Abstract
Background: Lymphopenic patients with community-acquired pneumonia (CAP) have shown high mortality rates. Corticosteroids have immunomodulatory properties and regulate cytokine storm in CAP. However, it is not known whether their modulatory effect on cytokine secretion differs in lymphopenic and non-lymphopenic patients with CAP. Therefore, we aimed to test whether the presence of lymphopenia may modify the response to corticosteroids (mainly in C reactive protein (CRP)) in patients with severe CAP and high inflammatory status). Methods: A post hoc analysis of a randomized controlled trial (NCT00908713) which evaluated the effect of corticosteroids in patients with severe CAP and high inflammatory response (CRP > 15 mg/dL). Patients were clustered according to the presence of lymphopenia (lymphocyte count below 1000 cell/mm3). Results: At day 1, 35 patients (59%) in the placebo group presented with lymphopenia, compared to 44 patients (73%) in the corticosteroid group. The adjusted mean changes from day 1 showed an increase of 1.19 natural logarithm (ln) cell/mm3 in the corticosteroid group and an increase of 0.67 ln cell/mm3 in the placebo group (LS mean difference of the changes in ln (methylprednisolone minus placebo) 0.51, 95% CI (0.02 to 1.01), p = 0.043). A significant effect was also found for the interaction (p = 0.043) between corticosteroids and lymphopenia in CRP values at day 3, with lower values in patients without lymphopenia receiving corticosteroids after adjustments for potential confounders. Conclusion: In this exploratory post hoc analysis from ramdomized controlled trial (RCT) data, the response to corticosteroids, measured by CRP, may differ according to lymphocyte count. Further larger studies are needed to confirm this data.
1. Introduction
Lymphopenic patients with community-acquired pneumonia (CAP) have shown high mortality rates [1]. The presence of prior conditions leading to immunosuppression, the recruitment/escape of these cells to sites of inflammation/infection, and/or apoptotic phenomena may explain the presence of low lymphocyte counts in patients with CAP [2]. Corticosteroids have immunomodulatory properties and regulate cytokine storm in CAP. However, it is not known whether their modulatory effect on cytokine secretion differs in lymphopenic and non-lymphopenic patients with CAP. Therefore, we aimed to test whether the presence of lymphopenia may modify the response to corticosteroids (mainly in C reactive protein (CRP)) in patients with severe CAP and high inflammatory status.
2. Methods and Patients
We performed a post hoc analysis of a randomized controlled trial [3] (NCT00908713) which evaluated the effect of corticosteroids in patients with severe CAP and high inflammatory response (CRP > 15 mg/dL). We clustered patients according to the presence of lymphopenia (lymphocyte count below 1000 cell/mm3). We reported the number and percentage of patients for categorical variables and the median (interquartile range) for continuous variables. Categorical variables were compared using the x2. Continuous variables were compared using the nonparametric Kruskal-Wallis test. Pairwise comparisons were carried out via the Bonferroni method in order to control the experiment-wise error rate. We fitted analysis of covariance (ANCOVA) model [4,5] to analyze the change from baseline in lymphocyte counts at day 3, adjusting for the treatment, PSI risk class, year of recruitment, and center. Each treatment effect was estimated by the Least Square (LS) mean, its standard error (SE), and 95% confidence interval (CI). Lymphocyte counts were log-transformed to fit the ANCOVA model. We also analyzed the CRP values at day 3 by means of ANCOVA models [4,5], adjusting for the baseline values, treatment, lymphocyte counts, lymphocyte counts x treatment interaction, PSI risk class, year of recruitment, and centre. Each treatment effect was estimated by the LS mean, its SE, and 95% CI. CRP values were log-transformed to fit the ANCOVA model. Further information on our study is provided elsewhere [3].
3. Results
Of the 120 patients, 57 received placebo and 54 received corticosteroids in the per protocol (PP) population. On day 1, 35 patients (59%) in the placebo group presented with lymphopenia compared to 44 patients (73%) in the corticosteroid group.
Baseline characteristics, CRP values, and clinical outcomes are summarized in Table 1. At day 3 adjusted mean increased from day 1 in natural logarithms (ln) of lymphocyte count for those with lymphopenia. This was higher for corticosteroid than for placebo patients. The adjusted mean changes from day 1 showed an increase of 1.19 ln cell/mm3 in the corticosteroid group and an increase of 0.67 ln cell/mm3 in the placebo group (LS mean difference of the changes in ln (methylprednisolone minus placebo) 0.51, 95% CI (0.02 to 1.01); p = 0.043).

Table 1.
Baseline characteristics and outcomes of the intention-to-treat population (n = 119) and outcomes of the per protocol population (n = 111).
A significant effect was also found for the interaction (p = 0.043) between corticosteroids and lymphopenia in CRP values at day 3 (Table 1), with lower values in patients without lymphopenia receiving corticosteroids after adjustments for potential confounders. Moreover, in patients who received corticosteroids, a negative correlation between lymphocyte count and the value of change in CRP was found (r = −0.29 p = 0.049). This correlation was not significant in patients who received placebo (Figure 1). Treatment failure rates did not differ between the four groups.
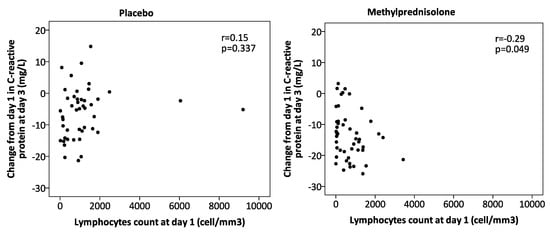
Figure 1.
Correlation between the change from day 1 in C-reactive protein at day 3 and lymphocyte count at day 1 for the per protocol population.
4. Discussion
Our study found that patients who received corticosteroids presented differences in the systemic anti-inflammatory effect as measured by CRP at day 3, with variations related to the presence or absence of lymphopenia. However, we did not find a clinical impact on treatment failure because the analysis was underpowered for this outcome. Previous studies have shown that levels of CRP at day 3 are associated with treatment failure in CAP [6]. Corticosteroids have immunomodulatory activity through the inhibition of NF-kappaB activity and activator protein 1, both of which are transcription factors that activate immunoregulatory genes [7] and might decrease the rates of treatment failure. Recently, a post hoc analysis in an RCT of corticosteroids in septic shock also showed a differential response according to previously described transcriptomic sepsis response signatures [8,9].
In this post hoc analysis, patients who received corticosteroids showed higher increases in lymphocytes at day 3, contrary to the expectations that corticosteroids might produce lymphopenia [10] due to apoptosis or by a redistribution of recirculating lymphocytes at an early stage. A greater response from bone marrow and redistribution from lymph nodes induced by corticosteroids may explain the increase at day 3. In spite of these results, the clinical impact of lymphocyte response to corticosteroids remains unknown.
There are two main limitations in our study. First, it is a post hoc analysis of a previous RCT without a specific sample size calculation for the current investigation purpose. Second, there were disbalances in the basal CRP with lower values in patients from the placebo group and lymphopenia compared with the other groups. However, and concerning this second point, what really matters when evaluating a biomarker is the delta differences, in this case between baseline and day 3 [11]. The major strength is the validity of data that proceeds from a RCT.
5. Conclusions
In conclusion, the response to corticosteroids, measured by CRP, may differ according to lymphocyte count. The low number of patients in each group does not allow an analysis of clinical outcomes. However, in view of our results, new studies are warranted to evaluate the effect of corticosteroids in non-lymphopenic CAP patients where higher benefits could be observed.
Author Contributions
Study concept and design: A.T., A.C.; data collection: A.T., A.C., M.F., O.S., C.C., R.M. (Raul Mendez), R.M. (Rosario Menendez); statistical analysis: A.G., analysis and interpretation of data: A.T., A.C., A.G., J.B.-M., M.S.N.; drafting of the manuscript: A.T., A.C.; critical revision of the manuscript for important intellectual content: A.T. and M.S.N.; and study supervision: A.T. A.T. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported by CIBERES and IDIBAPS.
Acknowledgments
We are indebted to all our medical and nursing colleagues for their assistance and cooperation in this study. We thank Michael Maudsley (University of Barcelona) for his assistance in reviewing the language of the manuscript. This study was supported by CIBERES and IDIBAPS. CC is the recipient of a Postdoctoral Grant PERIS 2016-2020 (Pla estratègic de recerca i innovació en salut), SEPAR 2018 grant and a grant from the Fondo de Investigación Sanitaria (grant PI19/00207). The funding sources had no role in the design or conduct of the study, in the collection, management, analysis, and interpretation of the data, in the preparation, review, or approval of the manuscript, or in decision to submit the manuscript for publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bermejo-Martin, J.F.; Cilloniz, C.; Mendez, R.; Almansa, R.; Gabarrus, A.; Ceccato, A.; Torres, A.; Menendez, R. NEUMONAC group Lymphopenic Community Acquired Pneumonia (L-CAP), an Immunological Phenotype Associated with Higher Risk of Mortality. EBioMedicine 2017, 24, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Martin, J.F.; Almansa, R.; Martin-Fernandez, M.; Menendez, R.; Torres, A. Immunological profiling to assess disease severity and prognosis in community-acquired pneumonia. Lancet Respir. Med. 2017, 5, e35–e36. [Google Scholar] [CrossRef]
- Torres, A.; Sibila, O.; Ferrer, M.; Polverino, E.; Menendez, R.; Mensa, J.; Gabarrús, A.; Sellarés, J.; Restrepo, M.I.; Anzueto, A.; et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015, 313, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Cochran WG, S.G. Statistical methods, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 1991. [Google Scholar]
- Brown DR, W.B. Statistical principles in experimental design; McGraw Hill: New York, NY, USA, 1991. [Google Scholar]
- Menéndez, R.; Cavalcanti, M.; Reyes, S.; Mensa, J.; Martinez, R.; Marcos, M.A.; Filella, X.; Niederman, M.; Torres, A. Markers of treatment failure in hospitalised community acquired pneumonia. Thorax 2008, 63, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Antcliffe, D.B.; Burnham, K.L.; Al-Beidh, F.; Santhakumaran, S.; Brett, S.J.; Hinds, C.J.; Ashby, D.; Knight, J.C.; Gordon, A.C. Transcriptomic Signatures in Sepsis and a Differential Response to Steroids: From the VANISH Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.E.; Burnham, K.L.; Radhakrishnan, J.; Humburg, P.; Hutton, P.; Mills, T.C.; Rautanen, A.; Gordon, A.C.; Garrard, C.; Hill, A.V.S.; et al. Genomic landscape of the individual host response and outcomes in sepsis: A prospective cohort study. Lancet Respir. Med. 2016, 4, 259–271. [Google Scholar] [CrossRef]
- Bromberg, L.; Roufosse, F.; Pradier, O.; Delporte, C.; Van Antwerpen, P.; De Maertelaer, V.; Cogan, E. Methylprednisolone-Induced Lymphocytosis in Patients with Immune-Mediated Inflammatory Disorders. Am. J. Med. 2016, 129, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Méndez, R.; Menéndez, R.; Cillóniz, C.; Amara-Elori, I.; Amaro, R.; González, P.; Posadas, T.; Gimeno, A.; España, P.P.; Almirall, J.; et al. Initial Inflammatory Profile in Community-acquired Pneumonia Depends on Time since Onset of Symptoms. Am. J. Respir. Crit. Care Med. 2018, 198, 370–378. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).