Abstract
Florists’ greens are a very important element of floral compositions, and their vase life must match that of the flowers, hence this review presents the results of research that has been conducted over the years in order to improve the post-harvest longevity of species that are grown for florists’ greens using growth regulators from groups of gibberellins (GAs) and cytokinins (CKs). Florists’ greens include foliage, the leafy and non-leafy stems of herbaceous plants, trees, bushes, and phylloclades. The post-harvest longevity of florists’ greens is influenced by genetics. Also strongly affected by the growing conditions and the conditions of the transport of the florists’ greens and the conditions when supplying them to markets are also significant. Moreover, florists’ greens are not supplied with growth regulators, which play a critical role in their ageing process. The CKs and GAs are considered to be inhibitors of ageing; however, unfortunately, their content in plant tissues decreases during the progressive ageing process, while the amount of regulators that accelerate ageing increases. International research is focusing on the use of growth regulators in the post-harvest treatment of florists’ greens. Their effectiveness has been shown to depend on the species, the cultivar, the concentration, and the method of application, therefore, there is no ready-made recipe that can be used for all species. The growth regulators from the CK and GA groups are used to condition the florists’ greens. Few studies to date point to the possibility of using topolines (Ts) and ionic liquids in order to extend the post-harvest longevity of florists’ greens. The standard cut flower medium containing 2% sucrose and hydroxyquinoline esters—sulphate or citrate (8HQS and 8HQC)—at a concentration of 200 mg·dm−3, which is used to conditioning, does not have a positive effect on florists’ greens of most species.
1. Introduction
‘Florists’ greens’ is a conventional term that describes all of the bouquet additions that are used in modern floristry. Florists’ greens include foliage, the leafy and non-leafy stems of herbaceous plants, trees, bushes, and phylloclades. It is often the case that florists’ greens extend to the fruiting and flowering shoots that end in inflorescences consisting of small flowers [1,2,3]. The commercially available florists’ greens include species that are grown under cover, such as in greenhouses and polytunnels, for cut flowers and as potted plants for indoor decoration. Annual and perennial outdoor plants are also frequently grown because their cultivation cost is much lower [1,2,3,4]. The post-harvest longevity of florists’ greens is influenced by genetics [1]. Of the species that are grown in greenhouses, those with leathery, succulent, or waxy cuticle-covered leaves are particularly durable, such as those that are found in the following species: Anthurium cultorum, Zantedeschia sp., Hippeastrum sp., Spatiphyllum sp., Monstera deliciosa, Philodendron selloum, and Syngonium podophyllum. The species with colorful leaves, including Cordyline terminalis and C. fruticosa, are becoming increasingly important in this regard. Phylloclades are particularly durable. They have modified lateral shoots that resemble the structure of true leaves and perform their functions, such as those that are found in Ruscus sp. and Asparagus sp. In a dry environment, they are less likely to lose water through transpiration, and it is this negative water balance that is created by the loss of moisture from the leaf blades, or by the blockage of the conducting vessels preventing water uptake, that is the most important reason for the ageing of florists’ greens [5,6,7]. Among garden perennials, the leaves of Hosta sp., Heuchera sp., Bergenia cordifolia, Paeonia sp., Stachys lanata, Vinca major, Pulmonaria saccharata, Dictamnus albus, and Astilbe x arendsii stand out for their good longevity. When it comes to annual plants, Molucella laevis and Bupleurum griffithii are widely cultivated. In the case of these species, entire shoots are used [7].
Yellowing, drooping, wilting, or withering leads to the loss of the ornamental value of the florists’ greens, which have great variation among species and cultivars in terms of the post-harvest longevity, the resistance to transport conditions, and the storability [6,8]. Florists’ greens are divided into three groups based on their storability [6]. The first group includes fern species of the Cyrtomium, Polystichum, and Anturium cultorum genera [6,9], as well as Ruscus [6,10], which can be stored more than 3 weeks. The second group covers species of the Asparagus and Pteris cretica genera, which can be stored for 2–3 weeks. The last group includes species that can be stored for 10–14 days, such as Asparagus virigatus, as well as Nepfrolepis exaltata, and its cultivars [6].
The post-harvest longevity of florists’ greens is strongly affected by the growing conditions—the more they match the requirements of each species, the better the longevity will be [5]. The conditions of transporting florists’ greens and the conditions when supplying them to markets are also significant. Dry transport exacerbates the water stress that is initiated when the leaves and the leafy shoots are cut from the parent plants [1]. Moreover, florists’ greens are not supplied with growth regulators, which play a critical role in their ageing process [5,6]. Cytokinins (CKs) and gibberellins (GAs) are considered to be ageing inhibitors; however, unfortunately, their content in the plant tissues decreases during the progressive ageing process, while the levels of the regulators that accelerate the ageing process—ethylene, salicylic acid (SA), brassinosteroids (BR), abscisic acid (ABA), and jasmonic acid (JA)—increase [11]. In ageing leaf cells, membrane-damaging enzymes become active [2], proteolysis [12,13] and the breakdown of chlorophyll accelerates [1,6,10], and free radicals are produced in large quantities in the cells, destroying the cell components [14]. The production of reactive oxygen species (ROS) is one of the earliest responses of plant cells to ageing [15,16]. The ROS are formed as by-products of the aerobic energy metabolism, as well as from plant exposure to various biotic and abiotic stresses [17,18,19]. Under normal conditions, the ROS production in cells is kept low by the antioxidant enzymes. This balance can be disrupted by antioxidant depletion or the excessive accumulation of ROS, leading to oxidative stress, and resulting in damage to cellular macromolecules and membranes, as well as increased lipid peroxidation [20,21]. One of the most important ROS is hydrogen peroxide (H2O2), due to its relative stability and its ability to diffuse through membranes. H2O2 plays an important role during ageing, as it is a signal molecule of the ageing process. Its accumulation is indicative of oxidative stress [22].
A decrease in the chlorophyll content is the first visual symptom of leaf ageing [23,24]. The accompanying degradation of proteins, due to the increased proteolytic enzyme activity [25], results in the accumulation of ammonium and free amino acids, including free proline, which maintains the osmotic balance between the cytoplasm and the vacuole, and affects the protein structure and synthesis [24,26,27]. Delaying proteolysis is fundamental to slowing down ageing. Specific endo- and exo-proteases hydrolyze the peptide bonds that release the amino acids. The final products of hydrolysis, including ammonia (NH3), are dangerous to the cells, therefore, they are converted into less toxic forms, such as amides, which are also easier to transport [28]. The protein and RNA degradation, on the other hand, coincide with the loss of photosynthetic activity, due to chlorophyll breakdown [23].
This review presents the results of research that has been conducted over the years in order to improve the post-harvest longevity of species that are grown for florists’ greens using growth regulators from the groups of GAs and CKs.
2. Harvest and Pre-Treatment of Florists’ Greens
The healthy and undamaged leaves or shoots are cut early in the morning, due to the good tissue turgor pressure [6,7,8,29,30], or before the evening, due to the accumulation of assimilates in the tissues. It is advisable to cut the fully mature, undamaged leaves. Cutting the young leaves is not advisable, as they do not maintain their ornamental value for long after cutting [7].
Immediately after harvesting, florists’ greens should be placed into containers with water or should be conditioned and cooled [6,7,8]. The temperature for cooling, storing, and transporting the florists’ greens is species-dependent. Most of the species of florists’ greens prefer a temperature range of 2–4 °C. However, a temperature of at least 7 °C must be ensured for topical plant species at all of the stages of marketing [6,7].
The conditioning of the florists’ greens is a treatment that is carried out by the producer of the plants. It prevents water loss from the tissues, protects against the damaging effects of ethylene, inhibits the growth of microorganisms, and prevents the tissues from losing too many reserves [7,31]. Cooling the leaves of perennials at 4 °C immediately after cutting has, in most cases, a beneficial effect on their longevity in compositions. At the same time, these leaves should be kept in water for at least 12 h in order to ensure their better condition and a longer post-harvest longevity (Bergenia and Dictamnus). For outdoor ferns, however, it is better to place the ends of the petioles into boiling water (for up to 10 s) [7]. The conditioning takes 4–24 h. Short conditioning (4 h) takes place at 18–20 °C, while long conditioning (24 h) is carried out in a freezer at 4–5 °C [31]. There was an attempt to use a standard cut flower medium containing 2% sucrose and hydroxyquinoline esters—sulphate or citrate (8HQS and 8HQC)—at a concentration of 200 mg·dm–3 in order to condition the florists’ greens, however it was not shown to have a positive effect on the florists’ greens of most species (Table 1, Figure 1) [3,7,32,33].

Table 1.
Vase life of florists’ greens after the application of 8HQC + 2% sucrose.
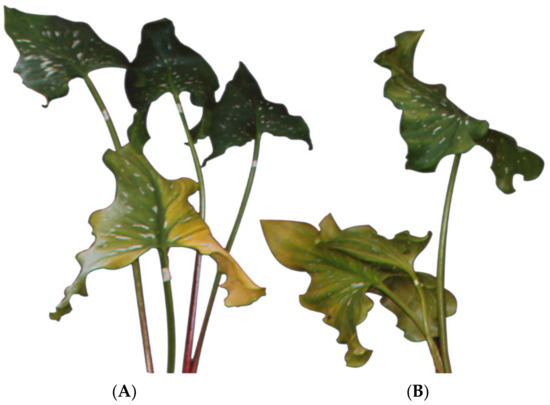
Figure 1.
Leaves of Zantedeschia elliottiana ‘Black Magic’. (A) control (B) conditioned in GA3 at a concentration of 300 mg dm−3 and stored in 8HQC.
3. Growth Regulators and Post-Harvest Longevity of Florists’ Greens
Research into the post-harvest longevity of florists’ greens started quite late, i.e., at the beginning of the last century. It can be linked to the increasing importance of green plant additions in floral compositions. As the ageing process of the florists’ greens is different to that of the flowers, in most cases the same measures that are used for the flowers cannot be used for them [29,34]. International research is focusing on the use of growth regulators in the post-harvest treatment of florists’ greens. Their effectiveness has been shown to depend on the species, the cultivar, the concentration, and the method of application, therefore, there is no ready-made recipe that can be used for all of the species and all recommendations must be backed up by research. The growth regulators from the group of CKs and GAs are used in order to conditioning the florists’ greens [1,3,4,6,8,11,29,30,31,32,34,35,36]. The most important GA is gibberellic acid (GA3). Of the CKs, benzyladenine (BA), which is a synthetic CK, is the most commonly used growth regulator. Few studies to date point to the possibility of using topolines (Ts) [1,28,37] and ionic liquids [1,38] in order to extend the post-harvest longevity of florists’ greens (Table 2).

Table 2.
Growth regulators that are used to extend the vase life of florists’ greens.
3.1. Effects of GA3 on Post-Harvest Longevity of Florists’ Greens
The effectiveness of GA3 in extending the post-harvest longevity of florists’ greens was demonstrated using species from different groups. Studies by Janowska and Schroeter-Zakrzewska [4] and Janowska et al. [36] have indicated that GA3 prolongs the post-harvest longevity of Limonium latifolium leaves. Arum italicum leaves also respond positively to GA3 [8]. Janowska et al. [31], on the other hand, have indicated that conditioning Geranium platypetalum leaves for 24 h in GA3 at 25 ppm and 50 ppm improves their post-harvest longevity by 9.4 and 10 days. Among the species that are cultivated for cut flowers, a positive effect on leaf longevity has been demonstrated in species of the Zantedeschia genus. In the study that was conducted by Janowska and Jerzy [32], GA3 had a favorable impact on the cut leaves of Zantedeschia with colorful spathes. In the ‘Florex Gold’ and ‘Black Magic’ cultivars, the leaves that were conditioned in GA3 at a concentration of 300 mg·dm−3 kept their ornamental values for the longest time. A comparable leaf longevity was recorded for the ‘Florex Gold’ cultivar that was placed in water after being conditioned in GA3 at a concentration of 200 mg·dm−3. In contrast, the results of the study by Skutnik et al. [34] indicated that GA3 is highly effective in extending the post-harvest longevity of Z. aethiopica leaves. Interesting results were also obtained in species of the Asparagus genus. In the study by Skutnik and Rabiza-Świder [40], soaking Asparagus falcatus shoots in a BA solution of 1 mmol∙dm−3 and in a GA3 solution of the same concentration increased their longevity by 1.5 times. Conditioning the shoots in the BA solution proved to be ineffective and conditioning them in the GA3 solution even reduced their post-harvest longevity. In an earlier study by Skutnik et al. [39], the effectiveness of the growth regulators depended on both the species and the cultivar of Asparagus and the method of the growth regulator application. The GA3 application prolonged the longevity of the cut shoots of Asparagus densiflorus ‘Myriocladus’, but only when they were subjected to 24 h conditioning in a 0.25 mmol∙dm−3 solution. In the case of A. densiflorus ‘Meyerii’, both of the forms of GA3 application, i.e., 24 h conditioning and short-term shoot soaking (a few seconds), prolonged their longevity. As for Asparagus setaceus, both conditioning and shoot soaking in the BA solution proved to be effective. In the experiment that was performed by Koziara and Sikora [41], it was shown that both conditioning and soaking Spatyphyllum leaves in GA3 and BA solutions prolonged their post-harvest longevity, while treating Dieffenbachia leaves with a GA3 solution of 1 mmol∙dm−3 had no effect on their longevity, with other treatments of growth regulators reducing their longevity. In contrast, there was no effect of either of the growth regulators on the post-harvest longevity of Syngonium leaves. According to Farahat and Gaber [45], GA3 at concentrations of 25 and 50 mg·dm−3 effectively extended the post-harvest longevity of Monstera deliciosa leaves to 51 and 59 days, respectively.
3.2. Effects of BA on Post-Harvest Longevity of Florists’ Greens
CKs are adenine derivatives. They are mainly produced in the roots, from which they are transported to the above-ground components. CKs play a key role in the different phases of plant growth and development, however, the underlying molecular mechanisms of their biosynthesis and their signal transduction have only recently been explained [46]. Synthetic BA is most commonly used in CK floriculture production [47]. In ornamental plants, BA is used primarily as a growth regulator that is responsible for the pullulation of in vitro propagated plants. In recent years, BA has also begun to be used in the cultivation of in vivo plants [48]. Ongoing research shows that BA effectively extends the post-harvest longevity of many species that are grown for florists’ greens. Janowska et al. [29] have demonstrated that the application of BA, at all of the tested concentrations, has a beneficial effect on the post-harvest longevity of Alchemilla mollis leaves. The leaves of Limonium latifolium [37], Arum italicum [33], Campsis radicans [42], and ×Heucherella respond similarly, with BA concentrations of 300 and 600 mg·dm−3 improving their longevity by 55.8% and 59.4% [30]. Koziara and Suda [43], on the other hand, have revealed that, of the Cordyline cultivars that they studied, BA had a positive effect on the post-harvest leaf longevity of the ‘Glauca’ cultivar only. Skutnik and Rabiza-Świder [49] have reported that the 24 h conditioning of cut Hosta ‘Undulata Erromena’ leaves in a BA solution extended their post-harvest longevity up to six times, compared to control leaves.
3.3. Effects of Ts on Post-Harvest Longevity of Florists’ Greens
Ts are a new group of endogenous, aromatic cytokinins that have been isolated from poplars at Palacky University Olomouc and at the Institute of Experimental Botany in the Czech Republic. The Ts are derivatives of benzyl amino purine. In their benzene ring, there is a hydroxyl group in an ortho or meta position. In the very few studies that have been conducted so far, Ts have only been used in order to assess their usefulness in in vitro cultures. It was determined that, in standard biological tests, these substances strongly prevent leaf ageing [50]. Recently, Ts have been used in research into the post-harvest longevity of florists’ greens. The published results indicate that Ts can also be used in vivo in order to improve the longevity of florists’ greens. According to Janowska et al. [36], meta-methoxytopoline (MemT) and its riboside (MemTR), at concentrations of 25–75 mg∙dm−3, have a beneficial effect on the post-harvest longevity of Z. albomaculata ‘Albomaculata’ leaves (Figure 2). When they are used to conditioning Limonium latifolium leaves for four hours, these CKs have been proven to be equally effective, extending their post-harvest longevity by 4.0–4.9 days [37]. In a later study, Janowska et al. [29] have shown that Ts also improved the post-harvest longevity of Alchemilla mollis leaves, but the beneficial effect of these growth regulators was only demonstrated when MemT was applied at a concentration of 75 mg∙dm−3 and MemTR at a concentration of 50–75 mg∙dm−3, confirming the different response of the species, not only to the growth regulators themselves, but also to their concentration. Recent studies have shown that MemTR, at concentrations of 50 and 100 mg∙dm−3, extend the leaf longevity of Hemerocallis × hybrida ‘Agata’, while MemTR and its riboside extend the leaf longevity of Heuchera × hybrida ‘Chocolate Ruffles’ [1].

Figure 2.
Leaves of Zantedeschia ‘Albomaculata’ 6 days after conditioning and insertion into water. (A)—from left—control leaves, from right—conditioned in MemT at concentration of 25 mg dm−3; (B)—from left—control leaves, from right—conditioned in MemT at concentration of 50 mg dm−3; (C)—from left—control leaves, from right—conditioned in MemT at concentration of 75 mg dm−3; (D)—from left—control leaves, from right—conditioned in MemTR at concentration of 25 mg dm−3; (E)—from left—control leaves, from right—conditioned in MemTR at concentration of 50 mg dm−3; (F)—from left—control leaves, from right—conditioned in MemTR at concentration of 75 mg dm−3.
3.4. Effects of Ionic Liquids and Quaternary Ammonium Salts with Gibberellate Anion on Post-Harvest Longevity of Florists’ Greens
Quaternary ammonium salts with selected organic cations and GA3 anions were obtained at the Faculty of Chemical Technology, Poznan University of Technology. The salts with choline (2-hydroxyethyl)dimethylethylammonium gibberellinate—[Chol][Gib]) and acetylcholine (ACh) (acetylcholine gibberellinate—[Gib][Ach]) cations were obtained by a methathesis reaction using the GA3 potassium salt in methanol (CH3OH). The salts with a 1-ethylquinine gibberellinate (1-ethylquinine gibberellinate—[Q-C2][Gib]) and 1-dodecylquinine (1-dodecylquinine gibberellinate—[Q-C12][Gib]) cation were obtained by a two-step reaction. In the first step, the bromide anion was exchanged for the hydroxyl anion using an ion-exchange resin, followed by a neutralisation reaction with GA3. The use of both of these methods allowed the halide anion to be exchanged for the gibberellate anion with high efficiency, ranging from 97 to 99%. The salts with the GA3 anion that were obtained were solids at an ambient temperature and did not exhibit a melting point in the studied range of the phase transitions. [Chol][Gib] and [Gib][Ach] at 25 °C were in the form of glassy solids and they belong to the group of ionic liquids. The melting point for [Q-C2][Gib]) and [Q-C12][Gib] was beyond that of the studied range and they are quaternary ammonium salts. Changing the active substance (choline or ACh) to the form of quaternary ammonium salts made it possible to obtain the growth regulators that have better biological activity, which are, therefore, more effective (Figure 3) [38]. Few studies to date have shown that quaternary ammonium salts with the gibberellate anion prolong the post-harvest longevity of Convallaria majalis leaves, while the use of acetylcholine gibberellinate ([Gib][Ach]) at a concentration of 100 mg·dm−3 causes the post-harvest leaf longevity to increase more than twofold [38]. In contrast, the four hour conditioning of Hemerocallis × hybrida ‘Agata’ leaves in [Chol][Gib] at 50 and 100 mg·dm−3, [Q-C2][Gib] at 100 mg·dm−3, and [Gib][Ach] at 50 mg·dm−3 extends the post-harvest longevity by seven to nine days. As for Limonium latifolium, the leaves with the best longevity are obtained after the application of [Gib][Ach] at 50 and 100 mg·dm−3 and [Q-C12][Gib] at 100 mg·dm−3 [1].

Figure 3.
Leaves of Convallaria majalis 8 days after conditioning and insertion into water. (A)—from left: control leaves, conditioned in [Q-C12][Gib] at concentration of 50 and 100 mg dm−3; (B)—from left: control leaves, conditioned in [Gib][Ach] at concentration of 50 and 100 mg dm−3; (C)—from left: control leaves, conditioned in [Q-C2][Gib] at concentration of 50 and 100 mg dm−3; (D)—from left: control leaves, conditioned in [Q-C12][Gib] at concentration of 50 and 100 mg dm−3.
4. Physiological Role of Growth Regulators in the Ageing Process of Florists’ Greens
The leaf ageing process is a genetically regulated and ordered series of events marking the last stage of leaf development before the organ dies. During ageing, photosynthesis declines, due to the breakdown of chlorophyll and the dismantling of the photosynthetic apparatus. The structure of the cell changes, the organelles are disassembled, and the proteins, the lipids, the nucleic acids, and the carbohydrates undergo hydrolysis. The factors that regulate leaf ageing are complex and they include both external and internal stimuli. These factors initiate the signal transduction pathways that inhibit photosynthesis and activate the expression of the genes that are involved in cell disassembly, recycling, and integral defense processes. The genes that increase in number during ageing are known as senescence-associated genes (SAGs) [5]. The degradation of chlorophyll and proteins is a completely natural process, but an unfavorable one, therefore, all post-harvest treatments aim to inhibit it. The proteins are an important component of plant cells. They regulate the vital processes, are the building blocks of the cellular structures and tissues, and are responsible for most biochemical reactions in living organisms. The ageing processes include the degradation of macromolecules, including a reduction in proteins through protease activation and the loss of carbohydrates through the activation of E-glucosidases [51]. The study by Rabiza-Świder et al. [52] on the post-harvest longevity of Hosta ‘Undulata Erromena’ leaves after BA and GA3 application has revealed that a decrease in the soluble protein content in control leaves is accompanied by an increase in the total proteolytic activity and in the activity of cysteine protease, which is the primary enzyme that is responsible for protein breakdown during ageing. The conditioning of Hosta leaves in BA prevents this increase, thus counteracting the loss of total protein. In Z. aethiopica, the conditioning with GA3 delays the protein degradation, but to a lesser extent than after BA treatment in Hosta ‘Undulata Erromena’ leaves. In the freshly cut leaves of both species, the authors found no expression of the cysteine protease gene. However, the presence of the gene transcript was detectable in the ageing leaves of both species that were stored in water. A significant increase in the cysteine protease activity was observed in these leaves. Still, no increase in cysteine protease mRNA levels was detected in the BA-conditioned Hosta leaves. In contrast, a slight increase in the transcript levels was observed in Z. aethiopica, regardless of whether the leaves were conditioned in GA3 or were stored in water. According to the authors, the different responses of the two species to the different growth regulators that were used to effectively inhibit the leaf ageing may point to a different mechanism for this process. In Hosta, BA also inhibits the ammonia and proline accumulation [44] and suppresses the increase in lipoxygenase activity [53]. The positive effect on the inhibition of protein degradation in florists’ greens following the application of growth regulators has been confirmed by the studies of other authors. Janowska et al. [1] have reported that the growth regulators that were used in their study inhibited the leaf protein breakdown to varying degrees in the analyzed species. In Hemerocallis × hybrida ‘Agata’, the application of MemT at 50 mg·dm−3, MemTR at 100 mg·dm−3, [Q-C2][Gib] and [Gib][Ach] at 100 mg·dm−3, and [Chol][Gib] at 50 mg·dm−3 inhibited the protein degradation in the leaves. In Limonium latifolium, this phenomenon was observed when the leaves were conditioned with [Chol][Gib] at concentrations of 50 and 100 mg·dm−3. Meanwhile, in Heuchera × hybrida ‘Chocolate Ruffles’, all of the conditioners, except for BA, inhibited the leaf protein breakdown, which was particularly evident for [Q-C2][Gib]. Janowska et al. [30] found that, in terms of the concentration, BA inhibits the protein degradation in the spring- and summer-harvested leaves of Heuchera ‘Purple Petticoats’ (600 mg·dm−3), in the leaves of the ‘Southern Comfort’ cultivar that were harvested in the spring (100–600 mg·dm−3) and the summer (600 mg·dm−3), and in the leaves of the ‘Plum Royale’ cultivar that were harvested in the summer (100 and 600 mg·dm−3). Meanwhile, the beneficial effect of GA3 on the protein content of Zantedeschia with colored inflorescences and leaves was reported by Janowska and Stanecka [35] and Janowska et al. [36].
Apart from inhibiting the protein degradation, the growth regulators also inhibit the chlorophyll breakdown, meaning that the leaves retain their green color for longer and have a better post-harvest longevity. This was confirmed in the study by Janowska and Schroeter-Zakrzewska [4], who used GA3 in order to condition Limonium latifolium leaves. Skutnik et al. [24], on the other hand, noted an inhibition of chlorophyll degradation after GA3 treatment in Z. elliottiana and Z. aethiopica leaves. The beneficial effect of GA3 on the chlorophyll content of Zantedeschia leaves with colored spathes has also been confirmed in a later study by Janowska and Stanecka [35]. Furthermore, the authors have shown that BA and a mixture of BA and GA3 were also effective in inhibiting the chlorophyll degradation in Zantedeschia leaves. The beneficial effect of BA on the greenness index for Hypericum ‘Magical Beauty’ shoots was shown by Janowska and Śmigielska [3]. The same effect was also observed in Asparagus falcatus by Skutnik and Rabiza-Świder [31] and by Skutnik et al. [40] in A. setaceus. In the few studies on the post-harvest longevity of florists’ greens after Ts application, it was shown that, for Limonium latifolium, only MemT had a beneficial effect on the greenness index [29], while for Z. albomaculata ‘Albomaculata’ leaves, Ts only inhibited the protein degradation without affecting the chlorophyll content. According to Janowska et al. [1], in Hemerocallis × hybrida ‘Agata’, the following substances are useful in inhibiting the chlorophyll degradation in the leaves: GA3, MemTR, [Gib][Ach], and [Q-C12][Gib] at both of the concentrations that were tested and [Q-C2][Gib] and [Chol][Gib] at 50 mg·dm−3. In Limonium latifolium, all of the conditioners, except for [Gib][Ach] and [Q-C12][Gib], inhibit chlorophyll breakdown, with GA3 at 100 mg·dm−3 being the most effective. In Heuchera × hybrida ‘Chocolate Ruffles’, only both Ts at 50 mg·dm−3 was ineffective in inhibiting the chlorophyll degradation. In this cultivar, [Chol][Gib] at a concentration of 50 mg·dm−3 is particularly effective.
As the leaves age, the sugar content changes. The sugars that are created during the photosynthesis process are the major structural and storage material in plant organisms. Intensive photosynthesis results in storing greater amounts of carbohydrates. Kozłowska et al. [54] have reported on the changes in the sugar content of the leaves of Z. elliottiana after the application of GA3 in rhizome soaking, depending on their development phase. The authors have reported that, during the initial phase of vegetative development, the leaf blades of the plants that were treated with GA3 were characterized by a higher content of hydrocarbons, especially fructose and glucose, compared to the plants from the control treatment. This content increased together with the development of the leaves in order to decrease when the plants entered the generative stage. At that time, the total content of the sugars in the leaves of the control plants was twice as high. The changes in the sugar content in the cut leaves of Z. aethiopica and Z. elliottiana were examined by Skutnik et al. [26]. During the advancing ageing process, the content of reducing sugars increased at the beginning and then fell to 60–80% of the initial level. Conditioning the leaves in a solution of BA did not have a retarding effect on this process. GA3 proved to be more effective, as it delayed the sugar degradation in the leaves of Z. aethiopica. In Z. elliottiana, it resulted in an increase in the sugar content. Having compared the sugar content of the ageing leaves of Heucherella cultivars, Janowska et al. [30] found that it depended significantly on both the leaf harvest date and the BA concentration. Irrespective of the BA concentration, ‘Solar Power’ and ‘Kimono’ cultivars had a significantly higher sugar content in the summer-harvested leaves. Irrespective of the leaf harvest date, it was shown that BA at the tested concentrations had a positive effect on the sugar content of the leaves for both of the cultivars. After comparing the interactions, both of the cultivars were found to have a significantly higher sugar content in the leaves in the treatments where BA was applied to the leaves of the parent plants at the tested concentrations. This effect was particularly noticeable in the leaves that were cut in the summer. In addition, Janowska et al. [31] report that conditioning Geranium platypetalum leaves in GA3 at the concentration of 25 ppm and 50 ppm causes the increase in the saccharide content.
5. Conclusions
‘Florists’ greens’ is a conventional term that describes all of the bouquet additions that are used in modern floristry. Florists’ greens include foliage, the leafy and non-leafy stems of herbaceous plants, trees, bushes, and phylloclades. It is often the case that florists’ greens extend to fruiting and flowering shoots that end in inflorescences consisting of small flowers. The commercially available florists’ greens include species that are grown under cover, such as in greenhouses and in plastic tunnels, for cut flowers and as potted plants for indoor decoration. Annual and perennial outdoor plants are also more frequently grown because their cultivation cost is much lower. The post-harvest longevity of florists’ greens is influenced by genetics. The post-harvest longevity of florists’ greens is also strongly affected by the growing conditions—the more they match the requirements of each species, the better the longevity will be. The conditions for transporting florists’ greens and the conditions for supplying them to markets are also significant. Dry transport exacerbates the water stress that is initiated when the leaves and the leafy shoots are cut from the parent plants. Moreover, florists’ greens are not supplied with growth regulators, which play a critical role in their ageing process. CKs and GAs are considered to be inhibitors of ageing; however, unfortunately, their content in the plant tissues decreases during the progressive ageing process, while the amount of regulators that accelerate ageing, increases. In the ageing leaf cells, membrane-damaging enzymes become active, proteolysis and the breakdown of chlorophyll accelerates, and free radicals are produced in large quantities in the cells, destroying the cell components. ROS production is one of the earliest responses of the plant cells to ageing. As the ageing process of the florists’ greens is different to that of the flowers, in most cases the same measures that are used for the flowers cannot be used for them. International research is focusing on the use of growth regulators in the post-harvest treatment of florists’ greens. Their effectiveness has been shown to depend on the species, the cultivar, the concentration, and the method of application, therefore, there is no ready-made recipe that can be used for all of the species and all recommendations must be backed up by research. The growth regulators from the CK and GA groups are used in order to condition florists’ greens. The most important GA is GA3. As for CKs, BA is the most commonly used growth regulator. Few studies to date point to the possibility of using Ts and ionic liquids to extend the post-harvest longevity of florists’ greens.
Author Contributions
Conceptualization, B.J. and R.A.; methodology, B.J. and R.A.; formal analysis, B.J. and R.A.; writing, B.J. and R.A.; funding acquisition, B.J. and R.A.; writing—original draft, B.J. and R.A.; writing—review and editing, B.J. and R.A. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was co-financed within the framework of Ministry of Science and Higher Education program “Regional Initiative Excellence” in the years 2019–2022, project no. 005/RID/2018/19, financing amount PLN 12,000,000.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Janowska, B.; Nowińska, M.; Andrzejak, R. The vase life of the leaves of selected perennial species after the application of growth regulators. Agronomy 2022, 12, 805. [Google Scholar] [CrossRef]
- Janowska, B.; Trelka, T. Effect of preparations from the Chrysal series and benzyladenine on the postharvest longevity of shoots of the St. John’s wort (Hypericum calycinum L.). Nauka Przyr. Technol. 2010, 4, 8. [Google Scholar]
- Janowska, B.; Śmigielska, M. Effect of growth regulators and 8-hydroxyquinoline sulphate on postharvest longevity of Hypericum inodorum L. ‘Magical Beauty’. Zesz. Probl. PostęPóW Nauk. Rol. 2010, 551, 103–110. [Google Scholar]
- Janowska, B.; Schroeter-Zakrzewska, A. Effect of growth regulators on the postharvest longevity of leaves of sea lavender (Limonium latifolium /Sm./ Kuntze). Nauka Przyr. Technol. 2010, 4, 3. [Google Scholar]
- Hayden, D.H. Characterization of senescence regulated gene expression in Anthurium. Ph.D. Thesis, University of Hawaii Library, Honolulu, HI, USA, 2003. [Google Scholar]
- Łukaszewska, A.; Skutnik, E. Przewodnik florysty; Wydawnictwo SGGW: Warszawa, Poland, 2003. [Google Scholar]
- Skutnik, E. Jak przedłużyć trwałość zieleni ciętej (cz. I). Hasło Ogrodnicze 2005, 7, 4–7. [Google Scholar]
- Janowska, B. Effect of conditioning on the longevity of leaves of the Italian arum (Arum italicum Mill.) kept at a low temperature. Nauka Przyr. Technol. 2010, 4, 12. [Google Scholar]
- Hansen, J.D.; Paull, R.E.; Hara, A.H.; Tenbrink, V.L. Predicting vase life in tropical cut flowers and foliage. Proc. Fla. State Hortic. Soc. 1991, 104, 61–63. [Google Scholar]
- Pacifici, S.; Burchi, G.; del Carlo, A.; Ferrante, A. Effect of storage temperature and duration on vase life of cut Ruscus racemosus L. foliage. Acta Hortic. 2013, 970, 69–74. [Google Scholar] [CrossRef]
- Asami, T.; Nakagawa, Y. Preface to the Special Issue: Brief review of plant hormones and their utilization in agriculture. J. Pestic. Sci. 2018, 43, 154–158. [Google Scholar] [CrossRef]
- Nam, H.G. The molecular genetic analysis of leaf senescence. Curr. Opin. Biotechnol. 1997, 8, 200–207. [Google Scholar]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.-S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Lee, S.; Seo, P.J.; Lee, H.J.; Park, C.M. A nac transcription factor ntl4 promotes reactive oxygen species production during drought-induced leaf senescence in arabidopsis. Plant J. Cell Mol. Biol. 2012, 70, 831–844. [Google Scholar]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar]
- Selote, D.S.; Khanna-Chopra, R. Drought acclimation confers oxidative stress tolerance by inducing co-ordinated antioxidant defense at cellular and subcellular level in leaves of wheat seedlings. Physiol. Plant. 2006, 127, 494–506. [Google Scholar]
- Silva, E.N.; Ferreira-Silva, S.L.; Fontenele, A.D.V.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Phsiol. 2010, 167, 1157–1164. [Google Scholar]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar]
- Río, L.A.D.; Pastori, G.M.; Palma, J.M.; Sandalio, L.M.; Sevilla, F.; Corpas, F.J.; Jiménez, A.; López-Huertas, E.; Hernández, J.A. The activated oxygen role of peroxisomes in senescence. Plant Physiol. 1998, 116, 1195–1200. [Google Scholar]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar]
- Jajic, I.; Sarna, T.; Strzalka, K. Senescence, stress, and reactive oxygen species. Plants 2015, 4, 393–411. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Earl, E.; Harrison, E.; Mathas, E.; Navabpour, S.; Page, T.; Pink, D. The molecular analysis of leaf senescence– a genomics approach. Plant Biolechnol. J. 2002, 1, 3–22. [Google Scholar]
- Skutnik, E.; Rabiza-Swider, J.; Wachowicz, M.; Łukaszewska, A. Senescence of cut leaves of Zantedeschia aethiopica and Z. elliottiana. Part I. Chlorophyll degradation. Acta. Sci. Pol. Hortorum Cultus 2004, 3, 57–65. [Google Scholar]
- Rabiza-Świder, J.; Skutnik, E.; Wachowicz, M.; Łukaszewska, A.J. Senescence of cut leaves of Zantedeschia aethiopica and Z. elliottiana. Part II. Free amino acids accumulation in relation to soluble protein content. Acta Sci. Pol. Hortorum Cultus 2004, 3, 67–174. [Google Scholar]
- Skutnik, E.; Rabiza-Swider, J.; Wachowicz, M.; Łukaszewska, A. Senescence of cut leaves of Zantedeschia aethiopica and Z. elliottiana. Part III. The reducing sugars content. Acta. Sci. Pol. Hortorum Cultus 2004, 3, 219–227. [Google Scholar]
- Yang, C.W.; Kao, C.H. Ammonium in relation to proline accumulation in detached rice leaves. Plant Growth Regul. 2000, 30, 139–144. [Google Scholar]
- Nooden, L.D.; Guiament, J.J. Genetic control of senescence and aging plants. Physiol. Plant 1996, 116, 416–421. [Google Scholar]
- Janowska, B.; Andrzejak, R.; Jakubowska, P.; Antkowiak, A.; Nawrot, D.; Krzaczkowska, A. The effect of growth regulators on the post-harvest longevity of leaves of the Alchemilla mollis (Bauser) Rothm. leaf longevity. Folia Hort. 2016, 28, 137–142. [Google Scholar]
- Janowska, B.; Czuchaj, P.; Rybus-Zając, M. Post-harvest longevity of ×Heucherella L. leaves after the application of benzyladenine sprayed on maternal plants. Acta Agrob. 2016, 69, 1649. [Google Scholar] [CrossRef]
- Janowska, B.; Rybus-Zając, M.; Deręgowska, P.; Kujawa, M.; Wróblewska, P.; Andrzejak, R. Post-harvest longevity of leaves of the Iberian cranesbills (Geranium platypetalum Fisch. et Mey.) after the application of gibberellic acid. Bul. J. Agric. Sci. 2015, 21, 579–584. [Google Scholar]
- Janowska, B.; Jerzy, M. Effect of gibberellic acid on the post-harvest Zantedeschia elliottiana (W.Wats) Engl. leaf longevity. J. Fruit Ornam. Plant Res. 2003, 11, 69–76. [Google Scholar]
- Janowska, B.; Schroeter-Zakrzewska, A. Effect of gibberellic acid, benzyladenine and 8-hydroxyquinoline sulphate on post-harvest leaf longevity of Arum italicum Mill. Zesz. Probl. PostęPóW Nauk. Rol. 2008, 525, 181–187. [Google Scholar]
- Skutnik, E.; Łukaszewska, A.J.; Serek, M.; Rabiza, J. Effect of growth regulators on postharvest characteristics of Zantedeschia aethiopica. Post. Biol. Technol. 2001, 21, 241–246. [Google Scholar] [CrossRef]
- Janowska, B.; Stanecka, A. Effect of growth regulators on the postharvest longevity of cut flowers and leaves of the Calla lily (Zantedeschia Spreng). Acta Agrobot. 2011, 64, 91–98. [Google Scholar] [CrossRef][Green Version]
- Janowska, B.; Stanecka, A.; Czarnecka, B. Postharvest longevity of the leaves of the Calla lily (Zantedeschia Spreng.). Acta Sci. Pol. Hortorum Cultus 2012, 11, 121–131. [Google Scholar]
- Janowska, B.; Grabowska, R.; Ratajczak, E. Post-harvest longevity of leaves of the Sea lavender (Limonium latifolium (Sm.) Kuntze) after application of growth regulators. Hort. Sci. 2013, 40, 172–176. [Google Scholar] [CrossRef]
- Szymaniak, D.; Pernak, J.; Rzemieniecki, T.; Kaczmarek, D.K.; Andrzejak, R.; Kosiada, T.; Janowska, B. Synthesis and characterization of bio-based quaternary ammonium salts with gibberellate or α-tryptophanate anion. Monatsh. Chem.–Chem. Mon. 2020, 151, 1365–1373. [Google Scholar] [CrossRef]
- Skutnik, E.; Rabiza-Świder, J.; Łukaszewska, A. Evaluation of several chemical agents for prolonging vase life in cut asparagus greens. J. Fruit Ornam. Plant Res. 2006, 14, 233–240. [Google Scholar]
- Skutnik, E.; Rabiza-Świder, J. Regulacja pozbiorczej trwałości ciętych pędów szparaga sierpowatego (Asparagus falcatus L.). Zesz. Probl. PostęPóW Nauk. Rol. 2008, 525, 389–396. [Google Scholar]
- Koziara, Z.; Sikora, E. Wpływ GA3, BA i preparatu Chrysal Clear na pozbiorczą trwałość wybranych gatunków roślin ozdobnych stosowanych na zieleń ciętą. Zesz. Probl. PostęPóW Nauk. Rol. 2006, 510, 309–315. [Google Scholar]
- Pogroszewska, E.; Woźniacki, A. Wpływ sposobu pozbiorczego traktowania na trwałość zieleni ciętej wykorzystywanej w kompozycjach kwiatowych. Zesz. Probl. PostęPóW Nauk. Rol. 2005, 504, 215–222. [Google Scholar]
- Koziara, Z.; Suda, B. Przedłużanie trwałości wybranych gatunków kordylin stosowanych na zieleń ciętą. Zesz. Probl. PostęPóW Nauk. Rol. 2008, 525, 203–210. [Google Scholar]
- Rabiza-Świder, J.; Skutnik, E.; Wachowicz, M. Wpływ substancji chemicznych na pozbiorcza trwałość liści barwnych odmian funkii (Hosta L.). Zesz. Probl. PostęPóW Nauk. Rol. 2006, 510, 559–565. [Google Scholar]
- Farahat, M.M.; Gaber, A. Influence of preservative materials on postharvest performance of cut window leaf foliage (Monstera deliciosa). Acta Hortic. 2010, 877, 1715–1720. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Kupke, B.M.; Tucker, M.R.; Able, J.A.; Porker, K.D. Manipulation of barley development and flowering time by exogenous application of plant growth regulators. Front. Plant Sci. 2022, 12, 694424. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R.; Kosiada, T.; Kwiatkowska, M.; Smolińska, D. The flowering and nutritional status of Gladiolus hybridus ‘ Black Velvet’ following a cytokinin treatment. J. Elementol. 2018, 23, 1119–1128. [Google Scholar] [CrossRef]
- Skutnik, E.; Rabiza-Świder, J. Effect of pulsing with growth regulators on senescence of the detached cold-stored leaves of Zantedeschia aethiopica Spr. and Hosta ‘Undulata Erromena’. Acta Sci. Pol. Hortorum Cultus 2005, 4, 101–110. [Google Scholar]
- Palavan-Ünsal, N.; Cağ, S.; Cetin, E.; Büyüktunçer, D. Retardation of senescence by meta-topolin in wheat leaves. J. Cell. Mol. Biol. 2002, 1, 101–108. [Google Scholar]
- Rubinstein, B. Regulation of cell death in flower petals. Plant Mol. Biol. 2000, 44, 303–318. [Google Scholar] [CrossRef]
- Rabiza-Świder, J.; Rybka, Z.; Skutnik, E.; Łukaszewska, A. Proteolysis and expression of the cysteine protease gene in senescing cut leaves of Hosta ‘Undulata Erromena’ and Zantedeschia aethiopica Spr. treated with BA or GA3. Acta Physiol. Plant. 2003, 25, 319–324. [Google Scholar] [CrossRef]
- Rabiza-Świder, J.; Łukaszewska, A.; Skutnik, E.; Rybka, Z.; Wachowicz, M. Lipoxygenase in senescing cut leaves of Zantedeschia aethiopica Spr. and Hosta ‘Undulata Erromena’ treated with GA3 or BA. Acta Physiol. Plant. 2004, 26, 411–415. [Google Scholar] [CrossRef]
- Kozłowska, M.; Rybus-Zając, M.; Stachowiak, J.; Janowska, B. Changes in carbohydrate contents of Zantedeschia leaves under gibberellin-stimulated flowering. Acta Physiol. Plant. 2007, 29, 27–32. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).