Abstract
This study evaluates the environmental impacts of different biomass pretreatment methods used for biomethane production using a life cycle assessment (LCA) approach. The three examined pretreatment technologies—CO2 injection, rumen fluid, and biological products—were applied to manure, alfalfa biomass, and winter wheat straw. The results indicate that cow manure pretreatment with CO2 increases fossil fuel depletion from 0.37 MJ/m3 to 17.31 MJ/m3 and increasing global warming potential by 1.08 kg CO2 eq/m3. Rumen fluid pretreatment moderately improves fossil fuel conservation but raises acidification (from 1.57 × 10−4 kg SO2 eq/m3 to 2.49 × 10−4 kg SO2 eq/m3) and eutrophication (from 2.67 × 10−5 kg PO4 eq/m3 to 5.3 × 10−5 kg PO4 eq/m3). Winter wheat straw CO2 pretreatment demonstrates the most favorable environmental profile, reducing human toxicity (from 0.1 kg 1,4-DB eq/m3 to 0.0058 kg 1,4-DB eq/m3) and minimizing fossil fuel depletion. The environmental trade-offs of biomethane production suggest that optimizing pretreatment strategies is essential to ensuring sustainable production.
1. Introduction
1.1. Background Information
Biogas production is increasingly recognized as a foundational component of sustainable energy solutions that can reduce the effects of climate change and facilitate the advancement of global environmental goals by transforming organic waste into clean, renewable energy. Biogas production is an increasingly vital component of sustainable energy strategies worldwide, offering both agricultural biowaste management solutions and renewable energy production. The conversion of this biomass into biogas, primarily biomethane, through anaerobic digestion, represents pathway for energy recovery and agricultural or industrial biowaste utilization. The transformation of biomass into biogas, utilizing a wide array of organic materials including agricultural residues [1], manures [2,3,4], and lignocellulosic waste [5,6,7], reduces emissions and the consumption of fossil fuels [8].
The Renewable Energy Directive (RED) of the European Union plays a crucial role in shaping the energy policies of member states, particularly in the promotion of renewable energy sources including biogas. Initially established in 2009, revised in 2018 (RED II) and 2023 (RED III) [9], the directive sets ambitious targets for the overall share of energy from renewable sources in the EU’s energy consumption. RED III raises the EU-wide renewable energy target from 32% to a minimum of 42.5% by 2030. Achieving this in the biogas energy sector can be approached in two primary ways. The first method involves expanding biogas production by increasing the input of agricultural or industrial biowaste in the existing biogas plants and increasing number of bioreactors. The second method focuses on enhancing the biogas production in the process itself, using advanced pretreatment methods for feedstocks to improve both the yield and the composition of the biogas produced.
Despite its potential, the efficiency of biogas production is often limited by the characteristics of the feedstocks used. Many biomass types are complex chemical and physical structures, particularly those containing high levels of lignin [10,11] and cellulose [12]. These materials are more resistant to microbial breakdown [13], and some of them can slow down the digestion process and reduce biogas yields [14].
Lignin and cellulose are integral components of plant cell walls and contribute to the structural integrity of biomass. However, their presence challenges for the anaerobic digestion process used in biogas production. Lignin effectively acts as a barrier [12], limiting the accessibility of cellulose and hemicellulose to the microorganisms that break these carbohydrates down into simpler compounds [15] that can be converted into biogas. Because of its complex structure, lignin is not only difficult to degrade but also binds [16] with other digestible organic materials, possibly further inhibiting [17] the overall biodegradation process.
Ammonia, particularly in its unionized form (NH3), is toxic to the methanogenic bacteria involved if increased to the certain amount in the anaerobic digestion process [18]. High concentrations of ammonia result from the breakdown of protein-rich materials such as certain manures, food wastes, and industrial effluents [19]. The toxicity primarily affects the archaea [20] that convert intermediate products like acetic acid into methane, thereby reducing the overall biogas yield. Managing ammonia levels in anaerobic digesters is crucial, especially in systems digesting high-protein feedstocks. Operational challenges include the need for careful feedstock selection and potential pretreatment to reduce protein content or dilute with water to reduce ammonia concentrations. Additionally, some digesters may require active ammonia removal or stripping [21,22] techniques to maintain ammonia at tolerable levels for microbial health.
Fats and proteins, while energy-rich substrates, may become challenge to the biogas production process when present in high concentrations. Fats (lipids) and proteins can inhibit microbial activity in the anaerobic digestion process and form of long-chain fatty acids (LCFAs) [23]. Fats can be hydrolyzed into glycerol and fatty acids, including LCFAs, which are known to be toxic to anaerobic bacteria, especially methanogens [24]. LCFAs can physically disrupt cell membranes of these microorganisms, inhibiting their function and reducing biogas production. Proteins break down into amino acids [25], which are further deaminated to produce ammonia and high ammonia levels can be toxic to methanogens and inhibit the overall methanogenesis process [26].
The variability in biomass composition depending on source and seasonality adds further complexity to optimizing the anaerobic digestion process, but this complexity can be managed by using carefully selected biomass pretreatment methods.
1.2. Biomass for Biogas Production Pretreatment Effect on Environment Pollution
Pretreatment methods have been developed to overcome these challenges, aiming to modify or break down the complex structures of biomass to enhance its biodegradability. Techniques such as mechanical maceration [27], supercritical carbon dioxide injection [28], chemical treatment with bases such as NaOH [29], and biological with hemicellulose degrading bacterium [29] pretreatment have been explored for their effectiveness in improving the anaerobic digestion process. These methods help expose the more digestible materials like cellulose and hemicellulose, increasing the rate and total yield of biogas production [30].
The environmental impact of biomass pretreatment is twofold [31]: while it is essential for increasing biogas yield, it also has potential to increase environmental pollution. The methods employed, ranging from the use of chemicals [32] to the application of high-energy [33] processes, can themselves consume resources and result in secondary emissions [34]. Understanding these impacts is critical, as the sustainability of biogas as a green energy source depends not only on its output but also on the ecological footprint of its production process.
Chemical pretreatments, such as acid or alkali treatments, are common for breaking down complex polymers like cellulose and lignin to increase biogas yields. For instance, sodium hydroxide (NaOH) pretreatment of wheat straw has been shown to improve methane production by up to 50% [35]. However, the use of chemicals for a biomass pretreatment can lead to environmental pollution through the generation of byproduct and the risk of chemical runoff into water bodies if managed incorrectly. Water transfer to solids pretreatment systems require energy for water transfer and product recovery and may affect aquatic ecosystems [36].
Physical pretreatments, including mechanical comminution and thermal processes like steam explosion, can enhance substrate digestibility but often require high energy inputs. Steam explosion pretreatment of corn stover [33], for example, increases biogas yields but consumes substantial energy [37], which may offset the environmental benefits due to associated greenhouse gas (GHG) emissions from energy production [4].
Biological pretreatments offer an eco-friendlier alternative by utilizing microorganisms or enzymes to degrade lignocellulosic biomass. The use of white-rot fungi, such as Trichoderma, can effectively break down lignin and improve methane yields without harmful chemical residues [38,39]. However, these methods are typically slower and more complex [14]. For instance, degradation products of lignin or hemicellulose can be toxic under certain conditions. Trichoderma can degrade lignin during pretreatment, leading to the release of phenolic compounds [40]. These compounds are toxic to methanogenic archaea, the microorganisms responsible for methane production in biogas systems [41]. Despite the experimental data showing positive results in biogas yield, the use in a real scale biogas plant may present challenges.
Each pretreatment method—mechanical, thermal, chemical, or biological—offers advantages in breaking down complex biomass structures but also presents potential drawbacks such as high energy consumption, generation of hazardous waste, and secondary emissions. Balancing these factors is critical to ensure that the production of biogas remains sustainable and does not inadvertently contribute to environmental pollution.
This article generalizes biomass pretreatment experiments performed by Žalys, Venslauskas, and Navickas using the life cycle assessment (LCA) technique. The aim of this study is to evaluate the environmental impacts associated with the utilization of chicken manure (ChM) [42], cow manure (CoM) [42], pig manure (PM) [42], alfalfa biomass (AB) [43], and winter wheat straw (WWS) [38] feedstocks in biogas production, focusing specifically on the effects of its pretreatment method. The selection of pretreatment methods was guided by the properties of each specific feedstock. For AB, a biological pretreatment using rumen fluid was implemented because the rumen microbiome contains a diverse array of lignocellulose-degrading microorganisms [44,45,46], such as cellulolytic bacteria and fungi [45], which facilitate the breakdown of complex plant polymers. In the case of manure feedstock, a chemical pretreatment involving carbon dioxide (CO2) injection was chosen due to the nitrogen present in manure [47,48]. CO2 can engage in multiple biochemical reactions of the AD process, such as the carbonic acid equilibrium [49]. The injection of CO2 can enhance the solubilization of nitrogenous compounds, improving the overall degradation process [42]. For wheat straw, biological product additives containing Trichoderma species were utilized. These fungi are known for their ability to secrete a wide spectrum of cellulolytic and hemicellulolytic enzymes [50], effectively degrading lignocellulosic materials and increasing the accessibility of fermentable sugars.
The aim of this study is to assess the environmental and energy-related impacts of different pretreatment methods—CO2 injection, rumen fluid, and biological product-based approaches—applied to agricultural feedstocks for enhancing biomethane production and to identify the most sustainable pretreatment method based on LCA results.
2. Materials and Methods
2.1. LCA Goal, Tasks, and Scope
The goal of this study is to assess the environmental and energy-related impacts of CO2, rumen fluid, and biological product-based methods applied to agricultural feedstocks for enhancing biomethane production, using an LCA approach. A life cycle assessment model was conducted in 2024 and 2025 using experimental data. This study focuses on evaluating how CO2 injection, rumen fluid, and a biological product affect the biogas production efficiency of feedstocks like ChM, CoM, and PM manures, along with AB and WWS biomass, while measuring improvements in biogas and methane yields and assessing environmental impacts using LCA. The environmental impact analysis of these pretreatment processes was performed using the SimaPro 9.1 process modeling software program. Information related to the necessary technological equipment and processes was extracted from the Ecoinvent v3 database [51], and the impacts were quantified using the CML-I baseline calculation model [52]. A scope of this study involved the 11 environmental impact categories—abiotic depletion potential (ADP); abiotic depletion (fossil fuels) potential (ADP(f)); global warming potential (GWP); ozone layer depletion potential (ODP); human toxicity potential (HTP); fresh water aquatic ecotoxicity potential (FWAEP); marine aquatic ecotoxicity potential (MAEP); terrestrial ecotoxicity potential (TEP); photochemical oxidation potential (POP); acidification potential (AP); eutrophication potential (EP)—resulting from the different pretreatment methods.
Each biomass type underwent a single pretreatment method, specifically selected based on the intrinsic properties and literature-supported compatibility with the method: CO2 injection was exclusively applied to manure substrates (cow, pig, and chicken manure) due to its effectiveness in enhancing nitrogen solubility and microbial accessibility. Rumen fluid pretreatment was specifically implemented for alfalfa biomass because of the rumen microbial consortium’s proven effectiveness in breaking down plant-based lignocellulosic structures. Biological products (Trichoderma spp.) were solely applied to winter wheat straw, chosen for their targeted enzyme-based lignocellulose degradation capabilities. This design choice was deliberate to assess each pretreatment’s efficacy in optimizing biomethane yield and reducing environmental impacts associated with specific biomass characteristics.
Pretreatment efficiency data for the injection of CO2 into feedstock efficiency were obtained from our series of experiments published by Žalys et. al. (2023) [42]. The summary of raw and pretreated feedstock (presented in the table as r—raw and p—pretreated) experiments results are presented in Table 1. A CO2 injection pretreatment of CoM, ChM, and PM was conducted in a separate continuously fed, agitated pretreatment reactor at 25 °C and ambient pressure. Results of raw and pretreated feedstock were recorded in parallel to evaluate process efficiency. Biogas yield was then assessed in an CSTR anaerobic digester under mesophilic conditions (37 °C).

Table 1.
Summary of pretreatment experiments results.
The rumen fluid pretreatment of AB feedstock efficiency data were obtained from the series of experiments published in Žalys et. al., (2023) [43]. The study evaluated the impact of rumen anaerobic bacteria inoculum on biogas yield and quality from AB. The study was performed on various pretreatment temperature ranges but for LCA, we used only raw feedstock versus the 3-day pretreatment. Both digester and pretreatment reactors were operated under mesophilic conditions (37 °C).
Data on winter wheat straw (WWS) pretreatment with selective biological products were obtained from a study published in Venslauskas et. al., (2024) [38]. The study evaluated the effect of a selective biological product (BP) on biogas production efficiency and biomethane potential, using WWS as a representative feedstock. The biological product, containing microorganisms from the Trichoderma genus, was applied to enhance microbial activity and improve the biodegradation of lignocellulosic material. Biogas yield was then assessed in an anaerobic digester under mesophilic conditions (37 °C).
The influence of pretreatment on biomethane yield is calculated as the percentage increase in biomethane yield resulting from pretreatment. This is determined by first calculating biomethane yield for both raw (r) and pretreated (p) feedstocks using the following formula:
For PM, pretreatment significantly enhanced biogas yields from feedstock, total solids, and volatile solids (p < 0.01), although methane concentration remained statistically unchanged (p = 0.486). CoM showed highly significant improvements (p < 0.001), with methane concentration approaching significance (p = 0.051), suggesting a possible trend. ChM exhibited statistically significant increases in all biogas yield metrics (p < 0.01) and a highly significant improvement in methane concentration (p < 0.001), indicating a strong response to pretreatment. AB also demonstrated robust improvements in yield metrics (p < 0.001), yet the methane concentration was not significantly affected (p = 0.644). Finally, WWS showed significant increases in biogas yield from feedstock and volatile solids (p < 0.01), while methane concentration changes were not statistically significant (p = 0.089).
2.2. Functional Unit
In LCA research, the functional unit (FU) represents the quantified function of the system to which all inputs and outputs are normalized, making its determination a crucial step in any LCA study [53]. This study focused on evaluating the environmental impacts associated with the pretreatment and anaerobic digestion of various feedstocks—including ChM, CoM, PM, AB, and WWS—each subjected to specific pretreatment methods based on their intrinsic properties.
Each pretreatment method causes electricity and heat consumption in the pretreatment processes, the construction and operation of pretreatment reactors (including materials like cement and steel reinforcement), and the associated infrastructure such as mixers, pumps, membranes, pipelines, and electrical installations. Because of the pretreatment process in all analyzed cases, biomethane yield was improved. For this reason, the FU as 1 m3 biomethane in the biogas production process was defined in the research.
2.3. Life Cycle Assessment System Boundaries
The LCA boundaries determine processes to be considered per the selected FU [54]. In our research, the biogas pretreatment process was chosen as the system’s primary function, and the system’s boundaries were limited to the pretreatment system’s onsite construction and production phases. Therefore, we incorporated the ‘gate-to-gate’ system boundary instead of ‘cradle-to-grave’, because we focused on the biogas pretreatment technology only (additional infrastructure and energy inputs) [55]. In this case, additional infrastructure includes the construction of additional concrete tanks, membrane for the roof and biogas storage, necessary pipelines, electrical installations, and other associated components. Electricity for operation is evaluated by additional electric motors power consumption necessary for pretreatment and it will be supplied from the grid, while heat will be provided by a biogas boiler, ensuring an integrated and sustainable approach to the pretreatment process. The CO2 used contains up to 1% methane [53]; however, in the baseline scenario, it will be emitted to atmosphere from biomethane equipment, and thus emissions from this source are not included in the life cycle analysis. Upstream processes associated with the pretreatment system have been excluded due to data constraints. Differences in feedstock delivery to the bioreactor technologies—whether using liquid feedstocks (e.g., manures) or dry feedstocks (e.g., AB or WWS)—were not taken into account because the LCA was performed in the pretreatment system boundaries.
The results presented in this study are valid only within the scope of the selected LCA approach and must be interpreted in light of its inherent limitations. Specifically, the adopted gate-to-gate system boundary excludes upstream processes such as biomass cultivation, transport, digestate handling, and downstream utilization, potentially underestimating the overall environmental impacts across the full life cycle. These methodological choices, while necessary for focus and comparability, introduce uncertainties that should be acknowledged when interpreting the findings.
2.3.1. CO2 Pretreatment LCA Boundaries
The LCA of the carbon dioxide pretreatment system was specifically applied to evaluate the construction and operation phases for ChM, CoM, and PM. The agitation system of pretreatment reactor consists of four submersible agitators, each with a power rating of 5 kW. These agitators programmed to operate in 15 min cycles, ensuring optimal mixing of the substrate within the reactor while minimizing energy usage. During each cycle, two agitators are active, consuming 10 kWh of energy for one hour of operation. The intermittent operation helps achieve effective mixing and prevents substrate stratification while maintaining an energy-efficient process. Substrate circulation pump with an electrical power of 7.5 kW is integrated into the system that provides pretreatment reactor with feedstock. Additionally, a gas blower with a power capacity of 11 kW is employed to circulate carbon dioxide within the system. This gas circulation enhances the efficiency of the pretreatment process by improving gas–liquid mass transfer, promoting microbial activity and also improving agitation. The pretreatment reactor itself is designed with a recessed configuration, providing a total volume of 990 m3, of which 900 m3 is the useful working volume. To complete the setup, a membrane for the roof is being constructed, alongside necessary pipelines, electrical installations, and other associated infrastructure. A structural diagram of the CO2 pretreatment LCA boundaries is illustrated in Figure 1.
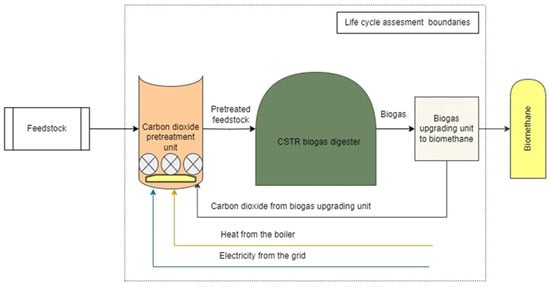
Figure 1.
Structural diagram of CO2 pretreatment LCA boundaries.
Electricity for the operation will be supplied from the national grid, while heat will be provided by a biogas boiler, ensuring an integrated and sustainable approach to the pretreatment process. The CO2 absorbed by the feedstock during the pretreatment process is excluded from life cycle analyses because the CO2 generated from biogas is regarded as carbon neutral [56].
2.3.2. Rumen Fluid Pretreatment LCA Boundaries
LCA boundaries for rumen fluid pretreatment of AB are shown in Figure 2. The agitation and feedstock input processes utilize the same resources as those required in the CO2 pretreatment reactor, with the exception of the biogas blower, which is not needed in this configuration. The infrastructure for the rumen fluid pretreatment also includes the installation of a gas-tight membrane roof. The reactor itself is designed with a total volume of 330 m3, of which 300 m3 serves as the useful working volume. The volume of pretreatment reactor designed using the retention time for the rumen fluid pretreatment process of 3 days, to be consistent with the conditions established in laboratory research [43], ensuring optimal treatment efficacy and scalability.
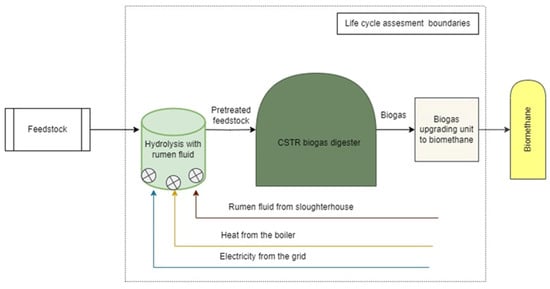
Figure 2.
Structural diagram of rumen fluid pretreatment LCA boundaries.
Electrical energy required for operating the mixers and circulation pump will be delivered from the national power grid. Thermal energy needs, however, will be met by a biogas boiler used for bioreactors heating.
It is important to note that the transportation of rumen fluid is excluded from the LCA. This is because the microbial community present in the rumen fluid serves as an inoculum for the pretreatment reactor, and once established, the system can sustain itself without the need for continuous addition of rumen fluid [57]. The initial inoculation provides a source of lignocellulose-degrading microorganisms, such as cellulolytic bacteria and fungi, which are critical for the effective breakdown of AB into fermentable substrates for biogas production [27,35].
2.3.3. Biological Product Pretreatment LCA Boundaries
In an up-scaled system, the pretreatment process for WWS with a BP (Figure 3) would involve a sealed, temperature-controlled pretreatment bioreactor designed for large-scale operations. A batch load pretreatment reactor is designed to cope with a total of 140 t feedstock. The straw would be incubated in the reactor for 48 h and then kept additional 5 days under partially sealed conditions, maintaining a 25.0 ± 0.5 °C temperature for bacteria to grow. Automated systems for temperature control and monitoring of pH and moisture levels would ensure consistency throughout the process. After 7 days, the pretreated straw would be unloaded using frontal loader and prepared for anaerobic digestion through direct feeding into biogas digesters.
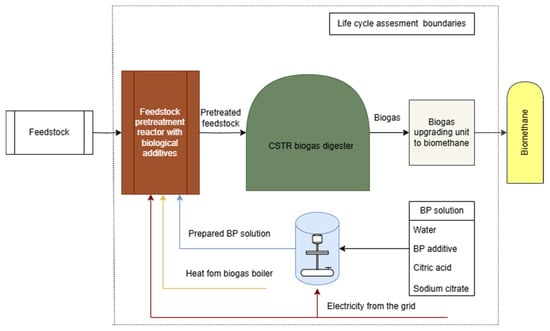
Figure 3.
Structural diagram of BP pretreatment LCA boundaries.
The BP solution is prepared in a dedicated mixing tank, where the biological product concentrate is diluted with deionized water and stabilized using citric acid and sodium citrate to achieve a pH of 5.10, maintaining the same 1:250 dilution ratio used in the experiment. The prepared BP solution is applied through spray nozzles or injectors to ensure even distribution. According to the data published in [38], 5 g of air-dried WWS was used, which was treated with 50 g of the prepared BP solution which gives a ratio of 1:10. For a 140 t of WWS feedstock, 1400 m3 of prepared BP solution would be necessary. A part of the BP liquid solution would be recirculated in the system.
2.4. Life Cycle Inventory
The Life Cycle Inventory (LCI) comprises the system inventory, considering within a described biogas pretreatment boundary for LCA. The LCI, as described in Table 2, shows the features with the quantitative terms against the selected FU. The activity data were quantified for 20 years by 8760 h/year, including operation energy consumption and biomethane upgrade equipment energy consumption. Additionally, the reduction in energy consumption in the biomethane purification process due to increased methane concentration in biogas using pretreatment was compared to raw feedstock and yearly biomethane production. The biomethane production process includes electrical energy for biogas compressing and cooling. The energy consumption for the biomethane upgrading unit is interpolated linearly between the provided experimental values for methane contents of 50%, 60%, and 70% from the research performed by Buivydas et.al. [53]. The experimental results presented in Section 2.1 were used for the unit process datasets.

Table 2.
Inventory for FU of 1 m3 biomethane.
The LCI also included consumption of the pretreatment inoculant and chemicals for BP. Over the lifetime of the biomethane production process, a total of 51,100 t of WWS was used as a feedstock. The pretreatment phase required 511,000 m3 of inoculant, composed of 1498.2 t of citric acid, 2912.7 t of sodium citrate, and 2044 t of a biological product. For the LCI analysis, the quantities of these inputs were normalized to its functional unit—their consumption per one cubic meter of biomethane produced. The calculated inoculant requirement was 2.04 L/m3 of biomethane, with contributions of 0.006 L/m3 for citric acid, 0.0116 L/m3 for sodium citrate, and 0.0081 L/m3 for the biological product. These values provide a quantitative basis for evaluating the environmental impacts and resource efficiency of the pretreatment process within the biomethane production system.
3. Results and Discussion
3.1. LCA of Manure CO2 Pretreatment Comparison to Raw Feedstock
Abiotic depletion, both in terms of elemental resources and fossil fuels, shows similar trend among the three manure types. Pretreatment technologies generally increase energy consumption, with CoM exhibiting the most substantial rise in ADP(f) from 0.37 MJ (rCoM) to 17.31 MJ (pCoM) (Table 3). This result shows that pretreatment processes demand additional energy inputs which may be larger than gain in biogas after pretreatment. ChM also showed an increase from 0.3 MJ to 4.93 MJ, while PM showed the same trend and increased by 6.1 MJ. These results also reflect the LCA results on grid electrical energy consumption. If the biogas plant were equipped with an on-site cogeneration unit operating in island mode—producing electricity solely from biogas—then any increase in biogas yield could potentially offset the energy requirements for the pretreatment process [58].

Table 3.
LCA results of CoM pretreatment with CO2.
Pretreated PM manure showed a significant increase in GWP values from 0.055 kg CO2 eq (rPM) to 0.45 kg CO2 eq (pPM) (Table 4). CoM followed a similar trend with a rise by 1.08 kg CO2 eq. The elevated GWP in pretreated manure may be linked to CO2 used during the process not being completely offset by enhanced biomethane yields. Studies such as Holm-Nielsen et al. (2009) [59] confirm that biomass pretreatment increases methane production but also emphasizes the role of biogas upgrading technologies in minimizing fugitive emissions.

Table 4.
LCA results of PM pretreatment with CO2.
HTP and FWAEP values differ widely among the three manure types. ChM exhibited a reduction in HTP after pretreatment, decreasing from 0.065 to 0.046 kg 1,4-DB eq. Conversely, CoM toxicity rose sharply by 6.63 × 10−2 following pretreatment. MAEP impacts were highest for CoM, with values increasing by 264.0 kg 1,4-DB eq after pretreatment.
AP increased for all pretreated manures. The highest increase was in the case of pCoM with a value of 1.05 × 10−3 kg SO2 eq. EP exhibited a similar pattern.
Given these results, CoM appears to be the least environmentally favorable feedstock for CO2 pretreatment (Table 5) due to its high energy demands, increased GWP, and severe ecotoxicity impacts.

Table 5.
LCA results of ChM pretreatment with CO2.
3.2. LCA of Alfalfa Pretreatment Using Rumen Fluid
The LCA results of rAB and pretreated AB with rumen fluid are presented in Table 6. Both feedstocks demonstrate negative values in the ADP category (−7.78 × 10−6 kg Sb eq forpAB and −7.42 × 10−6 kg Sb eq for rAB), indicating resource savings rather than depletion. These resource savings result from biomethane production displacing resource intensive energy sources, with slightly greater savings observed for pAB compared to rAB.

Table 6.
LCA results of AB pretreatment with rumen fluid.
Muhammad et. al. explained that preliminary studies on LCA highlighted that the lignocellulose-based bio-refiner process could help save up to 60% emissions of GHG in comparison with the fossil fuel system [60]. Our LCA evaluation data corroborate this statement, as pretreatment reduced ADP by −3.58 × 10−7 kg Sb eq.
Biomethane from both feedstocks contributes positively to emissions (0.17 kg CO2 eq for pAB and 0.099 kg CO2 eq for rAB), with rAB exhibiting a lower impact, suggesting that pretreatment increases greenhouse gas emissions. In terms of ODP, both samples exhibit negative impacts (−6.52 × 10−7 kg CFC-11 eq for pAB and −6.28 × 10−7 kg CFC-11 eq for rAB), reflecting beneficial displacement effects, with pAB achieving slightly higher savings resulting in improvement using pretreatment technology. HTP, reduced for both samples, showed a difference between pAB and rAB of −4.29 × 10−3 kg 1,4-DB eq.
FWAEP reveals small positive contributions to toxicity (0.029 kg 1,4-DB eq for pAB and 0.024 kg 1,4-DB eq for rAB), indicating that pretreatment increases this impact by 5.03 × 10−3. MAEP shows substantial positive impacts (122.8 kg 1,4-DB eq for pAB and 110.7 kg 1,4-DB eq for rAB), with pAB showing a larger impact. TEP demonstrates net reductions in toxic substances for both samples (−3.48 × 10−3 kg 1,4-DB eq for pAB and −3.34 × 10−3 kg 1,4-DB eq for rAB), with pAB performing slightly better. POP shows small contributions for both feedstocks (2.05 × 10−4 kg C2H4 eq for pAB and 1.96 × 10−4 kg C2H4 eq for rAB), with rAB showing better results.
AP and EP indicate the higher impacts pAB.
3.3. LCA of WWS Pretreatment with Biological Products Comparison to Raw Feedstock
The results of LCA are presented Table 7. Biological product pretreatment decreased ADP by −3.88 × 10−6 kg Sb eq. BP pretreatment has a minimal impact on ADP(f) with only a 1.05 × 10−2 MJ increase. This suggests that the pretreatment process does not significantly increase fossil energy demand, making it a sustainable step in terms of energy consumption.

Table 7.
LCA results of WWS pretreatment with BP.
ODP for WWS straw pretreatment decreases by −2.7 × 10−7 kg CFC-11 eq suggesting that pretreatment avoid release of substances that contribute to stratospheric ozone breakdown.
The LCA evaluation data reveals an increase in TEP after pretreatment, with an impact rising from 3.4 × 10−4 to 2.9 × 10−3 kg 1,4-DB eq. This rise suggests that the pretreatment process introduces or amplifies pollutants that adversely affect soil ecosystems.
The environmental benefits of WWS pretreatment with BP are most pronounced in categories such as HTP and MAEP, where reductions are achieved. Conversely, the increases in ADP and GWP raise concerns about the sustainability of the pretreatment process. These results suggest that the materials and energy inputs for BP pretreatment may outweigh its environmental benefits unless sourced sustainably.
3.4. Comparison of Pretreatment Technologies in LCA Perspective
ADP(f) (Figure 4) increased for CO2-pretreated manure, particularly for CoM, which showed an increase from 0.37 MJ (rCoM) to 17.31 MJ (pCoM). PM and ChM also exhibited increases of 6.1 MJ and 4.93 MJ, respectively. In contrast, rumen fluid pretreatment of AB displayed a relatively lower increase in ADP(f) (1.04 MJ), suggesting a more sustainable profile. BP pretreatment of WWS had the lowest increase (1.05 × 10−2 MJ), making it the most favorable option in terms of fossil energy conservation.
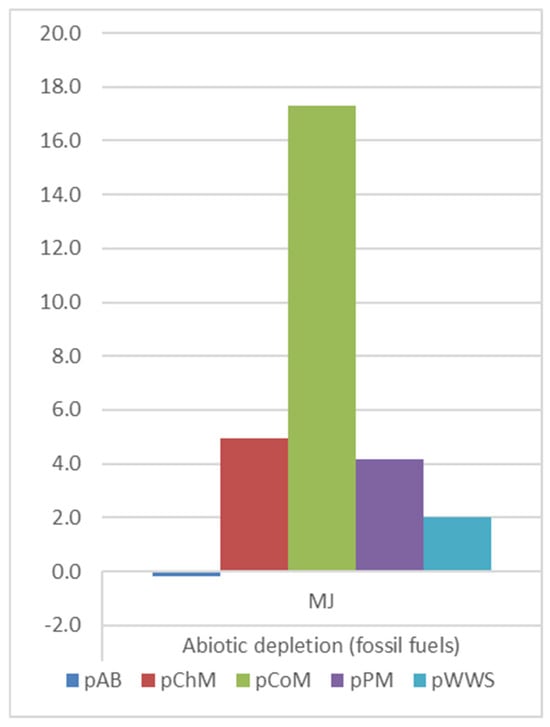
Figure 4.
ADP(f) comparison on pretreatment methods.
The GWP (Figure 5) values highlight increases in CO2 eq emissions for all pretreated manure feedstocks. CoM exhibited the highest increase, from 0.22 kg CO2 eq (rCoM) to 1.30 kg CO2 eq (pCoM), followed by PM (from 0.055 to 0.45 kg CO2 eq) and ChM (from 0.21 to 0.52 kg CO2 eq). Alfalfa biomass pretreatment showed an increase from 0.099 kg CO2 eq (rAB) to 0.17 kg CO2 eq (pAB), while BP pretreatment of WWS resulted in a moderate increase from 0.34 kg CO2 eq to 0.38 kg CO2 eq. The higher GWP associated with CO2 pretreatment suggests that additional energy inputs and fugitive emissions might offset the gains from increased biogas yields.
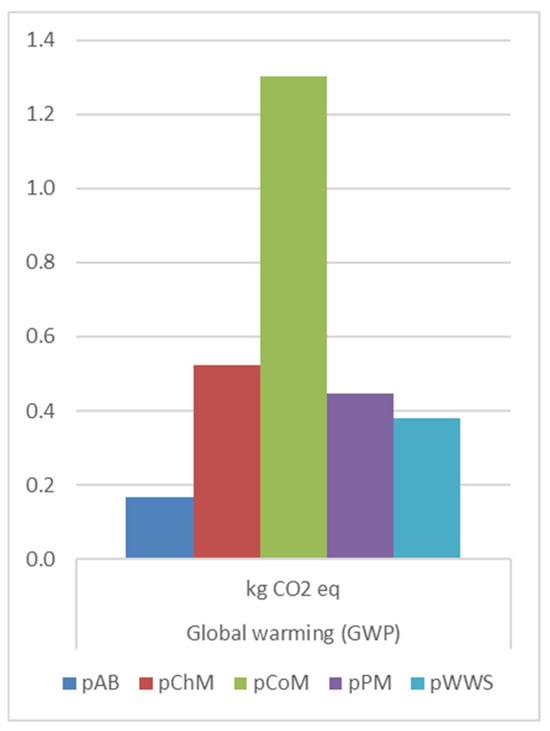
Figure 5.
GWP results comparison on pretreatment methods.
MAEP (Figure 6) showed the highest increase for CO2 pretreatment of CoM, with a rise of 264 kg 1,4-DB eq, followed by PM (increase of 1.3 × 102 kg 1,4-DB eq) and ChM (increase of 104 kg 1,4-DB eq). Alfalfa biomass pretreatment displayed a smaller increase (12.1 kg 1,4-DB eq). BP pretreatment of WWS showed a reduction in MAEP, making it the most environmentally beneficial method in this category.
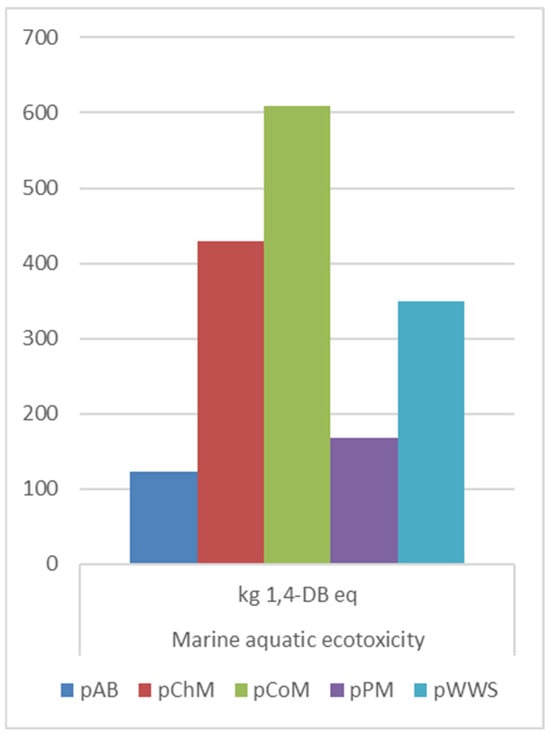
Figure 6.
MAEP comparison on pretreatment methods.
AP (Figure 7) increased across all pretreatment methods, with CoM displaying the highest increase (1.05 × 10−3 kg SO2 eq), followed by PM (4.6 × 10−4 kg SO2 eq) and ChM (2.5 × 10−4 kg SO2 eq). Alfalfa biomass and WWS BP pretreatment exhibited relatively lower increases, suggesting these technologies might be preferable in reducing acidification related impacts.
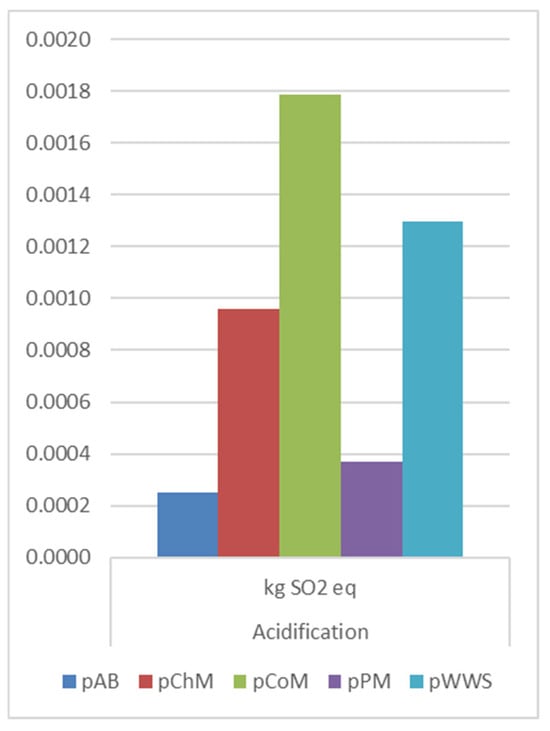
Figure 7.
AP comparison on pretreatment methods.
EP (Figure 8) also followed a similar trend, with the highest increase observed in CO2 pretreatment of CoM (5.07 × 10−4 kg PO4 eq). PM and ChM also exhibited increased eutrophication impacts (2.4 × 10−4 kg PO4 eq and 1.6 × 10−4 kg PO4 eq, respectively).
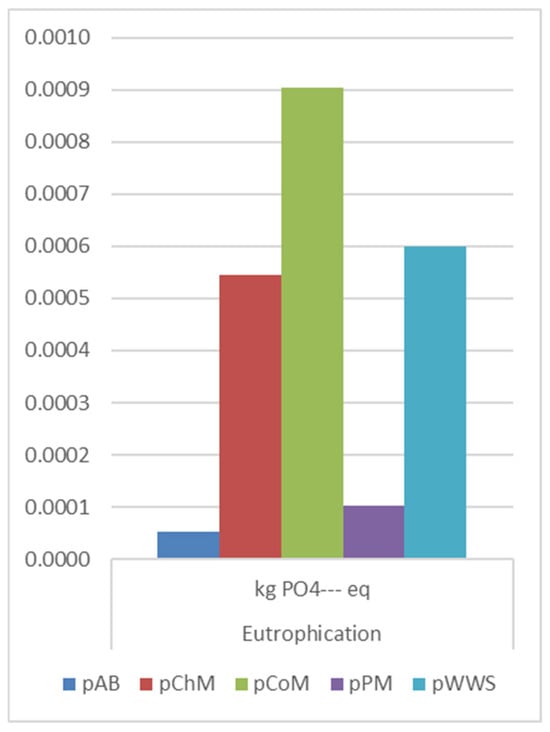
Figure 8.
EP comparison on pretreatment methods.
Alfalfa biomass pretreatment showed a moderate increase (2.62 × 10−5 kg PO4 eq), while WWS BP pretreatment displayed a reduction (−1.76 × 10−4 kg PO4 eq), making it the best alternative in this category.
3.5. Advancing and Optimizing Pretreatment Processes
Process optimization and energy integration should be considered for improving the efficiency of actual pretreatment systems. For example, finding optimal process parameters (e.g., temperature, residence time, chemical dosages) can reduce energy consumption and also bioreactors sizes necessary for pretreatment. Integrating energy recovery (e.g., using excess biogas in cogeneration systems operating in island mode or solar power) can offset energy demands, reducing the overall grid electricity input and improving the energy balance.
In this LCA, the electrical energy consumption of agitators and gas blowers was assessed based on actual installed power. However, the real power demand may be optimized through the implementation of frequency converters, which can reduce energy consumption by adjusting agitator or gas blower motor speed according to process requirements. This approach enhances energy efficiency and may contribute to lower overall environmental impacts in biogas production systems.
Heat recovery may also improve energy efficiency in feedstock pretreatment systems. An effective method is using digestate-feedstock heat exchangers, which transfer thermal energy from the outgoing warm digestate to the incoming feedstock. This process reduces the external heat demand for maintaining optimal pretreatment temperatures, improving overall energy efficiency.
To reduce chemical consumption in the real-scale BP pretreatment system, real-time pH monitoring and automated dosing systems should be implemented to precisely control the amount of chemical addition.
Environmental performance, techno-economic assessments should be conducted for further research to evaluate capital and operational costs, payback periods, and potential revenue from biomethane increase and byproducts (digestate) or energy recovery. This integrated approach would help to ensure that any process improvements are not only environmentally beneficial but also economically viable.
4. Conclusions
While all pretreatment methods enhance biogas production, their environmental trade-offs vary. CO2 pretreatment of manure exhibits the highest environmental burdens, making it a less sustainable choice. Rumen fluid pretreatment of alfalfa biomass presents a more balanced approach, whereas BP pretreatment of WWS emerges as the most favorable option, offering lower fossil fuel depletion, reduced human toxicity, and minimized ecotoxicity impacts. Future studies should focus on optimizing pretreatment techniques to maximize biogas yield and methane concentration while minimizing negative environmental externalities. Additionally, integrating energy recovery methods and improving process efficiencies can enhance the sustainability of biomethane production.
Author Contributions
Conceptualization, B.Ž. and K.N.; methodology, B.Ž. and K.V.; investigation, B.Ž.; resources, B.Ž. and K.V.; writing—original draft preparation B.Ž. and K.V.; writing—review and editing, B.Ž., K.N. and K.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge the support received from Vytautas Magnus University, Agriculture Academy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Su, H.; Tan, F.; Xu, Y. Enhancement of Biogas and Methanization of Citrus Waste via Biodegradation Pretreatment and Subsequent Optimized Fermentation. Fuel 2016, 181, 843–851. [Google Scholar] [CrossRef]
- Rafique, R.; Poulsen, T.G.; Nizami, A.S.; Asam, Z.-u.-Z.; Murphy, J.D.; Kiely, G. Effect of Thermal, Chemical and Thermo-Chemical Pre-Treatments to Enhance Methane Production. Energy 2010, 35, 4556–4561. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Q.; Chen, Y.; Sun, X.; Liu, X.; Li, D. Effects of Mixing and Sodium Formate on Thermophilic In-Situ Biogas Upgrading by H2 Addition. J. Clean. Prod. 2019, 216, 373–381. [Google Scholar] [CrossRef]
- Esteves, E.M.M.; Herrera, A.M.N.; Esteves, V.P.P.; Morgado, C. do R.V. Life Cycle Assessment of Manure Biogas Production: A Review. J. Clean. Prod. 2019, 219, 411–423. [Google Scholar] [CrossRef]
- Cata Saady, N.M.; Rezaeitavabe, F.; Espinoza, J.E.R. Chemical Methods for Hydrolyzing Dairy Manure Fiber: A Concise Review. Energies 2021, 14, 6159. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass Pretreatment: Fundamentals toward Application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Yang, F. Improving the Anaerobic Digestion of Switchgrass via Cofermentation of Rumen Microorganisms (Rumen Bacteria, Protozoa, and Fungi) and a Biogas Slurry. Energy Fuels 2019, 33, 1185–1195. [Google Scholar] [CrossRef]
- Weiland, P. Biogas Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- European Commission Directive (EU) 2023/2413 of the European Parliament and of the Council of 18 October 2023 Amending Directive (EU) 2018/2001, Regulation (EU) 2018/1999 and Directive 98/70/EC as Regards the Promotion of Energy from Renewable Sources. Off. J. Eur. Union 2023, 2413, 1–77.
- Ramos-Suárez, J.L.; Gómez, D.; Regueiro, L.; Baeza, A.; Hansen, F. Alkaline and Oxidative Pretreatments for the Anaerobic Digestion of Cow Manure and Maize Straw: Factors Influencing the Process and Preliminary Economic Viability of an Industrial Application. Bioresour. Technol. 2017, 241, 10–20. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Liu, Y.; Wang, Y.; Huo, F.; Fan, M.; Adidharma, H.; Li, X.; Zhang, S. Recent Progress in Theoretical and Computational Studies on the Utilization of Lignocellulosic Materials. Green Chem. 2019, 21, 9–35. [Google Scholar] [CrossRef]
- Anu; Kumar, A.; Rapoport, A.; Kunze, G.; Kumar, S.; Singh, D.; Singh, B. Multifarious Pretreatment Strategies for the Lignocellulosic Substrates for the Generation of Renewable and Sustainable Biofuels: A Review. Renew. Energy 2020, 160, 1228–1252. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of Lignocellulosic Biomass for Enhanced Biogas Production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.; Unalan, S.; et al. A Critical Review of Pretreatment Technologies to Enhance Anaerobic Digestion and Energy Recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Yu, B.; Xu, J.; Yuan, H.; Lou, Z.; Lin, J.; Zhu, N. Enhancement of Anaerobic Digestion of Waste Activated Sludge by Electrochemical Pretreatment. Fuel 2014, 130, 279–285. [Google Scholar] [CrossRef]
- Miron, J.; Ben-Ghedalia, D.; Morrison, M. Invited Review: Adhesion Mechanisms of Rumen Cellulolytic Bacteria. J. Dairy Sci. 2001, 84, 1294–1309. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The Critical Role of Lignin in Lignocellulosic Biomass Conversion and Recent Pretreatment Strategies: A Comprehensive Review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Serna-Maza, A.; Heaven, S.; Banks, C.J. In Situ Biogas Stripping of Ammonia from a Digester Using a Gas Mixing System. Environ. Technol. 2017, 38, 3216–3224. [Google Scholar] [CrossRef]
- Rubežius, M.; Venslauskas, K.; Navickas, K.; Bleizgys, R. Influence of Aerobic Pretreatment of Poultry Manure on the Biogas Production Process. Processes 2020, 8, 1109. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Azarbaijani, R.; Parsa Yeganeh, L.; Angelidaki, I.; Nizami, A.S.; Bhat, R.; Dashora, K.; Vijay, V.K.; Aghbashlo, M.; et al. Pretreatment of Lignocelluloses for Enhanced Biogas Production: A Review on Influencing Mechanisms and the Importance of Microbial Diversity. Renew. Sustain. Energy Rev. 2021, 135, 110173. [Google Scholar] [CrossRef]
- Fuchs, W.; Wang, X.; Gabauer, W.; Ortner, M.; Li, Z. Tackling Ammonia Inhibition for Efficient Biogas Production from Chicken Manure: Status and Technical Trends in Europe and China. Renew. Sustain. Energy Rev. 2018, 97, 186–199. [Google Scholar] [CrossRef]
- Yin, D.M.; Qiao, W.; Negri, C.; Adani, F.; Fan, R.; Dong, R.J. Enhancing Hyper-Thermophilic Hydrolysis Pre-Treatment of Chicken Manure for Biogas Production by in-Situ Gas Phase Ammonia Stripping. Bioresour. Technol. 2019, 287, 121470. [Google Scholar] [CrossRef] [PubMed]
- Nges, I.A.; Wang, B.; Cui, Z.; Liu, J. Digestate Liquor Recycle in Minimal Nutrients-Supplemented Anaerobic Digestion of Wheat Straw. Biochem. Eng. J. 2015, 94, 106–114. [Google Scholar] [CrossRef]
- Gianico, A.; Gallipoli, A.; Gazzola, G.; Pastore, C.; Tonanzi, B.; Braguglia, C.M. A Novel Cascade Biorefinery Approach to Transform Food Waste into Valuable Chemicals and Biogas Through Thermal Pretreatment Integration. Bioresour. Technol. 2021, 338, 125517. [Google Scholar] [CrossRef]
- Naujokienė, V.; Bagdonienė, I.; Bleizgys, R.; Rubežius, M. A Biotreatment Effect on Dynamics of Cattle Manure Composition and Reduction of Ammonia Emissions from Agriculture. Agriculture 2021, 11, 303. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia Inhibition and Toxicity in Anaerobic Digestion: A Critical Review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Methods for Increasing the Biogas Potential from the Recalcitrant Organic Matter Contained in Manure. Water Sci. Technol. 2000, 41, 189–194. [Google Scholar] [CrossRef]
- Kim, K.H.; Hong, J. Supercritical CO2 Pretreatment of Lignocellulose Enhances Enzymatic Cellulose Hydrolysis. Bioresour. Technol. 2001, 77, 139–144. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Methods for Increasing the Biogas Potential from the Recalcitrant Organic Matter Contained in Manure. In Proceedings of the 10th European Conference and Technology Exhibition on Biomass for Energy and Industry, Wurzburg, Germany, 8–11 June 1998; pp. 145–148. [Google Scholar]
- Costa, J.C.; Barbosa, S.G.; Alves, M.M.; Sousa, D.Z. Thermochemical Pre- and Biological Co-Treatments to Improve Hydrolysis and Methane Production from Poultry Litter. Bioresour. Technol. 2012, 111, 141–147. [Google Scholar] [CrossRef]
- Zdeb, M.; Bis, M.; Przywara, A. Multi-Criteria Analysis of the Influence of Lignocellulosic Biomass Pretreatment Techniques on Methane Production. Energies 2023, 16, 468. [Google Scholar] [CrossRef]
- Hasunuma, T.; Okazaki, F.; Okai, N.; Hara, K.Y.; Ishii, J.; Kondo, A. A Review of Enzymes and Microbes for Lignocellulosic Biorefinery and the Possibility of Their Application to Consolidated Bioprocessing Technology. Bioresour. Technol. 2013, 135, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Poddar, B.J.; Nakhate, S.P.; Gupta, R.K.; Chavan, A.R.; Singh, A.K.; Khardenavis, A.A.; Purohit, H.J. A Comprehensive Review on the Pretreatment of Lignocellulosic Wastes for Improved Biogas Production by Anaerobic Digestion. Int. J. Environ. Sci. Technol. 2022, 19, 3429–3456. [Google Scholar] [CrossRef]
- Kral, I.; Piringer, G.; Saylor, M.K.; Lizasoain, J.; Gronauer, A.; Bauer, A. Life Cycle Assessment of Biogas Production from Unused Grassland Biomass Pretreated by Steam Explosion Using a System Expansion Method. Sustainability 2020, 12, 9945. [Google Scholar] [CrossRef]
- Xue, Y.; Li, Q.; Gu, Y.; Yu, H.; Zhang, Y.; Zhou, X. Improving Biodegradability and Biogas Production of Miscanthus Using a Combination of Hydrothermal and Alkaline Pretreatment. Ind. Crops Prod. 2020, 144, 111985. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of Biogas Yield from Lignocellulosic Materials with Different Pretreatment Methods: A Review. Biotechnol. Biofuels 2021, 14, 359. [Google Scholar] [CrossRef]
- Venslauskas, K.; Navickas, K.; Rubežius, M.; Žalys, B.; Gegeckas, A. Processing of Agricultural Residues with a High Concentration of Structural Carbohydrates into Biogas Using Selective Biological Products. Sustainability 2024, 16, 1553. [Google Scholar] [CrossRef]
- Nagler, M.; Kozjek, K.; Etemadi, M.; Insam, H.; Podmirseg, S.M. Simple yet Effective: Microbial and Biotechnological Benefits of Rumen Liquid Addition to Lignocellulose-Degrading Biogas Plants. J. Biotechnol. 2019, 300, 1–10. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of Lignocellulosic Hydrolysates. II: Inhibitors and Mechanisms of Inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Sun, L.; Yuan, Q.; Cheng, G.; Argyropoulos, D.S. Extraction and Characterization of Lignin from Corncob Residue After Acid-Catalyzed Steam Explosion Pretreatment. Ind. Crops Prod. 2019, 133, 241–249. [Google Scholar] [CrossRef]
- Žalys, B.; Venslauskas, K.; Navickas, K.; Buivydas, E.; Rubežius, M. The Influence of CO2 Injection into Manure as a Pretreatment Method for Increased Biogas Production. Sustainability 2023, 15, 3670. [Google Scholar] [CrossRef]
- Žalys, B.; Venslauskas, K.; Navickas, K.; Buivydas, E.; Rubežius, M. The Influence of Dairy Rumen Anaerobic Bacteria Inoculum on Biogas Production. Eng. Proc. 2023, 37, 83. [Google Scholar] [CrossRef]
- Abbas, Y.; Jamil, F.; Rafiq, S.; Ghauri, M.; Khurram, M.S.; Aslam, M.; Bokhari, A.; Faisal, A.; Rashid, U.; Yun, S.; et al. Valorization of Solid Waste Biomass by Inoculation for the Enhanced Yield of Biogas. Clean Technol. Environ. Policy 2020, 22, 513–522. [Google Scholar] [CrossRef]
- Weimer, P.J. Degradation of Cellulose and Hemicellulose by Ruminal Microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef]
- Budiyono, B.; Widiasa, I.N.; Johari, S.; Sunarso, S. Increasing Biogas Production Rate from Cattle Manure Using Rumen Fluid as Inoculums. Int. J. Sci. Eng. 2014, 6, 31–38. [Google Scholar] [CrossRef]
- Park, H.S.; Jung, Y.M.; You, J.K.; Hong, W.H.; Kim, J.N. Analysis of the CO2 and NH3 Reaction in an Aqueous Solution by 2D IR COS: Formation of Bicarbonate and Carbamate. J. Phys. Chem. A 2008, 112, 6558–6562. [Google Scholar] [CrossRef]
- Andriani, D.; Wresta, A.; Atmaja, T.D.; Saepudin, A. A Review on Optimization Production and Upgrading Biogas Through CO2 Removal Using Various Techniques. Appl. Biochem. Biotechnol. 2014, 172, 1909–1928. [Google Scholar] [CrossRef]
- Muntau, M.; Lebuhn, M.; Polag, D.; Bajón-Fernández, Y.; Koch, K. Effects of CO2 Enrichment on the Anaerobic Digestion of Sewage Sludge in Continuously Operated Fermenters. Bioresour. Technol. 2021, 332, 125147. [Google Scholar] [CrossRef]
- Islam, S.M.M.; Li, Q.; Al Loman, A.; Ju, L.K. CO2-H2O Based Pretreatment and Enzyme Hydrolysis of Soybean Hulls. Enzym. Microb. Technol. 2017, 106, 18–27. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The Ecoinvent Database Version 3 (Part I): Overview and Methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Guinée, J.B.; Heijungs, R.; Huppes, G.; Kleijn, R.; de Koning, A.; van Oers, L.; Wegener Sleeswijk, A.; Suh, S.; Udo de Haes, H.A.; de Bruijn, H.; et al. I: LCA in Perspective. IIa: Guide. IIb: Operational Annex. III: Scientific Background. In Handbook on Life Cycle Assessment. Operational Guide to the ISO Standards; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Buivydas, E.; Navickas, K.; Venslauskas, K. A Life Cycle Assessment of Methane Slip in Biogas Upgrading Based on Permeable Membrane Technology with Variable Methane Concentration in Raw Biogas. Sustainability 2024, 16, 3323. [Google Scholar] [CrossRef]
- Filippa, F.; Panara, F.; Leonardi, D.; Arcioni, L.; Calderini, O. Life Cycle Assessment Analysis of Alfalfa and Corn for Biogas Production in a Farm Case Study. Processes 2020, 8, 1285. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Kim, S.; Overcash, M.R. Methodology for Developing Gate-to-Gate Life Cycle Inventory Information. Int. J. Life Cycle Assess. 2000, 5, 153–159. [Google Scholar] [CrossRef]
- Wiloso, E.I.; Heijungs, R.; Huppes, G.; Fang, K. Effect of Biogenic Carbon Inventory on the Life Cycle Assessment of Bioenergy: Challenges to the Neutrality Assumption. J. Clean. Prod. 2016, 125, 78–85. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic Digestion of Lignocellulosic Biomass: Challenges and Opportunities; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 178, ISBN 1808956354. [Google Scholar]
- Whiting, A.; Azapagic, A. Life Cycle Environmental Impacts of Generating Electricity and Heat from Biogas Produced by Anaerobic Digestion. Energy 2014, 70, 181–193. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The Future of Anaerobic Digestion and Biogas Utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Rehan, M.; Nizami, A.-S.; Rashid, U.; Naqvi, M.R. Waste Biorefineries: Future Energy, Green Products and Waste Treatment; Frontiers Research Topics; Frontiers Media SA: Lausanne, Switzerland, 2019; ISBN 9782889459933. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).