Abstract
The treatment for rhegmatogenous retinal detachment (RRD) is surgery, including pars plana vitrectomy (PPV) and scleral buckling (SB). Despite surgical advances, degeneration of the photoreceptors and post-operative complications, such as proliferative vitreoretinopathy (PVR), often occurs as the result of inflammation, preventing complete visual recovery or causing RRD recurrence. There is increasing evidence that in the presence of RRD, the activation of inflammatory processes occurs and the surgery itself induces an inflammatory response. This comprehensive review focuses on the use of different formulations of corticosteroids (CCS), as an adjunctive treatment to surgery, either PPV or SB, for RRD repair. The purpose was to review the efficacy and safety of CCS in improving functional and anatomical outcomes and in preventing postoperative complications. This review is organized according to the timing of CCS administration: preoperative, intraoperative, and postoperative. The evidence reviewed supported the role of the pre-operative use of CCS in the treatment of combined RRD and choroidal detachment (CD), reducing CD height. No solid consensus exists on intraoperative and postoperative use of CCS to treat and prevent postoperative complications. However, a large randomized clinical trial including more than 200 eyes suggested that oral prednisone after surgery decreases the rate of postoperative grade B PVR.
1. Introduction
Rhegmatogenous retinal detachment (RRD) is a common retinal disease with an incidence of one in 10,000 people per year [1] that often causes visual field defects and moderate to severe visual impairment. Surgery is the only therapeutic approach and the surgical techniques and instrumentation developed over the last decades have led to a very high primary reattachment rate, which is around 95%, according to various studies [2]. However, despite primary anatomic success, degeneration of the photoreceptors and post-operative complications, such as proliferative vitreoretinopathy (PVR) often occur as a result of inflammation, preventing complete functional recovery or causing RRD recurrence [3,4].
There is increasing evidence that in the presence of RRD, the activation of inflammatory processes occurs, in particular when it is associated with choroidal detachment [5,6]. The surgeries themselves, both scleral buckle (SB) and pars plana vitrectomy (PPV), induces an inflammatory response as demonstrated by an increase of aqueous flare values, from day one up to three months post-operatively with the peak value observed at post-operative day seven [7].
Corticosteroid (CCS) drugs are able to modulate inflammation by binding intracellular receptors and regulating cytokine synthesis [8]. Recently, the use of CCS in addition to surgery, either SB or PPV, has been introduced in the management of RRD to control inflammation, improving reattachment rates and visual recovery, and reducing the incidence of PVR [9]. Several studies have already investigated the efficacy of CCS prior, during, and after surgery for RRD repair, administered in different formulations: topical, subconjunctival, subtenon (ST), intravitreal (IVT) and systemic; however, there is no agreement about the most efficient formulation with the least side effects.
Ando et al. [10] in an experimental study, observed, after injection of 1 mg of intravitreal triamcinolone (TA), a reduction of both blood–retinal barrier (BRB) breakdown and incidence of tractional retinal detachment due to PVR development. Moreover, Bali et al. [11], in a prospective randomized clinical study, reported that preoperative subconjunctival injection of dexamethasone (DEX) significantly decreased postoperative laser flare in RRD eyes treated by SB.
Weijtens et al. [12,13,14,15] reported that the concentration of DEX disodium phosphate in both subretinal fluid and the vitreous of eyes affected by RRD was higher after a subconjunctival injection compared to a peribulbar injection or an oral administration [15]. Shen et al. [16], in a clinical study, and Kovacs at al. [17], in a theoretical model, showed an efficient delivery of Triamcinolone (TA) after an ST injection, measured either in the vitreous chamber or in the choroidal extracellular matrix, despite low serum levels of TA, which seemed to not alter patients’ metabolic balance.
However, other authors [18] observed a significantly higher vitreous concentration of TA after IVT than ST injection, beyond a greater effect on the reduction of the BRB breakdown.
Moreover, CCS use could cause systemic and ocular adverse events (AEs). In particular, systemic AEs include increased risk of serious infections, osteoporosis, Cushing’s syndrome, and insomnia strictly related to high dosage (linear dose-related pattern), depression and increased blood pressure (threshold dose-related pattern, with a high rate of AEs beyond a dose of 7.5 mg/day) [19]. A higher glucose level is another side effect of particular concern, especially in diabetic patients [20]. Ocular complications of corticosteroid use have been well reported after intravenous, oral, topical (ocular and cutaneous), and injected (sub-conjunctival, subtenon, and periocular) corticosteroids. In particular, oral corticosteroid use can cause glaucoma and cataract development with a threshold dose-related pattern (a mean value dosage of over 7.5 mg/day and 5 mg/day, respectively) [21]. The risk of cataract is reported either after intravitreal TA [22] or subtenon injection of TA [23] (up to 15–20%, and up to 2.1%, respectively). An increase in IOP is reported from 18% to 36% of the general population after topical CCS use, although a potentially damaging rise in IOP affects from 5 to 6% of the general population vs. 46% to 92% of patients with primary open-angle glaucoma [21]. Secondary ocular hypertension was also reported after subtenon and intravitreal TA injection (4.7% and up to 40%, respectively) [22,23,24,25] and DEX implant use (up 13%) [26].
The aim of this study was to systematically review the published literature on the use of different formulations of CCS as a therapeutic option in addition to surgery (prior, during, and after surgery), PPV and/or SB, for RRD repair, to investigate the efficacy and the safety of CCS in improving functional and anatomical outcomes and in preventing postoperative complications.
2. Methods
2.1. Search Methods for Identification of Studies
Literature from the PubMed search engine was analyzed to find current evidence on CSS use in addition to surgery for the management of RRD with or without choroidal detachment; papers published from 2005 up to December 2019 were analyzed. The search strategy used the following keywords and Mesh terms: “periocular, subconjunctival, subtenon, and intravitreal injection; systemic and topic administrations, preoperative, intraoperative, postoperative use of corticosteroids/dexamethasone/triamcinolone acetonide; fluocinolone acetonide; rhegmatogenous retinal detachment/choroidal detachment; SB; PPV; and inflammation”.
2.2. Eligibility Criteria
Inclusion criteria were English language and the use of CSS as a therapeutic option in addition to surgery for the treatment of RRD. Articles in which steroids were used as a dye for vitreous, posterior hyaloid or internal limiting membrane, were excluded, as well as articles concerning the use of CCS in the management of postoperative complications such as cystoid macular edema. The reference lists of the analyzed articles were also considered as a potential source of information.
2.3. Data Collection
The present review was structured in three sections based on the timing of CCS use in addition to surgery: preoperative, intraoperative, and postoperative.
The following mean characteristics were analyzed for each article:
- (1)
- Study design: retrospective, prospective, comparative and non-comparative, randomized and non-randomized, single-center and multicenter and case report
- (2)
- Clinical outcomes: anatomical and functional
- (3)
- 3Number of eyes studied
- (4)
- Primary treatment
- (5)
- Follow-up (duration of the study)
- (6)
- Main results
- (7)
- Side effects
Two investigators (VB and EO) independently assessed the articles for compliance with the inclusion criteria concerning the selection of the papers to be analyzed and resolved disagreements through consensus.
3. Results
A total of 245 articles were identified through database searching. After the exclusion of studies on the basis of irrelevant titles and abstracts (such as articles not written in English; studies on assessment of molecular or biomarker profiling, and on the estimation of body and ocular pharmacological concentration of CCS after their administration; evaluation of alternative pharmacological approaches to CCS in RRD surgery) or failure to meet inclusion criteria, 23 studies were assessed as eligible and included in our review (Figure 1).
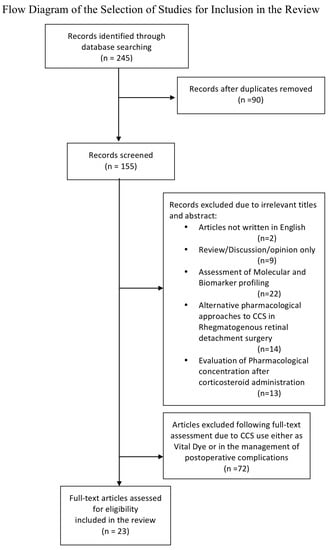
Figure 1.
Study selection process.
3.1. Preoperative Use
Six studies regarding the preoperative use of CCS were identified by our literature review. Their main characteristics and results are shown in Table 1. Four had a prospective design but only two of these were randomized; the other three of the seven studies were retrospective [27,28,29,30,31,32]. Our systematic review found that CCS are widely used preoperatively in addition to surgery to treat patients affected by RRD associated with choroidal detachment (CD) [27,28,29,30,31,32]. No articles concerning the use of preoperative CCS in RRD without CD surgery were found. Associations of retinal and choroidal detachment (RRDCD) were reported in 2%–4.5% of cases [33]. RRDCD was first, described in 1974 and is characterized by the detachment of the choroid and, often, of the ciliary body too [33].

Table 1.
Preoperative use of corticosteroid drugs in retinal detachment surgery.
RRDCD is an uncommon type of retinal detachment, characterized by rapid progression, poor functional and anatomic prognosis due to the more difficult visualization of the ocular fundus and retinal breaks as well as the high rate of postoperative PVR development. [34,35,36] The reattachment rate of RRDCD treated by SB (35.5%–52.4%) was lower compared to that of RRD without CD, according to Seelenfreund et al. [27] After the introduction of primary PPV, the reattachment rate of RRDCD improved by up to 90% [34,35,36].
Risk factors for the development of choroidal detachment are aphakia, pseudophakia, age > 50 years, low IOP, multiple and/or giant retinal breaks, especially when located posteriorly, high myopia, total retinal detachment, and macular hole [37].
The pathogenesis of RRDCD is unclear: vascular and inflammatory processes have been hypothesized. Regarding the vascular pathogenesis, the hypotony induced by the RD has been assumed to lead to CD by stimulating dilatation and hyper-permeability of choroidal blood vessels [38]. In addition, edema of the ciliary body itself could further reduce the production of aqueous humor with a positive feedback loop and consequently more hypotony.
Other authors [6] have shown inflammatory processes playing an important role in the development of CD associated with RRD and hypothesized that a severe uveitic process, secondary to RD, could occur and lead to the exudation of choroidal blood vessels, leakage of fluid and subsequent choroidal detachment with secondary hypotony, creating a vicious circle. This theory found support in the overexpression of inflammatory cytokines and proteins such as migration inhibitor factor (MIF) and soluble intercellular adhesion molecule1 (sICAM-1) observed by Dai et al. [39] in RRDCD eyes, compared with those affected by RRD without CD.
According to these pathogenic theories, the pre-operative use of CCS could play an important role in preparing patients affected by RRDCD for surgery, in order to increase IOP and reduce CD by reducing the permeability of choroidal blood vessels, inhibiting inflammatory reactions and cellular proliferation, and stabilizing BRB [27,28,29,30,31,32].
3.2. Intraoperative Use
From our systematic review of the literature, we identified 11 studies [40,41,42,43,44,45,46,47,48,49,50] investigating the effects of steroids as an intraoperative adjuvant in RRD surgery. Of the eleven studies, seven had a prospective design but only five were randomized; three studies were retrospective and one study was a case report.
Overall, in all of these studies, the main reason for the intraoperative use of CCS in combination with PPV was either the treatment of PVR, [41,42,44,48] when associated with RRD, or its prevention, in cases of RRD with a high risk of PVR development [45], and the prevention of postoperative complications such as macular pucker [43], persistent subretinal fluid (SRF) [46,48] and cystoid macular edema [46,48]. PVR is the main cause of surgery failure for RRD repair and its incidence is between 5-11% [51]. It can occur in cases of untreated RRD as well as after any retinal surgical procedure such as laser, criopexy, SB, and PPV [51,52,53]. Although the pathogenesis of PVR is complex and still remains partially unclear, the role of inflammation in the pathogenesis of PVR has been widely accepted and three overlapping biological processes have been identified as major triggers. These are: 1) Retinal pigment epithelial (RPE) and glial cellular migration (into the vitreal cavity and onto the retinal surface, respectively); 2) cellular proliferation (extravasations of blood components such as fibrin, elastin, fibronectin, growth factors, and cytokines secondary to blood–retinal barrier breakdown); and 3) cellular contraction (due to the deposition of the extracellular matrix and collagen synthesis). The final result is the formation of fibrocellular membranes located on the retinal surface and/or on the posterior hyaloid [54,55].
During RRD, the exposure of RPE cells to inflammatory cytokines and growth factors produces several effects. Following retinal detachment, RPE cells lose their polarity and undergo an epithelial-mesenchymal transformation stimulated by transforming growth factor beta (TGF- β). Platelet-derived growth factor (PDGF) stimulates proliferation and is also a chemoattractant for glial cells. Connective tissue factor (CTS-F) promotes cellular migration and proliferation and stimulates the production of extracellular matrix with the consequent formation of membranes. Blood retinal barrier breakdown causes serum growth factor and cytokines to be released into the vitreous, including vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), pigment epithelium-derived factor (PEDF), Platelet-Derived Growth Factor (PDGF), transforming growth factor β (TGFβ), tumor necrosis factor α (TNFα), Fibroblast growth factors (FGF), basic fibroblast growth factor (bFGF), insulin, insulin-like growth factor-1 (IGF-1), interleukin 1 (IL-1), IL-6, IL-8, IL-10, interferon γ (IFNγ), monocyte chemotactic protein, macrophage-colony stimulating factor, granulocyte colony-stimulating factor (G- CSF), chemokine ligand 2 (CCL2), CCL3, CCL4, CCL5, and protein [54,55]. All these factors stimulate cellular migration, cellular proliferation, deposition of cellular matrix, and creation of contractile membranes (growth factor and cytokines hypothesis) [55].
The role of macrophages has been recently highlighted. They have a multifactorial action that includes the secretion of growth factors such as PDGF and their differentiation into fibroblast-like cells (macrophage hypothesis) [55].
Several drugs have been proposed as an adjunctive treatment for preventing postoperative PVR. These include 5-Fluorouracil (5-FU) Low-Molecular-Weight Heparin (LMWH), Daunorubicin, 13-Cis-Retinoic Acid, Cyclin-Dependent Kinases, and Corticosteroids [55].
Corticosteroid treatment can modulate both the inflammatory and proliferative pathways of PVR by stabilizing the blood–retinal barrier and suppressing local growth factors and inflammatory cytokines. This could finally lead to the inhibition of the proliferation of RPE cells, fibroblasts, and myofibroblasts, [55,56].
TA, DEX, and fluocinolone acetonide (FA) implants are the only three CCS approved treatments for intravitreal use. These molecules differ from each other based on their pharmacokinetic and pharmacodynamic properties, with consequently different biological effectiveness, such as different glucocorticoid binding affinity (DEX > FA > TA) and different anti-inflammatory activities (DEX = FA and 5 times more active than TA) [57]. The advantage of a DEX implant is the slow release of active dexamethasone within the vitreous chamber for up to six months, with a single injection, and with a similar clearance in vitrectomized and non-vitrectomized eyes [58]. However, 4 mg TA intravitreal injection has been reported to have an effect that lasts up to three months [59] with a six times quicker clearance in vitrectomized than in non vitrectomized eyes [60].
The FA implant showed similar efficacy in the treatment of chronic diabetic macular edema in both vitrectomized and not vitrectomized eyes [61]. It has also been reported to have a longer action compared to the DEX implant in vitrectomized eyes [62]. Among the 11 studies reviewed, we identified 6 studies investigating the use of intravitreal triamcinolone (IVTA) and 3 studies assessing the use of the DEX implant in addition to surgery to treat RRD. Moreover, we found one study that investigated the use of intravenous dexamethasone during surgery. No studies regarding the intraoperative use of the FA implant were found. The main characteristics and results of these studies are shown in Table 2.

Table 2.
Intraoperative use of corticosteroid drugs in retinal detachment surgery.
Previous experimental and clinical research demonstrated that IVTA was not significantly retinotoxic when used during vitrectomy without silicone oil (SO) endotamponade [63,64,65,66]. On the other hand, toxicity is still controversial when IVTA (in different doses: 2, 4, 10, and 20 mg) is used during vitrectomy with SO endotamponade [67,68,69]. Kivilcim et al. [67] reported no toxic effects when TA was injected into silicone-filled eyes with a dose up to 5 mg. However, Perkins et al. [68] and Jonas J B [69] suggested that SO might increase TA absorption, prolonging its permanence in the vitreous cavity. Moreover, Spritzer [70], in an experimental model, observed that TA injected into the vitreous cavity filled with SO precipitated at the lower border of the endotamponade bubble, without mixing with it and causing a possible cytotoxic effect on the retina.
In conclusion, it is still unclear which is the best timing for TA injection: soon before silicone oil injection [40] or at the end of the operation into the silicone oil bubble filling the vitreous chamber [41].
Fewer data exist about a DEX implant into silicone oil-filled eyes. One author, using an in vitro model, showed that when DEX is injected into vitrectomized silicone oil-filled eyes, SO modified and increased DEX release over a one-year period [71]. To date, in vivo information is limited to a few case reports and only one recent randomized clinical trial (RCT), with the latter showing results that a DEX implant is generally well tolerated. On the other hand, Bakri and Alniemi [72] reported epiretinal fibrosis development around the implant at the 6th postoperative week, leading to recurrent retinal detachment that needed vitrectomy, removal of the implant, and peeling of epiretinal proliferation.
3.3. Postoperative Use
Five studies assessing the postoperative use of CCS (oral or topical) were identified by our literature review. [7,73,74,75,76] All of them had a prospective design but only two of these were randomized. Their main characteristics and results are shown in Table 3.

Table 3.
Postoperative use of corticosteroids in retinal detachment surgery.
The postoperative use of CCS was suggested to modulate postoperative pain and inflammation in order to obtain better compliance in terms of patient positioning and postoperative examinations [7]. Moreover, postoperative and intraoperative CCS use could improve surgical results decreasing postoperative complications including PVR, [73,75] macular pucker, [73,75] macular edema [73] and persistent subretinal fluid [74]. Persistent SRF occurs in 27–100% of cases, even four to six weeks after the operation, and is associated with poor vision [77,78].
Several factors have been postulated to contribute to the pathogenesis of persistent SRF, such as the height and extent of retinal detachment, clock hours of buckle placement, gender, age, refractive status, and SRF drainage. However, little evidence of any association has been shown between these factors and the presence of persistent SRF, except for the extent of retinal detachment [79]. According to previous reports, persistent macular SRF often lasts a long time before it is completely reabsorbed. Median duration of persistent SRF ranges from 5 to 10 months, and most SRF disappears within one year after surgery [79,80]. Previous studies found that the incidence of residual SRF was reduced by anti-inflammatory agents such as steroid and traditional non-steroid anti-inflammatory drugs showing that inflammation could play a part in the pathogenesis of persistent SRF. These drugs reduce the blood–retinal barrier breakdown that might be one of the possible mechanisms of persistent SRF after retinal detachment surgery [74,80].
4. Conclusions
In this systematic review, we examined the use of CCS in different formulations (topical, subconjunctival, ST, IVT, and systemic) in addition to surgery for RRD treatment using either SB or PPV.
The evidence suggested that preoperative CCS use could play a role only when RRD is associated with choroidal detachment thanks to its efficacy in decreasing the maximum CD height [29,30,31] and in reducing the complexity of surgery and potential complications related to CD. Dewattana et al. [31] reported a highly significant preoperative improvement (partial or complete) of CD in eyes treated with preoperative CCS (oral prednisolone or subtenon injection of triamcinolone), for a median of 7 days before surgery, compared with eyes that did not receive any preoperative CCS treatment. (30% vs. 82%; p > 0.001). However, preoperative CCS use did not provide better postoperative visual and anatomical outcomes [27,28,29,31].
On the contrary, Sharma et al. [27], in their prospective randomized study, reported better functional results in eyes treated immediately by PPV without CCS administration with respect to a group treated with oral prednisolone (1 mg/kg body weight) for 7 days prior to PPV, (improvement of visual acuity of two lines or better was 89% vs. 73%, respectively). The authors reported that preoperative CCS use could delay surgery (7.5 days vs. 3 days, in the steroid-group and no-steroid group, respectively; p < 0.001), increasing the risk of photoreceptor degeneration and apoptosis. Likewise, persistence of CD in 2 of 11 eyes was reported [27], despite preoperative CCS use, and no differences in terms of postoperative complications, including PVR, between the two groups were registered.
Taking all this into account, preoperative CCS administration was considered to promote preoperative resolution of CD, a single subtenon injection of TA [29] was more effective in CD improvement than endovenous CCS administration, with the advantage of inducing significantly lower blood sugar levels and lower suppression of plasmatic cortisol levels [29].
The most common reported adverse event after either preoperative periocular or subtenon injection of TA [29] was an elevated IOP that, however, was successfully treated by IOP-lowering medication, with no glaucoma surgery required in most cases. Cataract progression was also reported by Wei Y et al. [28] after both oral prednisolone administration (16.1%) and 40 mg methyl-prednisolone periocular injection (21.7%), given 3 to 7 days before surgery, however, it was very difficult to establish the role of vitrectomy, silicone oil tamponade and CCS in cataract progression.
Intraoperative intravitreal use of TA and DEX implant in addition to PPV were also investigated. In the literature, the aim of their use in eyes affected by RRD, was either the treatment of PVR itself [41,42,44,48] or the prevention of postoperative complications such as PVR [45], macular pucker, [44] persistent SRF and cystoid macular edema [46,48]. However, the evidence is limited and the results are still inconsistent. Comparative prospective controlled clinical trials [42,43] did not report any advantages in terms of both visual and anatomical outcomes or in prevention of macular pucker development and recurrent PVR rate in eyes treated by PPV and intraoperative IVTA injection [42,43]. In addition, an increased risk of IOP elevation was reported [18,42,43,46].
Mirshahi A et al. [46] in a prospective consecutive comparative study, investigating the effect of IVTA on the resolution of subretinal fluid after SB surgery, observed that a single IVTA injection increased the final BCVA in macula-off RRD patients despite persistent SRF (34% of SRF in IVTA eyes vs. 45% in placebo eyes), suggesting that anti-inflammatory drugs could play a role in the good results of SB surgery.
Banerjee PJ et al. [48], in a prospective randomized clinical trial, including a large sample of 140 eyes treated for RRD by PPV with silicone oil and intraoperative DEX implant, did not observe any benefits compared with the control group at a six-month follow-up, neither in terms of retinal reattachment stability, after SO removal, nor in final VA or PVR recurrence rate and quality of life. However, the authors reported a lower postoperative macular edema rate in the DEX implant group than the control group (42.7% vs. 67.2%) and a higher number of AEs in the control group, suggesting a beneficial effect of additional anti-inflammatory activity of the DEX implant and hypothesized that neural retinal remodeling, secondary to RD, could be the primary cause of the poor postoperative visual outcomes despite less postoperative macular edema. However, there were more episodes of intraocular high pressure in the DEX implant group than the control group, even if there was no difference in terms of diagnosis of glaucoma between the two groups.
Recently, Reibaldi et al. [50] suggested that combined intravenous therapy with dexamethasone and ondansetron, administered intraoperatively, could reduce the incidence and severity of suprachoroidal hemorrhage and intraocular bleeding that are well-known postoperative complications that could worsen anatomical and functional outcomes.
We also reviewed the use of postoperative CCS use in the treatment of RRD. In the literature, it has been reported that the use of systemic postoperative CCS could reduce inflammation and counteract the increased levels of inflammatory cytokines and VEGF associated with a breakdown of the blood–retina barrier responsible for postoperative PVR development.
In clinical practice, the efficacy of the postoperative use of systemic CCS after SB for RRD was tested in two randomized trials reporting controversial findings. Dehghan et al. [73] did not observe any visual or anatomical improvement or reduction in postoperative complications (CME, PVR, and CD) using oral prednisolone 1 mg/kg for 10 days after surgery.
On the contrary, Koener et al. [75] in 220 eyes, reported that oral prednisone at the initial dosage of 100 mg for six days, thereafter tapered to 50 mg for five days, and 12.5 mg for another four days, was effective in reducing the incidence of postoperative complications such as PVR stage B (26.7%, 23.6%, and 19.8% in the steroid group and 41.8%, 46.9%, and 39.1% in the placebo group, respectively, at one, three, and six months postoperatively).
Similarly, Wu et al. [74] reported the effectiveness of oral prednisolone for 3 days after SB, in significantly reducing SRF with respect to control eyes (56.6% vs. 80.0%). Additionally, the authors observed in patients with persistent SRF, a shorter period for fluid reabsorption in the CCS group rather than the control group (218.1 6 122.1 days vs. 286.5 6 141.0 days; p = 0.039).
The postoperative use of topical steroids could be used to reduce postoperative pain and flare, however, topical diclofenac sodium seems to be as potent as topical CCS in managing postoperative inflammation, either after PPV or SB, with better analgesic effects and lower intraocular hypertension rata [7,76].
The main limitation of this review was the heterogeneity of clinical variables among the included studies. Reviewed studies could have been widely different with regards to the specific characteristics of the detachment (advanced PVR, state of the macula, trauma, myopia, pseudophakic and recurrent RRD) as well as the patient’s general conditions (including underlying systemic or ocular inflammatory disease, diabetes mellitus). These different clinical characteristics could have had an influence on the baseline inflammatory state and the outcomes specifically related to steroid administration. Therefore, when the findings of the included studies were analyzed all together, the significance of steroid administration for any given primary pathology could have been partially masked by these confounding factors.
5. Recommendation
In conclusion, the available published evidence showed that the pre-operative use of CCS has a role only in the presence of combined rhegmatogenous retinal and choroidal detachment: it reduces CD height, even if no anatomical and functional gains were reported. Besides, its use could delay the operation, which, in turn, can lead to photoreceptor loss and reduced visual outcome. The intraoperative use of intravitreal TA or DEX implant either with or without silicone oil mainly aims to prevent postoperative PVR, but the results are still controversial, and no consensus exists for their use. Likewise, no solid consensus exists on the effectiveness of the systemic postoperative use of CCS with the purpose of reducing postoperative complications such as PVR, pucker, cystoid macula edema, and persistent SRF. However, a large RCT including more than 200 eyes suggested that oral prednisone after SB surgery decreased the rate of postoperative PVR grade B. Additionally, postoperative oral prednisolone could reduce the incidence of SRF after SB. Regarding topical postoperative CCS, topical diclofenac seems to be as potent as topical dexamethasone in managing postoperative inflammatory response induced by surgery for RRD with a better analgesic effect and more safety.
Author Contributions
Conceptualization, V.B. and E.O.; methodology, V.B., M.F., E.O., C.P. (Corrado Pizzo), A.P., and A.L.; investigation, V.B., E.O., M.R. (Michele Rinaldi); data curation M.R. (Michele Reibaldi), M.D.T., V.B., E.O.; writing—original draft preparation, R.R., K.N., A.R., I.M., S.C., G.C., M.V., and V.B.; writing—review and editing, all authors; supervision, C.P. (Clara Patane), T.A., V.B., E.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We wish to thank the Scientific Bureau of the University of Catania for language support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liao, L.; Zhu, X.H. Advances in the treatment of rhegmatogenous retinal detachment. Int. J. Ophthalmol. 2019, 12, 660–667. [Google Scholar] [PubMed]
- Sodhi, A.; Leung, L.S.; Do, D.V.; Gower, E.W.; Schein, O.D.; Handa, J.T. Recent trends in the management of rhegmatogenous retinal detachment. Surv. Ophthalmol. 2008, 53, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Kunikata, H.; Yasuda, M.; Aizawa, N.; Tanaka, Y.; Abe, T.; Nakazawa, T. Intraocular concentrations of cytokines and chemokines in rhegmatogenous retinal detachment and the effect of intravitreal triamcinolone acetonide. Am. J. Ophthalmol. 2013, 155, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.W.; Song, J.H.; Roh, M.I. Retinal detachment and proliferative vitreoretinopathy. Dev. Ophthalmol. 2016, 55, 154–162. [Google Scholar] [PubMed]
- Josifovska, N.; Lumi, X.; Szatmari-Tóth, M.; Kristóf, E.; Russell, G.; Nagymihály, R.; Anisimova, N. Clinical and molecular markers in retinal detachment from hyperreflective points to stem cells and inflammation. PLoS ONE 2019, 14, e0217548. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, W.H. Rhegmatogenous retinal detachment complicated by severe intraocular inflammation, hypotony, and choroidal detachment. Trans. Am. Ophthalmol. Soc. 1981, 79, 664–683. [Google Scholar]
- Ben, Y.S.; Kahloun, R.; Abroug, N.; Kalibi, I.; Laadhari, G.; Ielliti, B.; Khairallah, M. Comparative effect of topical diclofenac and topical dexamethasone on anterior chamber flare and postoperative pain following rhegmatogenous retinal detachment surgery. Int. Ophthalmol. 2016, 36, 623–628. [Google Scholar] [CrossRef]
- Barnes, P.J. Anti inflammatory mechanism of glucorticoids. Biochem. Soc. Trans. 1995, 23, 940–945. [Google Scholar] [CrossRef]
- Shi, H.; Guo, T.; Liu, P.C.; Wang, Q.Y.; Du, Y.R.; Liu, Q.Y.; He, M.M.; Liu, J.L.; Yu, J. Steroids as an adjunct for reducing the incidence of proliferative vitreoretinopathy after rhegmatogenous retinal detachment surgery: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2015, 9, 1393–1400. [Google Scholar]
- Ando, N.; Sen, H.A.; Berkowitz, B.A.; Wilson, C.A.; De Juan, E., Jr. Localization and quantitation of blood-retinal barrier breakdown in experimental proliferative vitreoretinopathy. Arch. Ophthalmol. 1994, 112, 117–122. [Google Scholar] [CrossRef]
- Bali, E.; Feron, E.; Peperkamp, E.; Veeckeneer Mulder, P.; Van Meurs, J. The effect of a preoperative subconjunctival injection of dexamethasone on blood- retinal barrier breakdown following scleral buckling retinal detachment surgery: A prospective randomized placebo-controlled double blind clinical trial. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Weijtens, O.; Schoemaker, R.C.; Lentjes, E.G.; Romijn, F.P.; Cohen, A.F.; Van Meurs, J.C. Dexamethasone concentration in the subretinal fluid after a subconjunctival injection, a peribulbar Injection or an oral dose. Ophthalmology 2000, 107, 1932–1938. [Google Scholar] [CrossRef]
- Weijtens, O.; Van der Sluijs, F.A.; Schoemaker, R.C.; Lentjes, E.G.; Cohen, A.F.; Romijn, F.P.; Van Meurs, J.C. Peribulbar corticosteroid injection:vitreal and serum concentrations after dexamethasone disodium phosphate injection. Am. J. Ophthalmol. 1997, 123, 358–363. [Google Scholar] [CrossRef]
- Weijtens, O.; Schoemaker, R.C.; Cohen, A.F.; Romijn, F.P.; Lentjes, E.G.; Van Rooij, J.; Van Meurs, J.C. Dexamethasone concentration in vitreous and serum after oral administration. Am. J. Ophthalmol. 1998, 125, 673–679. [Google Scholar] [CrossRef]
- Weijtens, O.; Feron, E.J.; Schoemaker, R.C.; Cohen, A.F.; Lentjes, E.G.; Romijn, F.P.; Van Meurs, J.C. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am. J. Ophthalmol. 1999, 128, 192–197. [Google Scholar] [CrossRef]
- Shen, L.; You, Y.; Sun, S.; Chen, Y.; Qu, J.; Cheng, L. Intraocular and systemic pharmacokinetics of triamcinolone acetonide after a single 40-mg posterior subtenon application. Ophthalmology 2010, 117, 2365–2371. [Google Scholar] [CrossRef]
- Kovacs, K.; Wagley, S.; Quirk, M.T.; Ceron, O.M.; Silva, P.A.; Singh, R.J.; Gukasyan, H.J.; Arroyo, J.G. Pharmacokinetic Study of Vitreous and Serum Concentrations of Triamcinolone Acetonide after Posterior Sub-Tenon’s Injection. Am. J. Ophthalmol. 2012, 53, 939–948. [Google Scholar] [CrossRef]
- Inoue, M.; Takeda, K.; Morita, K.; Yamada, M.; Tanigawara, Y.; Oguchi, Y. Vitreous concentrations of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am. J. Ophthalmol. 2004, 138, 1046–1048. [Google Scholar] [CrossRef]
- Huscher, D.; Thiele, K.; Gromnica-Ihle, E.; Hein, G.; Demary, W.; Dreher, R.; Zink, A.; Buttgereit, F. Dose-related patterns of glucocorticoid-induced side effects. Ann. Rheum. Dis. 2009, 68, 1119–1124. [Google Scholar] [CrossRef]
- Elena, C.; Chiara, M.; Angelica, B.; Chiara, M.A.; Laura, N.; Chiara, C.; Claudio, C.; Antonella, F.; Nicola, G. Hyperglycemia and Diabetes Induced by Glucocorticoids in Nondiabetic and Diabetic Patients: Revision of Literature and Personal Considerations. Curr. Pharm. Biotechnol. 2018, 19, 1210–1220. [Google Scholar] [CrossRef]
- Carnahan, M.C.; Goldstein, D.A. Ocular Complications of Topical, Peri-Ocular, and Systemic Corticosteroids. Curr. Opin. Ophthalmol. 2000, 11, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Veritti, D.; Di Giulio, A.; Sarao, V.; Lanzetta, P. Drug safety evaluation of intravitreal triamcinolone acetonide. Expert Opin. Drug Saf. 2012, 11, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Hirano, Y.; Nozaki, M.; Yoshida, M.; Ogura, Y. Incidence of posterior subcapsular cataract progression after triamcinolone acetonide administration. Nippon Ganka Gakkai Zasshi 2008, 112, 786–789. [Google Scholar]
- Reibaldi, M.; Avitabile, T.; Russo, A.; Bonfiglio, V.; Mariotti, C.; Romano, M.R.; Boscia, F.; Cennamo, G.; Fallico, M.; Parisi, G.; et al. Late onset ocular hypertension after vitrectomy: A multicenter study of 6048 eyes. Retina 2019, 39, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Ishikawa, H.; Nishikawa, H.; Shimizu, M.; Kinoshita, T.; Ogihara, R.; Kitano, S.; Yamanaka, C.; Mitamura, Y.; Sugimoto, M.; et al. Intraocular pressure elevation after subtenon triamcinolone acetonide injection; Multicentre retrospective cohort study in Japan. PLoS ONE 2019, 14, e0226118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kuppermann, B.D.; Bandello, F.; Lanzetta, P.; Zur, D.; Park, S.W.; Yu, H.G. Intraocular Pressure (IOP) After Intravitreal Dexamethasone Implant (Ozurdex) Amongst Different Geographic populations-GEODEX-IOP Study. Eye 2019, 30, 1–6. [Google Scholar] [CrossRef]
- Sharma, T.; Gopal, L.; Reddy, R.K.; Kasinathan, N.; Shah, N.A.; Sulochana, K.N.; Miriam, K.C.; Arvind, K.; Ramakrishnan, S.; Sukumar, B. Primary vitrectomy for combined rhegmatogenous retinal detachment and choroidal detachment with or without oral corticosteroids: A pilot study. Retina 2005, 25, 152–157. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, N.; Chen, F.; Wang, H.; Bi, C.; Zu, Z.; Yang, X. Vitrectomy combined with periocular/intravitreal injection of steroids for rhegmatogenous retinal detachment associated with choroidal detachment. Retina 2014, 34, 136–141. [Google Scholar] [CrossRef]
- Shen, L.J.; Mao, J.B.; Sun, S.M.; Dong, Y.G.; Chen, Y.Q.; Cheng, L.Y. Perioperative pharmacological management of choroidal detachment associated with rhegmatogenous retinal detachment. Acta Ophthalmol. 2016, 94, 391–396. [Google Scholar] [CrossRef]
- Alibet, Y.; Levytska, G.; Umanets, N.; Pasyechnikova, N.; Henrich, P.B. Ciliary body thickness changes after preoperative anti-inflammatory treatment in rhegmatogenous retinal detachment complicated by choroidal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1503–1508. [Google Scholar] [CrossRef]
- Denwattana, A.; Prahkunhungsit, S.; Thoongsuwan, S.; Rodanant, N.; Phasukkijwatana, N. Surgical outcomes of preoperative steroid for rhegmatogenous retinal detachment with associated choroidal detachment. Eye 2018, 32, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yue, Y.; Tong, N.; Zheng, P.; Liu, W.; An, M. Anatomic Outcomes and Prognostic Factors of Vitrectomy in Patients with Primary Rhegmatogenous Retinal Detachment Associated with Choroidal Detachment. Curr. Eye Res. 2019, 44, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Seelenfreund, M.H.; Kraushar, M.F.; Schepens, C.L.; Freilich, D.B. Choroidal detachment associated with primary retinal detachment. Arch. Ophthalmol. 1974, 91, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Harris, G. Choroidal detachment associated with retinal detachment as a presenting finding. Can. J. Ophthalmol. 1992, 27, 245–248. [Google Scholar]
- Loo, A.; Fitt, A.W.D.; Ramchadani, M.; Kirkby, G.R. Pars plana vitrectomy with silicon oil in the management of combined rhegmatogenous retinal and choroidal detachment. Eye 2001, 15, 612–615. [Google Scholar] [CrossRef]
- Gui, J.M.; Jia, L.; Liu, L.; Liu, J.D. Vitrectomy, lensectomy and silicone oil tamponade in the management of retinal detachment associated with choroidal detachment. Int. J. Ophthalmol. 2013, 6, 337–341. [Google Scholar]
- Yu, Y.; An, M.; Yang, Z.; Liu, W. Risk factors for choroidal detachment following rhagmatogenous retinal detachment in a chinese population. BMC Ophthalmol. 2016, 16, 140. [Google Scholar] [CrossRef]
- Sharma, T.; Gopal, L.; Badrinath, S.S. Primary vitrectomy for regmatogenous retinal detachment associated with coroideal detachment. Ophalmology 1998, 105, 2282–2285. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, Z.; Sheng, H.; Zhang, Z.; Yu, M.; Zhang, Q. Identification of inflammatory mediators in patients with rhegmatogenous retinal detachment associated with chroidal detachment. Mol. Vis. 2015, 21, 417–427. [Google Scholar]
- Munir, W.M.; Pulido, J.S.; Sharma, M.C.; Buerk, B.M. Intravitreal triamcinolone for treatment of complicated proliferative diabetic retinopathy and proliferative vitreoretinopathy. Can. J. Ophthalmol. 2005, 40, 598–604. [Google Scholar] [CrossRef]
- Cheema, R.A.; Peyman, G.A.; Fang, T.; Jones, A.; Lukaris, A.D.; Lim, K. Triamcinolone acetonide as an adjuvant in the surgical treatment of retinal detachment with proliferative vitreoretinopathy. Ophthalmic Surg. Lasers Imaging 2007, 38, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Admaideh, H.; Feghhi, M.; Tabatabaei, H.; Shoeibi, N.; Ramezani, A.; Mohebbi, M.R. Triamcinolone acetonide in silicone-filled eyes as adjunctive treatment for proliferative vitreoretinopathy: A randomized clinical trial. Ophthalmology 2008, 115, 1938–1943. [Google Scholar]
- Yamakiri, K.; Sakamoto, T.; Noda, Y.; Nakahara, M.; Ogino, N.; Kubota, T.; Yokoyama, M.; Furukawa, M.; Shibashi, T. One-year results of a multicenter controlled clinical trial of triamcinolone in pars plana vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 959–966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, W.; Chen, H.; Hou, P.; Fok, A.; Hu, Y.; Lam, D.S. Midterm results of low dose intravitreal triamcinolone as adjunctive treatment for proliferative vitreoretinopathy. Retina 2011, 31, 1137–1142. [Google Scholar] [CrossRef]
- Reibaldi, M.; Russo, A.; Longo, A.; Bonfiglio, V.; Uva, M.G.; Gagliano, C.; Toro, M.D.; Avitabile, T. Regmatogenous retinal detachment with a high risk of proliferative vitreoretinopathy treated with episcleral surgery and an intravitreal dexamethasone 0.7-mg implant. Case Rep. Ophthalmol. 2013, 4, 79–83. [Google Scholar] [CrossRef]
- Mirshahi, A.; Karkhaneh, R.; Zamani Amir, J.; Movassat, M.; Azadi, P. Influence of intravitreal triamcinolone acetonide injection in sclera buckling surgery for macula-off retinal detachment. Ophthalmic Res. 2014, 52, 160–164. [Google Scholar] [CrossRef]
- Sherif, M.; Wolfensberger, T.J. Intraocular dexamethasone implant as adjunct to silicone oil tamponade for proliferative vitreoretinopathy. Klin. Mon. Augenheilkd. 2017, 234, 501–504. [Google Scholar] [CrossRef]
- Banerjee, P.J.; Quartilho, A.; Bunce, C.; Xing, W.; Zvobgo, T.M.; Harris, N.; Charteris, D.G. Slow release dexamethasone in proliferative vitreoretinopathy:a prospective, randomized controlled clinical trial. Ophthalmology 2017, 124, 757–767. [Google Scholar] [CrossRef]
- Cho, A.R.; Yoon, Y.H. Adjunctive dexamethasone implant in patients with atopic dermatitis and retinal detachment undergoing vitrectomy and silicone oil tamponade: An interventional case series. BMC Ophthalmol. 2019, 19, 86. [Google Scholar] [CrossRef]
- Reibaldi, M.; Fallico, M.; Longo, A.; Avitabile, T.; Astuto, M.; Murabito, P.; Minardi, C.; Bonfiglio, V.; Boscia, F.; Furino, C.; et al. Efficacy of three different prophylactic treatments for postoperative nausea and vomiting after vitrectomy: A randomized Clinical Trial. J. Clin. Med. 2019, 8, 391. [Google Scholar] [CrossRef]
- Tseng, W.; Cortez, R.T.; Ramirez, G.; Stinnett, S.; Jaffe, G.J. Prevalence and risk factors for proliferative vitreoretinopathy in eyes with rhegmatogenous retinal detachment but no previous vitreoretinal surgery. Am. J. Ophthalmol. 2004, 137, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.; Conway, B.P.; Campochiaro, P.A.; Kaiser, D.; Gaskin, H. Clinical risk factors for proliferative vitreoretinopathy. Arch. Ophthalmol. 1989, 107, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Fleury, J.; Guenoun, S.; Yaniali, A.; Dumas, C.; Hajjar, C. Cryopexy in primary rhegmatogenous retinal detachment: A risk factor for postoperative proliferative vitreoretinopathy? Graefes Arch. Clin. Exp. ophthalmol. 1996, 234, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, C.; Toro, M.D.; Avitabile, T.; Stella, S.; Uva, M.G. Intravitreal steroids for the prevention of PVR After Surgery for Retinal Detachment. Curr. Pharm. Des. 2015, 21, 4698–4702. [Google Scholar] [CrossRef]
- Moysidis, S.; Thanos, A.; Vavvas, D.G. Mechanisms of Inflammation in Proliferative Vitreoretinopathy: From Bench to Bedside. Mediat. Inflamm. 2012, 2012, 815937. [Google Scholar] [CrossRef]
- Ricker, L.J.; Kessels, A.G.; De Jager, W.; Hendrikse, F.; Kijlstra, A.; la Heij, E.C. Prediction of proliferative vitreoretinopathy after retinal detachment surgery: Potential of biomarker profiling. Am. J. Ophthalmol. 2012, 154, 347–354. [Google Scholar] [CrossRef]
- Bonfiglio, V.; Reibaldi, M.; Fallico, M.; Russo, A.; Pizzo, A.; Fichera, S.; Rapisarda, C.; Macchi, I.; Avitabile, T.; Longo, A. Widening use of dexamethasone implant for the treatment of macular edema. Drug Des. Dev. Ther. 2017, 11, 2359–2372. [Google Scholar] [CrossRef]
- Chang-Lin, J.E.; Burke, J.A.; Peng, Q.; Lin, T.; Orilla, W.C.; Ghosn, C.R.; Zhang, K.M. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4605–4609. [Google Scholar] [CrossRef]
- Chin, H.S.; Park, T.S.; Moon, Y.S.; Oh, J.H. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina 2005, 25, 556–560. [Google Scholar] [CrossRef]
- Beer, P.M.; Bakri, S.J.; Singh, R.J.; Liu, W.; Peters, G.B.; Miller, M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology 2003, 110, 681–686. [Google Scholar] [CrossRef]
- Pessoa, B.; Coelho, J.; Correia, N.; Ferreira, N.; Beirão, M.; Meireles, A. Fluocinolone acetonide intravitreal implant 190 μg (ILUVIEN®) in vitrectomized versus nonvitrectomized eyes for the treatment of chronic diabetic macular edema. Ophthalmic Res. 2018, 59, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.; Malheiro, L.; Melo Beirão, J.; Meireles, A.; Pessoa, B. Real-world retropective comparison of 0.19 mg fluocinolone acetonide and 0.7 mg dexamethasone intravitreal implants for the treatment of diabetic macular edema in vitrectomized eyes. Clin. Ophthalmol. 2019, 13, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- McCuen, B.W., II; Bessler, M.; Tano, Y.; Chandler, D.; Machemer, R. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am. J. Ophthalmol. 1981, 91, 785–788. [Google Scholar] [CrossRef]
- Jonas, J.B.; Hayler, J.K.; Panda-Jonas, S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative vitreoretinopathy. Br. J. Ophthalmol. 2000, 84, 1064–1067. [Google Scholar] [CrossRef]
- Jonas, J.B.; Hayler, J.K.; Sofker, A.; Panda-Jonas, S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am. J. Ophthalmol. 2001, 131, 468–471. [Google Scholar] [CrossRef]
- Jonas, J.B.; Sofker, A.; Degenring, R. Intravitreal triamcinolone acetonide as an additional tool in pars plana vitrecto¬my for proliferative diabetic retinopathy. Eur. J. Ophthalmol. 2003, 13, 468–473. [Google Scholar] [CrossRef]
- Kivilcim, M.; Peyman, G.A.; El-Dessouky, E.S.; Kazi, A.A.; Cheema, R.; Hegazy, H. Retinal toxicity of triamcinolone acetonide in silicone-filled eyes. Ophthalmic Surg. Lasers 2000, 31, 474–478. [Google Scholar]
- Perkins, S.; Gallemore, R.; Yang, C.; Guo, H.; Ashton, P.; Jaffe, G. Pharmacokinetics of fluocinolone/5- fluorouracil Cudrug in the Gas- Filled Eye. Retina 2000, 20, 514–519. [Google Scholar] [CrossRef]
- Jonas, J.B. Concentration of intravitreally injected triamcinolone acetonide in intraocular silicone oil. Br. J. Ophthalmol. 2002, 86, 1450–1451. [Google Scholar] [CrossRef][Green Version]
- Spitzer, M.S.; Kaczmarek, R.T.; Yoeruek, E.; Petermeier, K.; Wong, D.; Heimann, H.; Jaissle, G.B. The distribution, release kinetics and biocompatibility of triamcinolone injected and dispersed in silicone oil. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2337–2343. [Google Scholar] [CrossRef]
- Flores-Villalobos, E.O.; Ramírez-Estudillo, J.A.; Robles-Contreras, A.; Oliva-Ramírez, J.L. Dexamethasone implant in silicone oil: In vitro behavior. Int. J. Retin. Vitr. 2018, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Bakri, S.J.; Alniemi, S.T. Fibrotic encapsulation of a dexamethasone intravitreal implant following vitrectomy and silicone oil for rhegmatogenous retinal detachment. Ophthalmic surgery, lasers and imaging retina. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.H.; Ahmadieh, H.; Soheilian, M.; Azarmina, M.; Moradian, S.; Ramezani, A.R.; Tavallal, A.; Naghibozakerin, J. Effect of oral prednisolone on visual outcomes and complications after scleral buckling. Eur. J. Ophthalmol. 2010, 20, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, C.J.; Hwang, J.F.; Chen, S.N. Influence of systemic steroids on subretinal fluid after scleral buckle surgery for macula-off retinal detachment. Retina 2011, 31, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Koerner, F.; Koener-Stiefbold, U.; Garweg, J.G. Systemic corticosteroids reduce the risk of cellophane membranes after retinal detachment surgery: A prospective randomized placebo-controlled double-blind clinical trial. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Motohashi, R.; Kotake, O.; Nakagawa, H.; Noma, H.; Shimura, M. Comparative effects of topical diclofenac and betamethasone on inflammation after vitrectomy and cataract surgery in various vitreoretinal diseases. J. Ocul. Pharmacol. Ther. 2016, 32, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Hagimura, N.; Lida, T.; Suto, K.; Kishi, S. Persistent foveal retinal detachment after successful regmatogenous retinal detachment surgery. Am. J. Ophthalmol. 2002, 133, 516–520. [Google Scholar] [CrossRef]
- Seo, J.H.; Woo, S.J.; Park, K.H.; Yu, Y.S.; Chung, H. Influence of persistent submacular fluid on visual outcome after successful scleral buckle surgery for macula-off retinal detachment. Am. J. Ophthalmol. 2008, 145, 915–922. [Google Scholar] [CrossRef]
- Benson, S.E.; Schlottmann, P.G.; Bunce, C.; Xing, W.; Charteris, D.G. Optical coherence tomography analysis of the macula after scleral buckle surgery for retinal detachment. Ophthalmology 2007, 114, 108–112. [Google Scholar] [CrossRef]
- Benson, S.E.; Ratclliffe, S.; Van Raders, P.; Schlottmann, P.G.; Khan, I.; Newsom, R.; Langford, R.M.; Charteris, D.G. A randomized comparison of parecoxib/valdecoxib and placebo for the prevention of cystoid macular edema after scleral buckling surgery. Retina 2009, 29, 387–394. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).