Abstract
Due to the specific requirements for low soil pH, new production methods are being introduced for highbush blueberry (Vaccinium corymbosum L.). Planting in pots has gained popularity in recent years due to the easier control of the substrate pH. This study was carried out on 2-year-old ‘Duke’, ‘Aurora’ and ‘Brigitta’ cultivar blueberry plants that were planted along a ridge or in pots. The substrate temperature reached higher values for the pots, while the substrate water content was higher for the ridge. In the ‘Duke’ and ‘Aurora’ plants, significantly higher sugar/organic acid ratios were obtained for fruit from the ridge. However, significantly higher fruit total phenolics content, greater plant volumes and lower yields per plant were obtained for ‘Aurora’ as potted plants compared to the ridge. The ‘Brigitta’ fruit harvested from potted plants had significantly higher total organic acid content; however, no significant difference was seen for the sugar/organic acid ratio between the conditions. This study is the first to compare the responses of different highbush blueberry cultivars in terms of production on a ridge and in pots, and the impact on the substrate microclimatic conditions, plant volume and fruit yield and primary and secondary metabolites content.
1. Introduction
Highbush blueberry (Vaccinium corymbosum L.) was domesticated in the 19th century in Florida and New England. For their optimal yield and vegetative growth, these plants need to be provided with low substrate pH (4.5–5.5), high organic matter (7%–10%) and good drainage [1,2,3]. Maintaining those three requirements at optimal levels can be challenging for growers, due to the great diversity seen for soil properties. Consequently, in recent years, growers have shown increased interest for alternative planting methods using custom substrates.
One alternative is the planting of blueberry plants along a ridge, and a second alternative that requires smaller amounts of substrate is planting in pots. Highbush blueberry production in pots thus represents an alternative to soil production, where their pH, organic matter and drainage demands can be maintained more easily using a selected substrate and controlled irrigation and fertilisation [4]. This pot production allows individual plants to be moved according to the plant volume, to thereby change plant density [2]. At the same time, production in pots can be carried out in areas with inappropriate soil properties, such as soil pests, salinity and residual toxic compounds or with poor or non-fertile soil [5,6]. A particular disadvantage of the pot method is limited root growth and consequently reduced absorption surface for water and nutrient uptake, with the possibility of effects on long-term production due to the shorter plant life. When pots with smaller volumes are used, strong wind can also lead to overturning of plants [2]. Planting in pots also represents additional cost for intensive blueberry production [7].
For blueberry production in pots, studies have so far investigated optimal pot volumes and their influence on yield [8], plant volume and physiological processes [9], along with water distribution in the substrate [10]. Peat, coir, pine bark and perlite have been the most frequently studied, and to date, further established substrates in variable proportions (depending on the cultivar) have also been tested for highbush blueberry production in pots [4,11]. Some studies have also examined the effects on potted highbush blueberry plants of different substrate pHs [12] and fertilisation [13] on plant volume, yield, ripening time, total soluble solids content in fruit and photosynthesis and microelement content in leaves.
To date, the published data on the cultivation of blueberry plants in pots have mainly reported on the differences in cultivar responses on such production methods, according to the yield and ripening time [14]. Therefore, a knowledge gap remains for comparisons of ridge and pot blueberry production. The aim of the present study was to determine how substrate properties are affected by ridge and pot conditions, and to compare the responses of three different cultivars on these two planting methods in relation to plant volume and fruit yield, and primary and secondary metabolites content.
2. Materials and Methods
2.1. Experimental Design
The experiment was performed in 2020 on 3-year-old blueberry plants of ‘Duke’, ‘Aurora’ and ‘Brigitta’ cultivars in the test field of the Biotechnical Faculty in Ljubljana (latitude: 46°05′ N; longitude: 14°47′ E; altitude: 295 m). Uniform plants for each cultivar were planted in May 2018 on 0.8 m-wide ridges (10–17 plants/treatment, i.e., individual planting method) at a distance of 0.6 m within the rows and 2 m between the rows, with north-south orientation and also in black 60 L pots.
The substrate was composed of peat, pine sawdust and soil (1:1:1, v/v/v). White polypropylene foil was placed on the ground on the ridge and under the pots to prevent weed growth and soil overheating. If necessary, the plants were drip irrigated with rainwater, and they were fertilised nine times during the growing season, which was from the beginning of April to the end of June. After flowering and until the beginning of leaf fall, the plants were covered with hail nets to protect the crop from hail and birds.
2.2. Substrate Properties
Substrate temperature and water content were monitored by continuous measurements with soil temperature sensors (DS18b20; Dallas Semiconductor, Dallas, TX, USA) and soil moisture sensors (EC5; Decagon Devices, Pullman, WA, USA). This made it possible for constant access for the temperature and substrate moisture measurements and conditions.
2.3. Plant Volumes and Harvest
Plant size was measured twice: once in spring (16 March 2020) and once in autumn (9 October 2020). A measuring tape was used to determine plant heights and two diameters (in the row direction; perpendicular to the row). Plant volumes (V) were calculated according to Equation (1):
V (〖dm〗^3) = (height (cm) × ½ width 1 (cm) × ½ width 2 (cm) × π)/1000
The fruits were harvested five to seven times for each cultivar, starting on 8 June 2020 for ‘Duke’ and ending on 24 August 2020 for ‘Aurora’. The fruits were harvested at their full maturity; that was when the fruit was fully dark blue. At each harvest, the fruits from the individual plants were counted and weighed in the laboratory using a precision scale (Kern ALS; Balingen, Germany). For total yield per plant, the fruit weights of all of the individual harvests were summed. Fruits of the same harvest and treatment (ridge and pots) were combined to obtain average samples, which were frozen in liquid nitrogen and stored at −20 °C.
2.4. Sugar, Organic Acid and Phenolics Extraction
Individual sugar and organic acid extractions were carried out for five replicates for each treatment. For the extraction, 1 g finely chopped thawed sample was weighed into a test tube and mixed with 4 mL bi-distilled water. The samples were mixed and left under extraction at room temperature for 30 min, with constant stirring (Unimax 1010 shaker; Heidolph, Schwabach, Germany). The samples were then centrifuged at 9000× g for 10 min at 4 °C and filtered into vials (0.2 μm cellulose filters; Chromafil A-20/25; Macherey-Nagel, Düren, Germany). The samples were then stored at −20 °C until further analysis.
For phenolics extraction, the samples were prepared the same way as for the primary metabolite extraction, with 2 g sample mixed with 4 mL of 70% methanol, 3% formic acid in bi-distilled water. The samples were mixed, left in a cooled (0 °C) ultrasonic bath for 1 h and then centrifuged at 9000× g for 10 min at 4 °C. The supernatant was transferred into the vials through 0.2 μm polyamide filters (Chromafil AO-20/25; Macherey-Nagel, Düren, Germany), with the samples stored at −20 °C until further analysis.
2.5. Analytical Methods
The HPLC system (Vanquish; Thermo Scientific, Waltham, Mass, USA) was connected to a refractive index detector (RefractoMax 520 plus; Thermo, Scientific, Waltham, MA, USA) to analyse the individual sugars. The analysis conditions were as follows: column, Rezex RCM-monosaccharide Ca + 2% (300 mm × 7.8 mm; Phenomenex, CA, USA); column temperature, 65 °C; flow rate, 0.6 mL min−1; injected sample volume, 20 μL; mobile phase, bi-distilled water; and run time, 30 min. Individual sugars were identified by comparison of their retention times with external standards of fructose, glucose and sucrose (Fluka Chemie GmbH, Buchs, Switzerland). Their contents were calculated from standard curves and are expressed as mg g−1 fresh weight (FW).
Organic acids were separated by HPLC (Vanquish; Thermo Scientific, Waltham, MA, USA). Their separation was performed using a Rezex ROA-Organic acid H + 8% column (150 mm × 7.8 mm; Phenomenex, CA, USA) at 65 °C. The UV detector was set to 210 nm, with sample injection volume of 20 μL analysed over 15 min at a flow rate of 0.6 mL min−1. The mobile phase was 4 mM sulfuric acid in bi-distilled water. Organic acids were identified according to the retention times of external standards for citric, tartaric, malic and shikimic acid, with calculation from standard curve equations, and expressed as mg g−1 FW.
Separation of the individual phenolics was performed by HPLC (Dionex UltiMate 3000; Thermo Scientific, Waltham, MA, USA) on a C18 column (Phenomenex Gemini, CA, USA; 150 mm × 4.6 mm, 3 μm), operating at 25 °C. Detection was performed at 280, 350 and 530 nm; the autosampler temperature was 10 °C; the flow rate was 0.6 mL min−1, and the injection volume was 20 μL. Two mobile phase solutions were used: solvent A, 3% acetonitrile, 0.1% formic acid in bi-distilled water (v/v/v); solvent B, 3% bi-distilled water, 0.1% formic acid in acetonitrile (v/v/v). The mobile phase gradient used was as follows: 0–15 min, 5% B; 15–20 min, 5%–20% B; 20–30 min, 20%–30% B; 30–35 min, 30%–90% B; 35–45 min, 90%–100% B; 45–50 min, 100%–5% B.
Individual phenolics were identified using mass spectrometry (LTQ XL; Thermo Scientific, Waltham, MA, USA), based on their mass fragmentation patterns and by comparison of retention times of samples and external standards. Sample analysis was performed in positive and negative ion mode, depending on the compound of interest, with electrospray ionization. The injected samples (10 μL) were analysed over 50 min, at flow rate of 0.6 mL min−1, with the same mobile phase solutions and linear gradient used as for the HPLC analyses. The capillary temperature was 250 °C, source voltage was 4 kV, m/z scanning was from 115 to 1600, sheath and auxiliary gases used were at 20 and 8 units, respectively. Phenolics contents were calculated from standard curve equations, expressed as mg kg−1 FW and presented as groups of phenolics.
2.6. Statistics
All of the data collected were evaluated statistically in R commander i386 4.0.4. Statistically significant differences between treatments were evaluated using one-way analysis of variance (ANOVA), t-tests and least significant difference (LSD) tests. Additionally, multivariate analysis was used for data visualisation and grouping.
3. Results
3.1. Substrate Properties
Maximal daily air and average substrate temperature for the individual planting methods from 1 January 2020 to 6 September 2020 are shown in Figure 1. In general, throughout this period, the substrate in the pots had higher average temperatures compared to the ridge, with the highest difference on 19 June 2020 when the difference was 4.7 °C. The highest recorded average temperatures were 26.1 °C for the ridge and 28.5 °C in the pots, at the beginning of August. At the same time, more pronounced fluctuations between minimal and maximal temperatures were seen for the pots compared to the ridge. The fluctuation trends of the substrate temperatures were in agreement with the fluctuations in maximal air temperatures, which increased slowly towards the warmer months of the year, reaching their highest on 1 August 2020 (36.7 °C).
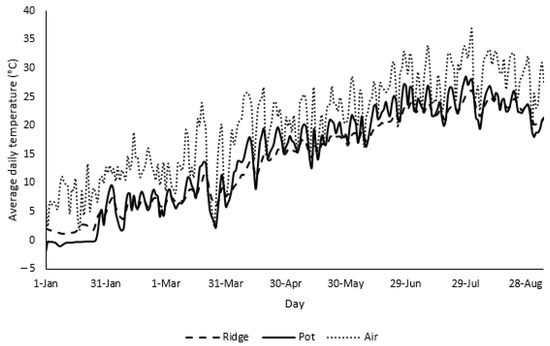
Figure 1.
Maximal air and average daily substrate temperatures for the ridge and the pots (as indicated) from 1 January 2020 to 6 September 2020.
The continuous measurements of substrate water content from the beginning of January 2020 to the beginning of September 2020 showed constantly higher water content for the ridge than for the pots (Figure 2). However, these differences were minor and the plants in the pots did not suffer from drought stress.
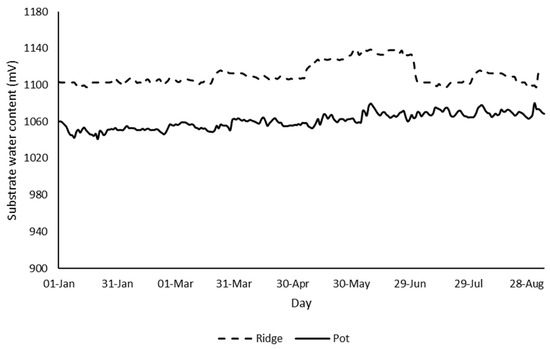
Figure 2.
Average daily substrate water content for the ridge and the pots from 1 January 2020 to 6 September 2020.
3.2. Plant Volume and Yield
The planting method, as rows versus pots, had no effects on plant volume of the ‘Duke’ and ‘Brigitta’ cultivars (Table 1). However, ‘Aurora’ responded positively to pot production (72.78 dm3), where the plant volume was significantly higher compared to the plants grown in the ridge (61.99 dm3). For the ridge production, ‘Brigitta’ had the highest plant volume, followed by ‘Aurora’ and ‘Duke’. No significant differences were seen between ‘Aurora’ and ‘Duke’. A similar trend was seen for the potted plants, where significant differences in plant volume were detected between all three cultivars, with the highest volume for ‘Brigitta’, followed by ‘Aurora’, and then ‘Duke’.

Table 1.
‘Duke’, ‘Aurora’ and ‘Brigitta’ plant volumes and yields for growth on the ridge and in pots.
The average yield per plant according to the planting methods and the cultivars is given in Table 1. There were no significant differences between the ridge and pot production for the ‘Duke’ and ‘Brigitta’ cultivars. On the contrary, pot production (289.6 g plant−1) significantly decreased yield quantity for ‘Aurora’ plants, compared to those grown in the ridge (492.1 g plant−1). Focusing only on the ridge production, the highest yields were seen for ‘Brigitta’ and ‘Aurora’ cultivars; however, no significant differences were seen between ‘Aurora’ and ‘Duke’. This leads to the conclusion that only ‘Duke’ and ‘Brigitta’ differ in yield quantity. Potted plants of ‘Duke’ and ‘Brigitta’ had the highest yield per plant, while the ‘Aurora’ yield was the lowest.
3.3. Sugar and Organic Acid Content
Considering the ridge and the pots separately, the data given in Table 2 define the individual and total sugars. These suggested that ‘Duke’ contained the significantly lowest contents compared to ‘Aurora’ and ‘Brigitta’. These last two had the highest contents of glucose (20.48 mg g−1 and 20.79 mg g−1 for the ridge and 19.14 mg g−1 and 19.25 mg g−1 for the pots, respectively), and no significant differences were observed between them. However, among all three of these cultivars, ‘Aurora’ fruit contained the significantly highest content of sucrose (9.12 mg g−1 for the ridge and 9.74 mg g−1 for the pots), fructose (33.91 mg g−1 for the ridge and 31.46 mg g−1 for the pots) and total sugars (63.52 mg g−1 for the ridge and 60.34 mg g−1 for the pots).

Table 2.
Sucrose, glucose, fructose and total sugars content in ‘Duke’, ‘Aurora’ and ‘Brigitta’ fruit harvested from plants grown on the ridge and in pots.
For the fruit of the ‘Duke’ cultivar, the individual and total sugar contents significantly differed between planting methods (Table 2). Sucrose content was significantly higher in fruit harvested from potted plants (4.21 mg g−1, while 3.25 mg g−1 on the ridge), while glucose (14.93 mg g−1), fructose (26.25 mg g−1) and total sugars content (44.43 mg g−1) were significantly higher in fruit from the ridge. The ‘Aurora’ and ‘Brigitta’ fruit from the ridge and the pots did not differ in individual nor in total sugars content.
Table 3 gives the average values of the individual and total organic acids contents. Considering only the ridge production, the individual organic acids contents varied greatly among the cultivars, where citric, tartaric and malic acid contents were highest in ‘Aurora’ and the lowest in ‘Brigitta’ or in ‘Duke’ for citric acid. The opposite was observed for shikimic acid, where the contents were highest in ‘Duke’ and the lowest in ‘Aurora’, with the same for the ridge as for the pots. In the fruit of the ‘Duke’ cultivar, there were significant differences in the total organic acids contents between the ridge (8.17 mg g−1) and the pots (9.68 mg g−1), due to the higher citric and malic acid contents in the fruit from the pots. The fruit of the ‘Aurora’ plants grown in pots produced significantly higher citric (15.05 mg g−1) and total organic acids (17.09 mg g−1) and lower shikimic acid (0.013 mg g−1), compared to the plants from the ridge (13.68 mg g−1, 15.69 mg g−1, 0.017 mg g−1, respectively), while the other individual acid contents did not differ between the fruit from the different planting methods. On the contrary, ‘Brigitta’ fruit contained the same amount of citric and total organic acids, while the fruit picked from the plants grown in the ridge contained significantly higher tartaric, malic and shikimic acid contents.

Table 3.
Citric, tartaric, malic and shikimic acids and total organic acid content in ‘Duke’, ‘Aurora’ and ‘Brigitta’ fruit harvested from the plants grown on the ridge and in pots.
Figure 3 illustrates the sugar/organic acid ratios in these blueberry fruit. Focusing on the ridge production, ‘Brigitta’ fruit showed the significantly highest ratio, while the other two cultivars did not differ significantly. For pot production alone, ‘Brigitta’, ‘Aurora’ and then Duke’ followed from highest to lowest sugar/organic acid ratios. For ‘Duke’ and ‘Aurora’, significantly higher ratios were seen for the fruit from the ridge, comparing with the pots, while no difference was seen for ‘Brigitta’.
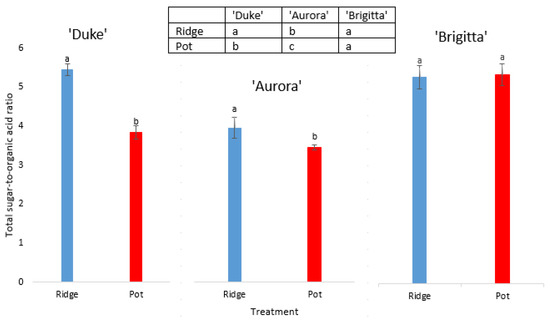
Figure 3.
Total sugar/organic acid ratios for ‘Duke’, ‘Aurora’ and ‘Brigitta’ fruit harvested from plants grown on the ridge and in pots. Data are means ± standard errors (five replicates per treatment). Different letters indicate statistically significant differences between planting methods for individual cultivar (t-test, α < 0.05) and letters in the box between cultivars for individual planting method (LSD test, α < 0.05).
3.4. Phenolics
The average phenolics content by groups of phenolics is given in Table 4, and the individual cultivar and planting method group classification according to total phenolics in Figure 4. According to the statistical analysis for the cultivars grown on the ridge, ‘Duke’ contained the significantly highest flavan-3-ol, anthocyanin and total phenolics content, followed by ‘Aurora’ and ‘Brigitta’. Phenolic acids content was the significantly lowest for ‘Aurora’ and flavonol for ‘Brigitta’. In the fruit from the potted plants, the significantly lowest values of all of the groups of phenolics were measured for ‘Brigitta’, followed by ‘Aurora’. The planting method did not affect the phenolic acids, flavonol, anthocyanin and total phenolics in ‘Duke’; however, flavan-3-ols content was significantly higher in the fruit harvested from the ridge. ‘Aurora’ fruit from the potted plants contained significantly higher phenolic acids, anthocyanin and total phenolic compounds, while no differences were seen for flavan-3-ol and flavonol contents between treatments. The phenolic acids content for the ‘Brigitta’ fruit was significantly higher for the ridge. However, the other phenolics identified did not differ between the planting methods.

Table 4.
Phenolic acid, flavan-3-ols, flavonols, anthocyanin and total phenolic compounds content in the ‘Duke’, ‘Aurora’ and ‘Brigitta’ fruit harvested from plants grown on the ridge and in pots.
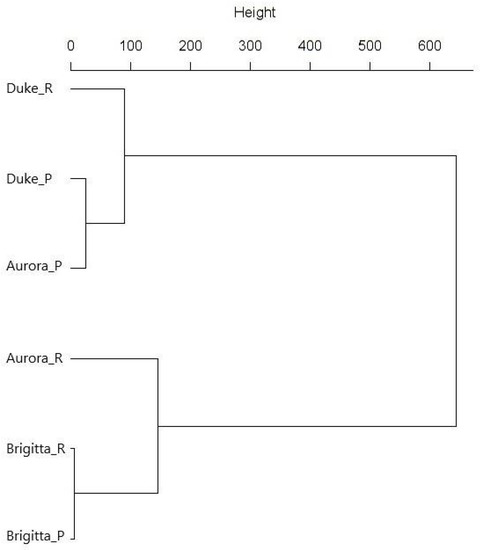
Figure 4.
Dendrogram for total phenolics content in the ‘Duke’, ‘Aurora’ and ‘Brigitta’ fruit for the different planting methods (R, ridge; P, pot), using Ward’s method based on Euclidian distances.
4. Discussion
Some studies have already been carried out on highbush blueberry production in pots, but none have compared ridge and pot production methods to date nor examined the impact of these planting methods on substrate temperature and water content. In general, the substrate temperatures in the pots reached higher average values compared to the ridge substrate. At the same time, the substrate in the pots warms up and cools down more quickly, which is indicated by the larger differences between the minimum and maximal temperatures, compared to the ridge. In relation to the volume of the substrate, the pots have a larger surface area exposed to the sun than the ridge, and therefore, the temperature of the substrate in the pots can fluctuate faster and more markedly [9]. Markham et al. (2011) reported that the black colour of the pots considerably contributes to these higher substrate temperatures, compared to the ridge, which in the present study was covered by white foil [15].
The pots used in the present study were comparable to the ridge in terms of the substrate volume and, consequently in root system development, which provided the plants in the pots with an adequate water supply. This led to equal plant volumes and yields. Cantliffe (2018) also reported that by increasing the pot volume, the growth of the shoots and roots also increases [16]. On the contrary, significantly higher plant volumes were seen for the ‘Aurora’ plants grown in the pots. As we can see, ‘Aurora’ responded positively to this pot planting method in terms of vegetative growth. Contradictory data were reported by Spiers (1995), who indicated that substrate temperatures of 27 °C and 38 °C negatively affected the growth of blueberry shoots [17]; this did not occur in the present study, with the highest substrate temperatures in the pots in the summer months not exceeding 32 °C. The pots had negative effects on the yield quantity in ‘Aurora’, as this was significantly lower in the potted plants. We can assume that plants invested more energy in vegetative and less in reproductive growth, which was reflected in the lower yield quantity. Cultivar comparisons according to the ridge and the pots separately have suggested that plant volumes and yields are first conditioned by genetic and then by environmental factors [14].
The sugar and organic acid contents affect the blueberry fruit sweetness/acidity, which is strongly correlated with the quality of the fruit [18]. Given that pot production methods did not significantly affect plant volume and yield, it is not surprising that there were no significant differences in the fruit sugars contents for ‘Aurora’ and ‘Brigitta’. As has already been reported, fruit sugar content is mainly dependent on the ripening stage [19]. Among individual sugars, sucrose, glucose and fructose were identified in blueberry fruit, with sucrose in the lowest proportion and fructose in the highest. Despite the higher sucrose content in the fruit of the ‘Duke’ plants in the pots, the total sugars content was significantly higher in fruit from the ridge. Moreover, despite the minor sucrose amount in the fruit from the ridge, the total sugars content arose from the significantly higher glucose and fructose contents. When comparing blueberry cultivars within these planting methods, the trends seen for the ridge and the pots were the same, with the lowest sugar content in ‘Duke’ and the highest in ‘Aurora’.
High organic acid content often reduces fruit quality, and therefore this is not desirable in blueberry fruit [18]. Among individual organic acids identified in blueberry fruit here, citric acid predominated, with the lowest proportions seen for shikimic acid. The blueberry pot production influenced the total organic acids in the fruit from ‘Brigitta’, which was significantly lower compared to the fruit from the ridge. Milivojevic et al. (2016) reported that the organic acid content depends to a large extent on the ripeness of the fruit and then on the environmental conditions [19], which is in part in agreement with the present study, with the exception of ‘Brigitta’. From the data given in Table 3, it can be concluded that blueberry organic acids content is primary a cultivar trait [14].
The total sugar/organic acid ratio affects the taste of the fruit; namely, the higher the ratio, the sweeter the fruit, and vice versa. The ratio itself is influenced by high sugar or low organic acid contents, which were clearly shown in the present study. The fruits from ‘Aurora’ are known for their acidic taste, so these need to be harvested at full maturity [20]. Although they contain the highest sugar content among the three cultivars here, their total sugar/organic acid ratio was not the highest, due to their high total organic acids content. The fruits in the present study were harvested at their optimum, in their full maturity, when the organic acids content has already decreased to the minimum. However, the lower total sugar/organic acid ratios in the fruit harvested from the pots of ‘Duke’ and Aurora’ might be attributed to slightly higher total organic acid content in these fruits, despite these data not reaching significance. On the other hand, fruit harvested from ‘Brigitta’ grown on the ridge and in pots showed significantly higher total sugar/organic acid ratio compared to the other two cultivars, due to the high total sugar and low total organic acid contents.
Anthocyanins provide the largest contributions to the total phenolics content, as they are the most abundant type of phenolics in blueberry fruit [21,22,23]. With no significant differences seen between the planting methods for anthocyanins (and consequently total phenolics content) in the fruit of ‘Duke’ and ‘Brigitta’, this suggests that the potted plants were probably not exposed to temperature or drought stress that can result from limited substrate capacity [17]. At the same time, the plants were only 2 years old at the time of this study and had not yet developed their full volume, so 60 L of the substrate more than met their needs [8]. On the contrary, for the fruit harvested from the ‘Aurora’ potted plants, significantly higher phenolic acids, anthocyanins and total phenolics content were seen. This indicates a greater susceptibility of ‘Aurora’ to the modified growing conditions, such as limited root growth or higher substrate temperature variations [17]. Focusing here only on the cultivar comparisons within the individual planting methods, we can come to the conclusion that the content of phenolics in blueberry fruit is primary determined by genetics [19].
5. Conclusions
The responses of highbush blueberry plants to ridge and pot production methods were monitored here during the first years after planting. The results of the present study suggest that growing these plants in 60 L pots can provide effective production for highbush blueberry plants, whereas for the ‘Duke’ and ‘Brigitta’ cultivars, the limited substrate capacity does not negatively affect the plant volume and yield nor the fruit primary and secondary metabolite contents. For the ‘Aurora’ plants, greater plant volumes were measured in the potted plants, although the yield per plant and sugar/organic acid ratio were significantly lower.
These comparisons of ‘Duke’, ‘Aurora’ and ‘Brigitta’ plant responses using the ridge and pot production methods showed that the use of these pots lead to higher substrate temperature variations and lower water contents, compared to the ridge method. However, further studies are necessary to monitor and compare these production methods over a period of several years.
Author Contributions
Conceptualization, J.J., R.V. and T.S.; data curation, T.S. and D.S.; formal analysis, T.S. and D.S.; funding acquisition, M.H.; investigation, T.S.; methodology, J.J.; project administration, J.J. and R.V.; resources, M.H.; supervision, J.J. and R.V.; visualization, T.S.; writing—original draft, T.S.; writing—review and editing, J.J., R.V. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support of the Slovenian Research Agency within the research program Horticulture (P4-0013).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd ed.; Cabi: Boston, MA, USA, 2018. [Google Scholar]
- Whidden, A. Commercial blueberry production methods in Hillsborough County. Proc. Fla. State Hort. Soc. 2008, 121, 36–37. [Google Scholar]
- Milivojević, J.M.; Radivojević, D.D.; Maksimović, V.M.; Dragišić Maksimović, J.J. Variation in health promoting compounds of blueberry fruit associated with different nutrient management practices in a soilless growing system. J. Agric. Sci. 2020, 65, 175–185. [Google Scholar] [CrossRef]
- Kingston, P.H.; Scagel, C.F.; Bryla, D.R. Suitability of sphagnum moss, coir, and douglas fir bark as soilless substrates for container production of highbush blueberry. HortScience 2017, 52, 1692–1699. [Google Scholar] [CrossRef]
- Olympios, C.M. Overview of soilless culture: Advantages, constraints, and perspectives. Prot. Cultiv. Mediterr. Reg. 1999, 31, 307–324. [Google Scholar]
- Voogt, W.; Van Dijk, P.; Douven, F.; Van Der Maas, R. Development of a soilless growing system for blueberries (Vaccinium corymbosum): Nutrient demand and nutrient solution. Acta Hortic. 2014, 1017, 215–221. [Google Scholar] [CrossRef]
- Fang, Y.; Nunez, G.H.; da Silva, M.N.; Phillips, D.A.; Munoz, P.R. A review for southern highbush blueberry alternative production systems. Agronomy 2020, 10, 1531. [Google Scholar] [CrossRef]
- Motomura, S.; Cho, A.; Hamasaki, R.; Akahoshi, K.; Kawabata, A.; Kawabata, A.; Nakamoto, S. Evaluation of pot size for greenhouse production of ‘Misty’ southern highbush blueberry in Volcano, Hawai‘i. Fruit, Nut, and Beverage Crops. 2016. 1–4. Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/F_N-48.pdf (accessed on 18 August 2021).
- Poorter, H.; Bühler, J.; Van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, J.S.; Altland, J.E. Container height and Douglas fir bark texture affect substrate physical properties. HortScience 2008, 43, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Kingston, P.H.; Scagel, C.F.; Bryla, D.R.; Strik, B.C. Influence of perlite in peat- and coirbased media on vegetative growth and mineral nutrition of highbush blueberry. HortScience 2020, 55, 658–663. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Q.; Wei, J.; Jiang, J.; Li, Y.; Chen, J.; Yu, H. Growth, fruit yield, photosynthetic characteristics, and leaf microelement concentration of two blueberry cultivars under different long-term soil pH treatments. Agronomy 2019, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Bi, G. Container production of southern highbush blueberries using high tunnels. HortScience 2019, 54, 267–274. [Google Scholar] [CrossRef]
- Ciordia, M.; Díaz, M.B.; García, J.C. Blueberry culture both in pots and under Italian-type tunnels. Acta Hortic. 2002, 123–127. [Google Scholar] [CrossRef]
- Markham, J.W.; Bremer, D.J.; Boyer, C.R.; Schroeder, K.R. Effect of container color on substrate temperatures and growth of red maple and redbud. HortScience 2011, 46, 721–726. [Google Scholar] [CrossRef]
- Cantliffe, D.J. Pre- and postharvest practices for improved vegetable transplant quality. Horttechnology 2018, 3, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Spiers, J.M. Substrate temperatures influence root and shoot growth of southern highbush and rabbit-eye blueberries. HortScience 1995, 30, 1029–1030. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, C.; Sun, J.; Jackson, A. Dynamic changes of enzymes involved in sugar and organic acid level modification during blueberry fruit maturation. Food Chem. 2020, 309, 125617. [Google Scholar] [CrossRef]
- Milivojević, J.; Radivojević, D.; Nikolić, M.; Maksimović, J.D. Changes in fruit quality of highbush blueberries (Vaccinium corymbosum) during the ripening season. Acta Hortic. 2016, 1139, 657–664. [Google Scholar] [CrossRef]
- Yang, W.Q.; Harpole, J.; Finn, C.E.; Strik, B.C. Evaluating berry firmness and total soluble solids of newly released highbush blueberry cultivars. Acta Hortic. 2009, 810, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. Changes in berry quality of northern highbush blueberry (Vaccinium corymbosum L.) during the harvest season. Turk. J. Agric. For. 2016, 40, 855–864. [Google Scholar] [CrossRef]
- Zorenc, Z.; Veberic, R.; Mikulic-Petkovsek, M. Are processed bilberry products a good source of phenolics? J. Food Sci. 2018, 83, 1856–1861. [Google Scholar] [CrossRef]
- Milivojevic, J.; Maksimovic, V.; Dragisic Maksimovic, J.; Radivojevic, D.; Poledica, M.; Ercili, S. A comparison of major taste-and health-related compounds of Vaccinium berries. Turk. J. Biol. 2012, 36, 738–745. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).