Abstract
Drip irrigation is an important way to alleviate the global water shortage. However, the emitter-clogging issue of drip irrigation directly affects irrigation uniformity and operation efficiency, even disabling the whole system and reducing crop production. Currently, with the widespread use of saline water and large-scale utilization of fertigation, the issue with the chemical clogging of emitters has become more prominent. The poor uniformity of irrigation and fertilization distribution caused by emitter clogging results in salt damage and fertilizer loss due to the complex clogging mechanism. However, no extensive information on chemical clogging is available. Herein, we surveyed the latest research on chemical clogging caused by saline water irrigation and fertigation in drip irrigation systems and described the clogging mechanisms of the emitter by analyzing the key factors, clogging rules, and substances. We also present a framework of the control technologies for clogging based on physical, chemical, and biological methods. Finally, we present the current challenges of fertigation with saline water and technical trends of emitter clogging in the drip irrigation system. To conclude, the efficient integration of these three methods is critical to prevent and eliminate chemical clogging.
1. Introduction
Water is critical for agricultural production, which plays an important role in global food security [1]. However, the shortage of water resources seriously restricts the sustainable development of the economy and society [2,3]. Agriculture is always the largest water user, accounting for more than 70% of global withdrawals of freshwater [4]. Thus, as the highest consumer of water, a lower return per unit of water than other economic sectors has put enormous pressure on the agricultural sectors and forced them to be more efficient in water utilization for more ‘crop per drop’ [5].
Micro-irrigation is an effective method to alleviate regional water shortage [6]. Statistics from the annual report of the International Commission on Irrigation and Drainage (ICID) show that both global total irrigation areas and micro-irrigation areas have increased in the past 10 years, especially for micro-irrigation, with a 43% increment in the past five years (Figure 1) [7,8,9]. As one of the micro-irrigation techniques, drip irrigation has an outstanding advantage in controllable precision, is widely used in the world, and regarded as the most efficient water-saving technique [10,11]. Moreover, drip irrigation technology including fertigation also changes the traditional fertilization mode. By applying fertilizers through an irrigation system, such as a drip or sprinkler system, fertigation technology considerably reduces the application of water and fertilizer.
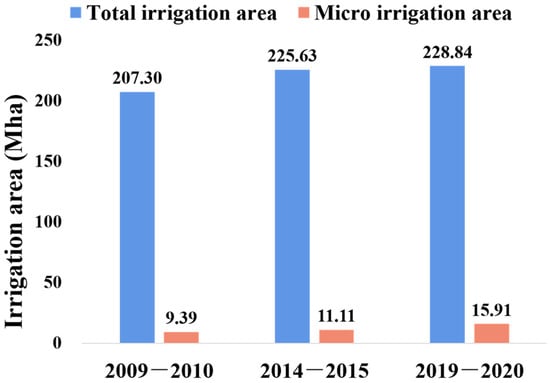
Figure 1.
Total irrigation area and micro irrigation area of the globe.
In a drip irrigation system, the emitter is the key component, consisting of a water inlet, a flow path, and an outlet. It can make the pressurized water flow through its internal flow channel to meet the requirements of energy dissipation and then drip into the soil with a uniform and stable water flow for crop absorption and utilization [12]. However, due to the small, narrow sized flow path (0.5–1.2 mm) of the emitter, it is easily clogged by solid particles, chemical precipitation, microorganisms, and other substances in irrigation water [12,13,14]. For example, for the flat emitters manufactured in Israel and China shown in Figure 2, the flow paths (length × width × depth, mm) were 34.0 × 0.59 × 0.67 and 53.5 × 0.48 × 0.80, respectively. Clogging substances can be deposited everywhere in an emitter. We summarized the three consequences of emitter clogging: (1) reducing the uniformity and utilization efficiency of irrigation water and fertilization, which in turn affects crop yield and quality [15,16]; (2) shortening the service life of drip irrigation systems and increasing investment costs [17]; (3) speeding up the pipeline replacement cycle, thereby increasing the plastic input, resulting in environmental pollution [18,19]. Emitter clogging has become a major issue and a worldwide challenge in drip irrigation research [20].
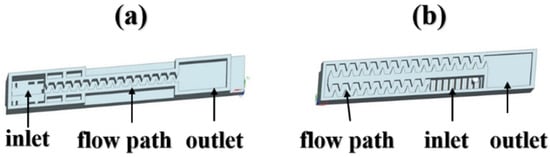
Figure 2.
Schematic of flat emitters were manufactured from Israel (a) and China (b).
Water quality is a direct factor that induces emitter clogging, and Bucks divided the types of emitter clogging into physical, chemical, and biological [21]. Since the first International Drip Irrigation Conference was held in 1971, there have been numerous attempts to solve clogging problems, such as the rational allocation of filtration systems, optimizing irrigation frequency, adding flushing equipment, water treatment (acidification and chlorination), microbial antagonism, and the optimal design of the emitter flow channel [22,23,24,25].
Currently, the shortage of water resources and pollution force the diversification of the sources of drip irrigation. As a result, reclaimed water, saline water, high sediment-water, and other low-quality water sources are used for irrigation [12,13,26]. Israeli scientists were the first to propose in 1966 that saline water could be used to irrigate crops [27]. This not only improved agricultural drought resistance ability and yield, but also contributed to the renewal of groundwater. Thereafter, the utilization of saline water was regarded as an important way to alleviate the contradiction between water supply and demand in the world [28]. However, saline water contains lots of salt, which can cause chemical clogging by the recombination and precipitate. Similarly, with the worldwide application of fertigation, the nutrient ions in fertilizers react with the ions in the irrigation water, forming insoluble precipitates that make the chemical clogging process of the emitters more complicated. Saline water for irrigation and fertigation are considered the most common factors for the chemical clogging of emitters [25,26,29,30]. We searched the core collection database of Web of Science from 1991 to 2021 on the theme of “Drip Irrigation and Clogging” and found 294 results (Figure 3). According to the references, “Physical clogging,” “Biological clogging,” and “Chemical clogging” are used as the theme for refining. Results also showed that chemical clogging became a hot topic especially after 2018, which may be related to the potential of saline water as an irrigation water source and the worldwide application of drip fertigation.
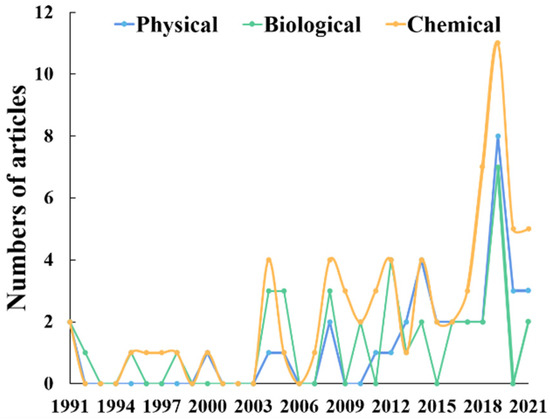
Figure 3.
Research articles on emitter clogging of drip irrigation published in 1991–2021.
Hence, the aims of this review were to (1) summarize the types and research progress of emitter clogging; (2) discuss the inducement, mechanism, as well as control methods for the chemical clogging of emitters caused by saline water and fertilizers; (3) raise a systematic solution to solve the chemical clogging of emitters; and (4) ascertain the current challenges and technical trends of emitter clogging caused by various water sources and fertigation.
2. Causes and Performance Evaluation Method of Emitters Clogging in Drip Irrigation
2.1. Types and Causes of Emitters Clogging
Emitter clogging is a phenomenon whereby solid particles, chemical precipitation, microorganisms, and other substances in irrigation water deposit in the lateral or emitters of drip irrigation, resulting in a decrease in irrigation flow rate and uniformity [13]. It is a complicated and unavoidable process in agricultural practices [31]. Generally, according to the recommendations given by the International Organization for Standardization, the standard for determining clogging is defined as the actual flow rate of the emitter less than 75% of the design [32]. According to the statistics of a consultant from the Food and Agriculture Organization, the probability of physical, chemical, and biological clogging in drip irrigation accounts for 31%, 22%, and 37%, respectively, while the other types account for 10% (Figure 4). Besides, due to the worldwide application of fertigation, the probability of chemical clogging of emitters is increasing.
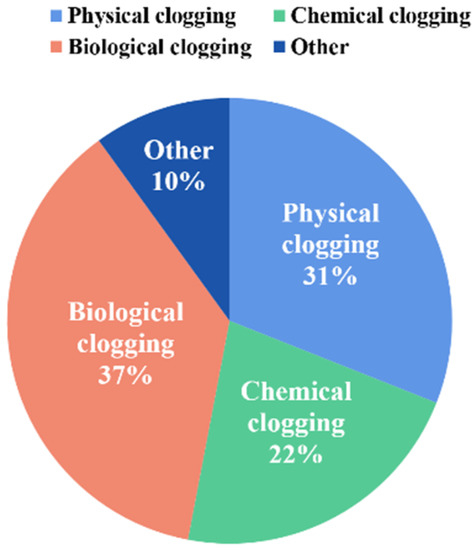
Figure 4.
The types and probability of emitter clogging in drip irrigation. Date from the statistics of consultant from the Food and Agriculture Organization.
2.1.1. Physical Clogging
Physical clogging is caused by organic or inorganic suspended matter, such as algae, phytoplankton, and zooplankton residues, plastic fragments, sand, silt, and clay particles, that cannot be filtered out in irrigation water using filtration equipment [21]. It also includes the clogging caused by negative pressure mud suction in subsurface drip irrigation systems [33]. Physical clogging caused by solid particles is considered the most common clogging type and is also the reason for emitter clogging [13,34], even if high-quality water is used [35]. Physical clogging is mainly caused by solid particles under different water sources, as shown in Table 1. However, physical clogging is hard to avoid in drip irrigation systems. The greater the content and size of particles, the more severe the physical clogging of emitters [36,37].

Table 1.
Emitter clogging caused by solid particles under different water sources.
2.1.2. Biological Clogging
The drip irrigation system can provide a favorable environment for algae, zooplankton, bacteria, and other organisms to grow and reproduce, resulting in slime accumulation. This slime can combine with mineral particles causing the biological clogging of emitters [11]. In addition, for subsurface drip irrigation, the reduction of irrigation flow rate caused by plant root invasion also belongs to the biological clogging category [42]. Biological clogging is common in drip irrigation systems that contain more microorganisms and organic matter in water sources. Table 2 summarizes the factors that cause the biological clogging of emitters, indicating that particulate matter is the main substance but not the initiating factor that causes emitter biological clogging. Secretions of microorganisms can increase the viscosity of the emitter wall and continuously adsorb the suspended particulate matter, which is considered a mechanism for inducing emitter clogging [43,44,45]. Currently, research has mainly focused on reclaiming water to relieve biological clogging. Actually, the active microbial species, water quality, and system operation methods can also directly affect the growth of biofilms [46]. Microorganisms play an important role in emitter clogging by secreting viscous substances to form biofilms [47]. The strong oxidation of chlorine can kill or inhibit microorganisms (bacteria) and prevent the formation of slime and clumps, so as to effectively reduce biological clogging [48]. Lateral flushing is another common method for removing and flushing biofilm out of the lateral and emitter by hydraulic shear force. Chlorination combined with lateral flushing could effectively reduce the microbial [49,50]. Recently, electromagnetic field treatment has been proven to solve biological clogging based on gene sequencing, which is expected to be an effective, chemical-free, and anti-biological treatment method [18].

Table 2.
The main factors causing emitter biological clogging.
2.1.3. Chemical Clogging
Chemical clogging is caused by the soluble substances in water sources, such as carbonates, phosphates, sulfates, silicates, hydroxides, Fe2+, Ca2+, Mg2+, and sulfides, that form chemical precipitates under certain conditions [21]. The use of groundwater, saline water, and fertigation for irrigation can cause chemical clogging. The formation of chemical clogging is influenced by system pressure, water temperature and pH, and concentration of ions in water [11]. Chemical precipitation is an important aspect of emitter clogging, especially in the use of saline water and drip fertigation. Table 3 shows the soluble substances in water sources that cause chemical clogging of the emitters. The mechanisms involved in chemical clogging are complex and diverse.

Table 3.
Emitter clogging caused by soluble substances in water sources.
2.1.4. Compound Clogging
Suspended particles, organic matter, salt, and microorganisms cannot be removed completely by the filtration systems. The type of emitter clogging varies with irrigation water quality. It is generally believed that the type of emitter clogging that occurs in saline water is chemical [25,26]. However, in addition to chemical precipitation, quartz and silicate are often detected in clogging substances, which indicates that both physical and chemical clogging coexist when using saline water for irrigation [56]. The particle size and concentration of sediments are two main factors in emitter clogging for high sediment water sources. In addition, researchers also found that the attachment of biofilm in the sediment surface aggravated the emitter clogging [57,58]. This indicated that the clogging of emitters using high sediment water is not a simple physical clogging but is accompanied by biological clogging. For the reclaiming water sources, researchers found that solid particles are still the main clogging substances and adsorbed by the biofilm, indicating that physical and biological clogging occur simultaneously [59]. Therefore, emitter clogging is often shown as the compound clogging caused by the synergy or coupling of two or three kinds of clogging: physical, chemical, and biological. Controlling any one of these factors can alleviate the clogging [60]. The clogging types of typical water sources are shown in Table 4.

Table 4.
The emitter clogging type for typical irrigation water.
2.2. Performance Evaluation Method of Emitters Clogging
The “field evaluation method for micro-irrigation system” issued by the American Society of Agricultural Engineers (ASAE) in 2003 is presently the most authoritative standard for evaluating the performance of drip irrigation systems. It summarizes the evaluation parameters commonly used, including manufacturing variation coefficient (Cv) for evaluating the production quality of emitters, relative outflow (Qr) for evaluating single emitter clogging, the average discharge variation ratio (Dra) for the overall clogging degree of multiple emitters, flow deviation (qvar), Christiansen of uniformity (CU), design emission uniformity (EU), and statistical uniformity coefficient (Us). Among them, Dra and CU are the key parameters usually used to evaluate emitter clogging and performance in drip irrigation (Figure 5). Popularly, clogging occurs once the actual flow rate of the emitter is less than 75% of the design [32]. Specifically, a single emitter is defined as unclogged if its outflow is higher than 95% of the design, 80–95% is slightly clogged, 50–80% is generally clogged, 20–50% is seriously clogged, and less than 20% is completely clogged [14] (Figure 5a). The performance of the emitter is defined as excellent if its CU is higher than 89%, and 71–89% is defined as medium, while less than 71% is defined as poor [61] (Figure 5b).
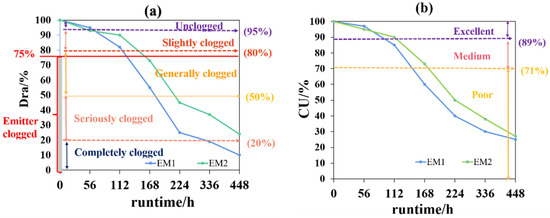
Figure 5.
Performance evaluation method of emitters clogging. (a) The average discharge variation ratio (Dra). An emitter Dra is higher than 95% of the design is defined as unclogged, 80–95% is slightly clogged, 50–80% is generally clogged, 20–50% is seriously clogged, and less than 20% is completely clogged [14]. 75% is the standard for determining clogging that given by the International Organization for Standardization [32]. (b)The christiansen of uniformity (CU). The performance of an emitter is defined as excellent if its CU is higher than 89%, 71–89% is medium, and 71% is poor [61]. EM1 and EM2 mean two types of flat emitters.
3. Mechanism of Emitters Chemical Clogging in Drip Irrigation
3.1. Research Progress and Mechanism on the Chemical Clogging of Emitters under Drip Irrigation System with Saline Water
With the potential application of saline water in agriculture, research about emitter clogging is increasing. Chemical clogging of emitters does not occur suddenly. It is caused by the long-term accumulation of the clogging substances [62,63]. There are randomness and fluctuations in the flow rate of a single emitter [26]. Chemical clogging processes can be categorized as gradual, fluctuating, or sudden reductions of emitter discharge [26,62,64], in which the sudden reductions in flow are normally observed in the high salinity water. Overall, the discharge of emitters shows a gentle fluctuation trend at first, and then a sharp decline with the operation of the system [20]. Similarly, Dra and CU have a slow, sharp downward trend, which is negatively correlated with the salinity of water [26]. The clogging degrees in a lateral are End > Middle > Head. The highest risk location for chemical clogging is the end of a lateral, as the velocity and water shear force are lower, which leads to greater accumulation of clogging substances. For the emitter, clogging substances can be deposited everywhere, such as the inlet, core flow path, and outlet of the emitter. The proportion of clogging position at the inlet accounts for 22–33%, 44–60% at the core flow path, and 7–34% at the outlet of the emitter, which indicates that the core flow path of the emitters is the main deposit position of clogging substances [26].
The clogging substances are normally calcium-magnesium carbonates, quartz, silicate, and a small quantity of sodium chloride [12,25,65]. Among them, calcium-magnesium carbonates account for 63.3–91.1% of the chemical clogging substances [66]. In addition, Dra, and CU decrease with the increase in clogging chemical precipitates, indicating the negative relationship among them [18,65,67]. Interestingly, SiO2 is mainly from the water source and hard to form in the emitter under normal temperature, which indicates that the physical clogging occurs apart from the chemical clogging [12].
Normally, chemical clogging caused by saline water in a drip irrigation system is always accompanied by physical clogging. The clogging mechanisms were summarized in Figure 6. First, as saline water containing a high concentration of cations (such as Ca2+ and Mg2+) and anions (such as CO32−, PO43−, SO42−, and OH−), often generate chemical precipitation deposited on the surface of the emitters flow path, and then crystallization fouling is formed, leading to chemical clogging of the emitters. Second, the crystallization fouling increases the roughness of the flow path surface and changes the transport process of unfiltered sediment particles in the irrigation water, which further aggravates the sedimentation of particles and results in physical clogging of the emitters. Finally, the formed clogging substances are deposited in the emitter and cause a reduction of Dra and CU, which in turn affects crop yield and quality.
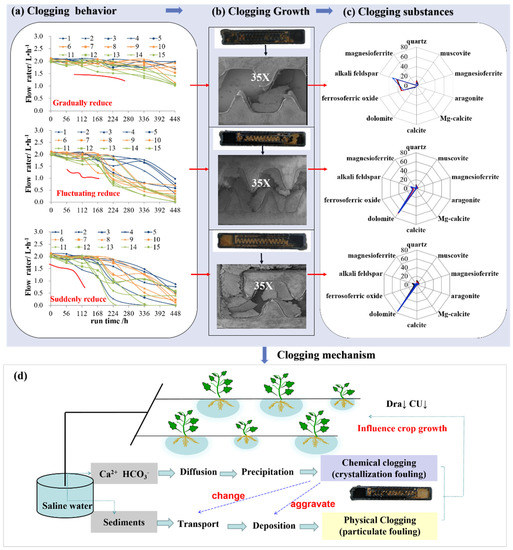
Figure 6.
The clogging behavior, growth, substances, and mechanism drip irrigation system using saline water. (a) The dynamic process of chemical clogging under the salinity levels of 1 ds·m−1 (upper), 2 ds·m−1 (middle), and 6 ds·m−1 (lower). A total of 15 emitters were selected along the laterals (five from the head of a lateral that labeled as 1–5, middle that labeled as 6–10, and tail sections that labeled as 11–15) to track changes in flow rates with time. (b) 35× magnification of field-emission scanning electronic microscopy micrographs of chemical fouling attached in emitters. (c) Chemical components of chemical fouling inside the drip irrigation emitter. (d) The clogging mechanism for drip irrigation system using saline water. These figures were derived from previous studies [25,26,66].
Moreover, many factors affect the formation of CaCO3, such as electrical conductivity, pH, temperature, and flow channel size. The Langelier saturation index (LSI) is usually used to evaluate the possibility of CaCO3 precipitation formation, which can characterize the equilibrium relationship between calcium carbonate solids and carbon dioxide-containing solutions [39]. If LSI < 0, CaCO3 is unsaturated, the solution can continue to dissolve. When LSI = 0, it is considered that the CaCO3 is in a saturated state, neither scaling nor dissolving, and if LSI > 0, it is considered that the CaCO3 of the solution is in a supersaturated state and can continue to scale formation [68]. The pH of the water can also influence the formation of CaCO3 precipitation. pH < 7.0, 7.0–8.0, and >8.0 can lead to slight, medium, and severe chemical clogging, respectively [21]. CaCO3 precipitation formation is also affected by temperature, which can reduce the solubility of calcium carbonate and cause further precipitation. Conductivity and hardness are the key indexes for saline water. Higher conductivity or hardness promotes precipitation reactions that can result in severe clogging [69]. A study demonstrated that saline water with EC higher than 4.0 dS/m is not recommended for use in irrigation [26]. In addition, emitters with larger discharge have a better anti-clogging capacity due to the larger flow path structure of the emitter allowing larger and more contaminants to pass through the flow path [59]. In general, the system under conditions with high ion contents, high pH water, high temperature, and small flow channel size tends to be at a higher risk of chemical clogging.
3.2. Research Progress and Mechanism on the Chemical Clogging of Emitters under Fertigation
Fertigation has been identified as another important factor that causes the chemical clogging of emitters [70,71]. Nutrients in fertilizers and ions in irrigation can be recombined to form chemical precipitation, especially when applying phosphorus-containing fertilizers with low-quality water as phosphate can be recombined with calcium, magnesium, and iron to form phosphate precipitates [69,72]. Previous studies found that the emitter clogging characteristics extensively varied with fertilizers and water quality [18,30,67,73]. Hence, nitrogen and phosphate fertilizers are commonly applied in agriculture, which is usually used for studying emitter clogging.
3.2.1. Nitrogen Fertilizer
Urea is commonly used in fertigation due to its high nitrogen content and good solubility. However, hydrolysis and adsorption will occur when urea is dissolved in irrigation water, which will alter the hydrodynamic properties of the water flow, flocculation, and precipitation of suspended particles in the flow channel, and the growth of the microorganism [74]. Furthermore, coupled with a higher viscosity coefficient and poor mobility, urea can easily cause emitter clogging [29]. The effect of urea on emitter clogging is also related to water quality. It is reported that the CU could reach 90% and Dra was stable at the level of 80–100% when applying urea with high-quality water [75], which indicates that the application of urea is not responsible for the induction of emitter clogging. Nevertheless, urea can enhance the agglomeration of solid particles and promote the formation of stable and compact clogging substances under muddy water conditions [74]. Moreover, the concentration of urea is a factor that affects emitter clogging. In general, the higher the concentration of urea, the greater the adhesion between urea molecules and the flow channel wall and the greater the number of urea molecules precipitated [29]. The mechanism of emitter clogging may be the physical clogging caused by the formation of aggregates between molecular urea precipitates and suspended particles in water sources [29]. Chemical clogging is also easily generated when urea is applied, which might promote the precipitation of Ca(OH)2, Mg(OH)2, CaCO3, and MgCO3. Applying urea with irrigation water can also change the water characteristics such as pH and nutrient concentration. The interaction process of urea in drip irrigation can be summarized into four categories: hydrolysis, dissociation, oxidation, and precipitation (Table 5) [76].

Table 5.
Mechanism reaction of urea in drip irrigation.
3.2.2. Phosphate Fertilizer
Phosphorus is easily immobilized by soil with low mobility and availability [77]. Drip irrigation is an effective technology for increasing P availability [18]. However, phosphorus fertilizer contains HPO42− that would react with Ca2+ and Mg2+, which can form the insoluble CaHPO4 or MgHPO4 precipitates and lead to drip irrigation clogging [53,54].
The types of phosphorus fertilizer and irrigation water sources greatly affect emitter clogging. Traditional phosphate fertilizers, such as diammonium phosphate [56], potassium phosphate monobasic [78], monopotassium phosphate (MKP) [65], calcium superphosphate [29], and potassium phosphate monobasic (PPM), can greatly worsen emitter clogging. They are not recommended for drip irrigation systems. In recent years, acidic water-soluble phosphate fertilizers, such as urea phosphate (UP) and neutral fertilizer ammonium polyphosphate (APP), have been identified as two potential anti-clogging phosphate fertilizers [79]. A previous study found that the application of UP and APP could effectively alleviate emitter clogging by inhibiting the formation of carbonate [56,67]. For UP, the decrease of carbonate is attributed to the acidity of the UP solution. When dissolved, 1 kg of UP can produce 6.3 moles of H+ and inhibit the formation of carbonate [80]. APP is often used as a scale inhibitor, which can chelate and shield Ca2+, Mg2+, and other metal ions and further reduce the probability of carbonate precipitate [81]. However, opposite results were also reported with irrigation by Yellow River water, where both APP and UP caused emitter clogging [18]. APP could drastically promote the deposition of carbonate and phosphate, which induced the most serious emitter clogging. Although UP reduced carbonate deposition, it could increase silicate content and cause emitter clogging. The reason for this is mainly attributed to the silicates in water. The reduction of pH by UP application could promote the condensation of silanol structure, impel the polymerization of silicate gel, and finally accelerate the deposition of silicates [18]. However, compared with other phosphate fertilizers, UP is suggested as the optimum anti-clogging phosphate fertilizer for drip irrigation systems [18,30,67,82]. It should be noted that UP should not be used in acidic soils to avoid the risk of soil acidification [18]. Moreover, fertilization mode is also a factor that affects emitter clogging. Low concentration and long-term fertilization or shortening the irrigation interval can effectively relieve emitter clogging [18,67,83].
3.2.3. Other Fertilizers
Currently, with the development of intensive agriculture, combined phosphorus and nitrogen fertigation is becoming more common, benefiting from the development of soluble fertilizer technology. Studies have shown that phosphorus coupled with nitrogen fertigation could accelerate the precipitation of phosphate sediments [82]. Except for urea or phosphorus fertilizers, Ca, S, Fe, and Mn fertilizers will also aggravate the clogging of the emitter. Hence, when using saline water for irrigation, the risk of clogging will be greatly increased [69,72]. The strategies for alleviating emitter clogging should be developed by comprehensively considering the given water source and fertilizer types.
4. Controlling the Chemical Clogging of Emitters in Drip Irrigation
According to the mechanism and influencing factors of emitter clogging, the prevention and control measures can be divided into three categories: improvement of emitter anti-clogging ability, pretreatment of irrigation water, and removal of clogging substances [13]. (1) Improvement of the emitter anti-clogging ability is important for alleviating the problem of emitter clogging using two approaches. The first approach involves optimizing the emitter flow channel structure to reduce the adhesion of clogging materials and promote the fall of the flow channel wall. The second approach involves developing new anti-clogging materials, mainly by adding special anti-bacterial materials to reduce the activity of microorganisms and inhibit the adhesion ability to clog substances, to reduce the formation and growth of clogging substances [20]. (2) The equality of irrigation water is the direct factor causing emitter clogging. The fewer the impurities in the water, the lower the emitter clogging risk [84]. Irrigation water pretreatment is mainly performed by strengthening water source filtration, adding acidic chemical reagents to reduce the pH, and adding chlorine to inhibit microbial growth. (3) Once the clogging substances are formed in emitters or laterals, they need to be cleaned out in time to avoid their accumulation, which would otherwise aggravate clogging. Flushing is suitable for cleaning most of the clogging substances, adding acidity reagents is suitable for removing chemical precipitation, and adding antagonistic microorganisms can inhibit the growth of the clogging-induced ones [18,85].
Currently, the main approach to controlling chemical clogging is treating with water to limit the formation of chemical precipitates or dissolve the precipitates that have already been generated. The control methods mainly depend on physical, chemical, and biological technologies (Figure 7a). Accordingly, we provide an efficient integration of all three methods to prevent and eliminate chemical clogging under different irrigation water quality during the crop growth cycle (Figure 7b).
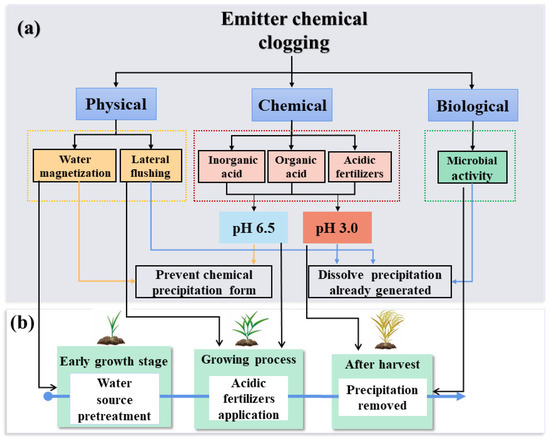
Figure 7.
The control method for emitter chemical clogging. (a)The control methods mainly depend on physical, chemical, and biological technologies to prevent and eliminate the chemical clogging. (b) Different methods to prevent and eliminate chemical clogging under different irrigation water quality during the crop growth cycle.
4.1. Physical
It was found in our previous studies that lateral ends were the highest risk positions for the chemical clogging of emitters [26]. Lateral flushing is one of the most commonly used physical methods, which can effectively reduce the accumulation of chemical precipitation [12] and increase the discharge of emitters by 5–8% [20]. In addition, new technologies emerge over time. At the end of the 19th century, magnetization technology was applied to heat exchangers, boilers, and other industrial descaling [86]. Magnetization treatment decreased the viscosity, surface tension, polymerization degree, and conductivity of water [87], while it increased the water osmotic force, ionic hydration reaction, and solubility [88]. These changes could affect the formation of scale crystals and improve the solubility of the scale substances. Studies have shown that the calcium carbonate in the water without magnetization treatment is calcite crystal with a dense and hard structure and strong adhesion to the pipe wall, while it is aragonite crystal with the loose and disordered arrangement of molecules and poor adhesion to the pipe wall after magnetization [89], which shows that magnetization treatment has a positive effect on removing calcium carbonate precipitation. Nowadays, magnetized water is applied in industry, agriculture, and medicine [90,91,92], and researches have shown that magnetized water irrigation has positive effects on the quality of crops and soil (Table 6). In recent years, some researchers innovatively introduced magnetization into the drip irrigation system and tried to solve the emitter clogging issue. They found that magnetization treatment could alleviate the clogging issue of saline water drip irrigation systems, but this depends on the irrigation water quality. The anti-clogging effect was better in water with low conductivity and high pH [85,87]. Nevertheless, magnetization technology can provide a reference for solving the problem of chemical clogging of the emitters under fertigation.

Table 6.
Regulation mechanism and effect of magnetized water in agricultural application.
4.2. Chemical
Higher pH water is more likely to cause chemical clogging. Chemical precipitation can be significantly prevented by injecting acidic chemical reagents that lower the solution pH [72,86]. There are two main approaches for injecting acidic chemical reagents. One is preventing chemical precipitation by injecting acidic chemical reagents into irrigation water to reduce the pH to slightly below 7.0 (commonly around 6.5) for a long period so that a weak acid drip irrigation state can prevent chemical precipitation. The second one is removing the precipitation substances that are already generated. Injecting acidic chemical reagents in a short time to reduce solution pH too far below 7.0 (usually around 3.0), makes the drip irrigation stay in a strong acidic state for a short time to dissolve chemical precipitation. However, it should be noted that safety is a key part of acidic chemical treatment, which requires expertise. Proper precautions for operators must be taken in handling and injecting chemicals into the irrigation lines. The standards developed by the American Society of Agricultural Engineers can be referenced for the use of safety devices used for applying liquid chemicals through irrigation systems. Meanwhile, it is necessary to note that low pH environment will corrode the pipe components [11]. Moreover, to avoid damaging crops, the amount of water irrigated during this period should be minimized and the irrigation system also needs to be flushed after the acid treatment [11]. From the current research, acid treatment is the most effective treatment used in preventing and dissolving alkaline precipitation substances in drip irrigation.
The commonly used acids can be summarized as inorganic acids, organic acids, and acidic fertilizers.
4.2.1. Inorganic Acids
Inorganic acids, such as sulfuric, hydrochloric, and phosphoric acids, are often used to control chemical clogging substances in drip irrigation [11]. It should be noted that injecting phosphoric acid into hard water may cause the precipitation of calcium phosphates. Sulfuric and hydrochloric acids are the other two acidic chemical reagents used for water treatment. It was found that emitter clogging could be effectively reduced by adding sulfuric or hydrochloric acid to reduce the pH of irrigation water to 6.5 and 6, respectively [86,106]. The pH of most irrigation waters is around 8.0, and injecting 1 mel/L of inorganic acid can reduce the pH to 6.0–6.5 [107].
4.2.2. Organic Acids
Presently, organic acids, mainly citric acid, and acid soil amendments are commonly used to control chemical clogging. The injection of organic acids into the drip irrigation system can improve the soil ion exchange and react with calcium and magnesium carbonate precipitation to form soluble substances [108]. Previous studies showed that Sper Sal, and Lineout could reduce the clogging of the emitter, and Lineout performed better than Sper Sal [109]. Clogging caused by CaCO3 precipitation can be prevented by adding a homopolymer of maleic anhydride into buried drip systems [110]. However, some studies showed opposite results. Severe clogging was found in emitters using organic acids as preventives [111]. Kreij et al. noted that the commercial anti-clogging agents containing organic acids could serve as substrates for the micro-organisms, and they suggested using the anti-clogging agents without organic acids [112]. These researches indicated that whether organic acids are anti-clogging remains an issue at the exploratory stage that needs to be studied further in the future.
4.2.3. Acidic Fertilizers
Calcium and magnesium precipitates are the main precipitates in the fertigation system [67,113]. The application of alkaline fertilizer can increase the pH of irrigation water, which will promote the formation of calcium and magnesium hydroxide and carbonate deposits. While acid fertilizer is a better choice for chemical regulation, it can reduce the precipitates generated by lowering the solution pH. Therefore, caution should be taken when applying phosphate fertilizer into hard water, as it may cause precipitation of calcium-magnesium phosphate. Acidic fertilizers include acid nitrogen fertilizer (urea sulfate), acid phosphate fertilizer (urea phosphate), acid potash fertilizer (potassium sulfate), and acid organic fertilizer. Among them, urea sulfate and urea phosphate are beneficial for crops and reduce emitter clogging. They can also increase the utilization of nutrients effectively by inhibiting soil fixation of phosphorus and potassium in alkaline soils.
4.3. Biological
Carbonate precipitation is the most common emitter-clogging factor among chemical precipitates in drip irrigation systems. Carbonates in nature have been continually dissolved directly or indirectly as a result of microbial activity to secrete acidic substances [114,115]. A previous study reports the application of microorganisms (Lactococcus spp.) in mineral processes to remove CaCO3 [116], which also provides a new environment-friendly solution for controlling the formation of chemical clogging substances in drip irrigation. Bacillus subtilis OSU-142 was found to dissolve calcium carbonate. The flow rate of the emitters increased by 10–20% after adding B. subtilis OSU-142 to the drip irrigation for 4 h. Thus, the OSU-142 strain exhibited good performance in dissolving calcium carbonate [117]. In addition, to relieve chemical clogging, microbial activity is also used in biological clogging. By adding three kinds of antagonistic bacteria into the clogged emitter, researchers found that the emitter was almost completely recovered within 14 days [85]. Li’s group selected a bacterial strain, Endophytic bacillus, as an antagonist to Arcicella sp., which causes clogging, to remove the clogging substances [118]. They also found that Pseudomonas was the most critical bacteria in affecting emitter biological clogging as it performed better in decomposing and utilizing organic matters [59]. However, only a few reports are available about the beneficial microorganisms that eliminate chemical clogging. Therefore, more studies need to be conducted to identify microorganisms as novel and environmentally friendly treatments to remove chemical precipitation for clogged emitters of drip irrigation systems.
4.4. Comprehensive Control Method of Chemical Clogging of Drip Irrigation Emitters during the Crop Growth Cycle
We put forward a set of control methods from the perspective of preventing chemical precipitation and removing the precipitation substances that are already generated in the drip irrigation based on the methods mentioned above for the control of chemical clogging of emitters (Figure 7b). (1) ‘Early growth stage’ water source pretreatment: Apart from water filtration treatment, magnetization can be used to change the characteristics of water, affect the formation and growth of scale crystals, and improve the solubility of scale substances. (2) ‘Growing process’-acidic fertilizer treatment: The application of acidic fertilizers reduces the formation of chemical clogging substances and provides nutrients for the crops. (3) ‘After harvest’ precipitation removed: At the end of the irrigation cycle, flushing can be used for perennial crops to remove the chemical precipitation. When crops are harvested, a strong acid reagent can be added to the drip irrigation system to make the laterals in a strong acid state dissolve the chemical precipitation. It would be better to screen out microorganisms that can both eliminate chemical deposits and benefit for crops in the future.
5. Summary and Outlook
In conclusion, we summarized the research concerning emitter clogging types. We deduced that the typical fouling formation in emitters is physical clogging caused by high sand content water, biological clogging caused by reclaimed water, and chemical clogging caused by saline water and fertigation. Regarding chemical clogging, this article elaborated on the inducement, mechanism, and control method for the chemical clogging of emitters caused by saline water and fertilizers. The reasons and mechanisms of saline water are the high concentration of cations (such as Ca2+ and Mg2+) and anions (such as CO32−, PO43−, SO42−, and OH−) that can easily interact, form chemical precipitation, then deposit on the surface of emitter flow path, leading to chemical clogging. Nutrients in fertilizers and ions in irrigation water can be recombined to form chemical precipitation, causing chemical clogging of the emitters, especially when applying phosphorus-containing fertilizers with low-quality water as phosphate can be recombined with calcium, magnesium, and iron to form phosphate precipitates. Carbonate, phosphate, silicate, and SiO2 are the main clogging substances for chemical clogging. The type of clogging substance is related to irrigation water quality, fertilizer type, and concentration. Generally, a high hardness of water, high concentration of ions, and high pH of irrigation water would cause chemical clogging. The prevention and control of clogging plays an important role in field production. For physical clogging, the solid particles are the direct reason that lead to emitter physical clogging. This study suggests that a proper water filtration system should be installed, especially using water with a high sediment load. For biological clogging, adding chlorine is commonly performed to inhibit microbial growth. For chemical clogging, adding acidic chemical reagents to lower the solution pH is a popular method. Considering the safety of soil and crops, more research should be carried out on beneficial microbial agents that have antagonistic effects against the key bacteria that cause biological clogging, and which can dissolve the chemical precipitate.
Although lots of meaningful findings were acquired from previous researches, some issues still are unaddressed and need to be investigated: (1) For emitter material, multiple anti-clogging functions need to be developed, such as reducing the activity of microorganisms and inhibiting weed growth and chemical precipitation; (2) For fertigation, in some areas, farmers still prefer to broadcast fertilizers into the soil rather than inject them into the irrigation system due to emitter clogging, which would increase the amount of fertilizer and lead to serious agricultural non-point source pollution and water eutrophication. Research also needs to be carried out on the new type of water-soluble fertilizer with anti-clogging, environmentally friendly, and high efficiency properties, as well as their mechanisms of anti-clogging; (3) For a monitoring method, with the wide application of modern information technology in agriculture, the rapid and real-time monitoring and testing of emitter clogging in drip irrigation systems requires modern technology to replace the current tedious operation process.
Author Contributions
Writing—original draft preparation, K.S.; data analysis, T.L.; review & editing original draft, W.Z.; performed the literature search, X.Z.; Writing—review & editing original draft, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The review was supported by the Program of the National Natural Science Foundation of China (NSFC) (Grant No. 51909007), the Key R & D Program of Yunnan Province (Grant No. 202002AE090010), and the Innovation ability construction project of Beijing academy of agriculture and forestry sciences (Grant No. KJCX20210411).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are already provided in the main manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, T.; Zou, Y.; Kisekka, I.; Biswas, A.; Cai, H. Comparison of different irrigation methods to synergistically improve maize’s yield, water productivity and economic benefits in an arid irrigation area. Agric. Water Manag. 2021, 243, 106497. [Google Scholar] [CrossRef]
- Hadadin, N.; Qaqish, M.; Akawwi, E.; Bdour, A. Water shortage in Jordan—Sustainable solutions. Desalination 2010, 250, 197–202. [Google Scholar] [CrossRef]
- Oktem, A. Effect of water shortage on yield, and protein and mineral compositions of drip-irrigated sweet corn in sustainable agricultural systems. Agric. Water Manag. 2008, 95, 1003–1010. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2020. Overcoming Water Challenges in Agriculture; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Monaghan, J.M.; Daccache, A.; Vickers, L.H.; Hess, T.M.; Weatherhead, E.K.; Grove, I.G.; Knox, J.W. More ‘crop per drop’: Constraints and opportunities for precision irrigation in European agriculture. J. Sci. Food Agric. 2013, 93, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Y.; Sheng, Z.; Manevski, K.; Andersen, M.N.; Han, S.; Li, H.; Yang, Y. Did water-saving irrigation protect water resources over the past 40 years? A global analysis based on water accounting framework. Agric. Water. Manag. 2021, 249, 106793. [Google Scholar] [CrossRef]
- ICID. Annual Report 2019–20. Agricultural Water Management for Sustainable Rural Development; Word Irrigated Area, Sprinkler and Micro Irrigated Area: New Delhi, India, 2020; Available online: https://icid-ciid.org/publication/info/33 (accessed on 10 November 2021).
- ICID. Annual Report 2014–15. Supporting Agricultural Water Management for Sustainable Development; Word Irrigated Area, Sprinkler and Micro Irrigated Area: New Delhi, India, 2015; Available online: https://icid-ciid.org/publication/info/33 (accessed on 10 November 2021).
- ICID. Annual Report 2009–10. 60 Years of Service in Managing Water for Sustainable Agriculture; Word Irrigated Area, Sprinkler and Micro Irrigated Area: New Delhi, India, 2010; Available online: https://icid-ciid.org/publication/info/33 (accessed on 10 November 2021).
- Zhou, B. Characteristics, Evalution and Mechanism of Bio-Clogging Process in Drip Irrigation Emitters. Ph.D. Thesis, China Agricultural University, Beijing, China, 2016. [Google Scholar]
- Pitts, D.J.; Haman, D.Z.; Smajstria, A. Causes and Prevention of Emitter Plugging in Micro Irrigation Systems; Bulletin 258; Institute of food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 1990. [Google Scholar]
- Li, Y.; Pan, J.; Chen, X.; Xue, S.; Feng, J.; Muhammad, T.; Zhou, B. Dynamic effects of chemical precipitates on drip irrigation system clogging using water with high sediment and salt loads. Agric. Water Manag. 2019, 213, 833–842. [Google Scholar] [CrossRef]
- Nakayama, F.S.; Bucks, D.A. Water quality in drip/trickle irrigation: A review. Irrig. Sci. 1991, 12, 187–192. [Google Scholar] [CrossRef]
- Pei, Y.; Li, Y.; Liu, Y.; Zhou, B.; Shi, Z.; Jiang, Y. Eight emitters clogging characteristics and its suitability under on-site reclaimed water drip irrigation. Irrig. Sci. 2014, 32, 141–157. [Google Scholar] [CrossRef]
- Talozi, S.; Hills, D. Simulating emitter clogging in a microirrigation subunit. Trans. ASAE 2001, 44, 1503. [Google Scholar] [CrossRef]
- Capra, A.; Scicolone, B. Recycling of poor quality urban wastewater by drip irrigation systems. J. Clean. Prod. 2007, 15, 1529–1534. [Google Scholar] [CrossRef]
- Li, J.S.; Li, Y.F.; Zhang, H. Tomato Yield and Quality and Emitter Clogging as Affected by Chlorination Schemes of Drip Irrigation Systems Applying Sewage Effluent. J. Integr. Agric. 2012, 11, 1744–1754. [Google Scholar] [CrossRef]
- Xiao, Y.; Seo, Y.; Lin, Y.; Li, L.; Muhammad, T.; Ma, C.; Li, Y. Electromagnetic fields for biofouling mitigation in reclaimed water distribution systems. Water Res. 2020, 173, 115562. [Google Scholar] [CrossRef] [PubMed]
- Enciso-Medina, J.; Colaizzi, P.D.; Multer, W.L.; Stichler, C.R. Cotton response to phosphorus fertigation using subsurface drip irrigation. Appl. Eng. Agric. 2007, 23, 299–304. [Google Scholar] [CrossRef]
- Li, Y.K.; Zhou, B.; Yang, P.L. Research advances in drip irrigation emitter clogging mechanism and controlling methods. J. Hydraul. Eng. 2018, 48, 103–114. [Google Scholar]
- Bucks, D.A.; Nakayama, F.S.; Gilbert, R.G. Trickle irrigation water quality and preventive maintenance. Agric. Water Manag. 1979, 2, 149–162. [Google Scholar] [CrossRef]
- Wei, Z.; Cao, M.; Liu, X.; Tang, Y.; Lu, B. Flow behaviour analysis and experimental investigation for emitter micro-channels. Chin. J. Mech. Eng. 2012, 25, 729–737. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Li, Y.; Yin, J.; Zhang, H. Effects of Chlorination Schemes on Clogging in Drip Emitters during Application of Sewage Effluent. Appl. Eng. Agric. 2010, 26, 565–578. [Google Scholar] [CrossRef]
- Duran-Ros, M.; Puig-Bargués, J.; Arbat, G.; Barragán, J.; de Cartagena, F.R. Effect of filter, emitter and location on clogging when using effluents. Agric. Water Manag. 2009, 96, 67–79. [Google Scholar] [CrossRef]
- Zhangzhong, L.L.; Yang, P.L.; Zheng, W.G.; Liu, Y.; Guo, M.; Yang, F. Effects of Drip Irrigation Frequency on Emitter Clogging using Saline Water for Processing Tomato Production. Irrig. Drain. 2019, 68, 464–475. [Google Scholar]
- Zhangzhong, L.L.; Yang, P.L.; Zheng, W.G.; Li, Y.K. Effects of water salinity on emitter clogging in surface drip irrigation systems. Irrig. Sci. 2020, 39, 209–222. [Google Scholar]
- Boyko, H. Saline Irrigation for Agriculture and Forestry; Dr. W. Junk N. V. Publishers: Hague, The Netherlands, 1968; p. 325. [Google Scholar]
- Sharma, B.R.; Minhas, P.S. Strategies for managing saline/alkali waters for sustainable agricultural production in South Asia. Agric. Water Manag. 2005, 78, 136–151. [Google Scholar] [CrossRef]
- Liu, L.; Niu, W.Q.; Wu, Z.G.; Guan, Y.H. Risk and Inducing Mechanism of Acceleration Emitter Clogging with Fertigation through Drip Irrigation Systems. Trans. Chin. Soc. Agric. Mach. 2017, 48, 228–236. [Google Scholar]
- Muhammad, T.; Zhou, B.; Liu, Z.; Chen, X.; Li, Y. Effects of phosphorus-fertigation on emitter clogging in drip irrigation system with saline water. Agric. Water Manag. 2021, 243, 106392. [Google Scholar] [CrossRef]
- Liu, L.; Niu, W.Q. Progress in Research on the Clogging and Prevention of Flow Pathin Drip Irrigation Emitter. J. Agric. Mech. Res. 2012, 4, 13–18. [Google Scholar]
- EP405.1. Design and Installation of Micro-Irrigation Systems; ASAE: Washington, DC, USA, 2003. [Google Scholar]
- Cheng, X.J.; Xu, D. R&D of Emitter for subsurface-drip-irrigation (SDI) and its preliminary application. Trans. CSAE 2001, 2, 51–54. [Google Scholar]
- Yan, D.; Bai, Z.; Rowan, M.; Gu, L.; Shumei, R.; Yang, P. Biofilm structure and its influence on clogging in drip irrigation emitters distributing reclaimed wastewater. J. Environ. Sci. 2009, 21, 834–841. [Google Scholar] [CrossRef]
- Taylor, H.D.; Bastos, R.K.X.; Pearson, H.W.; Mara, D.D. Drip irrigation with waste stabilisation pond effluents: Solving the problem of emitter fouling. Water Sci. Technol. 1995, 31, 417–424. [Google Scholar] [CrossRef]
- Bounoua, S.; Tomas, S.; Labille, J.; Molle, B.; Granier, J.; Haldenwang, P.; Izzati, S.N. Understanding physical clogging in drip irrigation: In situ, in-lab and numerical approaches. Irrig. Sci. 2016, 34, 327–342. [Google Scholar] [CrossRef]
- Niu, W.; Liu, L.; Chen, X. Influence of fine particle size and concentration on the clogging of labyrinth emitters. Irrig. Sci. 2013, 31, 545–555. [Google Scholar] [CrossRef]
- Feigin, A.; Ravina, I.; Shalhevet, J. Effect of irrigation with treated sewage effluent on soil, plant and environment. In Irrigation with Treated Sewage Effluent; Springer: Berlin/Heidelberg, Germany, 1991; pp. 34–116. [Google Scholar]
- Ge, L.X.; Wei, Z.Y.; Cao, M.; Tang, Y.P.; Lu, B.H. Deposition law of sand in labyrinth-channel of emitter. Trans. CSAE 2010, 26, 20–24. [Google Scholar]
- Jiang, S. Study on Aniti-Clogging Performance of Drip Tape and Critical Sand Water. Ph.D. Thesis, University of Chinese Academy of sciences, Beijing, China, 2010. [Google Scholar]
- Liu, L.; Niu, W.Q.; Zhou, B. Influence of sediment particle size on clogging performance of labyrinth path emitters. Trans. CSAE 2012, 28, 87–93. [Google Scholar]
- Zhao, H.F.; Li, G.Y. Water Quality Analysis and Evaluation Indexes for Micro-irrigation. Water Sav. Irrig. 2004, 6, 4–7. [Google Scholar]
- Ravina, I.; Paz, E.; Sofer, Z.; Marm, A.; Schischa, A.; Sagi, G.; Yechialy, Z.; Lev, Y. Control of clogging in drip irrigation with stored treated municipal sewage effluent. Agric. Water Manag. 1997, 33, 127–137. [Google Scholar] [CrossRef]
- Li, Y.K.; Wang, W.N.; Sun, H.S. Particle-Wall Collision Characteristics Influenced by Biofilms in Drip Irrigation Laterals with Reclaimed Water. Trans. Chin. Soc. Agric. Mach. 2015, 46, 159–166. [Google Scholar]
- Cararo, D.C.; Botrel, T.A.; Hills, D.J.; Leverenz, H.L. Analysis of clogging in drip emitters during wastewater irrigation. Appl. Eng. Agric. 2006, 22, 251–257. [Google Scholar] [CrossRef]
- Li, G.B.; Li, Y.K.; Xu, T.W.; Liu, Y.Z.; Jin, H.; Yang, P.L.; Yan, D.Z.; Ren, S.M.; Tian, Z.F. Effects of average velocity on the growth and surface topography of biofilms attached to the reclaimed wastewater drip irrigation system laterals. Irrig. Sci. 2012, 30, 103–113. [Google Scholar] [CrossRef]
- Hao, F.Z.; Li, J.; Wang, Z.; Li, Y.Z. Effect of chlorination and acidification on clogging and biofilm formation in drip emitters applying secondary sewage effluent. Trans. ASABE 2018, 64, 1351–1363. [Google Scholar] [CrossRef]
- Rav-Acha, C.; Kummel, M.; Salamon, I.; Adin, A. The effect of chemical oxidants on effluent constituents for drip irrigation. Water Res. 1995, 29, 119–129. [Google Scholar] [CrossRef]
- Mariscal, A.; Lopez-Gigosos, R.M.; Carnero-Varo, M.; Fernandez-Crehuet, J. Fluorescent assay based on resazurin for detection of activity of disinfectants against bacterial biofilm. Appl. Microbiol. Biot. 2009, 82, 773–783. [Google Scholar] [CrossRef]
- Song, P.; Li, Y.K.; Li, J.S.; Pei, Y.T. Chlorination with lateral flushing controlling drip irrigation emitter clogging using reclaimed water. Trans. Chin. Soc. Agric. Mach. 2017, 33, 80–86. [Google Scholar]
- Adin, A.; Sacks, M. Dripper-Clogging Factors in Wastewater Irrigation. J. Irrig. Drain. Eng. 1991, 117, 813–826. [Google Scholar] [CrossRef]
- Ravina, I.; Paz, E.; Sofer, Z.; Marcu, A.; Shisha, A.; Sagi, G. Control of emitter clogging in drip irrigation with reclaimed wastewater. Irrig. Sci. 1992, 13, 129–139. [Google Scholar] [CrossRef]
- Mikkelsen, R.L. Phosphorus Fertilization through Drip Irrigation. J. Prod. Agric. 1989, 2, 279–286. [Google Scholar] [CrossRef]
- Liu, C.; Dang, X.L.; Mayes, M.A.; Chen, L.L.; Zhang, Y.L. Effect of long-term irrigation patterns on phosphorus forms and distribution in the brown soil zone. PLoS ONE 2017, 12, e0188361. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985. [Google Scholar]
- Yang, X.; Wang, Z.; Li, J.; Li, Y.; Liu, H.; Wang, J. Effect of phosphorus fertigation on clogging in drip emitters applying saline water. In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019; p. 1. [Google Scholar]
- Wang, W.N.; Xu, F.P.; Zhou, B.; Feng, J.; Li, Y.K. Structural and fractal characteristics of biofilm attached on surfaces of sediments in Yellow River for drip irrigation. J. Drain. Irrig. Mach. Eng. 2014, 32, 914–920. [Google Scholar]
- Zhou, B.; Li, Y.K.; Song, P.; Xu, Z.C. Dynamic Characteristics and Inducing Mechanism of Emitter Clogging in the Drip Irrigation System Using Yellow River Water. J. Irrig. Drain. 2014, 33, 123–128. [Google Scholar]
- Zhou, B.; Wang, T.; Li, Y.; Bralts, V. Effects of microbial community variation on bio-clogging in drip irrigation emitters using reclaimed water. Agric. Water Manag. 2017, 194, 139–149. [Google Scholar] [CrossRef]
- Nakayama, F.S.; Bucks, D.A. Emitter Clogging Effects on Trickle Irrigation Uniformity. Trans. ASAE 1981, 24, 77–80. [Google Scholar] [CrossRef]
- Zhangzhong, L.L. Mechanism of the Emitters Chemical-Clogging under Drip Irrigation with Saline Water and Its Preventation Mehtods. Ph.D. Thesis, China Agricultural University, Beijing, China, 2016. [Google Scholar]
- Chen, H.; Liu, Y.; Chen, J.; Zhang, L.; Cai, Y.; Chen, H.; Wu, S.; Zhou, M. The Clogging Rules of Ceramic Emitter in Irrigation Using Saline Water with Different EC. Agronomy 2019, 9, 436. [Google Scholar] [CrossRef]
- Hills, D.J.; Nawar, F.M.; Waller, P.M. Effects of Chemical Clogging on Drip-Tape Irrigation Uniformity. Trans. ASAE 1989, 32, 1202–1206. [Google Scholar] [CrossRef]
- Han, S.; Li, Y.; Zhou, B.; Liu, Z.; Feng, J.; Xiao, Y. An in-situ accelerated experimental testing method for drip irrigation emitter clogging with inferior water. Agric. Water Manag. 2019, 212, 136–154. [Google Scholar] [CrossRef]
- Zhangzhong, L.L.; Peiling, Y.; Ren, S.M.; Li, Y.K.; Liu, Y.; Xia, Y.H. Chemical Clogging of Emitters and Evaluation of Their Suitability for Saline Water Drip Irrigation. Irrig. Drain. 2016, 65, 439–450. [Google Scholar]
- Zhangzhong, L.L.; Yang, P.; Zhen, W.; Zhang, X.; Wang, C. A kinetic model for the chemical clogging of drip irrigation system using saline water. Agric. Water Manag. 2019, 223, 105696. [Google Scholar] [CrossRef]
- Ma, C.; Xiao, Y.; Puig-Bargués, J.; Shukla, M.K.; Tang, X.; Hou, P.; Li, Y. Using phosphate fertilizer to reduce emitter clogging of drip fertigation systems with high salinity water. J. Environ. Manag. 2020, 263, 110366. [Google Scholar] [CrossRef]
- Langelier, W.F. The analytical control of anti-corrosion water treatment. J.-Am. Water Work. Assoc. 1936, 28, 1500–1521. [Google Scholar] [CrossRef]
- Shinde, D.; Patel, K.; Solia, B.; Patil, R.; Lambade, B.; Kaswala, A. Clogging behaviour of drippers of different discharge rates as influienced by different fertigation and irrigation water salinity levels. J. Environ. Res. Dev. 2012, 7, 917–922. [Google Scholar]
- Li, J.S.; Zhang, J.J.; Xue, K.Z. Principles and Applications of Fertigation through Drip Irrigation Systems; China Agricultural Science and Technology Press: Beijing, China, 2003. [Google Scholar]
- Bucks, D.A.; Nakayama, F.S. Injection of fertilizers and other chemicals for drip irrigation. In Proceedings of the Agri-Turf Irrigation Conference; The Irrigation Association: Silver Spring, MD, USA, 1980; pp. 166–180. [Google Scholar]
- Bozkurt, S.; Ozekici, B. The effects of fertigation managements on clogging of in-line emitters. J. Appl. Sci. 2006, 6, 3026–3034. [Google Scholar] [CrossRef]
- Hopkins, B.G.; Ellsworth, J.W.; Shiffler, A.K.; Cook, A.G.; Bowen, T.R. Monopotassium phosphate as an in-season fertigation option for potato. J. Plant Nutr. 2010, 33, 1422–1434. [Google Scholar] [CrossRef]
- Liu, L.; Niu, W.; Guan, Y.; Wu, Z.; Ayantobo, O.O. Effects of Urea Fertigation on Emitter Clogging in Drip Irrigation System with Muddy Water. J. Irrig. Drain. Eng 2019, 145, 04019020. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, W.E.; Hu, X.T. Research on the Emitter Anti-clogging Performance and System Uniformity Caused by Fertigation. China Rural. Water Hydropower 2015, 11, 1–5. [Google Scholar]
- Liu, G.; McAvoy, G. How to Reduce Clogging Problems in Fertigation; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2012; pp. 1–7. Available online: http://www.edis.ifas.ufl.edu/pdffiles/HS/HS120200.pdf (accessed on 10 November 2021).
- Garg, S.; Bahl, G.S. Phosphorus availability to maize as influenced by organic manures and fertilizer P associated phosphatase activity in soils. Bioresour. Technol. 2008, 99, 5773–5777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, Y.; Wang, Y.; Zhou, B.; Bhattarai, R. Composite fouling of drip emitters applying surface water with high sand concentration: Dynamic variation and formation mechanism. Agric. Water Manag. 2019, 215, 25–43. [Google Scholar] [CrossRef]
- Kafkafi, U.; Tarchizky, J. A Tool for Efficient Fertilizer and Water Management; International Potash Institute (IPI): Paris, France, 2011; pp. 1–123. [Google Scholar]
- Goyal, M.R. Water and Fertigation Management in Micro Irrigation; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Gryta, M. Polyphosphates used for membrane scaling inhibition during water desalination by membrane distillation. Desalination 2012, 285, 170–176. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Li, J. Effect of phosphorus-coupled nitrogen fertigation on clogging in drip emitters when applying saline water. Irrig. Sci. 2020, 38, 337–351. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Y.; Xiao, Y.; Liu, Z. Different operation patterns on mineral components of emitters clogging substances in drip phosphorus fertigation system. Irrig. Sci. 2019, 37, 691–707. [Google Scholar] [CrossRef]
- Capra, A.; Scicolone, B. Water Quality and Distribution Uniformity in Drip/Trickle Irrigation Systems. J. Agric. Eng. Res. 1998, 70, 355–365. [Google Scholar] [CrossRef]
- Sahin, U.; Tunc, T.; Eroğlu, S. Evaluation of CaCO3 clogging in emitters with magnetized saline waters. Desalin. Water Treat. 2012, 40, 168–173. [Google Scholar] [CrossRef]
- Aali, K.A.; Liaghat, A.; Dehghanisanij, H. The effect of acidification and magnetic field on emitter clogging under saline water application. J. Agric. Sci. 2009, 1, 132. [Google Scholar]
- Esmaeilnezhad, E.; Choi, H.J.; Schaffie, M.; Gholizadeh, M.; Ranjbar, M. Characteristics and applications of magnetized water as a green technology. J. Clean. Prod. 2017, 161, 908–921. [Google Scholar] [CrossRef]
- Amor, H.B.; Elaoud, A.; Salah, N.B.; Elmoueddeb, K.J.I.J.A.I.E. Effect of magnetic treatment on surface tension and water evaporation. Int. J. Adv. Ind. Eng. 2017, 5, 119–124. [Google Scholar]
- Alimi, F.; Tlili, M.; Ben Amor, M.; Gabrielli, C.; Maurin, G. Influence of magnetic field on calcium carbonate precipitation. Desalination 2007, 206, 163–168. [Google Scholar] [CrossRef]
- Silva, J.A.T.D.; Judit, D. Impact of magnetic water on plant growth. Environ. Exp. Biol. 2014, 12, 137–142. [Google Scholar]
- Simonič, M.; Urbancl, D. Alternating magnetic field influence on scaling in pump diffusers. J. Clean. Prod. 2017, 156, 445–450. [Google Scholar] [CrossRef]
- Awad, M.A.; Hindi, A.A.; Al-Wohiby, N.; Soliman, D.A.; Ortashi, K.M.O. Magnetic Treatment of Water: Properties and Prevention of the Growth of Bacteria. J. Comput. Theor. Nanosci. 2018, 15, 1312–1319. [Google Scholar] [CrossRef]
- Khoshravesh, M.; Mirzaei, S.M.J.; Shirazi, P.; Valashedi, R.N. Evaluation of dripper clogging using magnetic water in drip irrigation. Appl. Water Sci. 2018, 8, 81. [Google Scholar] [CrossRef]
- Ružič, R.; Vodnik, D.; Jerman, I. Influence of aluminum in biologic effects of elf magnetic field stimulation. Electro- Magnetobiol. 2000, 19, 57–68. [Google Scholar] [CrossRef]
- Aladjadjiyan, A. Study of the influence of magnetic field on some biological characteristics of Zea mais. J. Cent. Eur. Agric. 2002, 3, 89–94. [Google Scholar]
- Haq, Z.U.; Iqbal, M.; Jamil, Y.; Anwar, H.; Younis, A.; Arif, M.; Fareed, M.Z.; Hussain, F. Magnetically treated water irrigation effect on turnip seed germination, seedling growth and enzymatic activities. Inf. Process. Agric. 2016, 3, 99–106. [Google Scholar] [CrossRef]
- Miao, J.; Wang, S.; You, H.J.A.S. Effects of Magnetized Water on Seed Germination of Welsh Onion (Allium fistulosum L.). Agric. Sci. Technol. 2017, 18, 777–784. [Google Scholar]
- Sayed, H. Impact of magnetic water irrigation for improve the growth, chemical composition and yield production of broad bean (Vicia faba L.) plant. Am. J. Exp. Agric. 2014, 4, 476–496. [Google Scholar] [CrossRef]
- Maheshwari, B.L.; Grewal, H.S. Magnetic treatment of irrigation water: Its effects on vegetable crop yield and water productivity. Agric. Water Manag. 2009, 96, 1229. [Google Scholar] [CrossRef]
- Hozayn, M.; El-Monem, A.; Qados, A.A.; El-Hameid, E. Response of some food crops to irrigation with magnetized water under green house condition. Aust. J. Basic Appl. Sci. 2011, 5, 29–36. [Google Scholar]
- Hamdy, A.; Khalifa, S.; Abdeen, S. Effect of magnetic water on yield and fruit quality of some mandarin varieties. Ann. Agric. Sci. 2015, 53, 657. [Google Scholar]
- Surendran, U.; Sandeep, O.; Josenph, E.J. The impacts of magnetic treatment of irrigation water on plant, water and soil characteristics. Agric. Water Manag. 2016, 178, 21–29. [Google Scholar]
- Khoshravesh, M.; Mostafazadeh-Fard, B.; Mousavi, S.; Kiani, A. Effects of magnetized water on the distribution pattern of soil water with respect to time in trickle irrigation. Soil Use Manag. 2011, 27, 515–522. [Google Scholar] [CrossRef]
- Zlotopolski, V. The Impact of magnetic water treatment on salt distribution in a large unsaturated soil column. Int. Soil Water Conserv. Res. 2017, 5, 253–257. [Google Scholar] [CrossRef]
- Al-Ogaidi, A.A.M.; Wayayok, A.; Rowshon, M.K.; Abdullah, A.F. The influence of magnetized water on soil water dynamics under drip irrigation systems. Agric. Water Manag. 2017, 180, 70–77. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, L.; Wu, P.; Liu, Y.; Cai, Y.; Zhou, W. Clogging formation and an anti-clogging method in subsurface irrigation system with porous ceramic emitter. Agric. Water Manag. 2021, 250, 106770. [Google Scholar] [CrossRef]
- Lamm, F.R.; Ayars, J.E.; Nakayama, F.S. Microirrigation for Crop Production: Design, Operation, and Management; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Martins, C.C.; Soares, A.A.; Ramos, M.M.; Reis, E.F. The use of organic chlorine in clogging prevention in drip irrigation systems. In Proceedings of the International Conference of Agricultural Engineering, XXXVII Brazilian Congress of Agricultural Engineering, International Livestock Environment Symposium-ILES VIII, Iguassu Falls City, Brazil, 31 August–4 September 2008. [Google Scholar]
- Yuan, Z.M.; Waller, P.Y.; Choi, C. Effects of organic acids on salt precipitation in drip emitters and soil. Trans. ASAE 1998, 41, 1689–1696. [Google Scholar] [CrossRef]
- Meyer, J.L.; Snyder, M.J.; Valenzuela, L.H.; Harris, A.; Strohman, R. Liquid polymers keep drip irrigation lines from clogging. Calif. Agric. 1991, 45, 24–25. [Google Scholar] [CrossRef][Green Version]
- Van der Burg, N. Bacterieslijm blokkeert druppelslangen. Stop verslijming. (Bacteria slime blocks driplines. Stop slime formation). Vakbl. Bloem. 1996, 51, 40–41. [Google Scholar]
- Kreij, C.D.; Van der Burg, A.M.M.; Runia, W.T. Drip irrigation emitter clogging in Dutch greenhouses as affected by methane and organic. Agric. Water Manag. 2003, 60, 73–85. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wu, P.T.; Zhu, D.L.; Zhang, Y. Clogging of Labyrinth Emitters in Greenhouse Fertigation. Trans. Chin. Soc. Agric. Mach. 2014, 45, 50–55. [Google Scholar]
- Santelli, C.M.; Welch, S.A.; Westrich, H.R.; Banfield, J.F. The effect of Fe-oxidizing bacteria on Fe-silicate mineral dissolution. Chem. Geol. 2001, 180, 99–115. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Geomicrobiology: Its significance for geology. Earth-Sci. Rev. 1998, 45, 45–60. [Google Scholar] [CrossRef]
- Yanmis, D.; Orhan, F.; Gulluce, M.; Sahin, F. Biotechnological magnesite enrichment using a carbonate dissolving microorganism, Lactococcus sp. Int. J. Miner. Process. 2015, 144, 21–25. [Google Scholar] [CrossRef]
- Eroglu, S.; Sahin, U.; Tunc, T.; Sahin, F. Bacterial application increased the flow rate of CaCO3-clogged emitters of drip irrigation system. J. Environ. Manag. 2012, 98, 37–42. [Google Scholar] [CrossRef]
- Li, Y.K.; Wang, K.Y.; Wang, T.Z.; Zhang, Z.J. Microbial Antagonism Clear Drip Irrigation System Clogging Method. China Patent CN103752560B; Beijing, China, 2016. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).