Abstract
There has been an excitement toward novel eco-friendly alternatives to pest management, particularly formulations based on essential oils (EOs). Here, the biological activity of an EO-based emulsion derived from patchouli, Pogostemon cablin, was assessed against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), a devastating and invasive pest in tomato crops worldwide. Laboratory bioassays were carried out to determine and compare lethal doses, lethal times, oviposition of treated individuals, and oviposition of T. absoluta on treated leaves of tomato to pure patchouli essential oil or its emulsion containing 18% of oil. The LD50 were 10.06 and 2.57 µg of patchouli per mg of insect for the EO and emulsion, respectively. Oviposition was reduced in adults derived from the second instar treated with LD10 by 78.5% (EO) and 85.4% (emulsion). The EO and its emulsion conferred similar avoidance to adults when sprayed on tomato leaves. Therefore, both formulations present similar lethal and sublethal effects against T. absoluta. This study demonstrated a remarkable potential of an emulsion containing 18% of P. cablin oil to be employed for T. absoluta management on tomato crops. Further studies are needed to assess and guarantee open field applications.
1. Introduction
The tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), is an important pest of tomato crops in Brazil and Europe [1,2,3]. The larvae cause crop yield losses due to feeding activity on leaves, stems, flowers, and fruits [2,4]. In Brazil, T. absoluta was first reported in 1979 and two years later, it was already present in the central tomato-producing regions of the country, causing severe yield losses [1,5,6]. The tomato leafminer is native to South America, and its invasion and spread in Europe in 2006 changed its importance to a significant threat to global tomato production [1,7]. Currently, T. absoluta is found in approximately 80 countries [8].
Pest resistance against current insecticides and increased concern regarding the collateral effects of pesticides on human health and the environment are driving the search for novel alternatives to pest control [9]. Essential oils (EOs) have been considered an alternative in this sense, as they are derived from medicinal and aromatic plants, which are considered eco-friendly due to their low persistence in the environment and lower toxicity to non-target organisms, in addition to having biological activity against several pests [10,11,12]. However, water insolubility, chemical instability, high volatility, and short residual activity due to degradation by temperature and light impair its use [11].
The development of EO-based emulsion may be an alternative to overcome the drawbacks of EO since this formulation confers small particle size, controlled release in the environment and increased penetration in the target pest [10,11,13,14,15,16]. These characteristics protect the active ingredients from degradation and may also increase efficacy as per smaller particle size and higher solubility and mobility than pure EO [14,17].
Several studies showed the biological activity of essential oils derived from Pogostemon cablin against urban and crop pests [15,16,18,19,20]. Besides the noted lethal effect on numerous insect species [20], several sublethal effects have been attributed to this EO, such as impairment of reproduction and feeding [16], impairment of behavioral traits such as displacement and speed on some ant species [15], increased walking activity [15,16], and histopathological changes in the midgut [16,18]. In addition, repellent effect was also observed at some concentrations of EO and its main compound (patchoulol) [19].
In addition to lethal effects, essential oils can have sublethal effects on insect pests. The sublethal effects of these oils are due to volatile or non-volatile substances that can have a positive or negative effect on insects. Volatile substances can be attractive or repellent to insects, affecting the location of the host plant, oviposition and feeding of herbivorous insects. The non-volatile substances present in essential oils can exert deterrence or arrestance effects on insects, and thus affect the biological performance of insect pests. These sublethal effects, along with the lethal effects, contribute to pests causing less damage to plants in crops [21,22,23].
Despite the importance of T. absoluta as a pest in tomato crops and the ecotoxicological advantages of using essential oils to control pests, there are few commercial options for natural products to control this pest. In this context, there is no study on the use of patchouli essential oil in the control of T. absoluta. Thus, the objective of this work was to determine the lethal and sublethal effects of patchouli essential oil microformulation for T. absoluta.
2. Material and Methods
2.1. Insects
The population of T. absoluta used in this work was collected from tomato fields in Viçosa, state of Minas Gerais, Brazil. The T. absoluta insects used in this work were reared in wooden cages (45 × 45 × 45 cm) covered with organza. The larvae were fed with Aguamiel variety tomato leaves grown in greenhouses. A piece of white cloth (2 × 5 cm) containing honey was inserted in the rearing cages for the adults of T. absoluta. These creations were maintained in the laboratory at 25 ± 2 °C, relative humidity of 70 ± 10% and a 12 h photophase [4].
2.2. Emulsion
The P. cablin essential oil (98% pure) used in this work was purchased from Empório Laszlo Corp. in Belo Horizonte, state of Minas Gerais, Brazil. The emulsion was composed of 36% Tween 80 (surfactant), 36% ethanol (95% PA; co-surfactant), 18% Pogostemon cablin essential oil (oil phase) and 10% ultrapure water (aqueous phase), and it was obtained by the magnetic stirring method (Corning (Corning, NY, USA) PC-420D) [16]. After preparation, the emulsion was stored in a 200 mL amber bottle at 25 °C in the dark for 24 h for complete homogenization. The main compounds present in this emulsion were patchoulol (43.05%), α-Guaiene (16.06%), and α-Bulnesene (13.69%) [16]. The emulsion had a microparticle size of 3.73 ± 3.70 μm (mean ± SD), conductivity of 0.0472 ± 0.0353 mS/cm, pH (7.68 ± 0.35), and zeta potential of −20.27 ± 5.81 mV [16].
2.3. Bioassays
Bioassays were conducted to determine: (i) lethal doses, (ii) lethal times, (iii) oviposition and (iv) oviposition preference of T. absoluta individuals exposed to the essential oil and its emulsion.
Treatments were diluted in acetone (99.9% purity). The control treatment consisted of acetone only. Previous tests showed that acetone, the surfactant and co-surfactant present in the microemulsion, did not influence the mortality of T. absoluta.
2.4. Dose–Response Curve
Serial dilutions of patchouli EO and its emulsion were performed to obtain doses of 5, 10, 15, 20 and 30 µg of EO per mg of insect and 5, 10, 15, 20, 25 and 30 µg of emulsion per mg of insect.
A total of six replicates were performed for each dose. The experimental unit consisted of Petri dishes (6 × 1.5 cm) containing 10-s instar T. absoluta and untreated tomato leaflets (cultivar ‘Aguamiel’) for feeding.
Patchouli essential oil and emulsion were applied by topical application of 0.5 μL of the treatment in the dorsal region of the insects with the aid of a micro syringe (Hamilton® (Reno, NV, USA), 10 μL). Then, the plates were sealed with plastic film and placed in a BOD Incubator at 25 ± 2 °C, 70 ± 10% RH and underwent a 12 h photophase. Mortality was assessed 72 h after application. In the assessments, the insects were touched with the bristles of a brush, and those that did not move were considered dead.
2.5. Time-Mortality Bioassay
In the time-mortality bioassay, we used the LD90 for the emulsion, with acetone as a control treatment. Four replicates were used, each consisting of 10 s instar of T. absoluta. A tomato leaf was provided for feeding. Topical application was performed as described in the determination of the dose–response curve.
Mortality assessment was performed every 10 min until 2 h, every 1 h until 12 h, and then every 12 h until 72 h. Individuals that did not move after being touched by the bristles of a brush were considered dead.
2.6. Oviposition Rate
Second instar T. absoluta was subjected to LD10 of essential oil and emulsion by topical application. The surviving individuals that reached the pupal stage were sexed [24,25], and individual couples were placed in separate cages until emergence. A tomato leaf and a cotton cloth moistened with honey were placed inside each cage. To maintain turgidity, a bevel cut was made on the tomato leaf petiole, then placed in a glass bottle. Four replicates were used, each with one couple. The oviposition of T. absoluta females, derived from second instar larvae treated with the LD10, was evaluated after 72 h.
2.7. Oviposition Preference
Tomato leaves were sprayed with 0.1% v/v Patchouli EO or its emulsion and the control treatment with pure acetone. A total of eight replicates were performed, each consisting of a cage (40 × 40 × 40 cm) containing 10 couples of T. absoluta and a leaf treated with one of the treatments. The cages were maintained at room temperature. The number of eggs in each of the treatments was evaluated after 24 h with the aid of a magnifying glass (40×).
2.8. Statistical Analyses
All statistical analyses were performed in the R software version 4.2.1 [26]. Mortality data were submitted to PROBIT analysis using the ecotox package [23]. Models with p > 0.05 were accepted by the chi-square goodness-of-fit test (χ2). LC50 and LC90, as well as their 95% confidence intervals, were calculated.
Time mortality was analyzed using Kaplan–Meier estimators in the survival package [27]. LT50 and its 95% confidence intervals were calculated. Curves and LT50 were compared using the Log-rank test with Bonferroni correction.
Oviposition rate and oviposition data were fitted to generalized linear models (GLM) using the lme4 package [28], with treatment as the fixed effect and error distribution accordingly. Oviposition rate was fitted to the negative binomial model and oviposition preference was initially fitted to the Poisson error distribution, but as overdispersion was detected, this data was refitted to quasi-Poisson. Model fit was checked using the ‘performance’ package [29], and means were compared using least-square means at α = 0.05 (package ‘emmeans’) [30].
3. Results
3.1. Dose-Mortality Bioassay
Tuta absoluta presented similar susceptibility to the EO and EO-based emulsion. Estimated dose–response curves for the essential oil (χ2 = 5.82, df = 3, p = 0.12) and the EO-based emulsion (χ2 = 2.37, df = 5, p = 0.67) showed similar toxicity to T. absoluta based on overlapping 95% confidence intervals of the LD50 (Figure 1 and Table 1). The LD50 for the EO was 9.86 (6.95–12.50) and for the emulsion was 14.0 (12.1–16.0) μg per mg of insect. The estimated LD90 was 21.7 (16.4–38.7) for the EO and 36.2 (29.5–49.7) μg per mg of the insect for the emulsion (Figure 2). As the EO is 98% patchouli and the emulsion is 18% EO, then the LD50 in terms of μg patchouli mg of insect were 10.06 (7.09–12.76) for the EO and 2.57 (2.22–2.94) for the emulsion. The LD90 in terms of μg patchouli mg of insect were 22.14 (16.73–39.49) for the EO and 6.65 (5.42–9.13) for the emulsion. Therefore, in terms of μg patchouli mg of insect the LD50 of the emulsion was 290% lower than the LD50 of the EO. The LD90 of the emulsion was 230% lower than the LD90 of the EO in terms of μg of patchouli mg of insect.
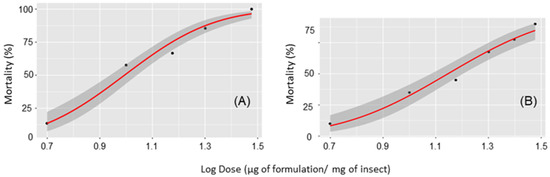
Figure 1.
Dose response curve for second instar larvae of Tuta absoluta exposed to the (A) essential oil of patchouli and the (B) EO-based emulsion (18%). Shaded areas depict the confidence intervals at 95% probability (CI95%).

Table 1.
Statical values.
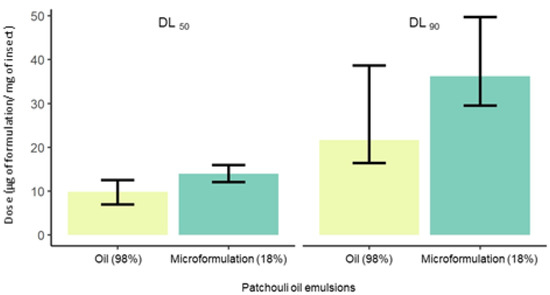
Figure 2.
Lethal doses (LD50 and LD90) for second instar larvae of Tuta absoluta exposed to the essential oil (EO) of patchouli and the EO-base emulsion (18%). The vertical line segments represent the LD50 and LD90 confidence intervals at 95% probability.
3.2. Time-Mortality Bioassay
Survival of second instar T. absoluta exposed to LD90 of the EO-based emulsion reduced according to time. High mortality was observed during the first hour of assessment, indicating this is a fast-acting pesticide. The time necessary to decrease half of the population (LT50) was 3.00 ± 0.08 h. The lethal time for the pure essential oil could not be calculated because all individuals died within the first 10 min of evaluation (Figure 3).
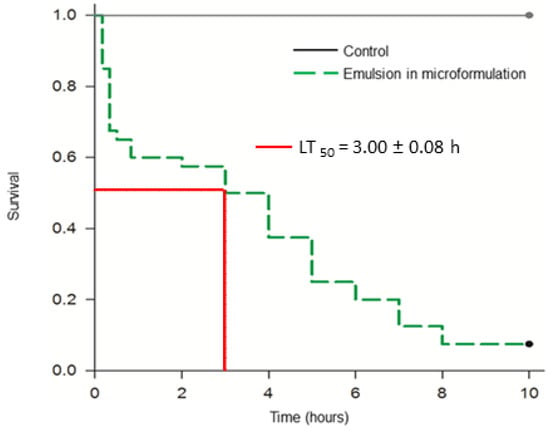
Figure 3.
Survival curve for second instar larvae of Tuta absoluta exposed to the LD90 of the essential oil emulsion (18%). Dotted line indicates the LT50. The Kaplan–Meier survival analysis was performed to determine statistical differences between the curves.
3.3. Oviposition
Oviposition of adult females exposed to the EO and EO-based formulation at the sublethal concentration (LD10) were negatively affected when compared to second instar larvae that received only acetone (control) (χ2 = 37.95, p < 0.001). The average number of eggs of females exposed to LD10 during the second instar was 5.75 ± 0.48 for the EO-base emulsion, 8.50 ± 0.87 for the EO, and 39.50 ± 1.71 eggs per plant for control treatment after 72 h of oviposition (Figure 4).
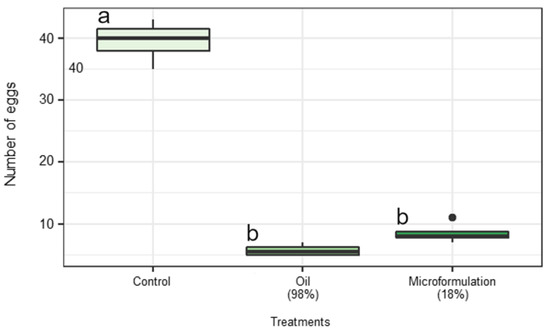
Figure 4.
Oviposition of Tuta absoluta females submitted to acetone (Control), LD10 of patchouli essential oil and emulsion containing 18% of the oil during second instar. The treatments followed by the same letter are not significantly different according to the Tukey’s test (α = 0.05).
3.4. Oviposition Preference
When exposed to sprayed leaves in a free-choice bioassay, females of T. absoluta preferred to oviposit on the control compared to the EO and EO-based emulsion (F2,21 = 7.60, p = 0.003). The average number of eggs on sprayed tomato leaves was 9.88 ± 1.55 for the EO, 7.50 ± 1.46 for EO-based emulsion, and 18.38 ± 3.05 eggs per female for control treatment after 24 h of oviposition (Figure 5).
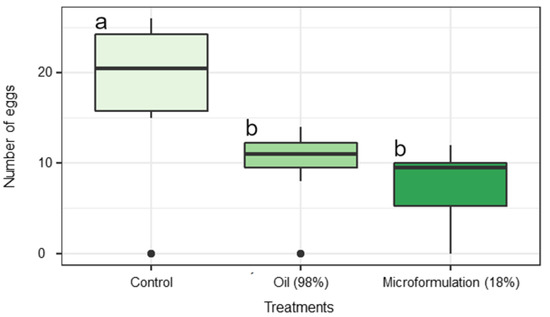
Figure 5.
Oviposition preference of Tuta absoluta in tomato leaves sprayed with the control (acetone), 0.1% of patchouli essential oil, or 0.1% of the emulsion containing 18% of the essential oil, in free choice bioassay after 24 h. The treatments followed by the same letter did not differ according to the Tukey’s test (α = 0.05).
4. Discussion
Innovative approaches that effectively reduce risks and hazards without losing efficacy and feasibility are of great appeal to attend agricultural concerns, as currently employed molecules are undoubtedly showing numerous drawbacks that have accelerated the replacement process. Therefore, essential oils have emerged as a promising, safe, and environmentally sustainable option for pest control [12]. However, several characteristics restrict the practical applications of this type of molecule, most notably their high volatility, low water solubility, and low chemical stability [31,32,33,34].
This study demonstrated that an EO-based emulsion of patchouli showed similar lethal and sublethal effects in comparison with the pure EO for T. absoluta. The EO-based emulsion was formulated in a micro-scale emulsion, which has been assumed to be a promising alternative to overcome the main drawback associated with the handling, storage and application of essential oils [12,35]. Formulation of oil-in-water emulsions confer more stability and availability of lipophilic molecules such as essential oils [12], ensuring sustained and controlled release of the active ingredients, solubilization of lipophilic substances, suitability for different ways of administration, protection from degradation, controlled volatilization, and reduction of side effects and dose [35].
The estimated lethal doses (LD50 and LD90) for the patchouli EO and its emulsion containing 18% of EO were equivalent. Thus, the emulsion has potential to be employed for the control of T. absoluta, since it had a similar toxicity with 5.5 times less active ingredients. Probably, the advantages related to the use of an emulsion-based formulation conferred this equivalent toxicity between the two formulations.
In our study, it was not possible to quantify the lethal time for the pure essential oil since all individuals died within the first 10 min of assessment. It can then be assumed that the LT50 for the pure essential oil is at most equal to 10 min, indicating that this compound has an extremely fast action. Based on this assumption, the emulsion would have a higher LT50 than the pure essential oil, that is, it has a comparatively slower action. However, the LT50 for the emulsion is only 3 h, which is also very fast acting, and considering the active ingredient savings and overcoming of several drawbacks conferred by the emulsion formulation, this slightly slower action becomes negligible. Another advantage of essential oils is the fact that many of them have a different mode of action than organosynthetic insecticides, which allows them to be used to control pests that are resistant to conventional insecticides in cities and crops [36,37,38] (Gaire et al., 2020, Oladipupo et al., 2022, Şengül Demirak and Canpolat 2022).
Second instar T. absoluta exposed to LD10 of the patchouli essential oil and its emulsion presented similar responses on the oviposition of emerging females. Both formulations significantly reduced the average number of eggs laid by females on tomato plants. In fact, these substances are known to be capable of causing effects to various organs and compromise feeding [16,39]. In a recent study, [16] several damages on the midgut of Hypothenemus hampei as a result of larval exposure to sublethal doses of essential oil and emulsion of P. cablin were observed. Moreover, these authors associated the reduced oviposition of adults emerged from treated larva because of lower feeding during larval development. EOs are also known to affect reproduction due to changes in the structure and number of gametes produced, resulting in failed fertilization and unviability of eggs [40,41].
In the free choice test, the mean numbers of eggs laid on tomato leaves treated with P. cablin EO and its emulsion were significantly lower than on control plants (acetone only), indicating both formulations conferred avoidance to oviposition. After exposing female adults of sweet potato whitefly, Bemisia tabaci biotype B, to plants sprayed with P. cablin oil [42], a similar response was observed. Reduced oviposition is observed in adult insect that settle on plants.
In conclusion, the essential oil of P. cablin and its microemulsion, containing 18% of oil, are promising to be used in the control of T. absoluta. This is because they have a high lethal effect on their larvae and reduce the oviposition of their adult females. In addition, oviposition inhibition by T. absoluta occurs in plants treated with them.
Author Contributions
Conceptualization, A.A.S. and L.B.; methodology, T.L.C., A.A.S., E.L., L.B. and M.C.P.; formal analysis, T.L.C. and A.A.S.; investigation, T.L.C., R.C.d.S., A.A.S., E.d.S.P., C.V.B. and D.R.d.F.; data curation, R.C.d.S., E.d.S.P., C.V.B. and D.R.d.F.; writing—original draft preparation, T.L.C., A.A.S. and M.C.P.; writing—review and editing, E.L., L.B. and M.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ‘Conselho Nacional de Desenvolvimento Científico e Tec-nológico’ (CNPq), ‘Fundação de Amparo à Pesquisa de Minas Gerais’ (FAPEMIG), ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil’ (CAPES)—Finance Code 001, INCT Se-mioquímicos na Agricultura, Processo 465511/2014-7.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to members of the Integrated Pest Management Laboratory at the Universidade Federal de Viçosa, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fun-dação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES). We thanks Eraldo Lima and the Laboratório de Semioquímicos e Comportamento de Insetos for found this search.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guedes, R.N.C.; Picanço, M.C. The tomato borer Tuta absoluta in South America: Pest status, management and insecticide resistance. EPPO Bull. 2012, 42, 211–216. [Google Scholar] [CrossRef]
- Campos, M.R.; Biondi, A.; Adiga, A.; Guedes, R.N.C.; Desneux, N. From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest Sci. 2017, 90, 787–796. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.-H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Picanço, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Muszinski, T.; Lavendowski, I.M.; Maschio, L.M.d.A. Constatação de Scrobipalpula absoluta (Meyrick, 1917) [=Gnorimoschema absoluta] (Lepidoptera, Gelechiidae), como praga do tomateiro (Lycopersicon esculentum Mill.), no litoral do Paraná. An. Soc. Entomológica Bras. 1982, 11, 291–292. [Google Scholar] [CrossRef]
- Galdino, d.S.T.V.; Picanço, M.C.; Ferreira, D.O.; Silva, G.A.R.; de Souza, T.C.; Silva, G.A. Is the performance of a specialist herbivore affected by female choices and the adaptability of the offspring? PLoS ONE 2015, 10, e0143389. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- EPPO. EPPO’s Plant Quarantine Data Retrieval System, Version 4.6. 2007. Available online: http://www.eppo.org/DATABASES/pqr/pqr.htm (accessed on 25 November 2022).
- Sousa, A.H.; Faroni, L.R.A.; Guedes, R.N.C. Locomotor behavior of Sitophilus zeamais populations under sublethal ozone exposure. J. Pest Sci. 2017, 90, 239–247. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Benelli, G.; Losic, D.; Rani, P.U.; Desneux, N. Nanoparticles for pest control: Current status and future perspectives. J. Pest Sci. 2018, 91, 1–15. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro- and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef]
- Lucia, A.; Guzmán, E. Emulsions containing essential oils, their components or volatile semiochemicals as promising tools for insect pest and pathogen management. Adv. Colloid Interface Sci. 2021, 287, 102330. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Chang, F.-Y.; Hung, D.-K. Terpene microemulsions for transdermal curcumin delivery: Effects of terpenes and cosurfactants. Colloids Surf. B Biointerfaces 2011, 82, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Werdin González, J.O.; Stefanazzi, N.; Murray, A.P.; Ferrero, A.A.; Band, B.F. Novel nanoinsecticides based on essential oils to control the German cockroach. J. Pest Sci. 2015, 88, 393–404. [Google Scholar] [CrossRef]
- Rocha, A.G.; Oliveira, B.M.S.; Melo, C.R.; Sampaio, T.S.; Blank, A.F.; Lima, A.D.; Nunes, R.S.; Araújo, A.P.A.; Cristaldo, P.F.; Bacci, L. Lethal effect and behavioral responses of leaf-cutting ants to essential oil of Pogostemon cablin (Lamiaceae) and its nanoformulation. Neotrop. Entomol. 2018, 47, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.A.; Farder-Gomes, C.F.; Ribeiro, A.V.; Costa, T.L.; França, J.C.O.; Bacci, L.; Demuner, A.J.; Serrão, J.E.; Picanço, M.C. Lethal and sublethal effects of an emulsion based on Pogostemon cablin (Lamiaceae) essential oil on the coffee berry borer, Hypothenemus hampei. Environ. Sci. Pollut. Res. 2022, 29, 45763–45773. [Google Scholar] [CrossRef]
- Montefuscoli, A.R.; González, J.O.W.; Palma, S.D.; Ferrero, A.A.; Band, B.F. Design and development of aqueous nanoformulations for mosquito control. Parasitol. Res. 2014, 113, 793–800. [Google Scholar] [CrossRef]
- Ga’al, H.; Fouad, H.; Mao, G.; Tian, J.; Jianchu, M. Larvicidal and pupicidal evaluation of silver nanoparticles synthesized using Aquilaria sinensis and Pogostemon cablin essential oils against dengue and zika viruses vector Aedes albopictus mosquito and its histopathological analysis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1171–1179. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Wang, Y.; You, C.-X.; Guo, S.-S.; Du, Y.-S.; Du, S.-S. Bioactivities of patchoulol and phloroacetophenone from Pogostemon cablin essential oil against three insects. Int. J. Food Prop. 2019, 22, 1365–1374. [Google Scholar] [CrossRef]
- Lima Santos, L.; Brandão, L.B.; da Costa, A.L.P.; Martins, R.L.; Rodrigues, A.B.L.; Lobato, A.A.; da Silva de Almeida, S.S.M. Bioinsecticidal and pharmacological activities of the essential oil of Pogostemon cablin benth leaves: A Review. Pharmacogn. Rev. 2022, 16, 139–145. [Google Scholar] [CrossRef]
- Gantner, M.; Najda, A.; Piesik, D. Effect of phenolic acid content on acceptance of hazel cultivars by filbert aphid. Plant Prot. Sci. 2019, 55, 116–122. [Google Scholar] [CrossRef]
- Piesik, D.; Wenda-Piesik, A.; Krasińska, A.; Wrzesińska, D.; Delaney, K.J. Volatile organic compounds released by Rumex confertus following Hypera rumicis herbivory and weevil responses to volatiles. J. Appl. Entomol. 2016, 140, 308–316. [Google Scholar] [CrossRef]
- Yarou, B.B.; Bawin, T.; Boullis, A.; Heukin, S.; Lognay, G.; Verheggen, F.J.; Francis, F. Oviposition deterrent activity of basil plants and their essentials oils against Tuta absoluta (Lepidoptera: Gelechiidae). Environ. Sci. Pollut. Res. 2018, 25, 29880–29888. [Google Scholar] [CrossRef] [PubMed]
- Genç, H. The tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae): Pupal key characters for sexing individuals. Turk. J. Zool. 2016, 40, 801–805. [Google Scholar] [CrossRef]
- Nayana, B.P.; Kalleshwaraswamy, C.M. Biology and external morphology of invasive tomato leaf miner, Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae). Pest Manag. Hortic. Ecosyst. 2015, 21, 169–174. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Therneau, T.M.; Lumley, T.; Elizabeth, A.; Cynthia, C. Survival: Survival Analysis. 2022. Available online: https://cran.r-project.org/web/packages/survival/survival.pdf (accessed on 25 November 2022).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Lenth, R.V.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, aka Least-Squares Means. 2023. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 27 November 2022).
- Gierer, F.; Vaughan, S.; Slater, M.; Thompson, H.M.; Elmore, J.S.; Girling, R.D. A review of the factors that influence pesticide residues in pollen and nectar: Future research requirements for optimising the estimation of pollinator exposure. Environ. Pollut. 2019, 249, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Megharaj, M. Local applications but global implications: Can pesticides drive microorganisms to develop antimicrobial resistance? Sci. Total Environ. 2019, 654, 177–189. [Google Scholar] [CrossRef]
- Upadhayay, J.; Rana, M.; Juyal, V.; Bisht, S.S.; Joshi, R. Impact of pesticide exposure and associated health effects. In Pesticides in Crop Production; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 69–88. [Google Scholar]
- Zhu, Q.; Yang, Y.; Zhong, Y.; Lao, Z.; O’Neill, P.; Hong, D.; Zhang, K.; Zhao, S. Synthesis, insecticidal activity, resistance, photodegradation and toxicity of pyrethroids (A review). Chemosphere 2020, 254, 126779. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemulsions as optimized vehicles for essential oils. In Sustainable Agriculture Reviews 44: Pharmaceutical Technology for Natural Products Delivery Vol. 2 Impact of Nanotechnology, Sustainable Agriculture Reviews; Saneja, A., Panda, A.K., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 115–167. [Google Scholar]
- Gaire, S.; Lewis, C.D.; Booth, W.; Scharf, M.E.; Zheng, W.; Ginzel, M.D.; Gondhalekar, A.D. Bed bugs, Cimex lectularius L., exhibiting metabolic and target site deltamethrin resistance are susceptible to plant essential oils. Pestic. Biochem. Physiol. 2020, 169, 104667. [Google Scholar] [CrossRef] [PubMed]
- Oladipupo, S.O.; Hu, X.P.; Appel, A.G. Essential oils in urban insect management—A review. J. Econ. Entomol. 2022, 115, 1375–1408. [Google Scholar] [CrossRef] [PubMed]
- Şengül Demirak, M.Ş.; Canpolat, E. Plant-based bioinsecticides for mosquito control: Impact on insecticide resistance and disease transmission. Insects 2022, 13, 162. [Google Scholar] [CrossRef]
- Shahzad, K.; Manzoor, F. Nanoformulations and their mode of action in insects: A review of biological interactions. Drug Chem. Toxicol. 2021, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.; Cruz, G.; Wanderley-Teixeira, V.; Teixeira, A.; Oliveira, J.; Correia, A.; Câmara, C.; Cunha, F. Effects of Piper hispidinervum on spermatogenesis and histochemistry of ovarioles of Spodoptera frugiperda. Biotech. Histochem. 2014, 89, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Cruz, G.; Teixeira, V.; Oliveira, J.; Teixeira, A.; Araújo, A.; Alves, T.; Magliano, F.; Breda, M. Histological and histochemical changes by clove essential oil upon the gonads of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Int. J. Morphol. 2015, 33, 1393–1400. [Google Scholar] [CrossRef][Green Version]
- Yang, N.-W.; Li, A.-L.; Wan, F.-H.; Liu, W.-X.; Johnson, D. Effects of plant essential oils on immature and adult sweetpotato whitefly, Bemisia tabaci biotype B. Crop Prot. 2010, 29, 1200–1207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).