Abstract
Reducing plant height improves lodging resistance and helps to obtain high grain yield under various environmental conditions. So far, the introduction and maintenance of the dwarfing allele of the Ddw1 gene is the most effective height-reducing genetic approach in rye and triticale breeding programs. However, the dominance of the dwarfing Ddw1 allele makes it difficult to select against heterozygous lines for further breeding based on plant phenotype. To assist breeders in the identification of the allele status of the Ddw1 gene, we developed a cleaved amplified polymorphic sequence (CAPS) marker that requires basic equipment and can be easily applied. The CAPS marker was tested on two F2 segregating populations of triticale, and the test showed the association of the Ddw1 genotype with plant height. The application of the marker for marker-assisted selection (MAS) for rye and triticale is discussed in detail.
1. Introduction
Triticale (x Triticosecale Wittmack) is a plant genus comprised of synthetic amphidiploid crops combining the genomes of wheat (Triticum) and rye (Secale). Triticale was created in order to obtain a crop with the productivity of wheat and the durability of rye. The chromosome set of triticale consists of wheat (AABB or AABBDD) and rye (RR) chromosomes and its ploidy varies from tetraploid (2n = 2x = 14, AARR) to octaploid (2n = 8x = 56, AABBDDRR). The hexaploid level (2n = 6x = 42, AABBRR) is the most common in commercial cultivars due to its excellent combination of traits and genomic stability [1]. Currently, triticale is grown in more than 40 countries around the world because it produces high and stable yields in a wide range of soil and climatic conditions [2,3]. As with other cereals, reduced plant height improves lodging resistance and it is a desirable trait in breeding programs. A number of height-reducing genes are currently known in wheat and rye genomes, and the most effective genes are exploited in the breeding of semi-dwarf cultivars of these cereals and triticale. Primarily, these are the dwarfing alleles Rht-B1b and Rht-D1b of the wheat gene Rht1 (from the B and D genomes, respectively), which encode DELLA protein participating in gibberellin signaling [4,5]. The dominant dwarfing allele of rye Ddw1 (formerly known as Hl) was discovered in the rye genome in a study of natural rye dwarf mutant EM-1 [6]. Recent data suggest that Ddw1 encodes gibberellin 2-oxidase that destroys gibberellin [7].
Ddw1 has attracted most attention in rye and triticale breeding programs because of its strong effect on plant height. However, beside reducing plant height, the dwarfing alleles Ddw1 may also reduce the grain weight per spike and the weight of 1000 grains. This negative effect of Ddw1 can be compensated for by the presence of Rht-B1b [8,9,10].
The dominant gain-of-function effect of Ddw1 complicates the reliable differentiation of homozygous pure lines from heterozygotes based on plant phenotype. Marker-assisted selection (MAS) is a powerful approach to cereal breeding, however there is currently no reliable marker for dominant Ddw1 homozygous plants. Currently, markers targeting microsatellite locus REMS1218 are used for Ddw1 genotyping. REMS1218 (GenBank accession BE587316) contains (AG)8 repeats and was originally discovered by the analysis of cDNA sequences in GenBank. It was mapped at the end of a long arm of chromosome 5R in rye population obtained by crossing cv. Steel and cv. Monstrous. Initially, several REMS1218 were revealed [11]. The position of REMS1218 is close to the restriction fragment length polymorphism (RFLP) marker Xwg199, which co-segregates with Ddw1. The distance between Xwg199 and Ddw1 has been estimated as 5.6 cM, and as 5.3 cM between Xwg199 and REMS1218 in the same direction [11,12]. In a study by Tenhola-Roininen and Tanhuanpa, a combination of SSR and SNP markers for REMS1218 was suggested to distinguish dominant dwarfing Ddw1 from recessive neutral ddw1 alleles, however, this was not able to distinguish Ddw1 homozygous from Ddw1 ddw1 heterozygotes is some cases. In addition, this approach involves analytical discrimination of PCR products with a difference of only 4 bp (317 and 321 bp), which requires either an expensive capillary electrophoresis system or labor-consuming manual polyacrylamide gel electrophoresis procedures [13].
Originally, PCR amplification of REMS1218 was found to produce two product (amplicons) patterns based on their size: a combination of 317 bp and 321 bp products associated with the dwarfing Ddw1 allele, and a 317 bp product associated with the neutral ddw1 allele [13]. Amplicon 317 bp in ddw1 ddw1 plants is shown as a single peak when analyzed using capillary electrophoresis. In fact, 317 bp amplicon consists of two products that are equal in size but polymorphic in nucleotide sequence. In our previous study using a collection of 86 triticale accessions in plants homozygous for neutral ddw1 alleles, we observed several REMS1218 PCR products of various lengths, but in all cases, they were produced together with 317 bp product [5]. Thus, all alleles of the Ddw1 gene found to date produce the conservative 317 bp product, which makes it impossible to distinguish heterozygous and dominant Ddw1 homozygous plants using capillary electrophoresis only. The purpose of this study was to (1) simplify the protocol for identification of Ddw1 alleles and make it useful for screening in conventional breeding laboratories, and (2) to improve the method so that it detects 100% of the dominant allele of the Ddw1 gene.
For this work, we used winter triticale Khongor and Avangard as cultivars homozygous for the dominant allele (Ddw1 Ddw1). The dwarfing Ddw1 allele in this cultivar was transferred from triticale cultivar AD-Zelenyi, the latter inherited Ddw1 from rye EM-1. Two spring triticale cultivars Solovei-Kharkovskii and Dublet were used as carriers of the recessive (ddw1 ddw1) genotype. Previously, in a study of 86 triticale cultivars and breeding lines we revealed that plant height of semi-dwarf cultivars of spring triticale is reduced due to only one gene, wheat-derived Rht-B1b, and they do not carry Ddw1 [5].
In this study, we applied a cleaved amplified polymorphic sequence (CAPS) approach and developed a marker that identifies Ddw1 dwarfing and ddw1 neutral alleles, and distinguishes reduced height heterozygous Ddw1 ddw1 plants from homozygotes Ddw1 plants.
2. Materials and Methods
2.1. Plant Material
Plants (names of cultivars are according to http://wheatpedigree.net) with known dominant dwarfing (Ddw1) or recessive neutral (ddw1) alleles of Ddw1 gene were used for Ddw1 CAPS marker development: (1) winter triticale Khongor (Ddw1 Ddw1 Rht-B1b Rht-B1b); (2) winter triticale Avangard (Ddw1 Ddw1 Rht-B1a Rht-B1a); (3) winter triticale Valentin 90 (Ddw1 Ddw1); (4) winter triticale AD Zelenyi (Ddw1 Ddw1); (5) spring triticale Solovei Kharkovskii (ddw1 ddw1 Rht-B1b Rht-B1b); (6) spring triticale Dublet (ddw1 ddw1 Rht-B1b Rht-B1b); (7) winter triticale Dozor (ddw1 ddw1).
Plant of two F2 populations were partially used for testing Ddw1 CAPS marker and for assessing the association of Ddw1 allele with the plant height. One population designated Kh/D segregating in ddw1/Ddw1 alleles was obtained by crossing Khongor (Ddw1 Ddw1 Rht-B1b Rht-B1b) and Dublet (ddw1 ddw1 Rht-B1b Rht-B1b). Another population designated A/SKh segregating in ddw1/Ddw1 and Rht-B1a/Rht-B1b was obtained by crossing Avangard (Ddw1 Ddw1 Rht-B1a Rht-B1a) and Solovei Kharkovskii (ddw1 ddw1 Rht-B1b Rht-B1b). F1 uniform heterozygotes and their F2 segregating progenies were grown in a greenhouse at 22–26 °C, with 5 plants per vegetative pot. Ninety-one F2 plants of Kh/D population and seventy-seven F2 plants of A/SKh population were genotyped and used to assess the association between theDdw1 allele and plant height.
2.2. DNA Isolation and PCR Amplification of REMS1218 Microsatellite Fragment
Genomic DNA was isolated from individual plant leaves using the cetyltrimethylammonium bromide (CTAB) method [14] with some modifications and adaptations for 96-well deep plates. Briefly, 15–50 mg of dried grinded leaves was extracted by 0.5 mL buffer containing 0.7 M NaCl, 1% CTAB, 25 mM Tris-HCl pH 8.0 and 10 mM EDTA. The suspension was incubated for 1 h at 65 °C with shaking, extracted at room temperature with 2/3 volume of chloroform/isoamyl alcohol (24:1), and centrifuged 30 min at 500× g using a swing-out rotor for deep-well plates. Aqueous phase (200 μL) was collected and DNA was precipitated with 360 µL isopropyl alcohol at −20 °C for 30 min followed by 1 h centrifuge at 500× g. The DNA pellet was rinsed with 300 μL 70% ethanol, dried and dissolved in 150 µL H2O.
The allelic variant of Ddw1 was determined by PCR using primers for microsatellite marker REMS1218 (forward: 5′-CGC ACA AAC AAA AAC ACG AC-3′, reverse: 5′-CAA ACA AAC CCA TTG ACA CG-3′) [13]. PCR reactions were performed using a DNA Engine Tetrad 2 (Bio-Rad, USA) thermocycler with the following conditions: initial denaturation at 94 °C for 5 min; 35 cycles of 94 °C for 30 s, 60 °C for 30 s and 72 °C for 1 min, and a final extension step of 5 min at 72 °C. PCR products were analyzed by capillary electrophoresis using ABI 3130xl DNA analyzer (Applied Biosystems, Life Technologies, CA, USA).
2.3. Cloning and Sequencing REMS1218 PCR Products
REMS1218 PCR products were cloned into pGEM-T Easy vector (Invitrogen, USA) according to the manufacturer’s instructions. The resulting plasmids were transformed into E. coli strain DH10B, bacterial colonies of interest were selected based on blue-white screening and based on the appropriate size of PCR product after amplification using M13 primer. DNA sequencing was performed using the BigDye Terminator v3.1 Cycle Sequencing Kit (Nimagen, Netherlands) and 3130xl Genetic Analyzer (Applied Biosystems, Life Technologies, CA, USA) according to the manufacturer’s instructions. Sequences were analyzed using GeneDoc software [15].
2.4. CAPS Analysis of REMS1218
REMS1218 PCR amplification products were cleaved by restriction endonucleases MnlI (New England Biolabs, MA, USA), Bso31I, RsaI (SibEnzyme Ltd., Moscow, Russian Federation) according to the instructions of the manufacturer of the enzymes. Restriction fragments were separated using 2% agarose gel in Tris-borate buffer with EDTA (TBE) and ethidium bromide at 6 V/cm and visualized in UV-transilluminator Gel Doc XR+ (Bio-Rad Laboratories, Inc., CA, USA). To evaluate our Ddw1 CAPS marker, we (1) compared the results of Ddw1 genotyping by capillary electrophoresis and by CAPS marker on the triticale cultivars (Khongor, Avangard, Valentin 90, AD Zelenyi, Solovei Kharkovskii, Dublet, and Dozor), the mix of DNA from two plants homozygous for alternative Ddw1 alleles, and F2 crosses of Khongor (Ddw1 Ddw1) and Dublet (ddw1 ddw1); and (2) performed two model MAS experiments on breeding two different triticale cultivars homozygous for Ddw1 with two different triticale cultivars homozygous for ddw1 with the analysis of the association of plant height with Ddw1 genotype using a 77-plant sample from A/SKh and a 91-plant sample from the Kh/D F2 population.
2.5. Rht-B1b Genotyping
Rht1 genotyping was carried out by PCR amplification using primer pairs BF, MR1 and BF, WR1 (Syntol Ltd., Russian Federation) and conditions previously reported in [16]. PCR products were run on 2% agarose gel. The presence of dwarfing Rht-B1b allele in plant genome was assigned if primers BF and MR1 yielded PCR product of 237 bp. The absence of Rht-B1b allele (the presence of Rht-B1a allele) was assigned if similar product was amplified using BF and WR1 primers.
2.6. Statistical Analysis
The differences in plant height between homozygous Ddw1, ddw1 and heterozygous Ddw1 ddw1 plants for the Kh/D F2 population, which is homogeneously homozygous in the Rht1 gene (Rht-B1b), were accessed using one-way ANOVA. Two-way ANOVA was used for the analysis of the plant height of the A/SKh F2 population, which segregated into two semi-dwarfing alleles, Ddw1 and Rht-B1b. The genotype for each of the genes (Ddw1 and Rht-B1b) was considered as a factor with 3 levels, depending on the allelic state of the gene (dominant homozygous, heterozygous, and recessive homozygous). No interactions between the factors were considered. Bonferroni post-hoc test was used for the comparisons between groups with different allelic states. Statistically significant difference was assumed for p < 0.05. The calculations were done using Statistica 10 software (Statsoft Inc., Tulsa, OK, USA).
3. Results
3.1. PCR Amplification of Microsatellite REMS1218 Marker Results in Several Product Patterns
In previous studies, REMS1218 was found to yield several types of PCR product patterns that we classified into three types: (i) Type 1: 317 bp + 317 bp (ddw1 ddw1); (ii) Type 2: 317 bp + 321 bp (Ddw1 Ddw1), and (iii) Type 3: 317 bp + 323 bp, 317 bp + 325 bp, 317 bp + 327 bp (ddw1 ddw1) [5,13]. For cloning and sequencing of the REMS1218 amplification products we selected triticale cultivars that represent each type of REMS1218 amplification pattern based on our previous research and pedigree data that reflects the origin of Ddw1. As a result of capillary electrophoresis, Type 1 (ddw1 ddw1) was observed in cv. Dublet and Solovei Kharkovskii, Type 2 (Ddw1 Ddw1) in cv. Khongor, Avangard, Valentin 90, and AD Zelenyi, and Type 3 (ddw1 ddw1) in cv. Dozor (Figure 1).
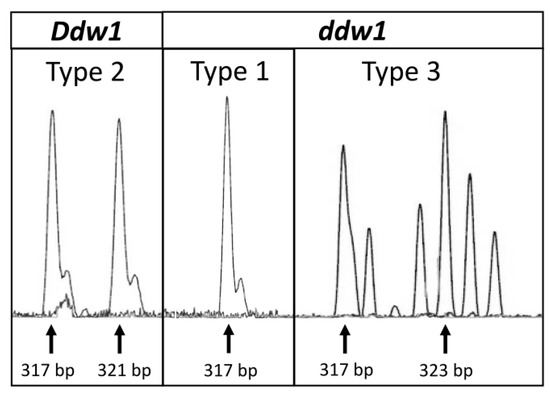
Figure 1.
Capillary electrophoresis profiles of PCR amplification products for REMS1218 marker. DNA from homozygote Ddw1 and ddw1 triticale cultivars was used for PCR amplification. Amplification for Ddw1 Ddw1 plants results in PCR products pattern Type 2, 317 bp + 321 bp. Amplification for ddw1 ddw1 plants results in the PCR products patterns Type 1, 317 bp + 317 bp, and Type 3, 317 bp + 323 bp.
3.2. Cloning and Sequencing of REMS1218 Amplicons Revealed a Common 257 bp Sequence with Six Polymorphic Nucleotides
The size of the PCR products of REMS1218 amplification from plants with different Ddw1 genotypes vary less than 10 bp (Figure 1), thus determination of the Ddw1 alleles cannot be done using regular agarose electrophoresis. An agarose electrophoresis-based CAPS marker would allow for Ddw1 genotyping in virtually any laboratory, and we decided to examine whether a CAPS marker for Ddw1 gene could be developed. In order to reveal possible restriction endonucleases suitable for a CAPS marker, several REMS1218 PCR amplification products of each type (Types 1–3) were isolated from 2% agarose gel, cloned into pGEM-T Easy vector, and sequenced. Multiple alignment for all cloned PCR products revealed polymorphic nucleotides at the relative positions 33, 68, 94, 147, 221, and 267. Any PCR product always had two variants of sequences, which we denoted as “a” and “b”: Type 1a (317 bp) and Type 1b (317 bp); Type 2a (321 bp) and Type 2b (317 bp); and Type 3a (323 bp) and Type 3b (317 bp) (Figure 2). The clones obtained from Type 1 product (which originated from ddw1 homozygote cultivars) gave sequences with nucleotide variants at relative positions 68 and 267. Clones originated from Type 2 products (Ddw1 homozygous plants) had variations at relative positions 94 and 221. Type 3 PCR product clones (from ddw1 homozygous plants) had variations at relative position 147 (Figure 2).

Figure 2.
Multiple alignment for sequences obtained from the cloned REMS1218 PCR products. T1a, T1b are two sequences for Type 1 PCR product pattern (ddw1), T2a, T2b are two sequences for Type 2 PCR product pattern (Ddw1), and T3a, T3b are two sequences for Type 3 PCR product pattern (ddw1). Restriction sites for endonucleases Mnl1 (GAGG), RsaI (GTAC) and Bso31I (GAGACC) are highlighted. Primer sequences are shown in grey.
3.3. Selection and Verification of a Set of Restrictases for CAPS Analysis of Ddw1 Alleles
The sequencing of REMS1218 PCR products allowed us to suggest a set of restriction endonucleases for CAPS analysis. According to the sequences, digestion with three restrictases, MnlI, RsaI, and Bso31I, can be used for identifying Ddw1 genotype (Figure 2). To verify this assumption, we performed PCR using DNA of the cultivars that we analyzed using capillary electrophoresis and sequenced. To simulate heterozygous plants, we mixed the DNA of two plants homozygous for alternative Ddw1 alleles. Additionally, for verification of the developed CAPS marker we used heterozygous F2 plants derived from crossing Khongor (Ddw1 Ddw1; Type 2) × Dublet (ddw1 ddw1; Type 1). The alleles for these plants were determined by capillary electrophoresis as Type 1 for ddw1 ddw1, Type 2 for Ddw1 Ddw1 or as Type 2 + Type 1 for Ddw1 heterozygotes. Aliquots of the PCR products were digested by restrictases and analyzed by 2% agarose gel electrophoresis (some results are shown in Figure 3).
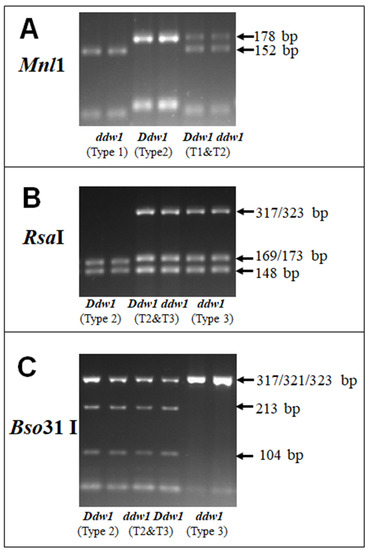
Figure 3.
Examination of Mnl1, RsaI, and Bso31I restriction endonucleases for CAPS marker: 2% agarose electrophoresis of REMS1218 PCR products of different genotypes after digestion by these restrictases. The types of REMS1218 PCR products were determined by capillary electrophoresis. The size of the restriction fragments are given based on our sequences of REMS1218 PCR products. (A) Digestion of homozygous Types 1 (T1) and 2 (T2), and heterozygous T1&T2 REMS1218 products by Mnl1 (Mnl1 does not cleave Type 3 and Type 2, so these results are not shown); (B) Digestion of homozygous Types 2 and 3 and heterozygous T2&T3 by RsaI (RsaI does not cleave Type 1 and Type 2, and these results are not shown); (C) Digestion of homozygous Types 2 and 3 and heterozygous T2&T3 by Bso31I (Type 1 is cleaved by Bso31I and gives 213 bp and 104 bp products only; the data is not shown).
Based on the nucleotide sequences and the results of the gel electrophoresis of digested products, we compiled a list of the digestion products depending on the Ddw1 genotype (Table 1). Mnl1 has four restriction sites that are common for all types of REMS1218, and one additional site starting at relative position 33, which is unique to Type 1 (Figure 2). At this unique site, Mnl1 cleaves both sequences of Type 1 (Type 1a and Type 1b), thus the digestion of Type 1 at this site is complete, and releases the only 152 bp fragment, while all other REMS1218 PCR products release 178 bp fragment (Figure 3). Other fragments of the digestion of REMS1218 PCR products by Mnl1 are much shorter, and not well observed in agarose gel. Mnl1 digestion results in two products, 152 bp and 178 bp, which indicates either the presence of both Type 1 and 2 (shown in Figure 3) or both Type 1 and 3 (not shown in Figure 3) heterozygous genotypes (Table 1).

Table 1.
Ddw1 genotype and DNA fragment sizes (in bp) after digestion of REMS1218 PCR products by an excess of the restriction endonucleases Mnl1, RsaI, or Bso31I. The fragments used for genotyping (“diagnostic fragments”) are highlighted by bold font; the smaller fragments are not essential for genotyping since they are out of the detection range in the agarose gel electrophoresis. Abbreviation T1a, T1b, etc. are the same as in Figure 2.
RsaI has the only site at relative position 147 (Figure 2). It cleaves all REMS1218 types with the exception of Type 3, which remains partially uncut because one of the two Type 3 sequences is not recognized by RsaI (Type3a) (Figure 3). Thus, the 323 bp band after RsaI digestion indicates the presence of Type 3 at least in one allele of REMS1218.
Bso31I has the only site at relative position 219. At this position, Bso31I cleaves Type 1 completely, which results in 104 and 213 bp fragments, Type 2 is partially cleaved (Type 2a is not recognized by Bso31I), which results in 104, 213 and 321 bp fragments, and leaves Type 3 uncut 317 + 323 bp PCR products (Figure 2). Table 1 summarizes the outcomes of the digestion of REMS1218 PCR products of different genotypes by Mnl1, RsaI, and Bso31I.
The agarose gel electrophoresis of digested PCR products was consistent with the fragment sizes expected from the sequences. All allelic states of Ddw1 had unique combinations of the restriction fragments (see Discussion). Thus, the results obtained using the developed CAPS marker coincides with the results of capillary electrophoresis, and we concluded that these three restrictases are suitable for the CAPS marker for the determination of Ddw1 genotype.
3.4. Comparison of CAPS Marker with Capillary Electrophoresis Analysis of REMS1218 PCR Products on 91 F2 Crosses of Khongor and Dublet
To validate the CAPS marker, we analyzed a larger number of DNA samples for consistency between the results in our CAPS assay and the results obtained by fragment analysis. For this purpose, 91 DNA samples of F2 crosses of Khongor (Ddw1 Ddw1; Type 2 of REMS1218 PCR product) and Dublet (ddw1 ddw1; Type 1 of REMS1218 PCR product) were analyzed by capillary electrophoresis to determine the type of REMS1218 PCR product. Digestion by MnlI is enough for CAPS analysis of these products: the only 152 bp fragment indicates homozygous Type 1 (ddw1), the only 178 bp fragment indicates Type 2 (Ddw1), and both fragments indicate heterozygous Type 1 + Type 2 (Ddw1 ddw1) (Table 1). Aliquots of REMS1218 PCR products were cleaved by the restrictase MnlI, and the restriction fragments were separated using 2% agarose electrophoresis. The results of genotyping using CAPS analysis were identical to the fragment analysis using capillary electrophoresis.
3.5. Association of Plant Height and Ddw1 Genotype Determined by Ddw1 CAPS Marker in Two Segregating Populations Obtained from Four Parent Cultivars
We suggest that the Ddw1 CAPS marker described in this work could be used as a tool for MAS in breeding triticale and rye. To examine whether the marker actually reveals the association of Ddw1 genotype and plant height, we measured the height in two samples taken from two F2 populations with homozygous and heterozygous Ddw1.
The first 77-plant sample was taken from a A/SKh F2 population that was obtained from the cross Avangard (Ddw1 Ddw1 Rht-B1a Rht-B1a) × Solovei Kharkovskii (ddw1 ddw1 Rht-B1b Rht-B1b) (hereafter A/SKh). In this A/SKh population sample, no statistically significant contribution of the Rht1 gene to plant height was determined by two-way ANOVA (p = 0.26, F = 1.38, degree of freedom = 2). Unlike Rht1, Ddw1 significantly contributed to the plant height: p = 0.004 (F = 5.87, degree of freedom = 2). Bonferroni post-hoc analysis revealed statistically significant differences between the (Ddw1 Ddw1) and (ddw1 ddw1) groups (p = 0.008) (Table 2). Also, less strict Fisher LSD post-hoc analysis shows statistical significance between the (Ddw1 ddw1) and (ddw1 ddw1) groups (p = 0.03).

Table 2.
The plant height in two plants samples derived from F2 triticale populations, and its association with Ddw1 genotype. The height is presented as average ± std dev.
The second 91-plant sample was taken from Kh/D F2 population that was obtained from the cross Khongor (Ddw1 Ddw1) × Dublet (ddw1 ddw1). The association of Ddw1 with plant height in the Kh/D population sample was significant: p = 0.00006 (F = 10.81, degree of freedom = 2). Bonferroni post-hoc analysis revealed statistically significant differences between the (Ddw1 Ddw1) and (ddw1 ddw1) groups (p = 0.00004), and between the (Ddw1 ddw1) and (ddw1 ddw1) groups (p = 0.0014) (Table 2).
4. Discussion
The introduction of the dwarfing Ddw1 allele increases lodging resistance and productivity of triticale cultivars. MAS significantly facilitates the breeding process, however, there is currently no cheap and reliable marker for the allelic state of the Ddw1 gene. REMS1218 marker, which is tightly linked to Ddw1 was improved by its combination with REMS1218 SNP marker by Tenhola-Roininen and Tanhuanpa, but was still unable to differentiate Ddw1 homozygotes from Ddw1 ddw1 heterozygotes in some cases [13]. Recently, fine mapping of Ddw1 in rye has been reported [7], however, no practical method for Ddw1 genotyping was provided. In our study we used more types, varieties and a higher number of plants compared to Tenhola-Roininen and Tanhuanpa [13], and by using capillary electrophoresis we observed one more pattern of REMS1218 PCR product (Type 3 in the current paper). Types 1 and 2 in our amplifications were consistent with the PCR products observed by Tenhola-Roininen and Tanhuanpa. Despite the differences in PCR product profiles on capillary electrophoresis between the dwarfing Ddw1 allele and neutral ddw1 alleles, the REMS1218 PCR marker cannot distinguish Ddw1 ddw1 heterozygote from Ddw1 Ddw1 homozygotes. Indeed, the peak 317 bp is present in all tested genotypes, thus it cannot differentiate dominant Ddw1 from recessive ddw1. Ddw1-specific peak 321 bp appears in both homo- and heterozygous plants.
In our study, we cloned and sequenced REMS1218 PCR products, and revealed three restriction endonucleases, Mnl1, RsaI and Bso31I, that in combination are able to distinguish polymorphisms of REMS1218. All variants of restriction fragments of REMS1218 PCR products that are well-detected in 2% agarose gel are summarized in Table 1. The REMS1218 CAPS marker, which is based on Mnl1, RsaI and Bso31I restrictases, allows us to unequivocally identify the Ddw1 genotype. An example of the universal algorithm for Ddw1 genotyping of plants with unknown parental lines is shown in Figure 4. In the case of MAS for Ddw1, the type of ddw1 allele (Type 1 or Type 3 of REMS1218 PCR products) for some cultivars is known. In the current study, Type 1 allele was observed in spring triticale (cv. Dublet and cv. Solovei Kharkovskii), and Type 3 allele was observed in winter triticale (cv. Dozor). For cultivars with an unknown type of ddw1 allele, this can be quickly determined by capillary electrophoresis since it requires examination of just a few plants. Then, depending on the ddw1 allele in the parent line, just Mnl1 (in case of breeding with Type 1) or two restrictases, RsaI and Bso31I (in case of breeding with Type 3) is enough for CAPS genotyping. The one-step protocol for Ddw1 genotyping for MAS is shown in Table 3. It is worth noting, that ddw1 ddw1 plants are easily phenotypically determined by height. Thus, in MAS of Type 3 ddw1 lines, the identification of Ddw1 homozygotes and Ddw1 ddw1 heterozygotes is essentially enough for further breeding. In this case, the restrictase, RsaI, is sufficient for MAS of Type 3 plants.
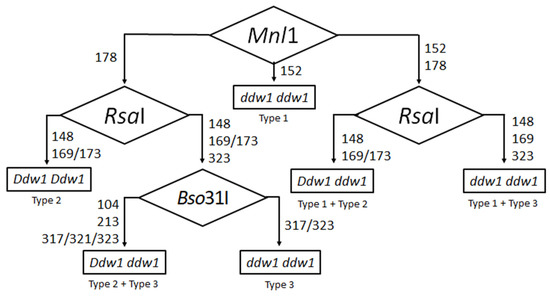
Figure 4.
REMS1218 CAPS analysis for Ddw1 genotyping of rye and triticale. Diamonds in the flowchart denote the digestion of the original REMS1218 PCR product by a restriction endonuclease; the numbers indicate the sizes of the fragments after the digestion in accordance with the rough estimate in Figure 3. In the case of plants that carry Type 1 ddw1 allele, restriction analysis by Mnl1 followed by RsaI distinguishes Ddw1, ddw1 homozygotes and Ddw1 ddw1 heterozygotes. If the analysis reveals a single product after Mnl1 digestion and three products after RsaI digestion, then Type 3 ddw1 allele is present, and additional digestion of REMS1218 PCR product by Bso31I is required to distinguish ddw1 homozygote and Ddw1 ddw1 heterozygote.

Table 3.
REMS1218 CAPS analysis for Ddw1 allele(s) for MAS. In some cases, RsaI is the only restriction enzyme required for MAS in breeding Type 3 lines (see discussion).
Genotyping by the Ddw1 CAPS marker was identical with genotyping by capillary electrophoresis, and low plant height was statistically significantly associated with homozygous dwarfing Ddw1 genotype in both model experiments, and with heterozygous Ddw1 ddw1 genotype in one of the experiments, perhaps because of the higher number of plants.
5. Conclusions
We developed a CAPS approach that enables determination of the allelic state of Ddw1 using common and cheap laboratory methods—PCR and digestion by restriction endonucleases followed by electrophoresis in 2% agarose gel. This approach overcomes the main drawback of REMS1218 microsatellite marker, that is, its dominant properties, and allows reliable identification of the dwarfing Ddw1 homozygotes even at early stages of plant growth. We suggest the use of this CAPS marker for MAS in breeding programs of Ddw1 plants.
Author Contributions
Conceptualization, M.G.D.; methodology, M.G.D.; validation, M.G.D., S.M.A.; formal analysis, D.Y.L. and M.S.B; investigation, A.G.C.; resources, M.G.D. and G.I.K.; writing—original draft preparation, D.Y.L.; writing—review and editing, P.Y.K., M.S.B., M.G.D.; visualization, D.Y.L., A.G.C. and P.Y.K.; supervision, M.G.D. and G.I.K.; project administration, M.G.D.; funding acquisition, M.G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 17-76-20023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ayalew, H.; Kumssa, T.T.; Butler, T.J.; Ma, X.-F. Triticale Improvement for Forage and Cover Crop Uses in the Southern Great Plains of the United States. Front. Plant Sci. 2018, 9, 1130. [Google Scholar] [CrossRef] [PubMed]
- Ammar, K.; Mergoum, M.; Rajaram, S. The history and evolution of triticale. In Triticale Improvement and Production. FAO Plant Production and Protection Paper No. 179; Mergoum, M., Gómez-Macpherson, H., Eds.; Food and Agriculture Organization of United Nations: Rome, Italy, 2004; pp. 1–9. [Google Scholar]
- Mergoum, M.; Pfeiffer, W.H.; Peña, R.J.; Ammar, K.; Rajaram, S. Triticale crop improvement: the CIMMYT programme. In Triticale Improvement and Production. FAO Plant Production and Protection Paper No. 179; Mergoum, M., Gómez-Macpherson, H., Eds.; Food and Agriculture Organization of United Nations: Rome, Italy, 2004; pp. 11–26. [Google Scholar]
- Van De Velde, K.; Ruelens, P.; Geuten, K.; Rohde, A.; Van Der Straeten, D. Exploiting DELLA Signaling in Cereals. Trends Plant Sci. 2017, 22, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Korshunova, A.D.; Divashuk, M.G.; Soloviev, A.; Karlov, G.I. Analysis of wheat and rye semidwarfing gene distribution in spring hexaploid triticale (Triticosecale Wittm.) cultivars and breeding lines. Russ. J. Genet. 2015, 51, 272–277. [Google Scholar] [CrossRef]
- Kobyliansky, V.D. On genetics of the dominant factor of short-strawed rye. Genetika 1972, 8, 12–17. [Google Scholar]
- Braun, E.-M.; Tsvetkova, N.; Rotter, B.; Siekmann, D.; Schwefel, K.; Krezdorn, N.; Plieske, J.; Winter, P.; Melz, G.; Voylokov, A.V.; et al. Gene Expression Profiling and Fine Mapping Identifies a Gibberellin 2-Oxidase Gene Co-segregating With the Dominant Dwarfing Gene Ddw1 in Rye (Secale Cereale L.). Front. Plant Sci. 2019, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Kroupin, P.Y.; Chernook, A.; Karlov, G.I.; Soloviev, A.; Divashuk, M.G. Effect of Dwarfing Gene Ddw1 on Height and Agronomic Traits in Spring Triticale in Greenhouse and Field Experiments in a Non-Black Earth Region of Russia. Plants 2019, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Kroupin, P.; Chernook, A.; Karlov, G.I.; Soloviev, A.; Korshunova, A.D.; Divashuk, M.G. Effects of Dwarfing Wheat (Triticum Aestivum L.) and rye (Secale Cereale L.) genes in spring triticale segregating population as studied in pot trials. Sel’skokhozyaistvennaya Boil. 2019, 54, 920–933. [Google Scholar] [CrossRef]
- Chernook, A.; Kroupin, P.Y.; Karlov, G.I.; Soloviev, A.; Korshunova, A.D.; Rubets, V.; Igonin, V.; Divashuk, M.G. Effects of Rht-B1b and Ddw1 Dwarfing Genes in Two Connecting Populations of Spring Triticale under Greenhouse Experiment Conditions. Agriculture 2019, 9, 119. [Google Scholar] [CrossRef]
- Khlestkina, E.; Than, M.H.M.; Pestsova, E.; Malyshev, S.V.; Korzun, V. Mapping of 99 new microsatellite-derived loci in rye (Secale Cereale L.) including 39 expressed sequence tags. Theor. Appl. Genet. 2004, 109, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Korzun, V.; Börner, A.; Melz, G. RFLP mapping of the dwarfing (Ddw1) and hairy peduncle (Hp) genes on chromosome 5 of rye (Secale Cereale L.). Theor. Appl. Genet. 1996, 92, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Tenhola-Roininen, T.; Tanhuanpää, P. Tagging the dwarfing gene Ddw1 in a rye population derived from doubled haploid parents. Euphytica 2009, 172, 303–312. [Google Scholar] [CrossRef]
- Murray, M.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, K.B.; Nicholas, H.B., Jr.; Deerfield, D.W., II. GeneDoc: Analysis and visualization of genetic variation. EMB Net. News 1997, 4, 1–4. [Google Scholar]
- Ellis, M.H.; Spielmeyer, W.; Gale, K.; Rebetzke, G.J.; Richards, R.A. “Perfect” markers for the Rht-B1b and Rht-D1b dwarfing genes in wheat. Theor. Appl. Genet. 2002, 105, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).