; Yoshida, H.; Hikosaka, S.; Goto, E. Effect of red and blue light versus white light on fruit biomass radiation-use efficiency in dwarf tomatoes. Front. Plant Sci. 2024, 15, 1393918. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates the inhibitory effect of low light on photosynthesis and enhances the accumulation of secondary metabolites in Digitalis purpurea. Plant Physiol. Biochem. 2019, 139, 52–61. [Google Scholar] [CrossRef]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants 2018, 10, ply052. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, X.; Xu, R.; Wang, J.; Lin, Y.; Pang, J.; Wu, S.; Zhong, F. Comprehensive analysis of photosynthetic characteristics and quality improvement of purple cabbage under different combinations of monochromatic light. Front. Plant Sci. 2016, 7, 1788. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. Hortic. Sci. 2015, 42, 291–298. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Zhiponova, M.; Paunov, M.; Anev, S.; Petrova, N.; Krumova, S.; Raycheva, A.; Goltsev, V.; Tzvetkova, N.; Taneva, S.; Sapunov, K.; et al. JIP-test as a tool for early diagnostics of plant growth and flowering upon selected light recipe. Photosynthetica 2020, 58, 399–408. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, Y.B.; Choi, I.-L.; Yoon, H.S.; Kim, J.; Kim, Y.; Kang, H.-M. Changes in Spectral Reflectance, Photosynthetic Performance, Chlorophyll Fluorescence, and Growth of Mini Green Romaine Lettuce According to Various Light Qualities in Indoor Cultivation. Horticulturae 2024, 10, 860. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J. Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiol. 2019, 179, 507–517. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of g-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurement. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Zagorchev, L.; Atanasova, A.; Albanova, I.; Traianova, A.; Mladenov, P.; Kouzmanova, M.; Goltsev, V.; Kalaji, H.M.; Teofanova, D. Functional Characterization of the Photosynthetic Machinery in Smicronix Galls on the Parasitic Plant Cuscuta campestris by JIP-Test. Cells 2021, 10, 1399. [Google Scholar] [CrossRef]
- Dimitrova, S.; Paunov, M.; Pavlova, B.; Dankov, K.; Kouzmanova, M.; Velikova, V.; Tsonev, T.; Kalaji, H.; Goltsev, V. Photosynthetic efficiency of two Platanus orientalis L. ecotypes exposed to moderately high temperature–JIP-test analysis. Photosynthetica 2020, 58, 657–670. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Effects of Red and Blue Light on Leaf Anatomy, CO2 Assimilation, and the Photosynthetic Electron Transport Capacity of Sweet Pepper (Capsicum annuum L.) Seedlings. BMC Plant Biol. 2020, 20, 318. [Google Scholar] [CrossRef] [PubMed]


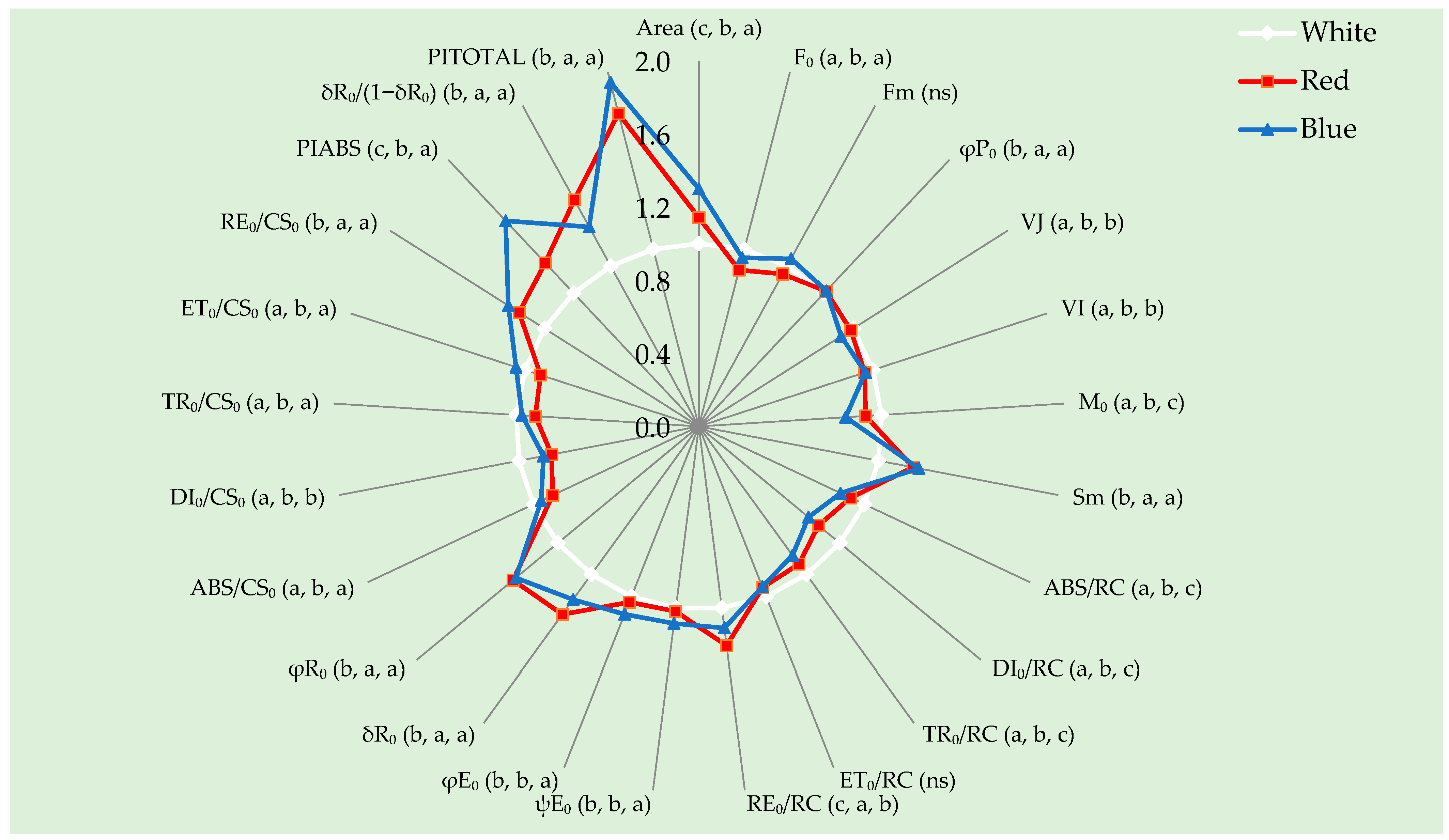
| Parameter | Formula | Description |
|---|---|---|
| Area | – | Total complementary area above the OJIP transient curve, proportional to the pool size of electron acceptors on the photosystem I (PSI) acceptor side. |
| F0 | Minimal fluorescence (O level) | Fluorescence intensity when all photosystem II (PSII) reaction centers (RCs) are open (dark-adapted state). |
| Fm | Maximal fluorescence (P level) | Fluorescence intensity when all PSII RCs are closed after a saturating light pulse. |
| φP0 | (Fm − F0)/Fm | Maximum quantum yield of PSII photochemistry is an indicator of the photosynthetic efficiency of PSII. |
| VJ | (FJ − F0)/(Fm − F0) | Relative variable fluorescence at the J-step (≈2 ms), reflecting primary quinone (QA) reduction. |
| VI | (FI − F0)/(Fm − F0) | Relative variable fluorescence at the I-step (≈30 ms), reflecting further electron transport beyond QA. |
| M0 | (ΔV/Δt)0 | Initial slope of the fluorescence rise; related to the rate of QA reduction. |
| Sm | Area/(Fm − F0) | Normalised area; proportional to the number of electron carriers per RC. |
| ABS/RC | (M0/Vj) × (1/φP0) | Absorption flux per PSII reaction center. |
| DI0/RC | (ABS/RC) − (TR0/RC) | Energy dissipated as heat per PSII reaction center. |
| TR0/RC | M0/VJ | Trapped energy flux leading to QA reduction per RC. |
| ET0/RC | (M0/VJ) × ψ(E0) | Electron transport flux beyond reduced QA (QA−) per RC. |
| RE0/RC | (M0/VJ) × δ(R0) | Electron flux reducing end acceptors of PSI per RC. |
| ψ(E0) | 1 − VJ | Probability that a trapped exciton moves an electron beyond QA−. |
| φ(E0) | (1 − F0/Fm) × (1 − VJ) | Quantum yield of electron transport beyond QA−. |
| δ(R0) | (1 − VI)/(1 − VJ) | Efficiency/probability that an electron moves from reduced intersystem carriers to PSI end acceptors. |
| φ(R0) | (1 − F0/Fm) × δ(R0) | Quantum yield for reduction of PSI end acceptors. |
| ABS/CS0 | F0 | Absorbed energy flux per excited cross-section (CS). |
| DI0/CS0 | ABS/CS0 − TR0/CS0 | Dissipated energy flux per excited cross-section. |
| TR0/CS0 | Fm − F0 | Energy trapped per excited cross-section. |
| ET0/CS0 | TR0/CS0 × ψ(E0) | Electron transport flux per cross-section. |
| RE0/CS0 | TR0/CS0 × δ(R0) | Electron flux reaching PSI per cross-section. |
| PIABS | (RC/ABS) × [φP0/(1 − φP0)] × [ψ(E0)/(1 − ψ(E0))] | Performance index on absorption basis; integrates PSII photochemical efficiency. |
| δ(R0)/(1 − δ(R0)) | δ(R0)/[1 − δ(R0)] | Ratio expressing the efficiency of PSI electron acceptor reduction. |
| PITOTAL | PIABS × [δ(R0)/(1 − δ(R0))] | Overall performance index including PSI activity (total photosynthetic performance). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkalec Kojić, M.; Varga, I.; Jović, J.; Stošić, M.; Đurić, M.; Vinković, T.; Ravnjak, B.; Parađiković, N.; Markulj Kulundžić, A. Blue Light Enhances Photosynthetic Efficiency and Antioxidant Capacity in Mullein (Verbascum phlomoides L.) Seedlings. Agriculture 2025, 15, 2385. https://doi.org/10.3390/agriculture15222385
Tkalec Kojić M, Varga I, Jović J, Stošić M, Đurić M, Vinković T, Ravnjak B, Parađiković N, Markulj Kulundžić A. Blue Light Enhances Photosynthetic Efficiency and Antioxidant Capacity in Mullein (Verbascum phlomoides L.) Seedlings. Agriculture. 2025; 15(22):2385. https://doi.org/10.3390/agriculture15222385
Chicago/Turabian StyleTkalec Kojić, Monika, Ivana Varga, Josipa Jović, Miro Stošić, Mario Đurić, Tomislav Vinković, Boris Ravnjak, Nada Parađiković, and Antonela Markulj Kulundžić. 2025. "Blue Light Enhances Photosynthetic Efficiency and Antioxidant Capacity in Mullein (Verbascum phlomoides L.) Seedlings" Agriculture 15, no. 22: 2385. https://doi.org/10.3390/agriculture15222385
APA StyleTkalec Kojić, M., Varga, I., Jović, J., Stošić, M., Đurić, M., Vinković, T., Ravnjak, B., Parađiković, N., & Markulj Kulundžić, A. (2025). Blue Light Enhances Photosynthetic Efficiency and Antioxidant Capacity in Mullein (Verbascum phlomoides L.) Seedlings. Agriculture, 15(22), 2385. https://doi.org/10.3390/agriculture15222385












