Abstract
Given the increasing concern about the presence of emerging contaminants in wastewater and their persistence in the environment, this study aimed to assess the effects of two anxiolytic pharmaceuticals commonly used in human therapy—Tranxilium (dipotassium clorazepate) and Zolpidem (zolpidem tartrate)—on plant development. Lettuce (Lactuca sativa L.) and wheat (Triticum aestivum) were selected as the biotest species. Phytotoxicity assays were also performed on Raphanus sativus. Greenhouse experiments were conducted using different concentrations of both pharmaceuticals, and several physiological and growth parameters were evaluated, including the germination rate, biomass accumulation, SPAD index, and spectrophotometrically measured contents of chlorophyll A, chlorophyll B, and carotenoids. The results indicated that both pharmaceuticals can affect plant growth, with stimulatory effects at intermediate concentrations and phytotoxic effects at higher levels. These findings highlight the importance of considering the impact of emerging contaminants on agricultural ecosystems and their potential risks to environmental and human health.
1. Introduction
In recent decades, concern about the presence of unregulated chemical substances in the environment has grown significantly. Among these substances are the so-called emerging contaminants (ECs), which include synthetic or natural compounds whose presence in the environment is not necessarily new but had not previously been identified as pollutants. Concern over their potential environmental and health consequences has led to their recognition as a relevant threat in recent years [].
ECs encompass a wide variety of substances such as personal care and hygiene products, antibiotics, hormones, plasticizers, pharmaceuticals, endocrine disruptors, perfluorinated compounds, and microplastics, among others. Most of these substances have complex chemical structures and various functional groups that give them characteristics such as high environmental persistence, mobility, and a tendency to bioaccumulate in organisms, increasing the risk they represent both for human health and ecosystems [].
However, some ECs, despite not being highly persistent in the environment, are continuously released, which offsets their rapid degradation and elimination rates. This enables their detection in surface waters (rivers, lakes, and seas) at concentrations ranging from ng L−1 to μg L−1.
One of the main pathways through which emerging contaminants enter the environment is urban wastewater and the effluents from wastewater treatment plants (WWTPs), despite including primary, secondary, and, in some cases, tertiary treatments. These treatment stages mainly remove solids, organic matter, and nutrients, but they are not specifically designed to eliminate emerging contaminants. As a result, many of these substances persist in treated effluent []. A significant portion of these substances and their metabolites, therefore, pass through treatment processes and reach aquatic environments via treated water discharges [].
This limitation in conventional treatments has been confirmed by European-level studies that have detected a wide variety of emerging contaminants in wastewater treatment plant (WWTP) effluents, including pharmaceuticals, pesticides, antibiotics, and personal care products, evidencing their continuous release into the aquatic environment even after treatment [].
An increasing number of populations rely on water resources that are directly or indirectly replenished with treated wastewater. This practice has raised growing concern about the potential impact of these substances on aquatic ecosystems and the quality of water intended for human consumption [].
Despite increasing concern, emerging contaminants are still not routinely included in environmental monitoring programs. Consequently, their environmental fate, behavior, and potential ecotoxicological effects remain insufficiently understood. These knowledge gaps underscore the need for further research to evaluate their risks and implications for both ecosystems and human health [].
Among ECs, pharmaceuticals have become one of the most studied substances. Pharmaceuticals found in surface water and groundwater originate from various pollution sources. One of the main types is the discharge of urban wastewater, which contains significant concentrations of pharmaceutical active ingredients (PAIs). After oral administration, these compounds undergo metabolic processes in the body, and a significant proportion is excreted either in their original (unmetabolized) form or as metabolites, which may or may not retain biological activity. These substances reach domestic wastewater systems, whether untreated or subjected to purification processes [].
Effluents generated in WWTPs are often discharged into surface water bodies or reused for agricultural irrigation. Additionally, residual sewage sludge (biosolids) obtained from treatment processes can be reused as fertilizer.
In livestock farming, the use of medicated feed is common, leading to the excretion of pharmaceutical residues. These residues, often reused as fertilizers on agricultural soils, can contribute to the entry of these substances into terrestrial and aquatic ecosystems []. For example, Żołnowski et al. [] demonstrated that the application of mineral–microbial deodorizing preparations to poultry manure can influence its properties as a soil amendment, highlighting how treated animal wastes can act as vectors for chemical compounds and microorganisms into agricultural soils.
Their presence in aquatic environments represents not only an environmental risk but also a public health concern, as they can enter the food chain through crop irrigation or the consumption of animal-derived products that have been indirectly exposed.
Drugs such as anxiolytics, hypnotics, antidepressants, and antibiotics can be present not only in untreated wastewater but also in treated effluents []. This highlights the limitations of current treatment processes in completely removing these contaminants.
This phenomenon is particularly relevant in the current context, where a significant increase in pharmaceutical consumption has been observed, especially those related to mental health, such as psychotropics, including antidepressants, tranquilizers, and hypnotics.
Recent studies in Spain show that the consumption of these pharmaceuticals has risen significantly in recent years, particularly among adults and middle-aged women. Specifically, the percentage of people consuming hypnotic-sedatives has nearly tripled in recent years, rising from less than 4% to nearly 10% of the adult population, contributing to the persistent presence of these compounds in wastewater and surface water bodies [].
The two pharmaceuticals evaluated in this study are Tranxilium and Zolpidem, both of which are of growing environmental concern due to their widespread use and persistence. Tranxilium, whose active compound is dipotassium clorazepate, is an anxiolytic medication from the benzodiazepine class, commonly prescribed for the treatment of anxiety disorders [].
Zolpidem, on the other hand, is a hypnotic pharmaceutical with benzodiazepine-like properties. Its active ingredient, zolpidem tartrate, is commonly prescribed for the short-term treatment of sleep disorders in adults [].
Previous studies have evidenced the presence of Zolpidem in water bodies at detectable levels, identifying it in wastewater samples from different geographical areas such as America, indicating its persistence in the aquatic environment and its potential as an emerging contaminant [].
Despite the lack of concrete research on the presence of Tranxilium in wastewater, its chemical composition and environmental behavior are similar to other benzodiazepines, such as diazepam and oxazepam, whose presence has been widely documented in WWTP effluents [].
In addition, the presence of benzodiazepines in the environment has been associated with adverse effects on aquatic organisms. For example, studies have shown that exposure to delorazepam can interfere with embryonic development in species such as Xenopus laevis, causing effects related to the production of reactive oxygen species and alterations in the expression of key developmental genes [].
Plants play a critical role as natural sensors of environmental conditions, as they respond sensitively to changes in air, water, and soil quality. Their capacity to absorb and accumulate pollutant compounds has long supported their use as bioindicators for detecting the presence of toxic substances.
This makes them valuable for assessing risks associated with chemical, radioactive, industrial, and agricultural pollution. Their widespread geographical distribution, physiological sensitivity, and ease of sampling further enhance their effectiveness as tools for environmental monitoring [].
In this study, wheat and lettuce (Triticum aestivum L. var. Fuego and Lactuca sativa L. var. Batavia) have been used as bioindicators to evaluate the effect of emerging contaminants in irrigation water. These species are representative of both leafy crops (dicotyledons) and cereals (monocotyledons), offering a broader perspective on the potential impact on food safety.
Both lettuce and wheat are sensitive to environmental changes. Lettuce, due to its rapid development, small size, and ease of handling, has become a widely used species in toxicological assessment studies []. Wheat, on the other hand, enables the evaluation of long-term effects and different phenological stages, which is relevant when studying the accumulation of contaminants in edible parts. It has been shown to accumulate ECs such as chlorinated organophosphate esters, which disrupt physiological functions like photosynthesis and induce oxidative stress []. In addition to lettuce and wheat, radish (Raphanus sativus) has been widely used in ecotoxicological bioassays due to its rapid growth and sensitivity to pollutants. For instance, Qi et al. [] compared the uptake and accumulation of antibiotics such as cephalexin in radish, lettuce, and celery, highlighting radish as an effective bioindicator for pharmaceutical contaminants. Moreover, Beltran and Sánchez [] demonstrated the potential of radish in phytoremediation studies, particularly for removing endocrine disruptors like 4-nonylphenol and bisphenol A from water. These studies support the feasibility of using radish as a relevant test species in bioassays evaluating the impact of pharmaceutical residues in the environment.
Research on the effects of these compounds remains limited. However, given their persistence and potential impact on the environment and human health, it is essential that we understand their behavior, presence, and mechanisms of action. This knowledge will enable the development of effective strategies to mitigate their presence, contributing to public health protection and the preservation of ecosystems.
This study aimed to assess the physiological effects of the pharmaceuticals Tranxilium (dipotassium clorazepate) and Zolpidem (zolpidem tartrate) on agriculturally relevant plant species. To this end, germination assays were conducted using Raphanus sativus L., and greenhouse experiments were performed with lettuce (L. sativa L.) and wheat (T. aestivum). The study evaluated key parameters such as the germination rate, biomass production, and chlorophyll content in response to various concentrations of the pharmaceuticals.
2. Materials and Methods
2.1. Plant Material
Three plant species were selected to evaluate the effects of the pharmaceuticals: Raphanus sativus L. (red round radish), used in a preliminary phytotoxicity assay; Triticum aestivum L. var. Fuego (wheat), representing monocotyledonous crop species; and Lactuca sativa L. var. Batavia (Batavia lettuce), representing dicotyledonous species. Seeds of R. sativus were purchased from Batlle S.A. (Barcelona, Spain), T. aestivum var. Fuego from KWS Momont España S.A. (Lleida, Spain), and L. sativa var. Batavia from Semillas Fitó S.A. (Barcelona, Spain). This combination of species enabled the evaluation of differential physiological responses between monocotyledonous and dicotyledonous plants, as well as the identification of potential species-specific sensitivities to pharmaceutical exposure.
2.2. Pharmaceuticals Used
Pharmaceuticals used in this study were Tranxilium® capsules (5 mg, clorazepate dipotassium) obtained from Sanofi-Aventis, S.A., Barcelona, Spain; and Zolpidem tablets (5 mg) purchased from Generfarma S.L., Valencia, Spain. Both pharmaceuticals were used as received without further modification. Due to their nature as strictly controlled anxiolytic pharmaceuticals, they were acquired under appropriate authorization for research purposes. To comply with confidentiality and legal regulations, the specific details of this authorization are not disclosed.
2.3. Germination Assay
The acute toxicity test using R. sativus seeds was employed to evaluate the phytotoxic effects of the two pharmaceuticals during the germination stage.
For each pharmaceutical, five treatment groups were established: a control group (distilled water, no treatment) and four groups exposed to increasing concentrations of the pharmaceutical (5, 10, 15, and 20 mg L−1), designated as T1, T2, T3, and T4. In parallel, the seed response to a reference toxicant, zinc sulfate heptahydrate (ZnSO4·7H2O), was also evaluated. In this case, two types of controls were included: a negative control (distilled water), used to ensure adequate germination (>90%) in the absence of toxic elements, and a positive control consisting of zinc sulfate heptahydrate (ZnSO4·7H2O) at concentrations of 1 M, 0.1 M, 0.01 M, 0.001 M, and 0.0001 M. The concentration range was selected to determine the inhibitory concentration (IC50) for the seed lot, defined as the concentration at which 50% of the seeds exhibited germination inhibition.
For this purpose, five seeds were placed in each Petri dish lined with Whatman™ Grade 1 Qualitative Filter Papers (Cytiva, Amersham, UK) paper impregnated with the test solutions, including a control and four increasing concentrations of the pharmaceuticals.
The test solutions were prepared from commercial tablets containing 5 mg of the active ingredient, dipotassium clorazepate (Tranxilium) and zolpidem tartrate (Zolpidem).
To obtain a final concentration of 20 mg L−1 in a total volume of 250 mL, one tablet (5 mg), previously ground into a fine powder, was dissolved in distilled water.
The mixture was stirred using a magnetic stirrer until complete homogenization was achieved. From this stock solution (20 mg L−1), serial dilutions were prepared to obtain lower concentrations of 5, 10, and 15 mg L−1, each adjusted to a final volume of 50 mL. The dilution volumes of stock solution and distilled water for each concentration were as follows:
- 5 mg L−1: 12.5 mL stock solution + 37.5 mL distilled water;
- 10 mg L−1: 25 mL stock solution + 25 mL distilled water;
- 15 mg L−1: 37.5 mL stock solution + 12.5 mL distilled water.
Each treatment, including the control and four concentrations, was conducted in triplicate, resulting in a total of 30 Petri dishes (5 treatments × 3 replicates × 2 pharmaceuticals). The dishes with seeds were placed in a growth chamber at 25 °C in darkness for 48 h to allow germination. After this period, the dishes were sealed with Parafilm and stored at −18 ± 2 °C until analysis, which was performed 3 weeks after sowing. Freezing the germinated seedlings facilitates measurement by modifying the texture of the hypocotyl and radicle, as described by Sobrero and Ronco [].
To assess the toxic impact, inhibition of germination rate, as well as radicle and hypocotyl length, were measured using millimeter paper as a precise measuring tool. This approach allowed for the detection of both severe toxic effects that impair germination and milder effects reflected by reduced root growth (Figure S1).
Based on the results obtained from the germinated seeds, Formulae (1)–(3) were used to calculate the relative germination percentage (RGP), relative root growth (RRG), and germination index (GI) []:
2.4. Experimental Design
The experiment investigated the effects of Tranxilium and Zolpidem on the growth and development of wheat and lettuce. The tests were conducted in the greenhouse of the Experimental Field at the Higher Technical School of Agricultural and Forestry Engineering and Biotechnology (ETSIAMB, Albacete, Spain).
For each pharmaceutical, five treatment groups were established: a control group (no treatment) and four groups exposed to increasing concentrations of the pharmaceutical (5, 10, 15, and 20 mg L−1).
Each treatment was arranged in a single row within a seedling tray, consisting of eight cells per row, with three seeds sown per cell. To ensure the reliability and validity of the results, three independent replicates were performed for each treatment and pharmaceutical (Figure S2).
2.4.1. Sowing and Growth Conditions
Sowing was performed on 10 March 2025, using a commercial substrate composed of Sphagnum peat, herbaceous peat (H8), coconut fiber, wood fiber, and perlite (Gramoflor GmbH & Co. KG, Vechta, Germany), purchased through a local horticultural supplier in Spain. The substrate was supplemented with a controlled-release fertilizer, PG Mix 14-16-18 (Yara International ASA, Oslo, Norway), at a concentration of 1.5 g L−1, following the manufacturer’s recommendations.
All plants were cultivated in a greenhouse, under natural light conditions without temperature control. Environmental parameters such as temperature and humidity were not strictly monitored but were consistent with typical regional spring conditions, ranging approximately between 10 °C and 22 °C. Irrigation was performed manually every 2–3 days, applying sufficient water to maintain the substrate consistently moist but not waterlogged. Volumes were adjusted based on visual assessment of substrate moisture and the specific water needs of each species during the seedling stage in standard seedling trays.
Once the seedlings developed at least two fully expanded true leaves, indicating adequate growth for transplanting, they were moved into larger pots (15 cm deep and 5 cm in diameter) to promote optimal root development and enhance growth. Transplanting occurred on 9 April (30 days after sowing, DAS) for wheat and on 28 April (49 DAS) for lettuce.
Pharmaceutical treatments were applied once most seeds had germinated and seedlings reached sufficient development. For each cell, 5 mL of the corresponding pharmaceutical solution was administered using a syringe to ensure precise dosing (Figure S3). Applications were performed on a previously moistened substrate to facilitate pharmaceuticals’ absorption.
The first dose was applied on 27 March (17 DAS) for wheat and on 30 April (51 DAS) for lettuce, reflecting the slower growth rate of the latter.
2.4.2. Evaluated Parameters
Monitoring of Plant Development:
Throughout the experiment, periodic monitoring of plant development was performed to assess the potential effects of pharmaceutical treatments on early growth stages. Monitoring took place under controlled greenhouse conditions and involved recording morphological variables associated with seedling emergence and vegetative development of the cultivated species.
Greenness Index (SPAD):
In this study, the SPAD-502 Plus portable chlorophyll meter (Konica-Minolta, Osaka, Japan) was employed to assess the relative chlorophyll content in crop leaves. This device provides an indirect, non-invasive estimation of chlorophyll levels, serving as a rapid and straightforward indicator of the plant’s nutritional status. The measurement is based on absorbance at two specific wavelengths: 650 nm (red), corresponding to chlorophyll’s maximum absorption, and 940 nm (infrared), which serves as a reference to compensate for factors such as leaf thickness and water content [].
The difference in transmittance between both wavelengths results in a value known as the SPAD index, which is directly related to chlorophyll content in the leaves [].
The measurement procedure involved taking readings directly from the leaf blade, selecting the most developed leaves of each plant, and avoiding damaged areas and the central vein (Figure S4). Measurements were taken twice a week throughout the experimental period, allowing the monitoring of chlorophyll content changes in response to the different pharmaceutical treatments.
Fresh Biomass Determination:
At the end of the experimental period, the plants were harvested to evaluate fresh biomass. In the case of lettuce, harvesting took place in the 14th week of growth, while, for wheat, it was in the 15th week. Plants were carefully removed from the substrate and cleaned with water to eliminate any remaining substrate particles (Figure S5).
Subsequently, each plant was divided into two parts: the aerial portion and the root system. Both fractions were weighed separately in their fresh state using an analytical balance with a precision of 0.001 g. This procedure allowed for an accurate estimation of vegetative development under the influence of the different pharmaceutical treatments, serving as an indicator of their impact on overall plant growth.
Photosynthetic Pigments (Chlorophyll and Carotenoids):
To evaluate the effect of the treatments, the quantification of photosynthetic pigments, specifically chlorophyll a, chlorophyll b, and carotenoids, was carried out. These compounds are key indicators of the physiological status of the plants, as they are closely related to photosynthetic efficiency and nutritional status [].
Samples were collected randomly by selecting healthy leaves from the middle portion of the plants, avoiding both the youngest and senescent leaves. This sampling strategy was applied within a single row per tray, collecting leaves from different plants to ensure representativeness. Three independent analytical replicates were performed per row, totaling nine analyses per pharmaceutical concentration, as three trays were used for each treatment. Leaves were processed immediately after collection to preserve the integrity of the compounds. Prior to extraction, leaves were rinsed with distilled water to remove any residues and then cut into small pieces to facilitate pigment release.
Pigment extraction was carried out using 96% ethanol as the organic solvent. For each sample, 1 g of fresh leaf tissue was weighed and placed into a test tube. Subsequently, 10 mL of ethanol was added, and the mixture was manually homogenized in a mortar until a uniform suspension was obtained. The samples were then centrifuged at 3000 rpm for 10 min, and the resulting supernatant containing the extracted pigments was collected for further analysis (Figure S6).
Chlorophylls and carotenoids were quantified using spectrophotometry by measuring absorbance at specific wavelengths: 663 nm (A663) for chlorophyll a (Chla), 646 nm (A646) for chlorophyll b (Chlb), and 470 nm (A470) for carotenoids (Car). Ninety-six percent ethanol was used as a blank to avoid solvent interference. Spectrophotometric measurements were performed using a Zuzi 4201/20 spectrophotometer (model Zuzi 4201/20) with a wavelength range of 325–1000 nm and a bandwidth of 5 nm.
Pigment concentrations were calculated using the formulae proposed [] for chlorophyll a (4), chlorophyll b (5), and total carotenoids (6):
Statistical Analysis:
Statistical analyses were performed using Statgraphics Centurion, version 18.1.16 (Statgraphics Technologies, Plains, VA, USA). Each plant species (lettuce Lactuca sativa and wheat Triticum aestivum) was analyzed separately due to differences in growth period and light exposure. For each species, a two-factor experimental design was implemented with the following factors:
- Pharmaceutical type (Tranxilium and Zolpidem);
- Dose (0, 5, 10, 15, and 20 mg L−1).
Two-way ANOVA was applied to assess the effects of pharmaceutical type, dose, and their interaction on all measured parameters. Where significant differences were found, post hoc comparisons were performed using appropriate multiple comparison tests. This approach allowed for the evaluation of dose-dependent responses and species-specific effects of the pharmaceuticals.
3. Results
3.1. Monitoring of Vegetative Development
During the experiment, no significant differences in initial development were observed between treatments. In wheat, emergence began on 19 March (9 days after sowing, DAS), with most plants reaching four to five leaves by 9 April (37 DAS). The tillering stage (the formation of lateral shoots) was observed on 21 April (49 DAS) in plants treated with Tranxilium and on 5 May (66 DAS) in those treated with Zolpidem, occurring at similar dates in the control group (Figure S7).
In lettuce, the first cotyledons appeared on 24 March (14 DAS), albeit only in some cells. Emergence became widespread around 9 April (37 DAS), with a progressive appearance of true leaves. By 30 April (58 DAS), most seedlings had developed three to four leaves, increasing to six to seven leaves by 6 May (67 DAS). The most developed plants reached up to eight leaves, with no visible differences among treatments, including the control, which followed the same developmental timeline (Figure S8).
3.2. Germination Index (GI)
The results obtained from the phytotoxicity test (Figure 1) present the Germination Index (GI) as a function of the different concentrations of the two pharmaceuticals tested, Tranxilium and Zolpidem. The GI reflects both the proportion of germinated seeds and the extent of radicle development during the bioassay.
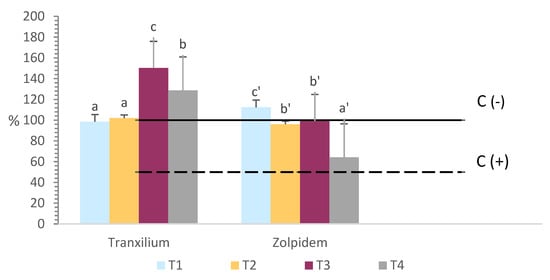
Figure 1.
Germination index (GI) of R. seeds at different concentrations of Tranxilium and Zolpidem. T1: 5 mg L−1, T2: 10 mg L−1, T3: 15 mg L−1, and T4: 20 mg L−1. Error bars indicate the standard deviation. Horizontal lines indicate the GI value for the negative control with distilled water (C) and the positive control containing sulfate heptahydrate (ZnSO4·7H2O), as reference toxicant (C (+)) with a GI of 50%. Different letters indicate statistically significant differences between parameters at a 95% confidence level for the same pharmaceutical.
For Tranxilium, an increasing trend in the GI was observed with rising compound concentrations, reaching a maximum value of 150.4% at 15 mg L−1, indicating a stimulatory effect on germination and root growth. At 20 mg L−1, the GI slightly decreased to 128.7%, but remained above the control level, suggesting a mild reduction in the positive effect at higher concentrations.
In contrast, Zolpidem displayed a different response. At low to moderate concentrations (5–15 mg L−1), the GI remained close to 100%, with no statistically significant differences compared to the control. However, at 20 mg L−1, the GI dropped markedly to 64.2%, indicating a phytotoxic effect at elevated concentrations. This adverse impact may be attributed to the accumulation of the compound or its metabolites in root tissues, negatively affecting germination and root development.
These findings suggest that Tranxilium may act as a biostimulant within moderate concentration ranges, whereas Zolpidem exhibits phytotoxicity at higher doses.
3.2.1. Radicle Length
The average radicle length of R. sativus varied depending on the type of pharmaceutical and the concentration applied (Figure 2).
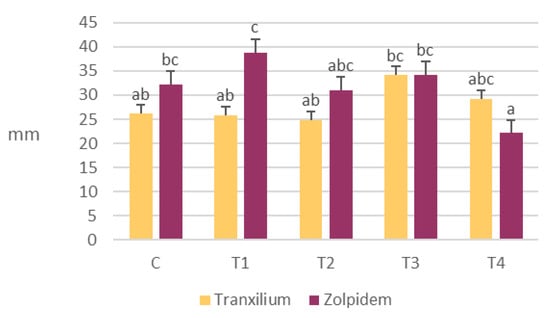
Figure 2.
Variation in average radicle length of R. sativus seeds at different concentrations of Tranxilium and Zolpidem. Error bars indicate the standard deviation. Different letters denote statistically significant differences between treatments at the 95% confidence level. C: control; T1: 5 mg L−1; T2: 10 mg L−1; T3: 15 mg L−1; and T4: 20 mg L−1.
Overall, treatment with Zolpidem resulted in greater root growth compared to Tranxilium. Specifically, concentrations of 5 and 15 mg L−1 of Zolpidem produced the longest radicle lengths, measuring 39 ± 16 mm and 34 ± 10 mm, respectively, both of which were statistically significantly greater than those observed in other treatments.
In contrast, the effect of Tranxilium on the radicle length was more variable, showing a moderate increase at 15 mg L−1 (34 ± 14.5 mm), but without a clear dose–response relationship.
3.2.2. Hypocotyl Length
Regarding hypocotyl length, the results showed greater differences between treatments (Figure 3).
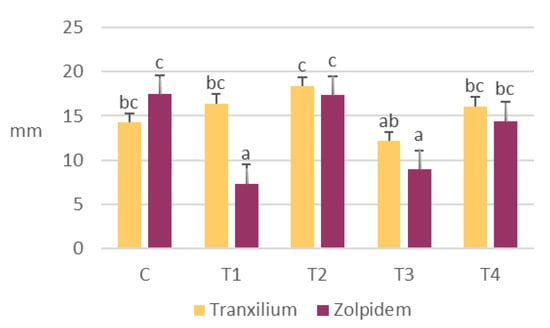
Figure 3.
Variation in mean hypocotyl length of R. sativus seeds at different concentrations of Tranxilium and Zolpidem. Error bars indicate the standard deviation. Different letters indicate statistically significant differences between parameters at a 95% confidence level. C: control; T1: 5 mg L−1; T2: 10 mg L−1; T3: 15 mg L−1; and T4: 20 mg L−1.
Zolpidem exhibited a more variable effect on hypocotyl development, with significant reductions at 5 and 15 mg L−1 (7 ± 3 mm and 9 ± 8 mm, respectively), both significantly lower than the control. In contrast, Tranxilium showed a more stable pattern, with values comparable to or slightly above the control, and a marked increase at 10 mg L−1 (18 ± 6 mm). However, a decrease at 15 mg L−1 (12 ± 7 mm) suggests an inhibitory effect at higher concentrations.
When comparing the effects of both pharmaceuticals on early seedling development, distinct responses were observed. Zolpidem significantly promoted radicle elongation at lower concentrations (5 and 15 mg L−1), while concurrently inhibiting hypocotyl growth, indicating a tendency to favor below-ground development. In contrast, Tranxilium exhibited a more balanced influence on both radicle and hypocotyl growth, though with less pronounced effects.
3.3. Greenness Index (SPAD)
3.3.1. Lettuce
The data collected reflects the evolution of SPAD readings for both pharmaceuticals across measurement dates. For plants treated with Tranxilium (Figure 4), a general trend of increasing chlorophyll content was observed with rising pharmaceutical concentration, particularly at moderate levels. Treatments with 5 and 10 mg L−1 showed a positive temporal progression, suggesting a cumulative physiological response to the compound.
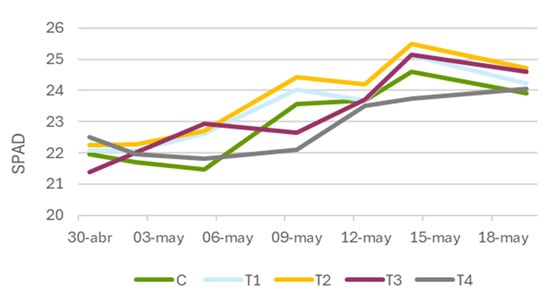
Figure 4.
Evolution of leaf greenness (SPAD) in Lactuca sativa (lettuce) treated with different concentrations of Tranxilium. C: control; T1: 5 mg L−1; T2: 10 mg L−1; T3: 15 mg L−1; and T4: 20 mg L−1.
The most significant difference from the control was recorded on 5 May (56 DAS), when treatment T2 (10 mg L−1) reached 22.7 SPAD units, compared to 21.48 in the control group. This supports the hypothesis that moderate Tranxilium doses may exert a biostimulant effect, potentially enhancing the biosynthesis of photosynthetic pigments.
However, the response was not linear. From 15 mg L−1 onward, SPAD values declined, and, although they occasionally remained above control levels, this drop suggests a toxicity threshold. Beyond this point, the compound may disrupt physiological processes, diminishing its stimulatory effect and potentially impairing chlorophyll production.
For plants treated with Zolpidem (Figure 5), a more moderate response was observed, though the general pattern mirrored that of Tranxilium at intermediate concentrations. An increase in SPAD values was recorded at 5 and 10 mg L−1 although these differences were not statistically significant. The highest difference occurred on 9 May (60 DAS), when treatment T2 (10 mg L−1) reached 23.3 SPAD units compared to 22.4 in the control. This suggests a mild stimulatory trend on chlorophyll biosynthesis at certain concentrations, though not significant.
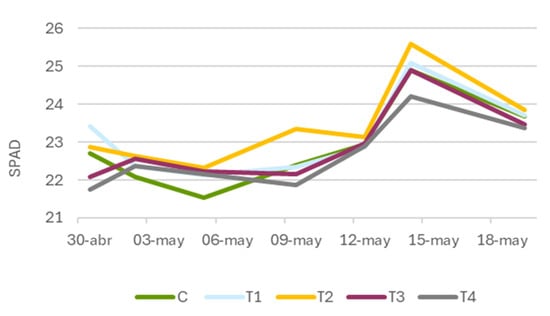
Figure 5.
Evolution of leaf greenness (SPAD) in Lactuca sativa (lettuce) treated with different concentrations of Zolpidem. C: control; T1: 5 mg L−1; T2: 10 mg L−1; T3: 15 mg L−1; and T4: 20 mg L−1.
At higher concentrations (≥15 mg L−1), SPAD values either stabilized or declined, indicating a threshold beyond which the compound’s positive effects diminish. This plateau or decrease may reflect phytotoxicity due to accumulation of the compound or its metabolites, ultimately interfering with normal chlorophyll production and photosynthetic function.
3.3.2. Wheat
The SPAD data for wheat showed a dynamic evolution depending on treatment and time.
The application of Tranxilium exhibited a stimulatory effect on the chlorophyll content at moderate doses (Figure 6), particularly at 10 mg L−1 (T2). From the beginning of the monitoring period (7 April), SPAD values in T2 progressively exceeded those of the control. This positive trend became more pronounced until 2 May, when the highest peak of the entire experiment was recorded: 43.8 SPAD units in T2, compared to 41.8 SPAD units in the control.
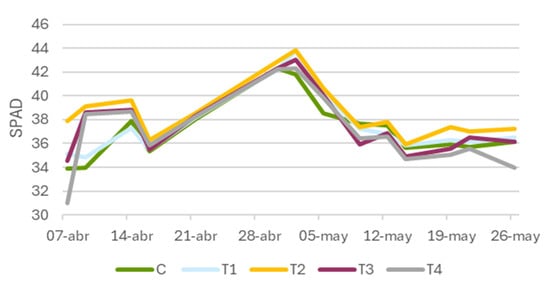
Figure 6.
Temporal evolution of leaf greenness (SPAD units) in Triticum aestivum (wheat) plants treated with different concentrations of Tranxilium. C: control; T1: 5 mg L−1; T2: 10 mg L−1; T3: 15 mg L−1; and T4: 20 mg L−1.
At higher concentrations (T3: 15 mg L−1 and T4: 20 mg L−1), the values remained relatively high, although they did not consistently exceed those of T2. For instance, on 26 May, the SPAD values in T2 still reached 37.3, whereas T4 dropped to 34.0, falling below the control (36.1 SPAD). These results suggest that, at higher doses, the compound may begin to interfere with cellular processes through metabolic disruption or physiological stress, affecting chlorophyll biosynthesis.
In the case of Zolpidem (Figure 7), similarly to Tranxilium, a clear upward trend was observed in all treatments during the initial stages. The peak of the trial occurred on 30 April (51 DAS), with 45.1 SPAD units in T1 (5 mg L−1) and 44.6 SPAD units in T2 (10 mg L−1), both significantly higher than the control (40.6 SPAD units). Notably, T2 showed the most stable and sustained response over time.
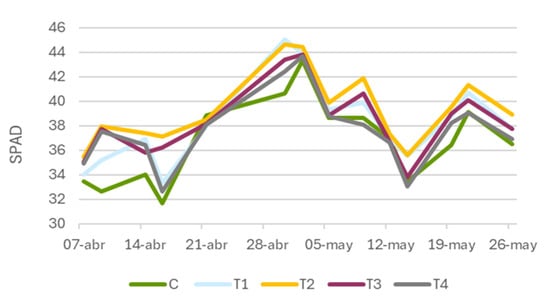
Figure 7.
Temporal evolution of leaf greenness (SPAD units) in Triticum aestivum (wheat) plants treated with different concentrations of Zolpidem. C: control; T1: 5 mg L−1; T2: 10 mg L−1; T3: 15 mg L−1; and T4: 20 mg L−1.
From 2 May (53 DAS), a gradual decline was observed in all treatments. Despite this, plants exposed to 10 mg L−1 better preserved their photosynthetic capacity during the decline phase, reinforcing the hypothesis that this concentration supports the maintenance of photosynthetic capacity in advanced developmental stages.
Higher concentrations (T3 and T4) did not produce further improvements and, on some dates, exhibited weaker responses, supporting the hypothesis of the possible accumulation of adverse effects at elevated doses.
3.3.3. Comparison Between Plant Species
This section presents a comparative interpretation of the physiological responses of wheat and lettuce to the same substances, rather than a formal statistical comparison, since both species differ in phenology and growth cycle.
Both crops responded positively to intermediate doses (especially 10 mg L−1) of the applied pharmaceuticals. In lettuce, the SPAD index increases were more gradual and sustained over time, especially with Tranxilium, showing an upward curve until the end of the trial. In contrast, in wheat, both pharmaceuticals caused an earlier and more pronounced increase, reaching maximum values by the end of April, followed by a gradual decline in the following weeks, showing an earlier but less stable reaction.
In both species, a loss of efficacy was observed at high concentrations (15–20 mg L−1), indicating a shared sensitivity to high doses. However, wheat’s response to Zolpidem was more variable than in lettuce, with less evident signs of toxicity even at 20 mg L−1. This suggests that wheat may have a greater physiological tolerance to these substances.
This response pattern is consistent with other studies reporting the existence of a hormetic-type curve, in which low concentrations may have neutral or slightly positive effects, but, beyond a specific threshold, adverse effects are induced [].
These findings highlight the importance of considering both concentration and exposure time when assessing the effects of emerging contaminants. Furthermore, they demonstrate that, although some new ECs may appear harmless at low doses, their progressive accumulation in ecosystems could compromise ecosystem health.
3.4. Fresh Biomass: Shoot and Root
3.4.1. Lettuce
Shoot biomass:
In the case of Tranxilium, the results (Table 1) show a significant increase in shoot weight. The highest value was recorded at 10 mg L−1 (12.48 ± 2.31 g), followed by 15 mg L−1 (12.02 ± 2.70 g). However, at 20 mg L−1, growth decreased sharply (6.37 ± 1.68 g), reaching levels similar to the control, indicating toxicity at high doses.

Table 1.
Aerial biomass (g), root biomass (g), and shoot–root biomass ratio of Lactuca sativa (lettuce) treated with different concentrations of Tranxilium and Zolpidem. Values are expressed as mean ± standard deviation. C: control; T1: 5 mg L−1; T2: 10 mg L−1; T3: 15 mg L−1; and T4: 20 mg L−1. Different lowercase letters within each column indicate statistically significant differences between doses for the same pharmaceutical at p < 0.05 (95% confidence level).
A similar pattern was observed with Zolpidem, where intermediate doses also promoted a significant increase in shoot weight, peaking at 10 mg/L (13.16 ± 2.80 g). At higher doses, inhibitory effects were again observed (7.10 ± 1.76 g), in this case causing a slight reduction compared to the control value (7.20 ± 1.68 g).
A multiple range test allowed the assignment of homogeneous groups. It was found that the control and 20 mg L−1 treatments shared the same letter (“a”), indicating no statistically significant differences between them.
Although both pharmaceuticals showed biostimulant effects at intermediate doses, no significant differences were detected between the two treatments, suggesting a similar physiological response of the plant to both compounds.
Root biomass:
The trend in the root biomass (Table 1) was parallel to the aerial biomass, with increases at moderate concentrations and a slight reduction at 20 mg L−1.
The highest root mass was recorded at 10 mg L−1 for both Tranxilium (2.02 ± 0.51 g) and Zolpidem (1.59 ± 0.53 g). Despite the decrease at 20 mg L−1, the values remained higher than the control in both cases (1.19 ± 0.35 g for Tranxilium and 0.96 ± 0.48 g for Zolpidem).
Although no statistically significant differences were found, possibly due to sample variability, differences in the root weight between treatments were observed. It is also clearly evident that the root biomass with Tranxilium was higher than that obtained with Zolpidem.
Shoot–root biomass ratio (SRBR):
The shoot–root biomass ratio is a useful indicator for assessing the balance in development between the shoot and root parts of the plant (Table 1).
In this analysis, Zolpidem showed significantly higher values than Tranxilium at most concentrations, indicating a greater allocation of resources toward shoot growth. The highest value was observed at 15 mg L−1 (9.40 ± 3.27), closely followed by the control (9.23 ± 3.95), both well above the values recorded with Tranxilium.
The statistical differentiation between treatments is reflected in the homogeneous groups: while the values corresponding to Zolpidem are grouped predominantly in the “c” category, those of Tranxilium are concentrated in the groups “a” and “ab”, showing a significant separation between the two pharmaceuticals.
These results reinforce the idea that Zolpidem more strongly promotes shoot biomass development over root growth, in contrast to Tranxilium, which induces a more balanced development between the two vegetative compartments.
3.4.2. Wheat
Shoot biomass:
In wheat, the shoot biomass (Table 2) also showed a dose-dependent response, particularly with Zolpidem, which reached its maximum value at 10 mg L−1 (2.60 ± 0.93 g), classified within homogeneous group “f”, statistically different from the control. Concentrations of 5 and 15 mg L−1 also promoted growth, though to a lesser extent, falling into the intermediate groups (“ef”, “cd”).

Table 2.
Aerial biomass (g), root biomass (g), and shoot–root biomass ratio of wheat treated with different concentrations of Tranxilium and Zolpidem. Values are expressed as mean ± standard deviation. C: control; T1: 5 mg L−1; T2: 10 mg L−1; T3: 15 mg L−1; and T4: 20 mg L−1. Different lowercase letters within each column indicate statistically significant differences between doses for the same pharmaceutical at p < 0.05 (95% confidence level).
Tranxilium also induced an increase in shoot weight up to the 10 mg L−1 dose (1.99 ± 0.54 g), classified in group “cd”, although the effects were more moderate compared to Zolpidem.
At 20 mg L−1, both treatments showed a marked decrease in shoot biomass, especially with Zolpidem (1.29 ± 0.47 g), under the control value, suggesting a potentially toxic effect. In contrast, Tranxilium showed a similar reduction (1.28 ± 0.48 g), but it was not statistically significant, as it shared the same group “a” with the control.
Root biomass:
As shown again (Table 2), at 10 mg L−1, both Zolpidem (1.40 ± 0.42 g) and Tranxilium (1.23 ± 0.20 g) significantly increased the root weight compared to the control, falling into distinct homogeneous groups (“d” and “c”, respectively). In this case, Zolpidem produced a more pronounced improvement, with a more consistent increase compared to Tranxilium.
The 20 mg L−1 dose in both treatments did not differ significantly from the control, sharing group “a”, supporting the existence of an optimal concentration range below which growth is promoted.
Shoot–root biomass ratio (SRBR):
The results (Table 2) show that Zolpidem tends to induce greater shoot development compared to Tranxilium, especially at low concentrations, although the highest value was recorded in the control (2.58 ± 1.51). However, as the concentration increased, index values progressively decreased, reaching levels similar to those observed with Tranxilium. From 10 mg L−1 onward, the treatments were statistically grouped in category “a”, indicating a loss of the differential effect.
These results reflect a greater ability of Zolpidem to favor shoot biomass development in wheat, particularly in the absence of stress caused by high doses. In contrast, Tranxilium exhibited a more uniform and balanced pattern.
3.4.3. Comparison Between Plant Species
At a comparative level, lettuce exhibited greater sensitivity in the shoot biomass, with more pronounced increases and a statistically homogeneous response in the root biomass, indicating that its aerial development is more susceptible to the presence of pharmaceuticals. In contrast, wheat showed a more balanced response between shoot and root parts, although with lower absolute increases.
Regarding the SRBR between lettuce and wheat, Zolpidem produced a more marked effect in lettuce, with significantly higher values (up to 9.40 ± 3.27) compared to those recorded in wheat (maximum of 2.58 ± 1.51). This indicates a greater promotion of shoot growth in lettuce. In both crops, Tranxilium showed a more stable and balanced pattern, with low values and no significant differences between concentrations. Overall, the results suggest that the response to Zolpidem is species-dependent, showing a more pronounced effect in leafy species such as lettuce compared to cereals like wheat.
3.5. Photosynthetic Pigments: Chlorophyll and Carotenoids
3.5.1. Lettuce
According to the analysis of variance (two-factor ANOVA), no statistically significant effects of the evaluated factors were found on the concentrations of chlorophyll a, chlorophyll b, and total carotenoids with a 95% confidence level (p > 0.05), as shown in Table 3.

Table 3.
p-values obtained from the analysis of variance (ANOVA) for each dependent variable in Lactuca sativa (lettuce) cultivation, considering the effects of pharmaceutical concentration (A), pharmaceutical type (B), and their interaction (A × B).
However, for chlorophyll a, a p-value close to the significance threshold was recorded (p = 0.0758) in relation to the pharmaceutical concentration, which may suggest a potential biological effect that does not reach statistical significance under the established experimental conditions. The type of pharmaceutical (p = 0.4212) and the interaction between both factors (p = 0.5507) did not show any influence on this variable.
In the case of chlorophyll b, neither concentration (p = 0.2384), type of pharmaceutical (p = 0.5797), nor interaction (p = 0.8386) showed statistically significant effects, suggesting that the observed variability cannot be attributed to the evaluated experimental factors, as occurred with total carotenoids, showing no statistically significant differences in any parameter.
The mean values of chlorophyll a (Table 4) show a slight upward trend in levels with an increasing concentration of both pharmaceuticals, especially with Zolpidem, with a maximum at 10 mg L−1 (21.50 ± 3.06). In the case of Tranxilium, the highest value also occurred at that concentration (18.06 ± 5.28), although with less difference with respect to the control. Despite these variations, the treatments were mostly grouped under the same groups (“a” and “ab”), which reinforces the absence of statistically significant differences.

Table 4.
Mean concentrations of chlorophyll a, chlorophyll b, and total carotenoids in Lactuca sativa (lettuce) grown under different concentrations of Tranxilium and Zolpidem. Values are expressed in μg mL−1 as mean ± standard deviation. C: control; T1: 5 mg L−1; T2: 10 mg L−1; T3: 15 mg L−1; and T4: 20 mg L−1. Different lowercase letters within each column indicate statistically significant differences between doses for the same pharmaceutical at p < 0.05 (95% confidence level). Total carotenoid values reported as <LOD indicate concentrations below the limit of detection.
The chlorophyll b content remained more stable. Slight increases were observed at 10 mg L−1 for both Tranxilium and Zolpidem (26.20 ± 4.49 and 25.81 ± 2.81, respectively), although these were not statistically significant compared to their respective controls.
As for total carotenoids, extremely low values were recorded. This may be due to measurement interference, as chlorophyll a and b absorb in the 470 nm region of the visible spectrum. Specifically, in protein complexes containing chlorophyll a and b, a secondary absorption peak has been observed around 472 nm. Therefore, if chlorophyll concentrations are overestimated or there is overlap in absorption bands, the correction applied in the formula may be excessive, resulting in erroneously low or even negative carotenoid values []. Furthermore, carotenoids are considered accessory pigments relative to chlorophylls, and, in mature leaves, their presence represents approximately one-third of the total chlorophyll (a + b) abundance. This supports the idea that their concentrations, even when real, may be close to the detection limit of the method, making them more susceptible to quantification errors [].
3.5.2. Wheat
For the wheat crop (Table 5), the analysis of variance (two-factor ANOVA) also revealed no statistically significant effects (p > 0.05) of the evaluated factors, pharmaceutical concentration, type, or interaction, on the concentrations of chlorophyll a, chlorophyll b, or total carotenoids.

Table 5.
p-values obtained from the analysis of variance (ANOVA) for each dependent variable in Triticum aestivum (wheat) cultivation, considering the effects of pharmaceutical concentration (A), pharmaceutical type (B), and their interaction (A × B).
Chlorophyll b showed a trend close to statistical significance regarding the concentration of the pharmaceutical (p = 0.0541), which could indicate an emerging biological response.
The mean values of chlorophyll a showed a slight increase at 10 mg L−1, particularly with Tranxilium (20.82 ± 6.72), compared to the respective controls. However, in the case of Zolpidem, the differences were not statistically significant and were grouped within the same homogeneous category (Table 6).

Table 6.
Mean concentrations of chlorophyll a, chlorophyll b, and total carotenoids in Triticum aestivum (wheat) grown under different concentrations of Tranxilium and Zolpidem. Values are expressed in μg mL−1 as mean ± standard deviation. C: control; T1: 5 mg L−1; T2: 10 mg L−1; T3: 15 mg L−1; and T4: 20 mg L−1. Different lowercase letters within each column indicate statistically significant differences between doses for the same pharmaceutical at p < 0.05 (95% confidence level). Total carotenoid values reported as < LOD indicate concentrations below the limit of detection.
Regarding chlorophyll b, the values were more variable among treatments, but positive peaks were noted with Zolpidem at the control concentration (25.46 ± 5.22) and 10 mg L−1 (24.4 ± 8.25), as well as with Tranxilium at 10 mg L−1 (24.52 ± 6.04).
As for total carotenoids, concentrations were very low across all treatments, as also reported for the lettuce crop.
4. Discussion
The observed trend, the stimulation of growth and physiological parameters at intermediate doses (10 mg L−1) and phytotoxic effects at higher concentrations (15–20 mg L−1), is consistent with a hormetic response pattern described in plants exposed to emerging contaminants and other bioactive compounds. In this context, Agathokleous [] reports that low doses of certain contaminants can activate signaling pathways associated with moderate stress, promoting photosynthesis and growth, whereas high doses induce oxidative stress and cellular damage, reducing plant performance.
In the germination assay with R. sativus, Tranxilium caused a significant increase in the germination index (GI), reaching up to 150.4% at 15 mg L−1, whereas Zolpidem remained close to control values at low doses but drastically reduced the GI at 20 mg L−1. These contrasting responses may be explained by the differences in root absorption and the interaction of each compound with the seedling’s primary metabolism. Previous studies have shown that certain benzodiazepine family pharmaceuticals can interact with ion channels and oxidative enzymes in plant tissues, affecting germination and root elongation processes []. Moreover, research on the uptake of pharmaceuticals by plants indicates that the absorption capacity can vary markedly between species and compounds. For instance, Rhodes et al. [] demonstrated that the accumulation of cephalexin in roots differed among lettuce, celery, and radish, with the root affinity and enzymatic transformation influencing overall uptake. These findings support the idea that the higher tolerance observed for Tranxilium compared to Zolpidem in R. sativus may be partly due to the differential absorption and metabolic handling within the seedlings.
Complementing these observations, a recent study on Lactuca sativa exposed to common pharmaceuticals including antibiotics and carbamazepine showed a similar concentration-dependent response: low to moderate concentrations had limited or slightly stimulatory effects on germination and root elongation, whereas higher concentrations produced clear phytotoxic effects []. This supports the generality of hormetic responses in plants subjected to pharmaceutical contaminants and underscores the importance of considering compound-specific toxicity thresholds when assessing the environmental risk.
Regarding the SPAD index, both cultivated species responded positively to intermediate doses, reaching maxima around 10 mg L−1, which suggests an enhancement in chlorophyll synthesis or preservation. This effect is consistent with observations by Liu et al. [] in wheat exposed to chlorinated organophosphate esters, where a transient increase in chlorophyll content was associated with antioxidant mechanisms and osmotic adjustment. However, at higher concentrations, the reduction in SPAD indicates potential damage to the photosynthetic machinery, likely due to the compound or the action thereof or their metabolites, whose effects on PSII through ROS compromise the repair of this vital structure [].
Consistent with SPAD changes, both the shoot and root biomass also exhibited a hormetic pattern. Lettuce showed greater sensitivity in the shoot biomass, whereas wheat displayed a more balanced response between above-ground and root tissues. This differential behavior may be linked to the morpho-anatomical traits of each species: dicotyledons such as lettuce typically have a larger leaf surface area and higher transpiration rates, which favor contaminant uptake and accumulation from irrigation water [], while monocotyledons like wheat possess deeper root systems and physiological adaptations that may buffer the initial impact of contaminants [].
Overall, the results reinforce the hypothesis that certain pharmaceuticals present as emerging contaminants, which can act as biostimulants at low doses, but also highlight that their accumulation or exposure at higher concentrations leads to adverse effects on plant growth and physiology. This dual response has also been documented by Herklotz et al. [] in plants exposed to pharmaceutical contaminants such as diclofenac, carbamazepine, or fluoxetine, suggesting that the phenomenon is generalizable across different classes of pharmaceutical compounds.
From an environmental perspective, the detection of physiological effects at relatively low concentrations is relevant, as levels ranging from ng L−1 to µg L−1 of these compounds have been routinely detected in WWTPs’ effluents and surface waters [,]. Although the concentrations used in this study are higher than those reported in natural environments, they serve as a model to understand the potential cumulative effects resulting from repeated exposures, considering that cultivated plants may be continuously irrigated with treated wastewater.
5. Conclusions
This study provides evidence that the pharmaceuticals Tranxilium and Zolpidem can significantly affect plant development, acting as emerging contaminants with physiological impacts on crop species. From early germination in Raphanus sativus to the later growth stages in lettuce (Lactuca sativa) and wheat (Triticum aestivum), both substances induced measurable alterations in morphological and physiological parameters.
At moderate concentrations (notably 10 mg L−1), both pharmaceutical exhibited biostimulant effects, enhancing traits such as leaf greenness (SPAD), root elongation, and fresh biomass accumulation. These findings are consistent with the hormetic response, where low to intermediate doses stimulate plant performance, while higher concentrations (15–20 mg L−1) caused phytotoxic effects, particularly in lettuce, indicating the existence of a dose-dependent threshold beyond which detrimental impacts occur.
The responses were clearly species-dependent. Zolpidem promoted shoot development more effectively, especially in dicotyledonous lettuce, whereas Tranxilium elicited more balanced effects on both the aerial and root biomass. The anatomical and physiological differences between dicots and monocots likely underlie these variations. A pigment analysis supported the SPAD measurements, showing increases in chlorophyll a and b at optimal doses, while carotenoids remained largely unaffected. These shifts reflect changes in plant metabolism, growth efficiency, and stress responses.
Overall, the results highlight the agronomic and ecological relevance of pharmaceutical contaminants in cropping systems. They emphasize the need for monitoring these compounds in agricultural soils and wastewater, and for establishing guidelines that define safe environmental concentrations. Given their potential for accumulation and persistence, further research is essential in order to assess the long-term impacts on crop productivity, food safety, and ecosystem resilience.
6. AI Tool Usage Statement
During the preparation of this manuscript/study, the author(s) used ChatGPT (OpenAI, GPT-4), Consensus (Consensus.app), and GitHub Copilot (GitHub, powered by OpenAI Codex) for the purposes of improving the clarity and style of the text, assisting with grammar and phrasing, and identifying relevant scientific literature. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15181916/s1.
Author Contributions
Conceptualization, P.M. and I.T.; methodology, P.M. and I.T.; software, P.M. and I.T.; validation, P.M.; formal analysis, P.M. and I.T.; investigation, P.M. and I.T.; resources, P.M.; data curation, I.T.; writing—original draft preparation, I.T.; writing—review and editing, P.M.; supervision, P.M.; project administration, P.M.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors would like to thank the ETSIAMB-UCLM for providing access to its practice fields, laboratory, and greenhouse facilities, which made this study possible.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ECs | Emerging contaminants |
| WWTPs | Wastewater treatment plants |
| PAIs | Pharmaceutical active ingredients |
| RGP | Relative germination percentage |
| RRG | Relative root growth |
| GI | Germination index |
| DAS | Days after sowing |
References
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive Review of Emerging Contaminants: Detection Technologies, Environmental Impact, and Management Strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef]
- Aleán Florez, J.D.; Márquez Mendez, D.S.; Burgos Nuñez, S.M.; Enamorado-Montes, G.H.; Marrugo Negrete, J.L. Presencia de Contaminantes Emergentes (CE) en los Distritos de Riego del Departamento de Córdoba, Colombia. Orinoquia 2021, 25, 57–63. [Google Scholar] [CrossRef]
- IDRICA. Las 4 Etapas de las Plantas de Tratamiento de Aguas Residuales. Available online: https://www.idrica.com/es/blog/plantas-de-tratamiento-de-aguas-residuales-etapas/ (accessed on 7 June 2025).
- Yang, W.; Bu, Q.; Shi, Q.; Zhao, R.; Huang, H.; Yang, L.; Tang, J.; Ma, Y. Emerging Contaminants in the Effluent of Wastewater Should Be Regulated: Which and to What Extent? Toxics 2024, 12, 309. [Google Scholar] [CrossRef]
- Montes, R.; Méndez, S.; Cobas, J.; Carro, N.; Neuparth, T.; Alves, N.; Santos, M.M.; Quintana, J.B.; Rodil, R. Occurrence of Persistent and Mobile Chemicals and Other Contaminants of Emerging Concern in Spanish and Portuguese Wastewater Treatment Plants, Transnational River Basins and Coastal Water. Sci. Total Environ. 2023, 885, 163737. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of Emerging Organic Contaminants on Freshwater Resources: Review of Recent Occurrences, Sources, Fate and Effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging Pollutants in the Environment: A Challenge for Water Resource Management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Wada, O.Z.; Olawade, D.B. Recent Occurrence of Pharmaceuticals in Freshwater, Emerging Treatment Technologies, and Future Considerations: A Review. Chemosphere 2025, 374, 144153. [Google Scholar] [CrossRef] [PubMed]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical Pollution in Aquatic Environments: A Concise Review of Environmental Impacts and Bioremediation Systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef]
- Żołnowski, A.C.; Bakuła, T.; Rolka, E.; Klasa, A. Effect of Mineral–Microbial Deodorizing Preparation on the Value of Poultry Manure as Soil Amendment. Int. J. Environ. Res. Public Health 2022, 19, 16639. [Google Scholar]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, Partition and Removal of Pharmaceuticals in Sewage Water and Sludge during Wastewater Treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef]
- Mourino, N.; Teijeiro, A.; Guerra-Tort, C.; Rey-Brandariz, J.; Candal-Pedreira, C.; Martín-Gisbert, L.; Mascareñas-García, M.; García, G.; Varela-Lema, L.; Pérez-Ríos, M. Consumption of Hypnosedatives in Spain: Characterization and Time Trends, 2005–2022. Gac. Sanit. 2024, 38, 102433. [Google Scholar] [CrossRef]
- Doctuo. Prospecto Tranxilium 50 Comprimidos Recubiertos. Available online: https://www.doctuo.es/prospectos/tranxilium-50-comprimidos-recubiertos (accessed on 7 June 2025).
- AEMPS. Zolpidem (Dalparan®, Stilnox®, Zolpidem EFG®): Riesgo de Somnolencia al Día Siguiente. Available online: https://www.aemps.gob.es/informa/notasinformativas/medicamentosusohumano-3/seguridad-1/2014/ni-muh_fv_05-2014-zolpidem/ (accessed on 8 June 2025).
- Adhikari, S.; Kumar, R.; Driver, E.M.; Bowes, D.A.; Ng, K.T.; Sosa-Hernandez, J.E.; Oyervides-Muñoz, M.A.; Melchor-Martínez, E.M.; Martínez-Ruiz, M.; Coronado-Apodaca, K.G.; et al. Occurrence of Z-Drugs, Benzodiazepines, and Ketamine in Wastewater in the United States and Mexico during the Covid-19 Pandemic. Sci. Total Environ. 2023, 857, 159351. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Perko, S.; Zupanc, M.; Zanoški Hren, M.; Landeka Dragičević, T.; Žigon, D.; Kompare, B.; Heath, E. Environmental Occurrence, Fate and Transformation of Benzodiazepines in Water Treatment. Water Res. 2012, 46, 355–368. [Google Scholar] [CrossRef]
- Fogliano, C.; Motta, C.M.; Venditti, P.; Fasciolo, G.; Napolitano, G.; Avallone, B.; Carotenuto, R. Environmental Concentrations of a Delorazepam-Based Drug Impact on Embryonic Development of Non-Target Xenopus laevis. Aquat. Toxicol. 2022, 250, 106244. [Google Scholar] [CrossRef]
- Tarish, M.; Ali, R.T.; Shan, M.; Amjad, Z.; Rui, Q.; Akher, S.A.; Al Mutery, A. Plant Tissues as Biomonitoring Tools for Environmental Contaminants. Int. J. Plant Biol. 2024, 15, 375–396. [Google Scholar] [CrossRef]
- Pernía, B.; Rojas-Tortolero, D.; Sena, L.; De Sisto, A.; Inojosa, Y.; Naranjo, L. Fitotoxicidad de HAP, Crudos Extra Pesados y sus Fracciones en Lactuca sativa: Una Interpretación Integral Utilizando un Índice de Toxicidad Modificado. Rev. Int. Contam. Ambient. 2018, 34, 79–91. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, M.; Wu, S.; Xiao, B.; Wang, X.; Sun, B.; Zhu, L. Metabolomics Reveals Antioxidant Stress Responses of Wheat (Triticum aestivum L.) Exposed to Chlorinated Organophosphate Esters. J. Agric. Food Chem. 2020, 68, 6520–6529. [Google Scholar] [CrossRef]
- Hoekstra, N.J.; Bosker, T.; Lantinga, E.A. Effects of Cattle Dung from Farms with Different Feeding Strategies on Germination and Initial Root Growth of Cress (Lepidium sativum L.). Agric. Ecosyst. Environ. 2002, 93, 189–196. [Google Scholar] [CrossRef]
- Zhu, J.; Tremblay, N.; Liang, Y. Comparing SPAD and atLEAF Values for Chlorophyll Assessment in Crop Species. Can. J. Soil Sci. 2012, 92, 645–648. [Google Scholar] [CrossRef]
- Callejas, R.; Kania, E.; Contreras, A.; Peppi, C.; Morales, L. Evaluación de un Método No Destructivo para Estimar las Concentraciones de Clorofila en Hojas de Variedades de Uva de Mesa. Idesia 2013, 31, 19–26. [Google Scholar] [CrossRef]
- Borowiak, K.; Gąsecka, M.; Mleczek, M.; Dąbrowski, J.; Chadzinikolau, T.; Magdziak, Z.; Goliński, P.; Rutkowski, P.; Kozubik, T. Photosynthetic Activity in Relation to Chlorophylls, Carbohydrates, Phenolics and Growth of a Hybrid Salix purpurea × triandra × viminalis 2 at Various Zn Concentrations. Acta Physiol. Plant 2015, 37, 1–12. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Agathokleous, E. The Rise and Fall of Photosynthesis: Hormetic Dose Response in Plants. J. For. Res. 2020, 32, 889–898. [Google Scholar] [CrossRef]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why Is Chlorophyll b Only Used in Light-Harvesting Systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Francisco, J.H. Archivos Latinoamericanos de Nutrición, Órgano Oficial de la Sociedad Latinoamericana de Nutrición. Arch. Latinoam. Nutr. 1966, 54, 149–155. [Google Scholar]
- Dudley, S.; Sun, C.; McGinnis, M.; Trumble, J.; Gan, J. Formation of Biologically Active Benzodiazepine Metabolites in Arabidopsis thaliana Cell Cultures and Vegetable Plants under Hydroponic Conditions. Sci. Total Environ. 2019, 662, 622–630. [Google Scholar] [CrossRef]
- Rhodes, G.; Chuang, Y.H.; Hammerschmidt, R.; Zhang, W.; Boyd, S.A.; Li, H. Uptake of Cephalexin by Lettuce, Celery, and Radish from Water. Chemosphere 2021, 263, 127916. [Google Scholar] [CrossRef]
- Tsytlishvili, K. Performing Acute Phytotoxicity of Widely Used Drugs on Germination and Root Elongation of Lactuca sativa L. J. Ecol. Eng. 2025, 26, 170–178. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yamamoto, H.; Allakhverdiev, S.I.; Inaba, M.; Yokota, A.; Murata, N. Oxidative Stress Inhibits the Repair of Photodamage to the Photosynthetic Machinery. EMBO J. 2001, 20, 5587–5594. [Google Scholar] [CrossRef]
- Sleight, H.; Boxall, A.B.A.; Toet, S. Uptake of Pharmaceuticals by Crops: A Systematic Review and Meta-Analysis. Environ. Toxicol. Chem. 2023, 42, 2091–2104. [Google Scholar] [CrossRef] [PubMed]
- Herklotz, P.A.; Gurung, P.; Vanden Heuvel, B.; Kinney, C.A. Uptake of Human Pharmaceuticals by Plants Grown under Hydroponic Conditions. Chemosphere 2010, 78, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Munzhelele, E.P.; Mudzielwana, R.; Ayinde, W.B.; Gitari, W.M. Pharmaceutical Contaminants in Wastewater and Receiving Water Bodies of South Africa: A Review of Sources, Pathways, Occurrence, Effects, and Geographical Distribution. Water 2024, 16, 796. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).