Effects of Nitrogen Nutrition on the Nutraceutical and Antinutrient Content of Red Beet (Beta vulgaris L.) Baby Leaves Grown in a Hydroponic System
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Experimental Design and Nutrient Solutions
2.3. Growth Analysis
2.4. Mineral Elements
2.5. Secondary Metabolites
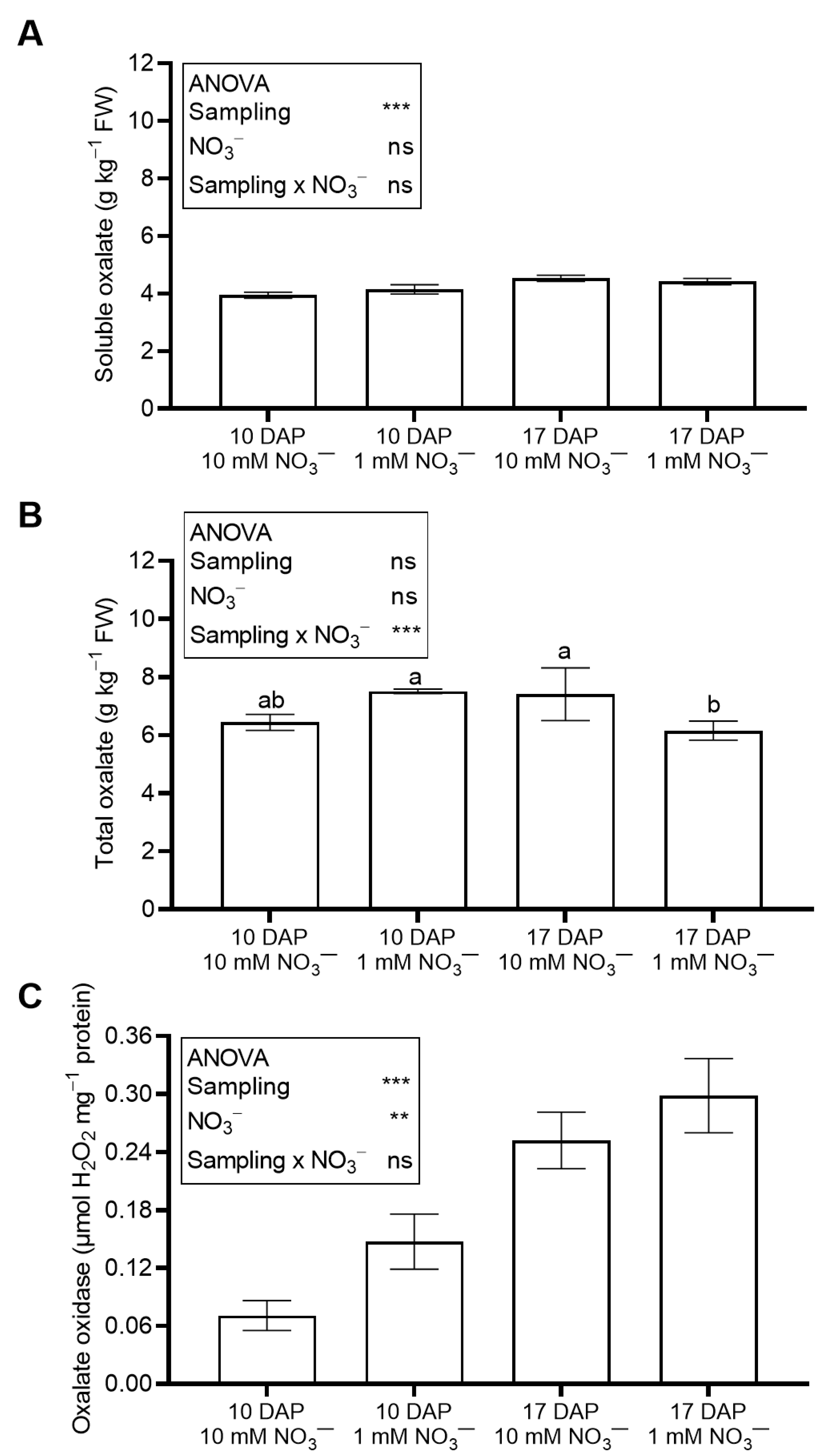
2.6. Ascorbic and Oxalic Acid
2.7. Oxalate Oxidase
2.8. Color Variation
2.9. Ethylene Evolution
2.10. Statistical Analysis
3. Results
3.1. Leaf Production and Quality
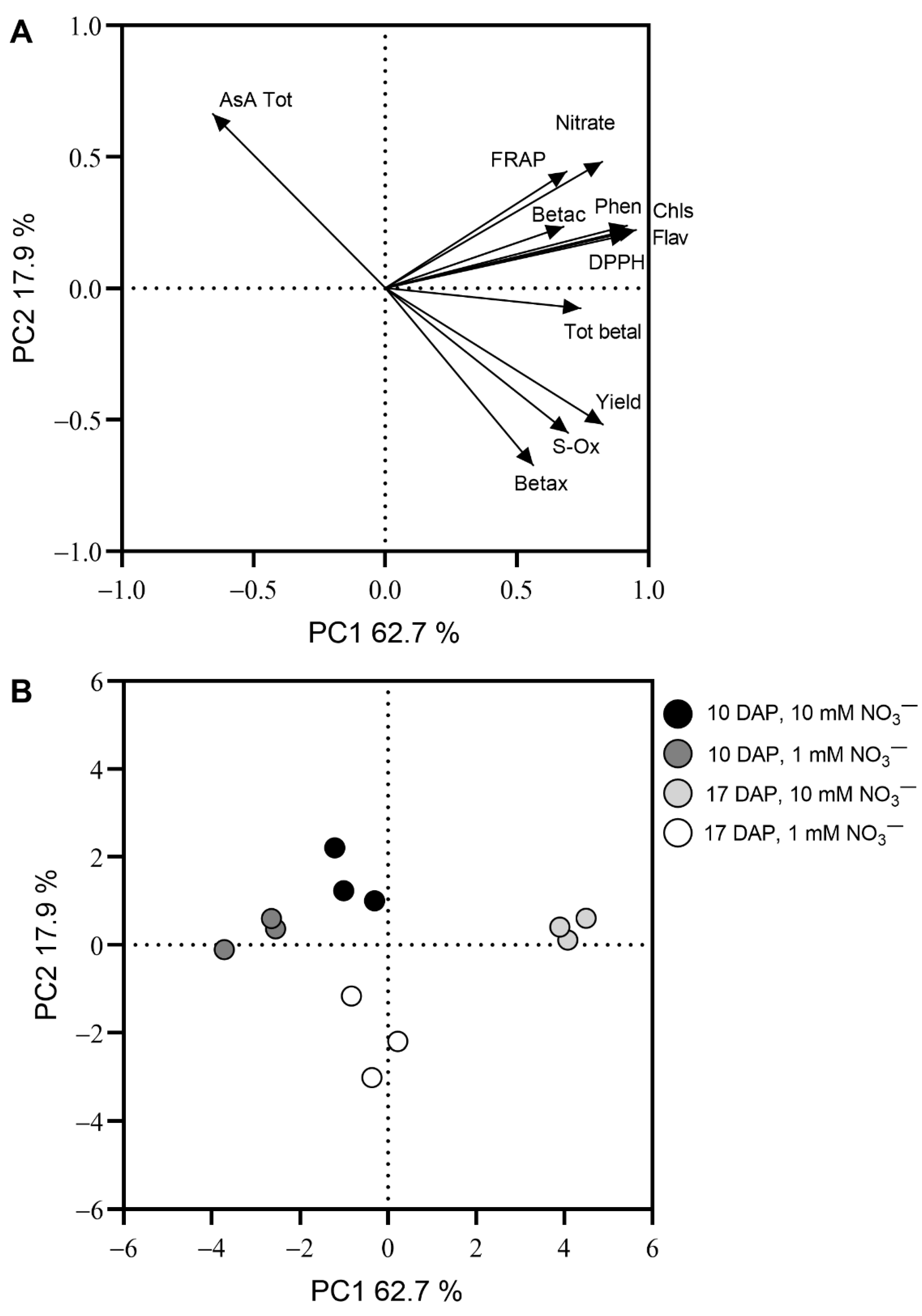
3.2. Principal Component Analysis
4. Discussion
4.1. Crop Growth and Yield
4.2. Leaf Organoleptic and Nutraceutical Quality
4.3. Leaf Antinutrient Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, M.; Rauf, M.; Akhtar, M.; Mukhtar, Z.; Saeed, N.A. Hazards of Nitrogen Fertilizers and Ways to Reduce Nitrate Accumulation in Crop Plants. Environ. Sci. Pollut. Res. 2020, 27, 17661–17670. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2024; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Y.; Zhang, X.; Li, T.; Grundy, S.; Yang, Q.; Cheng, R. A Review of Environment Effects on Nitrate Accumulation in Leafy Vegetables Grown in Controlled Environments. Foods 2020, 9, 732. [Google Scholar] [CrossRef]
- Gil, M.I.; Garrido, Y. Leafy Vegetables: Baby Leaves. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Academic Press: Cambridge, MA, USA, 2020; pp. 527–536. [Google Scholar] [CrossRef]
- Székely, D.; Máté, M.; Székely, D.; Máté, M. Red Beetroot (Beta vulgaris L.). In Advances in Root Vegetables Research; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Akbar Hussain, E.; Sadiq, Z.; Zia-Ul-Haq, M. Role of Betalain in Human Health. In Betalains: Biomolecular Aspects; Springer: Berlin/Heidelberg, Germany, 2018; pp. 97–107. [Google Scholar] [CrossRef]
- Fernández, M.V.; Jagus, R.J.; Agüero, M.V. Evaluation and Characterization of Nutritional, Microbiological and Sensory Properties of Beet Greens. Acta Sci. Nutr. Health J. 2017, 1, 37–45. [Google Scholar]
- Thiruvengadam, M.; Chung, I.M.; Samynathan, R.; Chandar, S.R.H.; Venkidasamy, B.; Sarkar, T.; Rebezov, M.; Gorelik, O.; Shariati, M.A.; Simal-Gandara, J. A Comprehensive Review of Beetroot (Beta vulgaris L.) Bioactive Components in the Food and Pharmaceutical Industries. Crit. Rev. Food Sci. Nutr. 2024, 64, 708–739. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; Petropoulos, S.A.; da Silveira, T.F.F.; Pires, T.C.S.P.; Ferreira, I.C.F.R.; Fernandes, Â.; Barros, L. Exploring the Biochemical Profile of Beta vulgaris L.: A Comparative Study of Beetroots and Swiss Chard. Plants 2025, 14, 591. [Google Scholar] [CrossRef] [PubMed]
- Ghanati, K.; Oskoei, V.; Rezvani- Ghalhari, M.; Shavali-Gilani, P.; Mirzaei, G.; Sadighara, P. Oxalate in Plants, Amount and Methods to Reduce Exposure; a Systematic Review. Toxin Rev. 2024, 43, 411–422. [Google Scholar] [CrossRef]
- Salgado, N.; Silva, M.A.; Figueira, M.E.; Costa, H.S.; Albuquerque, T.G. Oxalate in Foods: Extraction Conditions, Analytical Methods, Occurrence, and Health Implications. Foods 2023, 12, 3201. [Google Scholar] [CrossRef]
- Çalişkan, M. The Metabolism of Oxalic Acid. Turk. J. Zool. 2000, 24, 103–106. [Google Scholar]
- Kumar, V.; Irfan, M.; Datta, A. Manipulation of Oxalate Metabolism in Plants for Improving Food Quality and Productivity. Phytochemistry 2019, 158, 103–109. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Luo, Y.; Shi, H.; Li, Q.; Pinchu, C.; Li, X.; Yang, J.; Fan, W. Oxalate in Plants: Metabolism, Function, Regulation, and Application. J. Agric. Food Chem. 2022, 70, 16037–16049. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables; Springer: Boston, MA, USA, 2017; pp. 403–432. [Google Scholar]
- Carotti, L.; Pistillo, A.; Zauli, I.; Meneghello, D.; Martin, M.; Pennisi, G.; Gianquinto, G.; Orsini, F. Improving Water Use Efficiency in Vertical Farming: Effects of Growing Systems, Far-Red Radiation and Planting Density on Lettuce Cultivation. Agric Water Manag 2023, 285. [Google Scholar] [CrossRef]
- Jadhav, V.; Grondona, T.; Pistillo, A.; Pennisi, G.; Ghio, M.; Gianquinto, G.; Orsini, F. Optimizing Planting Density for Increased Resource Use Efficiency in Baby-Leaf Production of Lettuce (Lactuca sativa L.) and Basil (Ocimum basilicum L.) in Vertical Farms. Horticulturae 2025, 11, 343. [Google Scholar] [CrossRef]
- Martínez-Moreno, A.; Carmona, J.; Martínez, V.; Garcia-Sánchez, F.; Mestre, T.C.; Navarro-Pérez, V.; Cámara-Zapata, J.M. Reducing Nitrate Accumulation through the Management of Nutrient Solution in a Floating System Lettuce (Lactuca sativa L.). Sci Hortic 2024, 336, 113377. [Google Scholar] [CrossRef]
- Gustiar, F.; Septiani, D.; Agustina, H.; Adriansyah, F.; Ramadhani, F. Growth and Yield of Lettuce (Lactuca Sativa L.) Grown on Different Planting Media Volumes in a Floating Cultivation System. J. Lahan Suboptimal J. Suboptimal Lands 2025, 14, 44–50. [Google Scholar] [CrossRef]
- Faicán-Benenaula, M.A.; Hernández-Adasme, C.; Machuca, A.; Contreras, V.E. Survival and Internalization of Escherichia Coli in Baby Chard Subjected to Ozone Applications during Hydroponic System Cultivation. Eur J Hortic Sci 2024, 89, 1–10. [Google Scholar] [CrossRef]
- Puccinelli, M.; Carmassi, G.; Botrini, L.; Bindi, A.; Rossi, L.; Fierro-sañudo, J.F.; Pardossi, A.; Incrocci, L. Growth and Mineral Relations of Beta vulgaris Var. Cicla and Beta vulgaris ssp. Maritima Cultivated Hydroponically with Diluted Seawater and Low Nitrogen Level in the Nutrient Solution. Horticulturae 2022, 8, 638. [Google Scholar] [CrossRef]
- Abudureheman, A. Improving Sugar Beet (Beta vulgaris L.) Productivity by Inoculation with Gluconacetobacter spp.; Saint Mary’s University: Halifax, NS, Canada, 2012. [Google Scholar]
- Jones, J.B.; Wolf, B.; Mills, H.A. Plant Analysis Handbook; Micro–Macro Publishing: Athens, GA, USA, 1991. [Google Scholar]
- Olsen, S.R.; Sommers, E.L. Phosphorus. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. In Advances in Photosynthesis Research, Proceedings of the VIth International Congress on Photosynthesis, Brussels, Belgium, 1–6 August 1983; Springer: Berlin/Heidelberg, Germany, 1984; Volume 2, pp. 9–12. [Google Scholar]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Antioxidant Capacity of Lettuce Leaf Tissue Increases after Wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.G. HPLC-UV Method for the Simultaneous Determinations of Ascorbic Acid and Dehydroascorbic Acid in Human Plasma. Transl. Clin. Pharmacol. 2016, 24, 37–42. [Google Scholar] [CrossRef]
- Mirahmadi, S.F.; Hassandokht, M.; Fatahi, R.; Naghavi, M.R.; Rezaei, K. High and Low Oxalate Content in Spinach: An Investigation of Accumulation Patterns. J. Sci. Food Agric. 2022, 102, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Lateef, S.S. Analysis of Ascorbic Acid, Citric Acid and Benzoic Acid in Orange Juice. Agil. Appl. Solut. 2011, 1–12. [Google Scholar]
- Sathishraj, R.; Augustin, A. Oxalic Acid and Oxalate Oxidase Enzyme in Costus pictus D. Don. Acta Physiol. Plant. 2012, 34, 657–667. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Eyarkai Nambi, V.; Thangavel, K.; Shahir, S.; Geetha, V. Evaluation of Colour Behavior during Ripening of Banganapalli Mango Using CIE-Lab and RGB Colour Coordinates. J. Appl. Hortic. 2015, 17, 205–209. [Google Scholar] [CrossRef]
- Puccinelli, M.; Rosellini, I.; Malorgio, F.; Pardossi, A.; Pezzarossa, B. Hydroponic Production of Selenium-Enriched Baby Leaves of Swiss Chard (Beta vulgaris Var. Cicla) and Its Wild Ancestor Sea Beet (Beta vulgaris ssp. Maritima). Horticulturae 2023, 9, 909. [Google Scholar]
- Puccinelli, M.; Rosellini, I.; Malorgio, F.; Pardossi, A.; Pezzarossa, B. Iodine Biofortification of Swiss Chard (Beta vulgaris ssp. Vulgaris Var. Cicla) and Its Wild Ancestor Sea Beet (Beta vulgaris ssp. Maritima) Grown Hydroponically as Baby Leaves: Effects on Leaf Production and Quality. J. Sci. Food Agric. 2023, 103, 7888–7895. [Google Scholar] [CrossRef]
- Nemadodzi, L.E.; Araya, H.; Nkomo, M.; Ngezimana, W.; Mudau, N.F. Nitrogen, Phosphorus, and Potassium Effects on the Physiology and Biomass Yield of Baby Spinach (Spinacia oleracea L.). J. Plant Nutr. 2017, 40, 2033–2044. [Google Scholar] [CrossRef]
- Hessini, K.; Lachaâl, M.; Cruz, C.; Soltani, A. Role of Ammonium to Limit Nitrate Accumulation and to Increase Water Economy in Wild Swiss Chard. J. Plant Nutr. 2009, 32, 821–836. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Ma, B.; Ma, T.; Xian, W.; Hu, B.; Chu, C. Interplay between Ethylene and Nitrogen Nutrition: How Ethylene Orchestrates Nitrogen Responses in Plants. J. Integr. Plant Biol. 2023, 65, 399–407. [Google Scholar] [CrossRef]
- Imaseki, H. Control of Ethylene Synthesis and Metabolism. In New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 1999; Volume 33, pp. 209–245. [Google Scholar]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-Leaf and Multi-Leaf of Green and Red Lettuces Are Suitable Raw Materials for the Fresh-Cut Industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Damerum, A.; Chapman, M.A.; Taylor, G. Innovative Breeding Technologies in Lettuce for Improved Post-Harvest Quality. Postharvest Biol. Technol. 2020, 168, 111266. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Lončarić, Z.; Kristek, S.; Kulundžić, A.M.; Rebekić, A.; Antunović, M. Sugar Beet Root Yield and Quality with Leaf Seasonal Dynamics in Relation to Planting Densities and Nitrogen Fertilization. Agriculture 2021, 11, 407. [Google Scholar] [CrossRef]
- Bastos, E.L.; Schliemann, W. Betalains as Antioxidants. In Reference Series in Phytochemistry; Springer: Berlin/Heidelberg, Germany, 2022; pp. 51–93. [Google Scholar] [CrossRef]
- Dadáková, K.; Heinrichová, T.; Lochman, J.; Kašparovský, T. Production of Defense Phenolics in Tomato Leaves of Different Age. Molecules 2020, 25, 4952. [Google Scholar] [CrossRef]
- Khandaker, L.; Ali, M.B.; Oba, S. Influence of Cultivar and Growth Stage on Pigments and Processing Factors on Betacyanins in Red Amaranth (Amaranthus tricolor L.). Food Sci. Technol. Int. 2009, 15, 259–265. [Google Scholar] [CrossRef]
- Lefsrud, M.; Kopsell, D.; Wenzel, A.; Sheehan, J. Changes in Kale (Brassica oleracea L. Var. Acephala) Carotenoid and Chlorophyll Pigment Concentrations during Leaf Ontogeny. Sci. Hortic. 2007, 112, 136–141. [Google Scholar] [CrossRef]
- Sani, J.A.; Ibrahim, S.M.; Aisha, S.S.; Ali, M. Variation in Nutrient Contents between Early Growth and Mature Leaves of Spinacia oleracea (Spinach) and Lactuca sativa (Lettuce). Himal. J. Appl. Med. Sci. Res. 2023, 4, 24–28. Available online: https://himjournals.com/hjamr/948/948/articleID=1116/ (accessed on 1 July 2023).
- Salahas, G.; Papasavvas, A.; Giannakopoulos, E.; Tselios, T.; Konstantopoulou, H.; Savvas, D. Impact of Nitrogen Deficiency on Biomass Production, Leaf Gas Exchange, and Betacyanin and Total Phenol Concentrations in Red Beet (Beta vulgaris L. ssp. Vulgaris) Plants. Eur. J. Hortic. Sci. 2011, 76, 194–200. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent Advances in Betalain Research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Guillén-Román, C.J.; Guevara-González, R.G.; Rocha-Guzmán, N.E.; Mercado-Luna, A.; Pérez-Pérez, M.C.I. Effect of Nitrogen Privation on the Phenolics Contents, Antioxidant and Antibacterial Activities in Moringa Oleifera Leaves. Ind. Crops Prod. 2018, 114, 45–51. [Google Scholar] [CrossRef]
- Narvekar, A.S.; Tharayil, N. Nitrogen Fertilization Influences the Quantity, Composition, and Tissue Association of Foliar Phenolics in Strawberries. Front. Plant Sci. 2021, 12, 613839. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Olsen, K.M.; Slimestad, R.; Løvdal, T.; Bénard, C.; Verheul, M.; Bourgaud, F.; Robin, C.; Lillo, C. Influence of Repeated Short-Term Nitrogen Limitations on Leaf Phenolics Metabolism in Tomato. Phytochemistry 2012, 77, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chenard, C.H.; Kopsell, D.A.; Kopsell, D.E. Nitrogen Concentration Affects Nutrient and Carotenoid Accumulation in Parsley. J. Plant Nutr. 2005, 28, 285–297. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Zhang, Y.; Shao, J.Z.; Du, S. Effects of Nitrogen Levels and Nitrate/Ammonium Ratios on Oxalate Concentrations of Different Forms in Edible Parts of Spinach. J. Plant Nutr. 2005, 28, 2011–2025. [Google Scholar] [CrossRef]
- Linders, K.M.; Santra, D.; Schnable, J.C.; Sigmon, B. Variation in Leaf Chlorophyll Concentration in Response to Nitrogen Application Across Maize Hybrids in Contrasting Environments. Micropubl. Biol. 2024, 10, 17912. [Google Scholar] [CrossRef]
- Kulsum, M.U.; Baque, M.A.; Karim, M.A. Effects of Different Nitrogen Levels on the Leaf Chlorophyll Content Nutrient Concentration and Nutrient Uptake Pattern of Blackgram. Pak. J. Biol. Sci. 2007, 10, 250–254. [Google Scholar] [CrossRef][Green Version]
- Fathi, A.; Zeidali, E. Conservation Tillage and Nitrogen Fertilizer: A Review of Corn Growth, Yield and Weed Management. Cent. Asian J. Plant Sci. Innov. 2021, 1, 121–142. [Google Scholar] [CrossRef]
- Fathi, A. Role of Nitrogen (N) in Plant Growth, Photosynthesis Pigments, and N Use Efficiency: A Review. Agrisost 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Wang, N.; Fu, F.; Wang, H.; Wang, P.; He, S.; Shao, H.; Ni, Z.; Zhang, X. Effects of Irrigation and Nitrogen on Chlorophyll Content, Dry Matter and Nitrogen Accumulation in Sugar Beet (Beta vulgaris L.). Sci. Rep. 2021, 11, 16651. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Commission Regulation (EU) No 1258/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs. Off. J. Eur. Union 2011, 320, 15–17. [Google Scholar]
- Iammarino, M.; Berardi, G.; Vita, V.; Elia, A.; Conversa, G.; Di Taranto, A. Determination of Nitrate and Nitrite in Swiss Chard (Beta vulgaris L. Subsp. Vulgaris) and Wild Rocket (Diplotaxis tenuifolia (L.) DC.) and Food Safety Evaluations. Foods 2022, 11, 2571. [Google Scholar] [CrossRef]
- Hawkins-van der Cingel, G.; Walsh, S.B.; Eckardt, K.U.; Knauf, F. Oxalate Metabolism: From Kidney Stones to Cardiovascular Disease. Mayo Clin. Proc. 2024, 99, 1149–1161. [Google Scholar] [CrossRef]
- Bargagli, M.; Tio, M.C.; Waikar, S.S.; Ferraro, P.M. Dietary Oxalate Intake and Kidney Outcomes. Nutrients 2020, 12, 2673. [Google Scholar] [CrossRef]
- Abera, S.; Yohannes, W.; Chandravanshi, B.S. Effect of Processing Methods on Antinutritional Factors (Oxalate, Phytate, and Tannin) and Their Interaction with Minerals (Calcium, Iron, and Zinc) in Red, White, and Black Kidney Beans. Int. J. Anal. Chem. 2023, 2023, 6762027. [Google Scholar] [CrossRef]
- Simpson, T.S.; Savage, G.P.; Robert, S.; Vanhanen, L.P. Oxalate Content of Silver Beet Leaves (Beta vulgaris Var. Cicla) at Different Stages of Maturation and the Effect of Cooking with Different Milk Sources . J. Agric. Food Chem. 2009, 57, 10804–10808. [Google Scholar] [CrossRef]
- Solberg, S.O.; Yndgaard, F.; Axelsson, J. Nitrate and Oxalate in Germplasm Collections of Spinach and Other Leafy Vegetables. Emir. J. Food Agric. 2015, 27, 698–705. [Google Scholar] [CrossRef]
- Dembele, D.M.; Nguyen, T.T.A.; Bregard, A.; Naasz, R.; Jobin-Lawler, F.; Boivin, C.; Dorais, M. Effects of Growing Media and Fertilization Rates on the Organic Production of Baby Leafy Vegetables. Acta Hortic. 2022, 1348, 141–153. [Google Scholar] [CrossRef]
- Libutti, A.; Russo, D.; Lela, L.; Ponticelli, M.; Milella, L.; Rivelli, A.R. Enhancement of Yield, Phytochemical Content and Biological Activity of a Leafy Vegetable (Beta vulgaris L. Var. Cycla) by Using Organic Amendments as an Alternative to Chemical Fertilizer. Plants 2023, 12, 569. [Google Scholar] [CrossRef]
- Puccinelli, M.; Galati, D.; Carmassi, G.; Rossi, L.; Pardossi, A.; Incrocci, L. Leaf Production and Quality of Sea Beet (Beta vulgaris Subsp. Maritima) Grown with Saline Drainage Water from Recirculating Hydroponic or Aquaculture Systems. Sci. Hortic. 2023, 322, 112416. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhou, K.; Hu, Y.; Jin, R.; Lu, L.L.; Jin, C.W.; Lin, X.Y. Oxalate Synthesis in Leaves Is Associated with Root Uptake of Nitrate and Its Assimilation in Spinach (Spinacia oleracea L.) Plants. J. Sci. Food Agric. 2015, 95, 2105–2116. [Google Scholar] [CrossRef]
- Palaniswamy, U.R.; Bible, B.B.; McAvoy, R.J. Oxalic Acid Concentrations in Purslane (Portulaca oleraceae L.) Is Altered by the Stage of Harvest and the Nitrate to Ammonium Ratios in Hydroponics. Sci. Hortic. 2004, 102, 267–275. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ishii, Y.; Niimi, M.; Kawamura, O. Effect of Application Form of Nitrogen on Oxalate Accumulation and Mineral Uptake by Napiergrass (Pennisetum purpureum). Grassl. Sci. 2010, 56, 141–144. [Google Scholar] [CrossRef]
- Palaniswamy, U.R.; Bible, B.B.; Mcavoy, R.J. Effect of Nitrate: Ammonium Nitrogen Ratio on Oxalate Levels of Purslane. Trends New Crops New Uses 2002, 11, 453–455. [Google Scholar]
- Al Daini, H.; Norman, H.C.; Young, P.; Barrett-Lennard, E.G. The Source of Nitrogen (NH4+ or NO3−) Affects the Concentration of Oxalate in the Shoots and the Growth of Atriplex Nummularia (Oldman Saltbush). Funct. Plant Biol. 2013, 40, 1057–1064. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Gu, K.D.; Cheng, L.; Wang, J.H.; Yu, J.Q.; Wang, X.F.; You, C.X.; Hu, D.G.; Hao, Y.J. BTB-TAZ Domain Protein MDBt2 Modulates Malate Accumulation and Vacuolar Acidification in Response to Nitrate. Plant Physiol. 2020, 183, 750–764. [Google Scholar] [CrossRef]
- Wany, A.; Kumar Gupta, A.; Kumari, A.; Mishra, S.; Singh, N.; Pandey, S.; Vanvari, R.; Igamberdiev, A.U.; Fernie, A.R.; Gupta, K.J. Nitrate Nutrition Influences Multiple Factors in Order to Increase Energy Efficiency under Hypoxia in Arabidopsis. Ann. Bot. 2019, 123, 691–705. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y.; Zhang, Z.; Zhang, Y.; Jiang, Y.; Wang, S.; Wang, Y.; Sun, Z.; Lei, Z.; Gou, J.; et al. Transcriptomics and Metabolomics Reveal the Primary and Secondary Metabolism Changes in Glycyrrhiza Uralensis with Different Forms of Nitrogen Utilization. Front. Plant Sci. 2023, 14, 1229253. [Google Scholar] [CrossRef]
- Fontana, E.; Hoeberechts, J.; Nicola, S.; Cros, V.; Palmegiano, G.B.; Peiretti, P.G. Nitrogen Concentration and Nitrate/Ammonium Ratio Affect Yield and Change the Oxalic Acid Concentration and Fatty Acid Profile of Purslane (Portulaca Oleracea L.) Grown in a Soilless Culture System. J. Sci. Food Agric. 2006, 86, 2417–2424. [Google Scholar] [CrossRef]

| Nutrient Solution | ||
|---|---|---|
| 10 mM NO3− | 1 mM NO3− | |
| EC (mS/cm) | 2.4 | 2.3 |
| N-NO3 (mM) | 10.0 | 1.0 |
| P-PO4 (mM) | 1.5 | 1.5 |
| K (mM) | 9.0 | 8.0 |
| Ca (mM) | 4.5 | 4.5 |
| Mg (mM) | 2.0 | 2.0 |
| S-SO4 (mM) | 7.0 | 9.0 |
| Na (mM) | 0.8 | 0.8 |
| Cl (mM) | 0.7 | 0.7 |
| Fe (µM) | 40.0 | 40.0 |
| B (µM) | 40.0 | 40.0 |
| Cu (µM) | 3.0 | 3.0 |
| Zn (µM) | 10.0 | 10.0 |
| Mn (µM) | 10.0 | 10.0 |
| Mo (µM) | 1.0 | 1.0 |
| Sampling Time (DAP) | NO3− (mM) | Leaf FW (g m−2) | Leaf DW (g m−2) | Root DW (g m−2) | Total DW (g m−2) | LAI |
|---|---|---|---|---|---|---|
| 10 | 10 | 936.0 ± 50.7 | 38.4 ± 2.7 | 2.97 ± 0.39 | 41.3 ± 3.1 | 1.24 ± 0.16 c |
| 1 | 741.3 ± 26.8 | 33.3 ± 1.8 | 4.44 ± 0.47 | 37.8 ± 1.6 | 1.19 ± 0.02 c | |
| 17 | 10 | 2764.0 ± 225.3 | 146.7 ± 14.4 | 10.44 ± 0.90 | 157.1 ± 15.2 | 5.97 ± 0.64 a |
| 1 | 2264.0 ± 343.0 | 115.6 ± 14.9 | 13.98 ± 2.22 | 129.6 ± 15.3 | 4.04 ± 0.47 b | |
| MAIN EFFECT | ||||||
| 10 | 838.6 ± 50.5 b | 25.9 ± 1.8 b | 3.70 ± 0.43 b | 39.6 ± 1.7 b | 1.21 ± 0.07 b | |
| 17 | 2514.0 ± 214.9 a | 127.8 ± 11.6 a | 12.21 ± 1.33 a | 143.3 ± 11.4 a | 5.01 ± 0.56 a | |
| 10 | 1850.0 ± 421.6 a | 92.5 ± 25.1 a | 6.70 ± 1.73 b | 99.2 ± 26.8 a | 3.61 ± 1.10 a | |
| 1 | 1502.6 ± 373.6 b | 74.5 ± 19.6 b | 9.21 ± 2.36 a | 83.7 ± 21.6 b | 2.61 ± 0.67 b | |
| ANOVA | ||||||
| DAP | *** | *** | *** | *** | *** | |
| NO3− | * | * | * | * | * | |
| DAP x NO3− | ns | ns | ns | ns | * | |
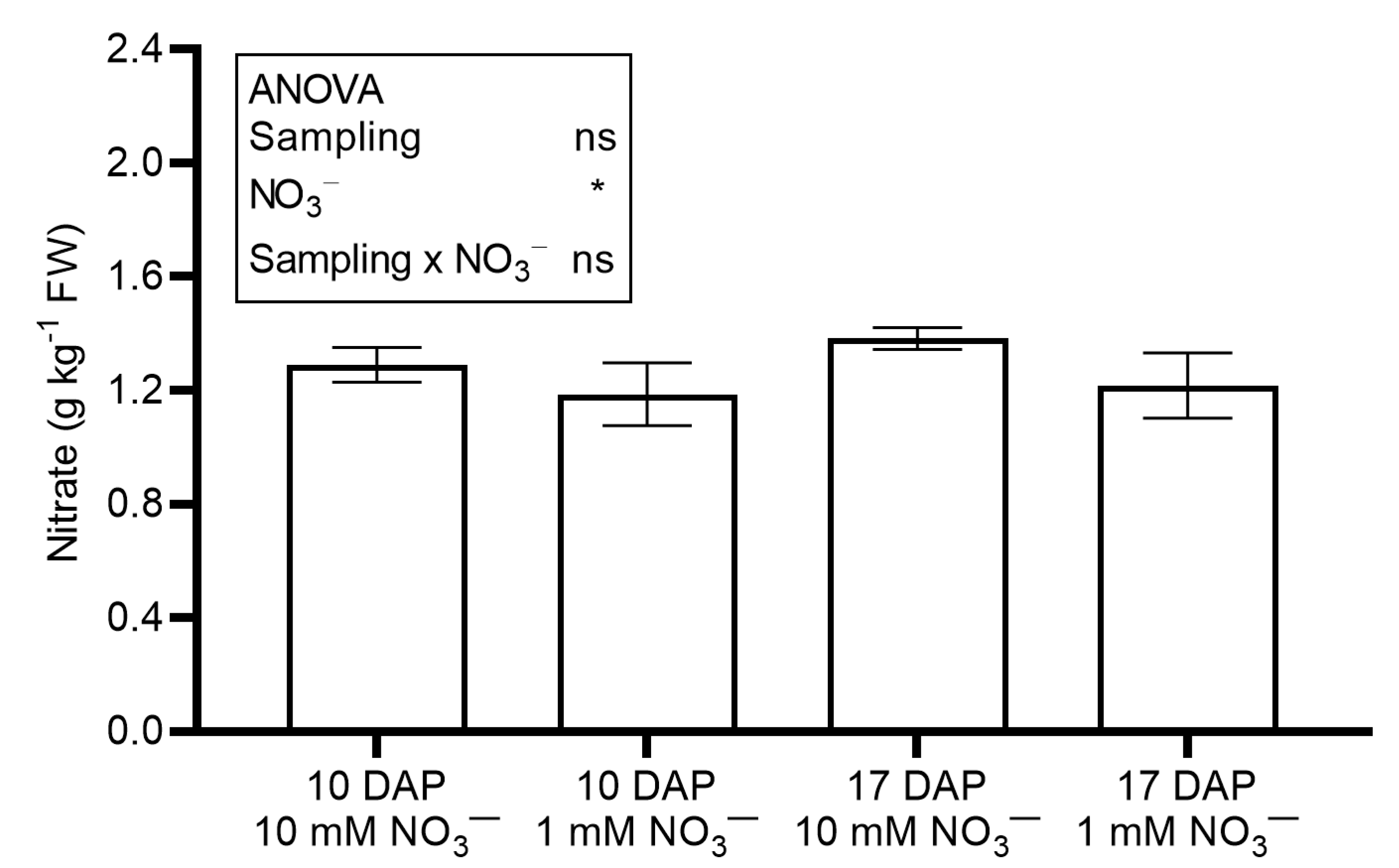
| Sampling Time (DAP) | NO3− (mM) | Moisture Content (%) | Succulence (kg m−2 FW) | Total Ascorbic Acid (g kg−1 FW) | Phenols (g kg−1) | Flavonoids (g kg−1) | FRAP (mmol Fe (II) kg−1) | DPPH (mmol TE kg−1) |
|---|---|---|---|---|---|---|---|---|
| 10 | 10 | 95.9 ± 0.1 | 0.737 ± 0.056 | 0.132 ± 0.009 | 1.84 ± 0.04 b | 0.552 ± 0.035 b | 28.0 ± 0.8 b | 7.96 ± 0.27 b |
| 1 | 95.5 ± 0.1 | 0.597 ± 0.018 | 0.108 ± 0.007 | 1.59 ± 0.05 b | 0.487 ± 0.039 b | 21.4 ± 1.3 c | 6.38 ± 0.39 bc | |
| 17 | 10 | 94.7 ± 0.1 | 0.441 ± 0.011 | 0.051 ± 0.002 | 2.76 ± 0.08 a | 0.854 ± 0.033 a | 45.0 ± 0.2 a | 11.54 ± 0.62 a |
| 1 | 94.9 ± 0.1 | 0.549 ± 0.1214 | 0.58 ± 0.002 | 1.63 ± 0.18 b | 0.518 ± 0.025 b | 24.5 ± 0.4 bc | 5.37 ± 0.22 c | |
| MAIN EFFECT | ||||||||
| 10 | 95.7 ± 0.07 a | 0.667 ± 0.037 a | 0.120 ± 0.007 a | 1.71 ± 0.06 b | 0.520 ± 0.028 b | 24.7 ± 1.6 b | 7.17 ± 0.41 b | |
| 17 | 94.8 ± 0.04 b | 0.495 ± 0.054 b | 0.0546 ± 0.002 b | 2.19 ± 0.27 a | 0.686 ± 0.077 a | 34.8 ± 4.6 a | 8.46 ± 1.41 a | |
| 10 | 95.3 ± 0.3 | 0.589 ± 0.071 | 0.091 ± 0.019 | 2.30 ± 0.21 a | 0.703 ± 0.071 a | 36.5 ± 3.8 a | 9.75 ± 0.86 a | |
| 1 | 95.2 ± 0.2 | 0.573 ± 0.056 | 0.088 ± 0.012 | 1.61 ± 0.09 b | 0.503 ± 0.022 b | 23.0 ± 0.9 b | 5.88 ± 0.30 b | |
| ANOVA | ||||||||
| DAP | *** | * | *** | ** | ** | *** | * | |
| NO3− | ns | ns | ns | *** | *** | *** | *** | |
| DAP x NO3− | ns | ns | ns | ** | ** | *** | *** | |
| Sampling Time (DAP) | NO3− (mM) | Chlorophylls (g kg−1) | Carotenoids (g kg−1) | Betacyanins (g kg−1) | Betaxantins (g kg−1) | Total Betalains (g kg−1) | Lightness | Chroma | Hue Angle |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 10 | 0.794 ± 0.046 b | 0.182 ± 0.012 b | 0.211 ± 0.006 a | 0.053 ± 0.005 | 0.263 ± 0.006 a | 26.4 ± 1.1 | 7.50 ± 0.12 | 15.6 ± 1.3 |
| 1 | 0.721 ± 0.039 b | 0.252 ± 0.015 ab | 0.135 ± 0.008 b | 0.039 ± 0.003 | 0.174 ± 0.005 b | 27.9 ± 0.9 | 8.93 ± 0.29 | 24.5 ± 2.0 | |
| 17 | 10 | 1.069 ± 0.013 a | 0.258 ± 0.025 a | 0.210 ± 0.002 a | 0.065 ± 0.004 | 0.275 ± 0.002 a | 27.5 ± 0.4 | 7.88 ± 0.31 | 37.9 ± 0.8 |
| 1 | 0.743 ± 0.002 b | 0.210 ± 0.011 ab | 0.185 ± 0.006 a | 0.075 ± 0.008 | 0.260 ± 0.002 a | 29.1 ± 1.1 | 8.73 ± 0.23 | 41.5 ± 0.5 | |
| MAIN EFFECT | |||||||||
| 10 | 0.758 ± 0.032 b | 0.217 ± 0.018 | 0.173 ± 0.018 b | 0.046 ± 0.004 b | 0.219 ± 0.020 b | 27.1 ± 0.7 | 8.22 ± 0.35 | 20.1 ± 2.2 b | |
| 17 | 0.906 ± 0.073 a | 0.234 ± 0.016 | 0.197 ± 0.006 a | 0.070 ± 0.004 a | 0.268 ± 0.004 a | 28.3 ± 0.6 | 8.30 ± 0.26 | 39.7 ± 0.9 a | |
| 10 | 0.932 ± 0.065 a | 0.220 ± 0.021 | 0.210 ± 0.003 a | 0.059 ± 0.004 | 0.269 ± 0.004 a | 26.9 ± 0.6 | 7.69 ± 0.17 b | 26.8 ± 5.0 b | |
| 1 | 0.732 ± 0.018 b | 0.231 ± 0.012 | 0.160 ± 0.012 b | 0.057 ± 0.009 | 0.217 ± 0.019 b | 28.5 ± 0.7 | 8.83 ± 0.17 a | 33.0 ± 3.9 a | |
| ANOVA | |||||||||
| DAP | ** | ns | ** | ** | *** | ns | ns | *** | |
| NO3− | *** | ns | *** | ns | *** | ns | ** | ** | |
| DAP x NO3− | ** | ** | ** | ns | *** | ns | ns | ns | |
| Sampling Time (DAP) | NO3− (mM) | N (g kg−1) | P (g kg−1) | K (g kg−1) | Ca (g kg−1) | Mg (g kg−1) | Na (g kg−1) | Fe (mg kg−1) | Mn (mg kg−1) | Zn (mg kg−1) | Cu (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 10 | 57.1 ± 0.3 | 21.01 ± 1.09a | 167.7 ± 5.9 b | 6.25 ± 0.10 a | 10.16 ± 0.16 a | 70.6 ± 3.7 b | 347.4 ± 9.8 a | 91.6 ± 6.0 | 68.3 ± 4.4 | 22.9 ± 2.3 |
| 1 | 51.1 ± 0.3 | 9.82 ± 0.45 b | 249.7 ± 7.5 a | 3.75 ± 0.09 c | 8.11 ± 0.09 c | 166.8 ± 1.9 a | 196.9 ± 11.6b | 115.5 ± 3.1 | 67.7 ± 1.7 | 26.1 ± 0.5 | |
| 17 | 10 | 55.3 ± 0.0 | 7.03 ± 0.15 b | 138.0 ± 2.6 c | 5.28 ± 0.18 b | 9.33 ± 0.24 b | 13.0 ± 0.6 d | 249.4 ± 4.2 b | 142.7 ± 5.1 | 96.1 ± 5.9 | 33.3 ± 1.3 |
| 1 | 50.8 ± 0.6 | 9.18 ± 0.43 b | 99.5 ± 2.4 d | 3.47 ± 0.10 c | 6.74 ± 0.30 d | 51.8 ± 1.9 c | 262.0 ± 30.1b | 167.5 ± 3.0 | 103.0 ± 3.5 | 38.7 ± 1.7 | |
| MAIN EFFECT | |||||||||||
| 10 | 54.1 ± 1.1 a | 15.42 ± 2.56a | 208.7 ± 18.8 a | 5.00 ± 0.56 a | 9.14 ± 0.46 a | 118.7 ± 21.6 a | 272.2 ± 34.3a | 103.5 ± 6.2 b | 68.0 ± 2.1 b | 24.5 ± 1.3 b | |
| 17 | 53.1 ± 0.9 b | 8.11 ± 0.52 b | 118.8 ± 8.8 b | 4.37 ± 0.41 b | 8.04 ± 0.60 b | 32.4 ± 8.7 b | 255.7 ± 13.9b | 155.1 ± 6.1 a | 99.6 ± 3.4 a | 36.0 ± 1.6 a | |
| 10 | 56.2 ± 0.4 a | 14.20 ± 3.16a | 152.9 ± 7.2 b | 5.76 ± 0.23 a | 9.74 ± 0.23 a | 41.8 ± 13.0 b | 298.4 ± 22.4 | 117.2 ± 12.0b | 82.2 ± 7.1 | 28.1 ± 2.6 b | |
| 1 | 51.0 ± 0.4 b | 9.50 ± 0.31 b | 174.6 ± 33.8 a | 3.61 ± 0.09 b | 7.43 ± 0.34 b | 109.3 ± 25.7 a | 229.4 ± 20.5 | 141.5 ± 11.8a | 85.4 ± 8.1 | 32.4 ± 2.9 a | |
| ANOVA | |||||||||||
| DAP | * | *** | *** | *** | *** | *** | ns | *** | *** | *** | |
| NO3− | ** | *** | ** | *** | *** | *** | ** | *** | ns | * | |
| DAP x NO3− | ns | *** | *** | * | ns | *** | ** | ns | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puccinelli, M.; Cuccagna, S.; Maggini, R.; Carmassi, G.; Pardossi, A.; Trivellini, A. Effects of Nitrogen Nutrition on the Nutraceutical and Antinutrient Content of Red Beet (Beta vulgaris L.) Baby Leaves Grown in a Hydroponic System. Agriculture 2025, 15, 1914. https://doi.org/10.3390/agriculture15181914
Puccinelli M, Cuccagna S, Maggini R, Carmassi G, Pardossi A, Trivellini A. Effects of Nitrogen Nutrition on the Nutraceutical and Antinutrient Content of Red Beet (Beta vulgaris L.) Baby Leaves Grown in a Hydroponic System. Agriculture. 2025; 15(18):1914. https://doi.org/10.3390/agriculture15181914
Chicago/Turabian StylePuccinelli, Martina, Simone Cuccagna, Rita Maggini, Giulia Carmassi, Alberto Pardossi, and Alice Trivellini. 2025. "Effects of Nitrogen Nutrition on the Nutraceutical and Antinutrient Content of Red Beet (Beta vulgaris L.) Baby Leaves Grown in a Hydroponic System" Agriculture 15, no. 18: 1914. https://doi.org/10.3390/agriculture15181914
APA StylePuccinelli, M., Cuccagna, S., Maggini, R., Carmassi, G., Pardossi, A., & Trivellini, A. (2025). Effects of Nitrogen Nutrition on the Nutraceutical and Antinutrient Content of Red Beet (Beta vulgaris L.) Baby Leaves Grown in a Hydroponic System. Agriculture, 15(18), 1914. https://doi.org/10.3390/agriculture15181914









