Real-Life Benefit of OCT Imaging for Optimizing PCI Indications, Strategy, and Results
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. OCT Acquisition Technique, Analysis, and Indications
2.3. Statistical Analysis
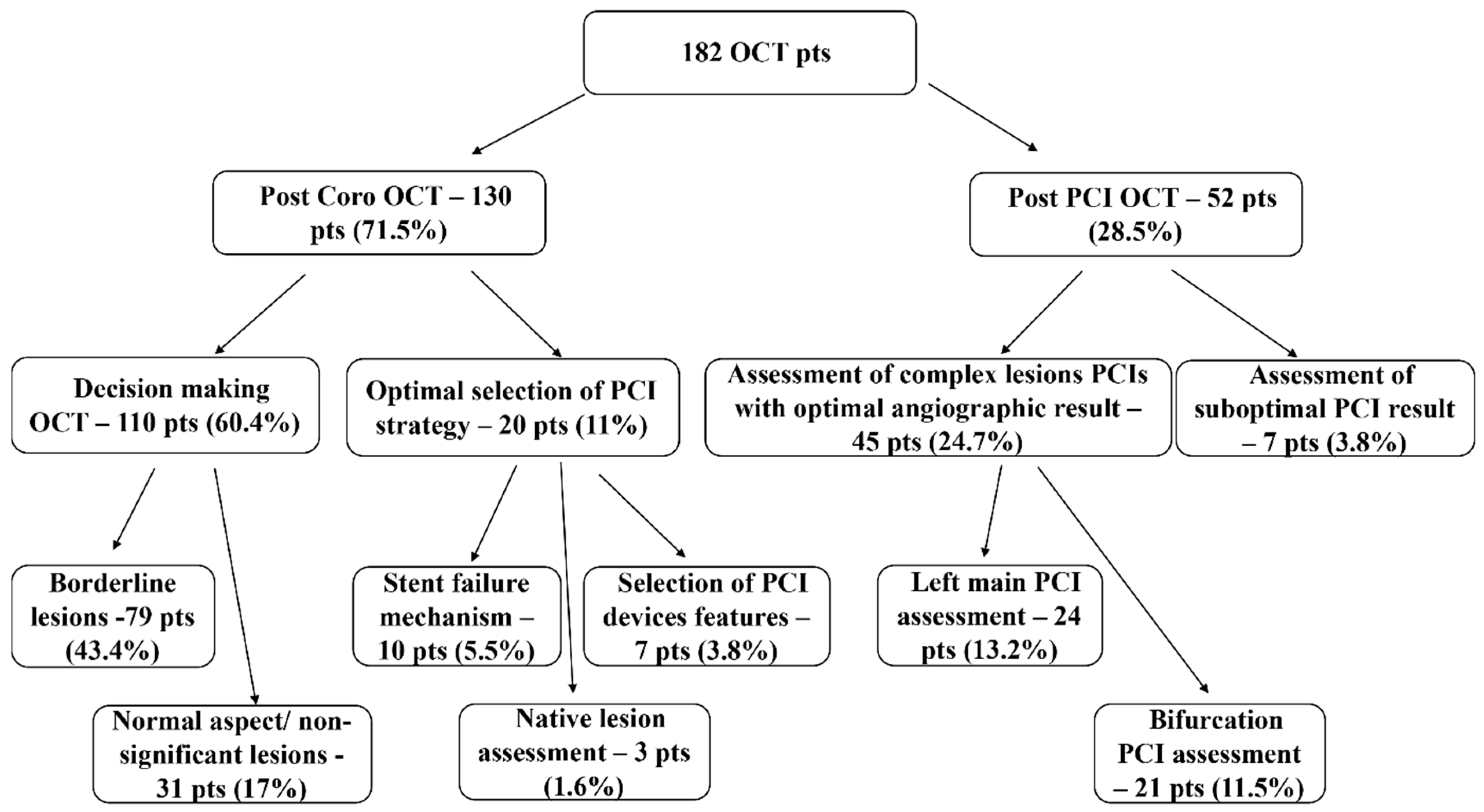
3. Results
3.1. Demographic, Clinical and Angiographic Data
3.2. Post Coronarography OCT
3.2.1. “Decision-making OCT Group”
Borderline CA Lesions
Nonsignificant Lesions or Normal Coronary Aspect
3.2.2. OCT for Optimal Selections of PCI Strategy
- -
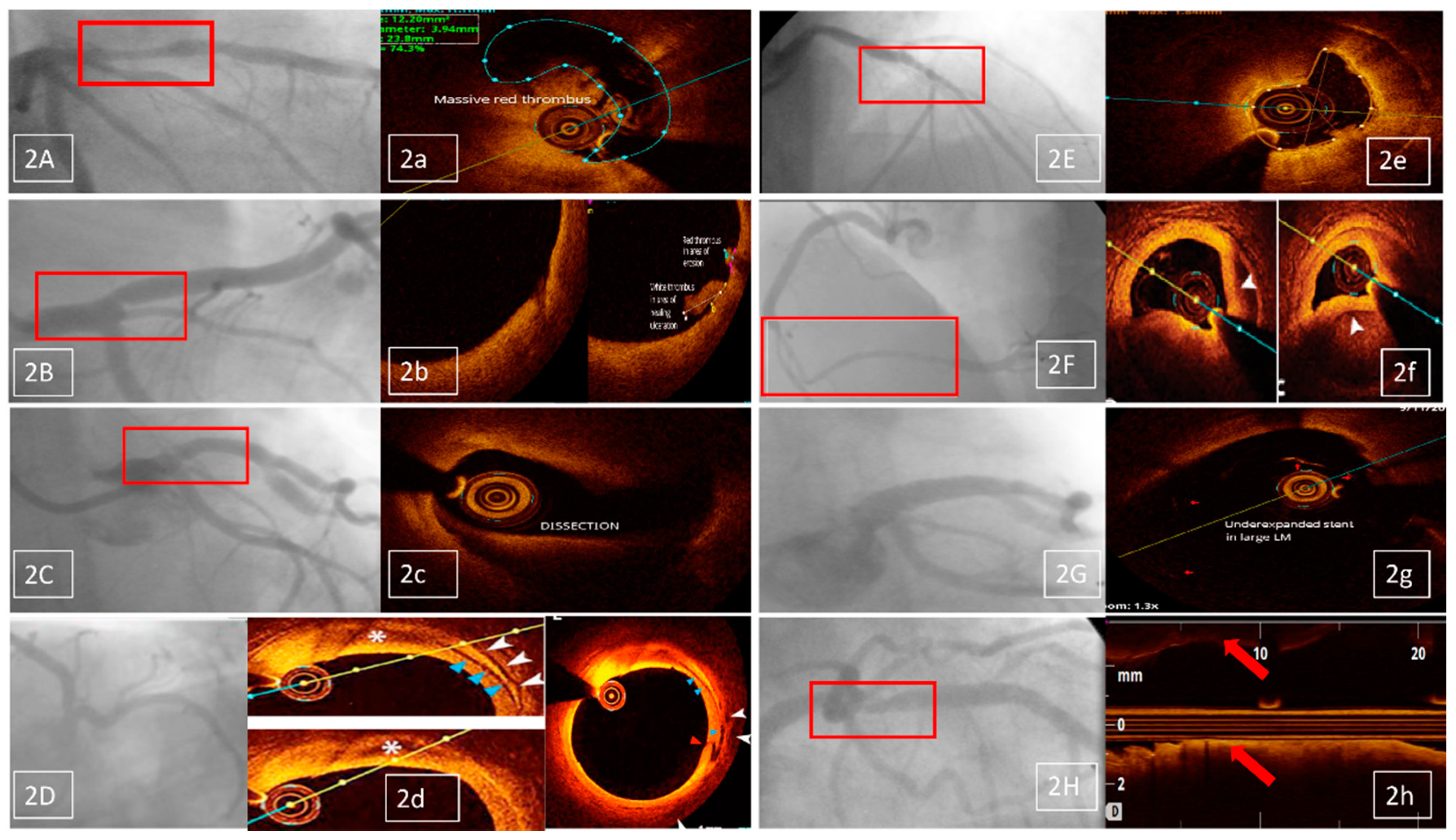
- identification of stent failure mechanism (10 patients, 5.5% of the OCT investigations); OCT proved restenosis in eight and neoatherosclerosis in two patients (Figure 2E/e);
- -
- selection of PCI devices features (seven patients, 3.8% of the OCT investigations); there were three cases of long lesions PCI (Figure 2F/f), two of left main (LM) PCI and another two of bifurcation PCI;
- -
- comprehensive assessment of native lesions suspected of complications (three patients, 1.6% of the OCT investigations); OCT confirmed complicated atherosclerotic plaques in all three of them, with identification of thrombus in one patient.
3.3. Post-PCI OCT Group
3.3.1. Assessment of Complex Lesions PCIs with Optimal Angiographic Result Included:
Left Main Lesions PCI Assessment
Bifurcation Lesions PCI Assessment
3.3.2. Assessment of Suboptimal PCI Results
- -
- angiographic suspicion of edge dissection (three cases); only one was confirmed by OCT, leading to PCI optimization;
- -
- angiographic suspicion of extensive coronary dissection (one case)—unconfirmed by OCT;
- -
- angiographic suspicion of coronary thrombus (two cases), confirmed by OCT in both cases and followed by PCI optimization;
- -
- angiographic suspicion of significant residual stenosis in the native coronary, proximal to the stented lesion (one case), unconfirmed by OCT (Figure 2H/h).
3.4. Decision Change after OCT
- -
- in all the 130 patients having OCT imaging as a complement to CA, for assessing the indication of revascularization and
- -
- in 27 of the 52 patients investigated post-PCI (51.9% of this subgroup).
3.5. OCT Investigations not Included in This Study
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Non-Communicable Diseases Country Profiles 2018. World Health Organization: Geneva, Switzerland, 2018. Available online: apps.who.int/iris/bitstream/handle/10665/274512/9789241514620-eng.pdf (accessed on 8 October 2018).
- Olinic, D.M.; Spinu, M.; Olinic, M.; Homorodean, C.; Tataru, D.A.; Liew, A.; Schernthaner, G.H.; Stanek, A.; Fowkes, G.; Catalano, M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int. Angiol. 2018, 37, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Johnson, T.W.; Akasaka, T.; Jeong, M.H. The role of optical coherence tomography in the setting of acute myocardial infarction. J. Cardiol. 2018, 72, 186–192. [Google Scholar] [CrossRef]
- Ha, F.J.; Giblett, J.P.; Nerlekar, N.; Cameron, J.D.; Meredith, I.T.; West, N.E.J.; Brown, A.J. Optical Coherence Tomography Guided Percutaneous Coronary Intervention. Heart Lung Circ. 2017, 26, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. International Working Group for Intravascular Optical CoherenceTomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef]
- Spînu, M.; Olinic, D.M.; Olinic, M.; Homorodean, C. In vivo imaging of complicated atherosclerotic plaque-role of optical coherence tomography (OCT). Rom. J. Morphol. Embryol. 2018, 59, 469–478. [Google Scholar] [PubMed]
- Otsuka, F.; Joner, M.; Prati, F.; Virmani, R.; Narula, J. Clinical classification of plaque morphology in coronary disease. Nat. Rev. Cardiol. 2014, 11, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.S.; Vengrenyuk, Y.; Yoshimura, T.; Matsumura, M.; Pena, J.; Baber, U.; Moreno, P.; Mehran, R.; Maehara, A.; Sharma, S.; et al. Fibrous cap thickness by optical coherence tomography in vivo. J. Am. Coll. Cardiol. 2017, 69, 644–657. [Google Scholar] [CrossRef]
- Di Mario, C.; Mattesini, A. Will Optical Coherence Tomography Become the Standard Imaging Tool for Percutaneous Coronary Intervention Guidance? JACC Cardiovasc. Interv. 2018, 11, 1322–1324. [Google Scholar] [CrossRef]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2018, 14, 656–677. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Nakamura, M.; Räber, L.; Colleran, R.; Kadota, K.; Capodanno, D.; Wijns, W.; Akasaka, T.; Valgimigli, M.; Guagliumi, G.; et al. Current use of intracoronary imaging in interventional practice—Results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) Clinical Practice Survey. EuroIntervention 2018, 14, e475–e484. [Google Scholar] [CrossRef]
- Homorodean, C.; Ober, M.C.; Iancu, A.C.; Olinic, M.; Tataru, D.; Spinu, M.; Olinic, D.M.; Burzotta, F.; Trani, C.; Erglis, A. How should I treat this mini-crush stenting complication? EuroIntervention 2017, 13, 1248–1252. [Google Scholar] [CrossRef]
- Olinic, D.M.; Spinu, M.; Homorodean, C.; Olinic, M. Vasa vasorum induced LAD dissection and haematoma in an anterior STEMI patient with nearly normal angiography: The role of OCT. Kardiol. Pol. 2017, 75, 504. [Google Scholar] [CrossRef] [PubMed]
- Homorodean, C.; Spinu, M.; Ober, M.C.; Olinic, M.; Olinic, D.M. Spontaneous coronary dissection: Optical coherence tomography insights before and after stenting. Cardiol. J. 2017, 24, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Karanasos, A.; Ligthart, J.; Witberg, K.; van Soest, G.; Bruining, N.; Regar, E. Optical Coherence Tomography: Potential Clinical Applications. Curr. Cardiovasc. Imaging Rep. 2012, 5, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.M.; Cialdella, P.; Lassandro Pepe, F.; Basile, E.; Zimbardo, G.; Arioti, M.; Ciriello, G.; D’Amario, D.; Buffon, A.; Burzotta, F.; et al. Fractional flow reserve in acute coronary syndromes and in stable ischemic heart disease: Clinical implications. Int. J. Cardiol. 2019, 277, 42–46. [Google Scholar] [CrossRef]
- Hakeem, A.; Almomani, A.; Uretsky, B.F. Role of fractional flow reserve in the evaluation and management of patients with acute coronary syndrome. Curr. Opin. Cardiol. 2017, 32, 767–775. [Google Scholar] [CrossRef]
- Wolfrum, M.; De Maria, G.L.; Banning, A.P. Optical coherence tomography to guide percutaneous treatment of coronary bifurcation disease. Expert Rev. Cardiovasc. Ther. 2017, 15, 705–713. [Google Scholar] [CrossRef]
- Onuma, Y.; Okamura, T.; Muramatsu, T.; Uemura, S.; Serruys, P.W. New implication of three-dimensional optical coherence tomography in optimising bifurcation PCI. EuroIntervention 2015, 11 (Suppl. V), V71–V74. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Katagiri, Y.; Burzotta, F.; Holm, N.R.; Amabile, N.; Okamura, T.; Mintz, G.S.; Darremont, O.; Lassen, J.F.; Lefèvre, T.; et al. Joint consensus on the use of OCT in coronary bifurcation lesions by European and Japanese bifurcation clubs. EuroIntervention 2019, 14, e1568–e1577. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Rathod, K.S.; Koganti, S.; Hamshere, S.; Astroulakis, Z.; Lim, P.; Sirker, A.; O’Mahony, C.; Jain, A.K.; Knight, C.J.; et al. Angiography Alone Versus Angiography Plus Optical Coherence Tomography to Guide Percutaneous Coronary Intervention: Outcomes From the Pan-London PCI Cohort. JACC Cardiovasc. Interv. 2018, 11, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Meneveau, N.; Souteyrand, G.; Motreff, P.; Caussin, C.; Amabile, N.; Ohlmann, P.; Morel, O.; Lefrançois, Y.; Descotes-Genon, V.; Silvain, J.; et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: Results of the multicenter, randomized DOCTORS study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation 2016, 134, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Wijns, W.; Shite, J.; Jones, M.R.; Lee, S.W.; Price, M.J.; Fabbiocchi, F.; Barbato, E.; Akasaka, T.; Bezerra, H.; Holmes, D. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur. Heart J. 2015, 36, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
| OCT Indication | Specific Area of Interest |
|---|---|
| Post-Coronary Angiography and before a first PCI procedure | |
| Lesion evaluation | Culprit lesion evaluation in ACS patients without coronary angiography significant stenosis |
| Evaluation of lesions with angiographic haziness (suspected dissection/thrombus/calcification) | |
| Pre-PCI assessment | Measurements of lumen diameter and lesion length for PCI devices selection (balloon/stent dimensions) |
| Lesion assessment for PCI technique/strategy selection (for left main and large bifurcations) | |
| Evaluation of “landing zones” | |
| Evaluation of guide wire position (in cases of coronary dissection/chronic occlusion) | |
| Post-PCI | |
| Immediate assessment of PCI result | Evaluation of stent expansion (identification of under-expansion/residual stenosis) |
| Evaluation of potential vascular injury (identification of edge dissection/intra-stent thrombus/tissue protrusion) | |
| Late evaluation of suspected stent failure | Identification and characterization of restenosis |
| Identification of in stent thrombosis | |
| Identification of neoatherosclerosis | |
| STEMI (No. of Patients) | NSTEMI (No. of Patients) | UA (No. of Patients) | SCAD (No. of Patients) | Total (No. of Patients/%) | ||
|---|---|---|---|---|---|---|
| 50 | 39 | 85 | 8 | 182 | ||
| Gender | Male | 34 | 29 | 55 | 6 | 124 (68.1%) |
| Female | 16 | 10 | 30 | 2 | 58 (31.9%) | |
| Age | <40 | 2 | 0 | 3 | 0 | 5 (2.7%) |
| ≤40–49 | 22 | 3 | 10 | 2 | 37 (20.3%) | |
| ≤50–59 | 9 | 13 | 20 | 2 | 44 (24.2%) | |
| ≤60–69 | 8 | 10 | 36 | 3 | 57 (31.3%) | |
| ≤70–79 | 8 | 12 | 16 | 1 | 37 (20.3%) | |
| ≥80 | 1 | 1 | 0 | 0 | 2 (1.1%) | |
| Coronary Angiography Results | Normal | 2 | 1 | 3 | 1 | 7 (3.8%) |
| LM | 5 | 6 | 11 | 0 | 22 (12.1%) | |
| 1 Vessel Disease | 29 | 8 | 30 | 2 | 69 (37.9%) | |
| 2 Vessels Disease | 6 | 7 | 17 | 3 | 33 (18.1%) | |
| 3 Vessels Disease | 8 | 17 | 24 | 2 | 51 (28%) | |
| OCT Examined Vessel | LM | 9 | 18 | 30 | 2 | 59 (32.4%) |
| LAD | 31 | 18 | 49 | 6 | 104 (57.1%) | |
| LCX | 3 | 2 | 2 | 0 | 7 (3.8%) | |
| RCA | 7 | 0 | 4 | 0 | 11 (6.1%) | |
| Venous Graft | 0 | 1 | 0 | 0 | 1 (0.5%) | |
| Hypertension | 143 (78.6%) |
| Diabetes mellitus | 46 (25.3%) |
| Dyslipidemia | 105 (57.7%) |
| Renal Insufficiency | 11 (6%) |
| Smoking habit | 52 (28.6%) |
| Overweight | 35 (19.2%) |
| Indications | Conservative Treatment No. of Patients/% | Revascularization No. of Patients/% | Total No. of Patients |
|---|---|---|---|
| Borderline lesions | 28(35.5%) | 51(64.5%) | 79 |
| Nonsignificant lesions or normal coronaries | 19(61.3%) | 12(38.7%) | 31 |
| Stent failure mechanism | 10 | 10 | |
| Native lesion assessment | 3 | 3 | |
| Selection of PCI devices | 7 | 7 | |
| LM PCI control, after optimal angiography | 11 (45.8%) | 13 (54.2%) | 24 |
| Bifurcation PCI control, after optimal angiography | 11 (52.3%) | 10 (47.7%) | 21 |
| Suboptimal PCI result on angiography | 4 (57.1%) | 3 (42.9%) | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olinic, D.M.; Spinu, M.; Homorodean, C.; Ober, M.C.; Olinic, M. Real-Life Benefit of OCT Imaging for Optimizing PCI Indications, Strategy, and Results. J. Clin. Med. 2019, 8, 437. https://doi.org/10.3390/jcm8040437
Olinic DM, Spinu M, Homorodean C, Ober MC, Olinic M. Real-Life Benefit of OCT Imaging for Optimizing PCI Indications, Strategy, and Results. Journal of Clinical Medicine. 2019; 8(4):437. https://doi.org/10.3390/jcm8040437
Chicago/Turabian StyleOlinic, Dan Mircea, Mihail Spinu, Calin Homorodean, Mihai Claudiu Ober, and Maria Olinic. 2019. "Real-Life Benefit of OCT Imaging for Optimizing PCI Indications, Strategy, and Results" Journal of Clinical Medicine 8, no. 4: 437. https://doi.org/10.3390/jcm8040437
APA StyleOlinic, D. M., Spinu, M., Homorodean, C., Ober, M. C., & Olinic, M. (2019). Real-Life Benefit of OCT Imaging for Optimizing PCI Indications, Strategy, and Results. Journal of Clinical Medicine, 8(4), 437. https://doi.org/10.3390/jcm8040437





