Abstract
The adaptability of phytophagous insects to different host plants is a key determinant of their population propagation. Understanding the feeding behaviour and population dynamics of insects is crucial for elucidating host adaptability and screening for insect-resistant germplasms. Here, we investigated Toxoptera aurantii (Hemiptera: Aphididae) adaptability using electropenetrography and assessed its population growth on seven tea cultivars: Huangjinya (HJY), Qianmei601 (QM601), Fudingdabaicha (FD), Longjing43 (LJ43), Qiancha1 (QC1), Qiancha8 (QC8) and Qiancha10 (QC10). The results showed that the feeding behaviour of T. aurantii differed significantly depending on the tea cultivars. The initial probing of T. aurantii on HJY was the earliest among the seven host plants. Aphids on QC1 and QC10 displayed an increased duration of stylet probing and decreased ingestion of phloem sap, whereas a contrasting trend was observed for aphids on HJY. In addition, the mechanical resistance of T. aurantii fed on HJY and QM601 during the probing phase was significantly lower than that of aphids fed on other cultivars. Population dynamic parameters revealed that the growth rate of T. aurantii reared on HJY was the fastest, and its population quantity within 15 days was markedly higher than that of aphids fed on other cultivars. These findings demonstrate that HJY is the most suitable host plant, whereas QC1 and QC10 are less suitable hosts for T. aurantii, although the aphids successfully survived on all the selected tea cultivars. Our results provide valuable information for the biological control of T. aurantii using resistant tea varieties.
1. Introduction
Plants in natural ecosystems have developed complex and effective defence systems to resist infestation by phytophagous insects following their long-term coevolution with the insects, which, in turn, has affected insect behaviour, population dynamics and distribution [1,2]. Previous studies have shown that there is specificity between insects and plants, and the adaptability of insects to host plants is usually affected by the physical structure, nutrient composition and the content of secondary substances of the host [3,4,5]. Phytophagous insects exhibit various adaptation strategies to host plants, such as through their feeding habits, physiological responses and fecundity [6,7]. Generally, insects preferentially settle and oviposit on the most suitable hosts to facilitate larval growth [8]. Adaptability determines the performance of insects under the pressure of natural selection. The evaluation of insect adaptability involves various ecological indicators, including larval development time, mortality rate, pupal weight and fecundity [9]. Thus, investigating the biological characteristics of insects on various host plants enhances our understanding of their adaptability, which is also the premise of screening for insect-resistant germplasm resources.
The tea plant (Camellia sinensis [L.] O. Kuntze) is a unique beverage crop globally which is frequently attacked by insect pests during its growing period. The tea aphid, Toxoptera aurantii Boyer de Fonscolombe (Hemiptera: Aphididae), a destructive insect pest with a piercing–sucking mouthpart, seriously threatens tea plants around the world [10]. Owing to its miscellaneous feeding habits and strong invasion capability, T. aurantii has a wide host range, such as tea-oil camellia (Camellia oleifera), citrus, coffee, cocoa and mango, among others, consequently causing substantial economic losses in each production season [10]. Aphids constitute a major group of crop pests that have excellent host adaptability. However, the adaptability of aphids varies greatly among different host plants [11]. Significant differences in developmental time, reproductive capacity and adult longevity have been observed in Aphis fabae Scopoli (Hemiptera: Aphididae) fed on different sugar beet cultivars [12]. According to Han et al., the duration of phloem sap ingestion of T. aurantii on tea plants is considerably longer than that on soybean and wheat plants [13]. Insect–host plant interactions are known to substantially affect the biological characteristics of related organisms. Therefore, the implications of such interactions in integrated pest management have attracted considerable attention among researchers. Unfortunately, there is limited knowledge on the performance of T. aurantii on tea plants.
The use of resistant tea germplasm is the most effective, economical and eco-friendly method of controlling insect pests. The level of plant resistance can be reflected by the adaptability of insects towards hosts. Previous studies have shown that insects improve their adaptive ability to host plants primarily through their selection behaviour and feeding habits; therefore, elucidating the feeding behaviour of insects is essential for revealing host resistance mechanisms [14,15]. To overcome the defensive traits of plants, phytophagous insects have evolved different types of mouthparts to adapt to complex environments. For example, aphids are insects with small piercing–sucking mouthparts that feed by inserting their slender mouthparts into phloem cells, the food conduits of plants. The electrical penetration graph (EPG) technique can accurately record the duration and frequency of each behaviour of aphid stylets within plant tissues, such as cell puncture, salivation, xylem sucking and phloem sucking [16]. EPG waveforms can reveal the position of the piercing–sucking insect stylet in plant tissues, thereby providing detailed information regarding plant resistance [17]. Currently, the EPG technique has been widely used to assess the host specificity of phytophagous insects because of its accuracy and efficiency [18,19,20]. Population dynamics is a key index in determining insect fitness on host plants. The adaptability of insects to host plants can be comprehensively evaluated by combining feeding behaviour with population dynamics [5]. Our recent study showed variations in the feeding selectivity of T. aurantii for different tea varieties [21]. However, T. aurantii adaptability to various tea cultivars remains unknown.
Therefore, this study evaluated T. aurantii adaptability by recoding its feeding behaviour on seven tea cultivars using the EPG technique. Furthermore, the population growth rates of T. aurantii on the different tea cultivars were analysed. This study provides valuable insights for screening aphid-resistant tea germplasm resources and formulating management strategies for T. aurantii.
2. Materials and Methods
2.1. Aphids
T. aurantii specimens were collected from tea plantations in Guiyang, China (26°45′ N, 106°66′ E) in May 2020 and reared on tea seedlings in a controlled environment chamber maintained at 25 °C ± 1 °C, 70 ± 5% RH, 150–200 μmol m−2 s−1 light intensity (LI) and a 14:10 h light:dark (L:D) photoperiod. Before tests, a wingless adult aphid from the above-mentioned population was transferred onto ‘Fudingdabaicha’ (FD) seedlings (a predominant tea variety in China). After three generations of reproduction, the newly moulted (within 6 h) T. aurantii adults were collected for analysis.
2.2. Plants
Seven tea cultivars, including Huangjinya (HJY), Qianmei601 (QM601), FD, Longjing43 (LJ43), Qiancha1 (QC1), Qiancha8 (QC8) and Qiancha10 (QC10), were used in the present study. The cultivars were generously provided by Prof. Zhengwu Chen (Institute of Tea, Guizhou Academy of Agricultural Sciences). All hosts were 2-year-old plants cultivated in plastic pots (D 16 cm × H 28 cm) under controlled conditions in an environment chamber (25 °C ± 1 °C, 70 ± 5% RH, 150–200 μmol m−2 s−1 LI and a 14:10 h L:D photoperiod) during spring.
2.3. EPG Recording
Feeding activities of T. aurantii on the different tea cultivars were recorded using a DC-EPG device (Giga-8 EPG amplifier; Wageningen, The Netherlands). Same-sized apterous adult aphids that emerged within 6 h of moulting were selected for the experiment. Aphids and tea plants were connected to the insect and plant electrodes of the EPG device, respectively. The insect electrode was a gold wire (12.5 µm diameter and 2–3 cm length). The gold wire terminal was dipped in silver glue drops when connecting aphids and then carefully attached to the pronotum of adult aphids. After 1 h of starvation treatment, the wired aphids were subsequently connected to the DC-EPG probe and placed individually on the abaxial surface of the tender tea leaves. The plant electrode was a copper nail (2 mm diameter and 10 cm length) and was inserted directly into the potting soil, where the tea seedlings were grown. The EPG system was placed in a Faraday cage to prevent interference due to external electrical noise. The feeding behaviour of T. aurantii was recorded at 09:00 every day for 6 h. The experiment was performed at a constant temperature of 25 °C ± 1 °C, 70 ± 5% RH and 150–200 μmol m−2 s−1 LI. Fifteen effective repetitions were obtained for EPG parameter analysis. Seven tea cultivars were stochastically arranged in the Faraday cage. EPG signals were stored on a computer through A/D card (DI-710, Dataq Instruments Inc., Akron, OH, USA) and WinDaq Lite Acquisition software ver 2.40 (Dataq Instruments Inc., Akron, OH, USA). The EPG waveforms were recorded and analysed during 6 h of T. aurantii feeding using the software Stylet+ (downloaded from http://www.epgsystems.eu/, accessed on 6 August 2022). EPG parameters were processed automatically using Excel Workbook 4.4.3 [22].
2.4. Population Dynamics
The population dynamics of T. aurantii on different tea cultivars were investigated using a non-free choice method as described by Jiang et al. [5]. Briefly, each plant was kept in a separate plastic tray (20 cm in diameter) filled with water (3 cm in depth) to prevent aphids from spreading among the host plants. Five newly moulted apterous T. aurantii adults were placed on the abaxial surface of the tender leaves of one plant from each tea cultivar (10 plants per variety) using a small hair paintbrush. The population of aphids on tea seedlings was recorded every 3 days for 15 days. The experiment was performed in a greenhouse, as mentioned previously, with three replicates for each tea cultivar.
2.5. Statistical Analysis
The waveform patterns of T. aurantii were identified using a previously described method [23]. All statistical analyses were performed using IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA). Prior studies have shown that some EPG parameters do not conform to the normal distribution [24,25]. Thus, normality and homogeneity of variances were checked before analysis. The duration and percentage of EPG parameters that did not conform to the normal distribution were log10(n + 1)- and arcsine-transformed, respectively. Statistical comparisons of variables of the seven host plants were performed using one-way analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) test at 0.05 level (p = 0.05).
3. Results
3.1. Feeding Behaviour of Toxoptera aurantii on Seven Tea Cultivars
According to the results, seven distinct EPG waveforms associated with T. aurantii piercing and sucking activities were observed on all host plants tested: non-penetration behaviour (waveform np), intercellular stylet pathway (waveforms A, B and C), potential drop (waveform pd), stylet penetration difficulties (waveform F), xylem ingestion (waveform G), phloem saliva secretion (waveform E1) and phloem sap ingestion (waveform E2) (Figure 1). A total of 20 EPG parameters were selected for comparison in this study (Table 1 and Table 2). The results showed significant differences in the feeding activities of T. aurantii among seven tea cultivars.
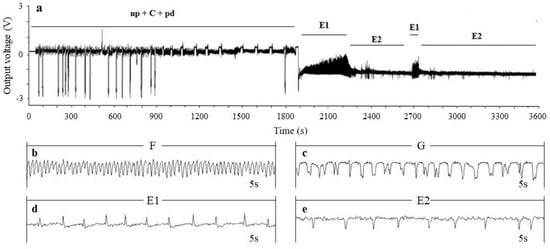
Figure 1.
Electrical penetration graph (EPG) waveforms of Toxoptera aurantii. (a) An overview of waveforms during the probing process; (b) stylet penetration difficulties (waveform F); (c) xylem ingestion (waveform G); (d) phloem saliva secretion (waveform E1); (e) phloem sap ingestion (waveform E2).

Table 1.
Non-phloem-phase electrical penetration graph (EPG) parameters of Toxoptera aurantii fed on seven tea cultivars.

Table 2.
Phloem-phase electrical penetration graph (EPG) parameters of Toxoptera aurantii fed on seven tea cultivars.
3.1.1. Probing Stage EPG Parameters of Toxoptera aurantii
The spying path phase parameters presented in Table 1 indicate that QC1 and QC10 exhibited a remarkable effect on the probing activities of T. aurantii. The number of probes on QC1 and QC10 was significantly higher than those on HJY, QM601, FD, QC8 and LJ43. T. aurantii on QC8 had the longest total duration of waveform np. The time to first probe from the start of the EPG recording was significantly shortened on HJY compared with that on others. There were no differences in the duration of the first probe, number of pd, average duration of pd, number of G and total duration of G among the seven tea cultivars (Table 1). The aphids on QC1 and QC10 displayed more frequent numbers of probes to the first E1 than those on HJY and QM601. Moreover, the number of short probes increased when aphids fed on QC1 and QC10. The total duration of C on QC1 was the longest, whereas the opposite was found on HJY. The number and duration of waveform F was significantly lower on HJY and QM601 compared with the other tea cultivars (Table 1).
3.1.2. Phloem-Stage EPG Parameters of Toxoptera aurantii
The T. aurantii stylet could reach the phloem to ingest sap on all seven host plants within 6 h of the EPG recording period. No significant differences were observed in the number of E1, total duration of E1 and number of sustained E2 (Table 2). The duration of first E was the shortest on QC10, which was significantly shorter in comparison with that on HJY, QM601, FD and QC8. The number of E2 on QC1 and QC10 was significantly lower than that on HJY. T. aurantii took significantly more time from the first probe to the first E2 on QC1 than on HJY and QM601. The total duration of waveform E2 of aphids on HJY was the longest, whereas that of aphids on QC1 was the shortest (Table 2).
3.1.3. Proportion of Each Waveform of Toxoptera aurantii
In the current study, the proportions of average (n = 15) durations of the different feeding waves of tea aphids on the seven test plants are illustrated in Figure 2. The proportion of waveform C when T. aurantii fed on QC1 and QC10 was significantly higher (51.00% and 47.76%, respectively) than that when fed on HJY (29.82%). The proportion of waveform G and E1 did not differ among the seven tea cultivars. The proportion of waveform F of aphids on HJY and QM601 was obviously lower than that of aphids on other tea cultivars. However, HJY emerged with the highest value of 55.68% in waveform E2, and QM601 appeared with the value of 37.64%, both of which were significantly higher than QC1 (23.52%), QC10 (25.66%) and LJ43 (32.25%).
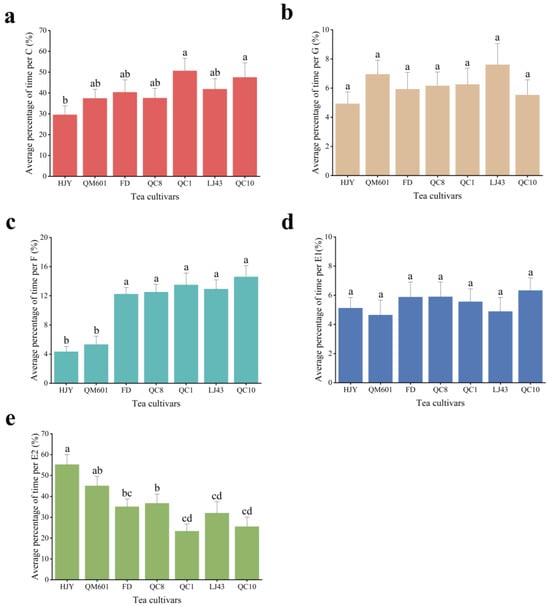
Figure 2.
The average proportion of time for each waveform of Toxoptera aurantii probing on seven tea cultivars for 6 h. (a) Proportion of duration of C wave; (b) proportion of duration of G wave; (c) proportion of duration of F wave; (d) proportion of duration of E1 wave; (e) proportion of duration of E2 wave. Different letters in the column chart indicate significant differences between treatments at p < 0.05 (Tukey’s HSD test), whereas the same letters indicate non-significant differences.
3.2. Population Dynamics of Toxoptera aurantii on Seven Tea Cultivars
The population quantity of aphids fed on the seven tea cultivars within 15 days are shown in Figure 3. The X- and Y-axes represent the time of T. aurantii infestation and the aphid population count, respectively. The results showed that T. aurantii populations on different tea cultivars increased with time, with the aphid population on HJY exhibiting the fastest growth rate. On day 3, the T. aurantii population on HJY significantly exceeded that on other tea plants. Additionally, the T. aurantii population on the seven tea cultivars increased rapidly on day 9, with the population on HJY being the highest. The aphid populations on all cultivars peaked on day 12, with a total of 370.67 ± 11.02 aphids observed on HJY, a count significantly higher than that observed on QC1 (149.33 ± 11.61) and QC10 (153.67 ± 5.61). On day 15, the population number on all test plants decreased slightly, but there were significant differences among the seven tea cultivars.
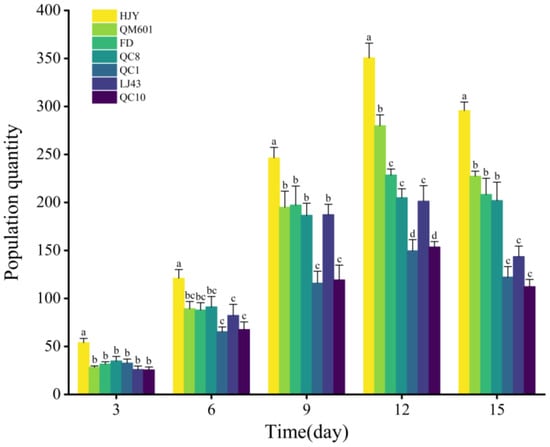
Figure 3.
Population dynamics of Toxoptera aurantii on seven tea cultivars. Different letters in the column chart indicate significant differences between treatments, which were detected with Tukey’s HSD test at p < 0.05.
4. Discussion
Prior studies have revealed that a broad host range plays an essential role in outbreaks of phytophagous insects [26,27]. However, the mechanism of insects’ extensive host adaptability is still unclear. Studying the adaptation mechanism of phytophagous insects to hosts provides novel concepts for comprehensive pest management, which is of great significance in the integration of agriculture, chemical and biocontrol measures and in the maintenance of ecological balance. Different plant species have substantial impacts on insect performance [28,29,30]. Therefore, it is essential to accurately understand the adaptability of insects to different hosts to design effective, economical and environmentally friendly pest control strategies. In the present study, the adaptability of T. aurantii to seven tea cultivars was evaluated based on their feeding activities and population dynamics.
Suitable host plants are fundamental for insect settlement and reproduction, and the feeding selectivity of phytophagous insects for different host plants is a crucial behavioural adaptation strategy [31]. The EPG technique plays an important role in investigating the correlation between sucking insects and host plants. The techniques can reveal the subtle variations in the feeding behaviour of sap-sucking insects within plant tissues. This study used the EPG technique to monitor the feeding activities of T. aurantii on seven tea cultivars. The results showed that T. aurantii on the tea plants used in our experiment exhibited seven EPG waveform types, including the np, C, pd, F, G, E1 and E2 waveforms. The EPG waveform pattern of T. aurantii was consistent with that observed by Han et al. [13]. Owing to the complex relationship between piercing–sucking insects and host plants, new waveforms are continually being identified and described. These findings confirm that EPG waveforms are not only stable but are also diverse [32]. According to a study by Zhou et al., the F waveform was not observed in either Eriosoma lanigerum Hausmann (Hemiptera: Aphididae) or Aphis citricola van der Goot (Hemiptera: Aphididae) when sucking sap from apple seedlings [33]. Therefore, further studies are required to determine whether the variations in aphid EPG waveforms are closely associated with the host plants or aphid species.
Understanding the feeding behaviour of insects on different host plants could facilitate the elucidation of insect-resistance mechanisms in plants [34,35]. Various studies have indicated that the probing behaviour of aphids on plants could be directly affected by external morphological characteristics and the internal organisational structure of the host plant [36,37]. If the resistance factors are located in the epidermis or mesophyll tissues, aphids exhibit a relatively high frequency of interruption during the probing process, and the probing pathway duration is markedly prolonged [38]. Our results demonstrated that the prying feeding behaviour of T. aurantii differed significantly among the seven tea cultivars. Aphids feeding on QC1 and QC10 increased the number of probes as well as significantly extended the total duration of the C wave, but the opposite was observed on HJY. The F-wave represents the waveform that occurs when the aphid stylet encounters mechanical resistance from the host tissue during the probing process [23]. In this study, both the number and total duration of F waveforms exhibited by T. aurantii during the pathway stage were lower on HJY than on QC1 and QC10. According to these results, we speculate that there are physical resistance factors in the leaf epidermal cells or mesophyll tissues in QC1 and QC10, which inhibited the penetration of T. aurantii stylets.
In previous studies, the focus on the interaction between plants and insect pests was primarily directed toward identifying plant resistance to pests, with little attention given to understanding the mechanisms underlying pest adaptation strategies in various plant–pest systems [39]. Aphids are typical phloem-sucking insects; consequently, their feeding behaviours during the phloem phase are crucial indicators for evaluating host suitability [40]. Generally, a short duration of phloem sap absorption by aphids is considered a sign of poor adaptation to the host [41]. In the current study, T. aurantii on QC1 and QC10 exhibited an evidently shorter duration of the waveform E2 than that on HJY. Furthermore, the duration of the first E waveform of T. aurantii on QC10 was the shortest and was obviously lower than that of aphids on other tea cultivars, except for QC1. The feeding activities of aphids can serve as indicators of their preference for specific plant hosts [27], and population dynamics are frequently used to assess the impact of host resistance on aphid behaviours [42]. The findings suggest that integrating these two indexes can improve the accuracy of aphid host adaptability assessments [43]. In the population experiment, our results also revealed that the growth rate of tea aphids on HJY was the fastest, and its population was higher than that observed on other tea cultivars within 15 days. A comparison of the EPG and population parameters indicated that QC1 and QC10 exhibited strong resistance to T. aurantii, whereas HJY was susceptible to attack by T. aurantii. There have been previous reports on the resistance of different tea germplasms to T. aurantii; however, the germplasm materials used by different researchers exhibit marked variation [17,44].
Structure, volatile compounds, nutrients and other factors of host plants can influence the feeding activities of phytophagous insects, with the physicochemical properties of plant leaves playing key roles [45,46]. Gao et al. reported that the inherent physical properties of the tea plant were the primary barriers to attack by T. aurantii and that the aphid population ratio was significantly negatively correlated with the thickness of the lower epidermis, cuticle and palisade tissues [47]. Aphids have a habit of tentatively feeding when selecting host plants, and the nutrient composition of plant tissues is a key factor that influences the process during aphid colonisation [48]. Presently, most studies demonstrated that insect feeding behaviour is positively correlated with the amino acid and sugar contents in host plant tissues; however, caffeine and polyphenols are anti-feeding compounds that deter insect feeding, inhibiting their growth and development, in turn [49,50,51]. In our previous study, marked differences were found in the physicochemical properties of leaves among the seven tea cultivars. The palisade tissue and lower leaf epidermis of QC1 and QC10 were thicker, and the caffeine and polyphenol contents were higher than those of the other tea cultivars; however, the free amino acid contents in QC1 and QC10 were lower than those observed in other tea cultivars [21]. Thus, we infer that tea plant resistance to T. aurantii is closely associated with the physicochemical properties of its leaves; the palisade tissue and lower epidermis thickness are the main physical barrier factors of tea plants against aphid feeding stress; and the contents of caffeine, tea polyphenol and free amino acids are the main nutritional factors.
5. Conclusions
This study evaluated the host adaptability of T. aurantii to seven tea cultivars. Analysis of the EPG parameters and population dynamics of T. aurantii revealed that HJY was the most suitable host plant, whereas QC1 and QC10 were less suitable hosts, although the aphids successfully survived on all seven of the tea cultivars. The findings of the current study provide valuable insights for the selection of effective germplasms for breeding aphid-resistant tea plant varieties. Tea is a perennial crop, and its resistance to insect pests is influenced by a complex regulatory network. Therefore, studying the resistance mechanism of tea plants to aphids requires further attention to relevant resistance traits and associated genes.
Author Contributions
Conceptualisation and methodology, Y.H. and D.Z.; software, W.C. and W.J.; validation, J.F. and W.J.; formal analysis, Y.H. and S.N.; investigation, Y.H., W.J. and D.Z.; resources, Y.H. and D.Z.; data curation, Y.H.; writing—original draft preparation, Y.H.; writing—review and editing, Y.H., D.Z., W.J., C.L. and W.C.; visualisation, Y.H.; supervision, D.Z.; project administration, D.Z.; funding acquisition, W.C. and D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Research Project of Science and Technology Department of Guizhou Province (QKHJC-ZK [2022]055), the National Guidance of Local Science and Technology Development Fund of China (QKHZYD [2023]009) and the Forestry Reform and Development Fund ([2022] YJ018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent can be acquired from all participants in this study.
Data Availability Statement
The datasets presented will be made available on request from D.Z.
Acknowledgments
We are extremely grateful to Zhengwu Chen (Institute of Tea, Guizhou Academy of Agricultural Sciences) for providing the seven tea cultivars.
Conflicts of Interest
The authors declare the absence of any conflicts of interest.
References
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant responses to herbivory, wounding, and infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.X.; Chi, H.; Zhang, J.; Zhou, Q.; Zhang, R.J. Life-table analysis of the performance of Nilaparvata lugens (Hemiptera: Delphacidae) on two wild rice species. J. Econ. Entomol. 2010, 103, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q. The Population Dynamics and Different Expressed Genes of Myzus persicae (Sulzer) on Different Host Plants. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2013. [Google Scholar]
- Jiang, W.B.; Cheng, Q.; Lu, C.H.; Chen, W.L.; Zhao, D.G.; He, Y.Q. Different host plants distinctly influence the adaptability of Myzus persicae (Hemiptera: Aphididae). Agriculture 2022, 12, 2162. [Google Scholar] [CrossRef]
- Zheng, X.M.; Tao, Y.T.; Chi, H.; Wan, F.H.; Chu, D. Adaptability of small brown planthopper to four rice cultivars using life table and population projection method. Sci. Rep. 2017, 7, 42399. [Google Scholar] [CrossRef] [PubMed]
- Chesnais, Q.; Couty, A.; Uzest, M.; Brault, V.; Ameline, A. Plant infection by two different viruses induce contrasting changes of vectors fitness and behavior. Insect Sci. 2017, 26, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, J.Y.; Xu, P.J.; Liu, Y.J.; Dong, Y.H.; Zang, Y.; Cai, X.J.; Ren, G.W. Changes in the nutrient composition of tobacco plants after Potato virus Y infection and their effects on the growth and development of the vector Myzus persicae (Hemiptera: Aphididae). Acta Entomol. Sin. 2020, 63, 181–190. [Google Scholar] [CrossRef]
- Li, C.M. Adaptability of Bemisia tabaci (Gennadius) to the Host Plants and Plant Secondary Substances. Master’s Thesis, Yangzhou University, Yangzhou, China, 2009. [Google Scholar]
- Wang, Y.X.; Chen, H.F.; Yin, Z.Y.; Chen, W.L.; Lu, L.T. The genetic adaptations of Toxoptera aurantia facilitated its rapid multiple plant hosts dispersal and invasion. Genomics 2022, 114, 110472. [Google Scholar] [CrossRef]
- Fang, Y.; Qiao, G.X.; Zhang, G.X. Morphological adaptation of aphid species on different host plant leaves. Acta Entomol. Sin. 2011, 54, 157–178. [Google Scholar] [CrossRef]
- Golizadeh, A.; Abedi, Z.; Borzoui, E.; Golikhajeh, N.; Jafary, M. Susceptibility of five sugar beet cultivars to the black bean aphid, aphis fabae scopoli (Hemiptera: Aphididae). Neotrop. Entomol. 2016, 45, 427–432. [Google Scholar] [CrossRef]
- Han, B.Y.; Chen, Z.M. The differences between probing behaviour of tea aphids on different parts of tea tree. J. Plant Prot. 2001, 28, 7–11. [Google Scholar]
- Helden, M.V.; Tjallingii, W.F. Tissue localization of lettuce resistance to the aphid Nasonovia ribisnigri using electrical penetration graphs. Exp. Appl. 1993, 68, 269–278. [Google Scholar] [CrossRef]
- Zhao, R.N.; Wu, C.X.; He, Y.Q.; Yu, C.; Liu, J.F.; Li, T.S.; Zhou, C.Y.; Chen, W.L. Different host plants distinctly influence the feeding ability of the brown citrus aphid Toxoptera citricida. Insects 2021, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Tjallingii, W.F. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 2006, 57, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.Y.; Liu, L.F.; Yu, X.P.; Han, B.Y. Evaluation of the resistance of different tea cultivars to tea aphids by EPG technique. J. Integr. Agric. 2012, 11, 2028–2034. [Google Scholar] [CrossRef]
- Garzo, E.; Soria, M.L.; Gómez-Guillamon, M.L.; Fereres, A. Feeding behavior of Aphis gossypii on resistant accessions of different melon genotypes (Cucumis melo). Phytoparasitica 2002, 30, 129–140. [Google Scholar] [CrossRef]
- Peng, H.C.; Walker, G.P. Sieve element occlusion provides resistance against aphis gossypii in TGR-1551 melons. Insect Sci. 2018, 1, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Chang, W.J.; Zhan, Y.D.; Liu, Z.; Liu, Y. Investigation on the resistance of wheat germplasm resources to aphid based on fuzzy recognition and electrical penetration graph (EPG) techniques. Chin. J. Appl. Ecol. 2020, 31, 3248–3254. [Google Scholar] [CrossRef]
- Ouyang, Y.T.; Tian, S.; Hu, L.; Zhao, D.G.; Chen, Z.W.; He, Y.Q. Effects of physical and chemical properties of tea leaves on feeding selectivity of Toxoptera aurantii Boyer de Fonscolombe. Plant Prot. 2023. submitted. [Google Scholar] [CrossRef]
- Sarria, E.; Cid, M.; Garzo, E.; Fereres, A. Excel workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 2009, 67, 35–42. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical nature of recorded signals during stylet penetration by aphids. Entomol. Exp. Appl. 1985, 38, 177–186. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhang, Y.Q.; Chen, J.N.; Chen, W.L.; Zeng, X.Y.; Chen, H.T.; Ding, W. Effects of Aphidius gifuensis on the feeding behavior and potato virus Y transmission ability of Myzus persicae. Insect Sci. 2017, 25, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Kanissery, R.; Ammar, E.-D.; Cabral, I.; Markle, L.T.; Patt, J.M.; Stelinski, L.L. Feeding behavior of Asian citrus psyllid [Diaphorina citri (Hemiptera: Liviidae)] nymphs and adults on common weeds occurring in cultivated citrus described using electrical penetration graph recordings. Insects 2020, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Guo, Z.; Yang, Z.; Han, H.; Wang, S.; Xu, H.; Yang, X.; Yang, F.; Wu, Q.; Xie, W.; et al. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 2021, 184, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Q.; Jiang, W.B.; Ding, W.; Chen, W.L.; Zhao, D.G. Effects of PVY-infected tobacco plants on the adaptation of Myzus persicae (Hemiptera: Aphididae). Insects 2022, 13, 1120. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.L.; Qiao, C.Y.; Kong, H.L.; Lu, M.; Mao, N.; Zhu, S.D. Adaptation of Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) to different tomato cultivars. J. Plant Prot. 2015, 42, 734–740. [Google Scholar] [CrossRef]
- Guo, J.F.; Zhang, M.D.; Gao, Z.P.; Wang, D.J.; He, K.L.; Wang, Z.Y. Comparison of larval performance and oviposition preference of Spodoptera frugiperda among three host plants: Potential risks to potato and tobacco crops. Insect Sci. 2021, 28, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Malekera, M.J.; Dhungana, S.K.; Sharma, S.R.; Lee, K.Y. Impact of rice and potato host plants is higher on the reproduction than growth of corn strain Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 256. [Google Scholar] [CrossRef]
- Brito, N.F.; Moreira, M.F.; Melo, A.C.A. A look inside odorant-binding proteins in insect chemoreception. J. Insect Physiol. 2016, 95, 51–65. [Google Scholar] [CrossRef]
- Luo, C.; Yue, M.; Xu, H.F.; Zhang, Z.L. Application of electrical penetration graph (EPG) in entomological studies and new findings. Acta Entomol. Sin. 2005, 48, 437–443. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Y.; Teng, Z.W.; Du, L.D.; Wan, F.H.; Zhou, H.X. EPG-based comparison of feeding behaviors of three piercing-sucking pests on apple seedlings. Acta Entomol. Sin. 2020, 63, 1207–1214. [Google Scholar] [CrossRef]
- Kempema, L.A.; Cui, X.; Holzer, F.M.; Walling, L.L. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol. 2007, 143, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, G.H.; Ding, W.B.; Zhang, Y.J.; Li, Y.Z. EPG analysis of feeding behavior of Sogatella furcifera (Hemiptera: Delphacidae) on different rice varieties. Acta Entomol. Sin. 2014, 57, 335–342. [Google Scholar] [CrossRef]
- Zehnder, C.B.; Hunter, M.D. Effects of nitrogen deposition on the interaction between an aphid and its host plant. Ecol. Entomol. 2008, 33, 24–30. [Google Scholar] [CrossRef]
- Pobozniak, M.; Gaborska, M.; Wojtowicz, T. Resistance and tolerance of ten carrot cultivars to the hawthorn-carrot aphid, Dysaphis crataegi Kalt., in Poland. PLoS ONE 2021, 16, e0247978. [Google Scholar] [CrossRef] [PubMed]
- Lantos, E.; Schliephake, E.; Krämer, R.; Will, T.; Nothnagel, T. Feeding behavior of Myzus persicae on asparagus species susceptible and resistant to Asparagus virus 1. Entomol. Exp. Appl. 2019, 167, 360–369. [Google Scholar] [CrossRef]
- Shih, P.Y.; Sugio, A.; Christophe-Simon, J. Molecular mechanisms underlying host plant specificity in aphids. Annu. Rev. Entomol. 2023, 68, 431–450. [Google Scholar] [CrossRef]
- Cao, H.H.; Wu, J.; Zhang, Z.F.; Liu, T.X. Phloem nutrition of detached cabbage leaves varies with leaf age and influences performance of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2018, 166, 452–459. [Google Scholar] [CrossRef]
- Ahmed, N.; Darshanee, H.L.C.; Fu, W.; Hu, X.; Fan, Y.; Liu, T. Resistance of seven cabbage cultivars to green peach aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2018, 111, 909–916. [Google Scholar] [CrossRef]
- Feng, H.; Han, H.L.; Pu, P.; Wei, D.; Wang, J.; Liu, Y.H. Effects of five host plant species on the life history and population growth parameters of Myzus persicae (Hemiptera: Aphididae). J Insect Sci. 2019, 19, 15. [Google Scholar] [CrossRef]
- Zhao, R.N. CTV-Mediated Host Selection and Population Fitness of Brown Citrus Aphid, Toxoptera citricida (Kirkaldy) (Homoptera: Aphidiae). Doctoral Thesis, Guizhou University, Guizhou, China, 2022. [Google Scholar]
- Lu, C.; Shen, N.; Jiang, W.; Xie, B.; Zhao, R.; Zhou, G.; Zhao, D.; He, Y.; Chen, W. Different tea germplasms distinctly influence the adaptability of Toxoptera aurantii (Hemiptera: Aphididae). Insects 2023, 14, 695. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Plant secondary metabolites modulate insect behavior-steps toward addiction. Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Korada, R.R. Plant volatiles and insect herbivore interactions: An overview. J. Appl. Zool. Res. 2017, 28, 122–137. [Google Scholar]
- Gao, X.F.; Li, H.L. The relationship between tea leaf structure and selectivity of tea aphids on tea germplasms. Fujian J. Agric. Sci. 2014, 29, 256–260. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, L.Y.; Gao, X.K.; Zhang, K.X.; Li, D.Y.; Luo, J.Y.; Cui, J.J.; Yang, J. Screening of EPG-based parameters for measuring resistance to Aphis gossypii. Chin. J. Biol. Control 2022, 38, 1193–1201. [Google Scholar] [CrossRef]
- Douglas, A. Phloem-sap feeding by animals: Problems and solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Liu, H.R.; Zhang, Z.F.; Liu, T.X. The green peach aphid Myzus persicae perform better on pre-infested Chinese cabbage Brassica pekinensis by enhancing host plant nutritional quality. Sci. Rep. 2016, 6, 21954. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.M.; Zhang, J.X.; Sun, Q.Y.; Ye, T.; Xia, X.J.; Zhang, R.; Ding, Y. Feeding preference and adaptation of Ectropis grisescens (Lepidoptera: Geometridae) to different tea cultivars and their relationship with nutritional components in leaves of tea plants. Acta Entomol. Sin. 2018, 61, 1300–1309. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).