Abstract
Background/Objectives: Down syndrome (DS) is the most common chromosomal abnormality in live births in the United States. Children with DS often require anesthesia for surgery or diagnostic imaging in their lives. These children present a unique perioperative risk profile due to a combination of anatomic and physiological alterations, along with associated comorbid conditions. There are limited studies on the perioperative outcomes of children with DS. This retrospective study assesses perioperative complications in pediatric patients with DS undergoing non-cardiac surgery or diagnostic imaging under anesthesia at a single tertiary pediatric hospital. Methods: The electronic medical record at a tertiary pediatric hospital was queried for children with DS who received anesthesia for non-cardiac surgery or diagnostic imaging from May 2016 to April 2021. The primary outcomes were complications defined as readmission, reoperation, or unexpected respiratory, cardiovascular, neurologic, surgical, or gastrointestinal issues. Exclusion criteria were cardiac surgery, age > 18 years, and records with incomplete or missing data. Results: A total of 1713 anesthetic records from 711 unique patients over five years were included in the final analysis. The study found a low overall complication rate (2.98%), with respiratory events being the most common (43.1%). While most complications are short term and resolved with treatment and time; there were also several severe, life-threatening complications. Increased procedural complexity, multiple procedures, and increased procedure duration were associated with higher complication rates, whereas patient age, sex, weight, and case urgency were not associated with higher complication rates. Conclusions: Children with DS often have comorbid conditions and require multiple life-improving surgeries. Our study found the perioperative complication rate for children with Down syndrome receiving anesthesia for non-cardiac surgery or diagnostic imaging is low, comparable to the general pediatric population. The findings indicate that anesthesia is well tolerated by children with DS. However, given patients’ unique anatomic and physiological differences, careful perioperative risk assessment and planning is essential. Clinical Implications: (a) What is already known about the topic: Pediatric patients with DS often require anesthesia for surgical procedures or medical imaging. They have anatomic and physiological alterations and comorbid conditions that may influence perioperative risk. (b) What new information this study adds: In a retrospective study at a tertiary pediatric hospital, patients with DS were found to have a low overall complication rate after anesthesia for non-cardiac surgery or diagnostic imaging. Increased procedural complexity, multiple procedures, and increased procedure duration were associated with higher complication rates.
1. Introduction
Down syndrome (DS) is the most common chromosomal abnormality in live births in the United States occurring at a rate of approximately 1 in 1000 births [1]. The French physicians Jérôme Lejeune, Gautier, and Turpin first described the association between DS and a third chromosome 21 in 1959 [2]. Since the 1950s, advances in healthcare have greatly improved life expectancy for individuals with DS. Estimated survival in the first year of life has increased from 46–71% in the 1950s [3,4] to 91% in the 1990s [5]. Life expectancy has increased to 53–60 years in the 2000–2010s [6,7]. The increase in life expectancy is attributable to advances in corrective cardiac surgery and overall improved healthcare [8].
Children with DS often require anesthesia for surgery or diagnostic imaging throughout their lives. As individuals with DS live longer, an increase in the number of anesthetic encounters is likely. These children present a unique perioperative risk profile due to a combination of anatomic and physiological alterations, along with associated comorbid conditions. Airway abnormalities including macroglossia, glossoptosis, subglottic stenosis, and atlantoaxial instability may contribute to increased difficulty with airway management [9,10,11,12,13,14,15,16]. Airway obstruction is very common and exacerbated by adenoid and tonsillar hypertrophy, a higher prevalence of obesity, and high incidence of obstructive sleep apnea [17,18,19,20,21]. Additionally, congenital heart disease is present in approximately 40% of children with DS [22,23] and certain specific variants are associated increased mortality as compared to children without DS [24]. Other health concerns that influence perioperative management include sensitivity to anesthetic agents [25,26], gastrointestinal abnormalities [22,27,28], impaired immune response [29], endocrine disorders [12,27], hematologic conditions [12,27], and intellectual disability affecting patient understanding and cooperation [12,27].
While large, prospective, multicenter studies have reported perioperative outcomes in the general pediatric population [30,31,32,33,34], data specific to children with DS remain limited. Much of the existing research is retrospective, small, and focused on a specific subset of procedures [35,36,37,38,39]. Some studies report no increased complication rates [36], while others suggest a higher risk [40]. Consequently, there is a need for updated, comprehensive research on perioperative risks and outcomes in this unique population across a broad range of surgical and imaging procedures. This is relevant to both general and pediatric anesthesiologists since greater than 80% pediatric surgeries are performed as ambulatory surgery [41]. The success of ambulatory surgery depends on careful selection, screening, and optimization [42,43]. It is important to understand perioperative complications in patients with DS to develop strategies to minimize complications and improve care.
The present study aims to address this gap by reporting on the incidence and types of perioperative complications in children with DS undergoing anesthesia at a single pediatric tertiary care center. Specifically, we seek to identify the most common perioperative adverse events and analyze factors associated with these complications.
2. Methods
The Ann and Robert H. Lurie Children’s Hospital of Chicago Institutional Review Board deemed this study as exempt from review, with a waiver of signed patient consent. This was a single center retrospective cohort study of patients with DS. A chart review of all anesthetics occurring in patients with DS between May 2016 and April 2021 was performedusing the Epic Systems electronic medical records platform (Verona, WI, USA) and a dataset was created with the following information: age, patient weight, gender, American Society of Anesthesiologists (ASA) physical status, surgical specialty, relative value units (Centers for Medicare and Medicaid Services Resource Based Relative Value Scale or RVUs), length of case, length of stay, complications, procedures performed, and urgence of case booking. Work RVUs [44] were summed based on the procedures recorded. Work RVUs were used as a proxy for case complexity [45]. Each anesthetic record was reviewed for perioperative adverse events by one of the authors. Complications were defined as readmission, reoperation, or unexpected respiratory, cardiovascular, neurologic, surgical, or gastrointestinal issues. Exclusion criteria were cardiac surgery, age > 18 years, and records with incomplete or missing data. Continuous variables are presented as mean (standard deviation) for normally distributed data or median [interquartile range] for non-normal distributions. Categorical variables are reported as frequencies (percentages). Group comparisons (complications vs. no complications) were performed using independent t-tests or Wilcoxon rank-sum tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables, as appropriate. Two-sided p-values < 0.05 were considered statistically significant. A univariate logistic regression model was used to assess the association between each characteristic and complications. Surgical specialties that did not have complications were excluded from the logistic regression model because calculating an odds ratio was not possible. Due to collinearity between the covariates and specialties, we could only adjust for age and gender in the multivariate logistic regression model. In this, the univariate model is sufficient, as age and gender are not associated with specialty line in the results. All statistical analyses were conducted using R version 4.4.0 (R Foundation for Statistical Computing, Vienna, Austria). As this was a descriptive study where the primary goal is to describe and summarize data without hypothesis testing, a formal power analysis was not performed.
3. Results
The final cohort consisted of 1713 anesthetic procedures with 711 unique patients that met the inclusion criteria (Figure 1). Of these 711 unique patients, 366 patients (51.5%) received a single anesthetic, while 345 (48.5%) patients received two or more anesthetics over the study period. Thirty-five cases (2.0%) were performed with sedation while the remainder were performed under a general anesthetic. There were 380 planned admissions (22%), 293 in-patient encounters (17%), 1029 same-day discharge encounters (60%), and 11 unanticipated admissions (0.64%). Table 1 lists the characteristics of patients with and without complications. Table 2 lists cases by specialty. Otorhinolaryngology (ENT) had the most numerous cases (n = 769, 45%) followed by medical imaging (n = 214, 12%). Fifty-one patients (2.92%) had a complication, as defined by the study metrics. Table 3 and Table 4 list the complication rate by specialty and complication type, respectively.
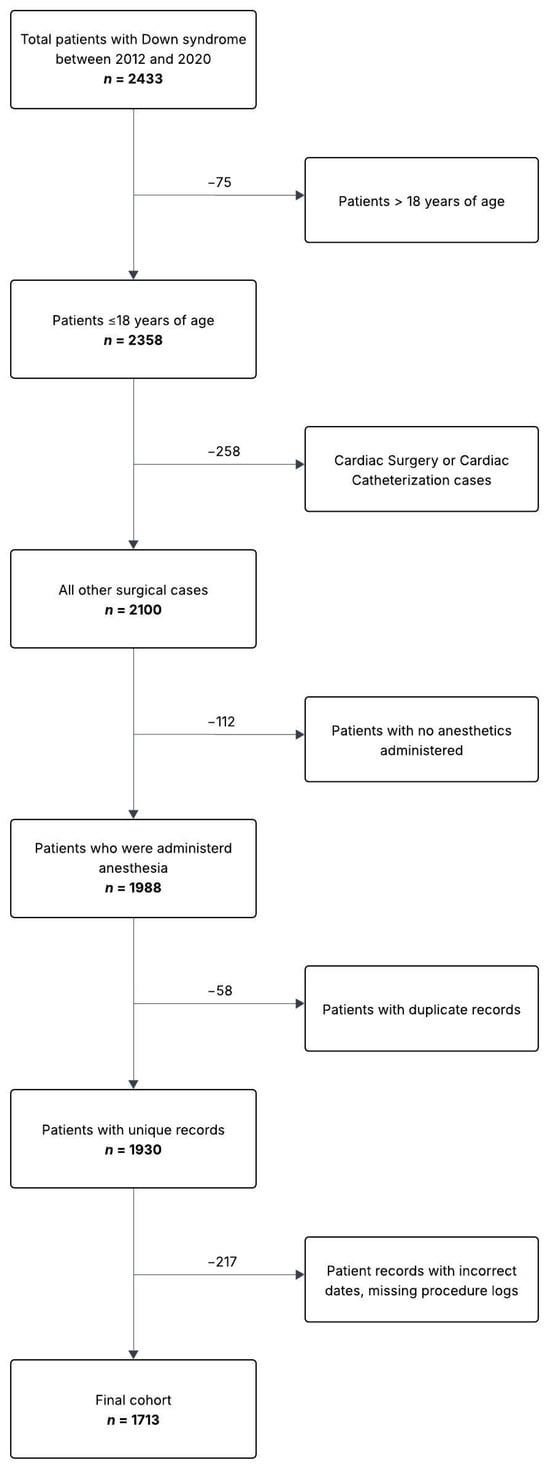
Figure 1.
Patient flow diagram.

Table 1.
Characteristics of patients with and without complications.

Table 2.
Cases by specialty.

Table 3.
Complication Rate by Specialty.

Table 4.
Complication Types.
3.1. Respiratory Complications
In our data, respiratory events were the most common complication (n = 22, 43%). Of the respiratory events, hypoxemia was the most common (n = 12, 0.7%) and typically resolved within hours to days. Only one of the patients with hypoxemia (0.06%) also displayed severe stridor requiring several doses of racemic epinephrine and steroids. Two patients (0.12%) required reintubation after surgery for respiratory failure. Four patients (0.23%) had a new initiation of noninvasive positive pressure ventilation (CPAP or BiPAP that was continued on discharge). Two patients (0.12%) remained intubated after surgery for airway protection due to airway narrowing and/or edema. Two patients (0.12%) presented to the Emergency Department with hypoxemia and respiratory distress after being discharged home. None of the respiratory events occurred in anesthetics for diagnostic imaging.
3.2. Gastrointestinal Complications
Twelve patients (0.7%) had poor oral intake leading to delayed discharge from the hospital. Two patients (0.12%) had delayed return of bowel function, one of whom had to be initiated on total parenteral nutrition.
3.3. Surgical Complications
Three patients (0.18%) required a second operation for hemorrhage. Two patients (0.12%) had hematemesis or bleeding needing admission and monitoring. One patient (0.06%) had new dysphagia and aspiration as a consequence of the surgery. One patient (0.06%) had a wound infection. One patient (0.06%) had surgical findings that warranted intensive care unit admission for monitoring.
3.4. Cardiovascular Complications
One patient (0.06%) experienced persistent bradyarrhythmia that required admission and monitoring. There were two patients (0.12%) with intraoperative cardiac arrests, both of whom had a return of spontaneous circulation. There were no reports of perioperative deaths in our data. Additionally, one patient (0.06%) was diagnosed intraoperatively with coarctation of the aorta necessitating admission and workup.
3.5. Neurologic
Two patients (0.12%) with a history of seizures had perioperative status epilepticus requiring intervention.
4. Discussion
Children with DS often have comorbid conditions [46,47] requiring multiple anesthetics in their lifetime. While the known airway, cardiac, pulmonary, and neuromuscular anomalies of DS might be expected to place these patients at higher risk for perioperative events, our data did not demonstrate this. At our institution, the perioperative complication rate for non-cardiac surgery or medical imaging was 2.98% for children with DS. There is no standardized definition of perioperative complications in the general pediatric literature and reported complication rates vary widely based on these differences in defining criteria, anywhere from 0.3% to nearly 50% [31,48,49]. Our complication rate is comparable to previously reported rates at institutions with dedicated pediatric anesthesiology providers taking care of complex or sicker patients (4.4% and 3.3%) [50,51]. There are studies demonstrating that children with DS are not at additional risk of perioperative complications in both cardiac and non-cardiac surgery [36,37,52,53,54,55]. However, there are certain surgeries where their outcomes are notably worse [24].
Perioperative respiratory events are one of the major causes of morbidity and mortality in pediatric patients undergoing anesthesia [31,32,49]. In our data, respiratory events were the most common complication (43.1%). Of the respiratory events, hypoxemia was the most common and typically resolved within hours to days. Several patients had more critical events such as needing reintubation postoperatively or readmission for respiratory failure. Complications reported in Borland et al. [35] (bradycardia, obstruction, difficult airway, post intubation croup, and bronchospasm) did not figure prominently in our data. This difference in the types of complications may be attributed to the advancement of pediatric anesthesia practice with the routine use of low-pressure cuffed endotracheal tubes and the increased utilization of supraglottic airway devices.
Inadequate oral intake was the second most common complication in our dataset (24%). The refusal to eat or drink postoperatively is multifactorial. It may be due to oropharyngeal pain and dysphagia, as in after airway surgery, and compounded by intellectual disability. While inadequate oral intake may not pose immediate life-threatening risks, it impacts postoperative recovery and patient well-being and may lead to unplanned admissions, lengthened hospital stays, and increased costs.
There was a notable difference in complication rates between outpatients and inpatients (3.4% vs. 1.0%) and 3.38 increased odds of a perioperative adverse event (p = 0.042, Table 5). This is a surprising finding as previous studies have demonstrated that outpatient surgery is safe in pediatric patients [56,57]. This discrepancy may be due to the relatively few number of inpatient surgeries (n = 293) or the additional time available to optimize inpatients preoperatively compared to outpatients. This may also represent an opportunity to enhance our outpatient preoperative medical optimization processes.

Table 5.
Univariate logistic regression model predicting complication.
There was also a large variability in complication rates between specialties. For instance, orthopedic and pediatric surgery had much higher complication rates than the overall group (15.6% and 9.3%, respectively). And on both univariable and multivariate logistic regression, orthopedic surgical cases had a significantly increased odds ratio of suffering a perioperative adverse event (3.65 [1.07–10.9], p = 0.11; Table 6). Diagnostic medical imaging was the second most common anesthetic encounter with a significantly lower odds ratio of a perioperative adverse event (0.12 [0.01–0.55], p = 0.034; Table 6), and only one complication (0.5%). Even so, the single complication was severe and life-threatening brady-arrhythmia, highlighting that anesthesia for low-risk procedures is not without risk.

Table 6.
Multivariate logistic regression model predicting complications.
A significant finding in our data was that an increasing number of procedures, procedural complexity, and total intraoperative minutes was associated with perioperative complications. Our finding is consistent with that previously reported in the literature [58,59]. Sometimes efforts are made by providers to combine multiple procedures into a single anesthetic to reduce cost, parental time burdens, to limit anesthetic exposures, and to limit healthcare costs. Yet, one must balance the potential risk of a perioperative complication following a long anesthetic with the theoretical cost savings of combining procedures.
Patient age, sex, weight, and case urgency were not associated with higher complication rates, which was unexpected given the existing literature suggesting higher risks for severe cardiovascular and respiratory complications in neonates, infants, and patients with higher ASA score [34,60,61]. Several factors may explain this incongruity. First, we excluded high-risk procedures such as cardiac surgery and cardiac catheterization, which are associated with higher morbidity and mortality. Second, the complication rate observed in the present study likely underestimates the true incidence of events because it relied on self-reported data, which tend to underreport adverse clinical events when compared with automated data collection by anesthesia information management systems (AIMS) or an EMR [62]. In children, perioperative adverse events such as hypotension, desaturation, bradycardia, and laryngospasm are relatively common and often regarded as “normal” occurrences associated with to pediatric anesthesia. These events are often considered uneventful as they are easily managed with no sequelae. Finally, the experience of anesthesia providers plays a crucial role in patient safety [31,63]. These cases were performed at tertiary pediatric hospital by experienced pediatric anesthesiologists and their expertise may have mitigated risk.
This study has several limitations. There are potential biases and limitations inherent to a retrospective single center study due to center practices that can disproportionately affect outcomes. Small sample sizes for certain specialties limit the generalizability of the data. Each encounter was considered as a unique event; however, some patients received multiple anesthetics during the study period. In longitudinal data collection, cofactors such as age and weight will change from one encounter to the next, while other intrinsic patient factors will not. Further, there are no matched cohort data for children without DS undergoing non-cardiac surgery or diagnostic imaging. Also, modifiable patient factors which affect perioperative outcomes were not captured well in the EMR (i.e., recent upper respiratory tract infection, asthma, smoking/passive smoking) [2].
Reducing perioperative adverse events requires a multifaceted approach beginning with the identification of at-risk patients. This allows for effective planning, resource allocation, and decisions regarding the location of surgery, staffing, and postoperative disposition [64,65]. A thorough preoperative evaluation, including medical history, physical examination, and clinical assessment, should be completed. Using risk prediction tools can further guide clinical decisions [66,67,68,69]. While some risk factors, such as age, physical status, and type of surgery, are unmodifiable, several modifiable risk factors can reduce the incidence of perioperative adverse events. These include preoperative optimization of asthma, delaying elective surgery following recent respiratory illness, and addressing abnormal lab findings [31,48,49,70]. Our study found that certain specialties were associated with an elevated risk of perioperative adverse events, and that outpatients faced a higher risk overall. This highlights a potential opportunity to improve outpatient preoperative medical optimization at our center.
Intraoperative anesthetic management, including choices between general and regional anesthesia, neuromuscular blockade, airway management (supraglottic airway vs. endotracheal tube), and ventilation strategy, also play an important role in outcomes [31,48,64,71,72]. With the high incidence of obesity and obstructive sleep apnea in children with DS, judicious titration of opioids and the use of multimodal analgesia is recommended [30,64]. And though it is not feasible to have all pediatric cases managed by pediatric anesthesiologists, it is important to consider the experience of the anesthesia provider particularly for high-risk surgeries in this vulnerable group. Studies have shown that both provider experience and pediatric case volume affect the rate of severe critical events [31,32,63]. The type of center (community hospital vs. free-standing children’s hospital) does not appear to affect the incidence of perioperative adverse events. However, children with DS and other high-risk conditions should receive appropriate postoperative monitoring and be cared for at a center capable of providing an elevated level of care if needed [31,64,65,71]. While it is impossible to eliminate all perioperative adverse events, comprehensive preoperative preparation and thoughtful anesthetic management can reduce their frequency and severity.
5. Conclusions
In line with previous reports, children with DS appear to tolerate anesthesia well for non-cardiac surgery or diagnostic imaging. This is significant as our study is the first to examine a large number of ENT and diagnostic imaging cases—two of the most common reasons for children with DS to receive anesthesia. Further research is needed to explore the relationship between complications and perioperative variables, such as the type of surgery, prior respiratory history, or preexisting conditions, to better understand the true risks associated with anesthesia and surgery in children with DS. By better characterizing the perioperative risk profile for this group, our findings may help inform anesthetic management strategies, improve perioperative care, and ultimately enhance safety and outcomes for children with DS surgery or medical imaging.
Author Contributions
M.T. had full access to all the data in the study and takes responsibility for the integrity and accuracy of the data; M.T. was involved in literature search, data collection, data cleaning, data analysis, data interpretation, and manuscript writing. F.Y. was involved in literature search, data collection, data cleaning, data interpretation, and data analysis. E.C. was involved in data analysis, data interpretation, manuscript writing, and manuscript reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The Ann and Robert H. Lurie Children’s Hospital of Chicago Institutional Review Board (IRB) approved this study (2021-4517) on 5 April 2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from Ann and Robert H. Lurie Children’s Hospital of Chicago. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of Ann and Robert H. Lurie Children’s Hospital of Chicago.
Acknowledgments
The authors thank John Hajduk, for his contributions to conception of the work and data curation and to Amy Yang, and Chunyi Wu, for their contributions in statistical analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Graaf, G.; Buckley, F.; Skotko, B.G. Estimates of the live births, natural losses, and elective terminations with Down syndrome in the United States. Am. J. Med. Genet. A 2015, 167A, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, J.; Gautier, M.; Turpin, R. Etude des chromosomes somatiques de neuf enfants mongoliens. Comptes Rendus Hebd. Séances L’académie Sci. 1959, 248, 1721–1722. [Google Scholar]
- Penrose, L.S. The incidence of mongolism in the general population. J. Ment. Sci. 1949, 95, 685–688. [Google Scholar] [CrossRef]
- Collmann, R.D.; Stoller, A. A life table for mongols in Victoria, Australia. J. Ment. Defic. Res. 1963, 7, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.; Bower, C.; Petterson, B.; Leonard, H. Survival of infants born with Down’s syndrome: 1980-96. Paediatr. Perinat. Epidemiol. 2000, 14, 163–171. [Google Scholar] [CrossRef]
- de Graaf, G.; Buckley, F.; Skotko, B.G. Estimation of the number of people with Down syndrome in the United States. Genet. Med. 2017, 19, 439–447. [Google Scholar] [CrossRef]
- Glasson, E.J.; Sullivan, S.G.; Hussain, R.; Petterson, B.A.; Montgomery, P.D.; Bittles, A.H. The changing survival profile of people with Down’s syndrome: Implications for genetic counselling. Clin. Genet. 2002, 62, 390–393. [Google Scholar] [CrossRef]
- Glasson, E.J.; Dye, D.E.; Bittles, A.H. The triple challenges associated with age-related comorbidities in Down syndrome. J. Intellect. Disabil. Res. 2014, 58, 393–398. [Google Scholar] [CrossRef]
- de Jong, A.L.; Sulek, M.; Nihill, M.; Duncan, N.O.; Friedman, E.M. Tenuous airway in children with trisomy 21. Laryngoscope 1997, 107, 345–350. [Google Scholar] [CrossRef]
- Nakazawa, K.; Ikeda, D.; Ishikawa, S.; Makita, K. A case of difficult airway due to lingual tonsillar hypertrophy in a patient with Down’s syndrome. Anesth. Analg. 2003, 97, 704–705. [Google Scholar] [CrossRef]
- Belanger, J.; Kossick, M. Methods of identifying and managing the difficult airway in the pediatric population. AANA J. 2015, 83, 35–41. [Google Scholar] [PubMed]
- Bull, M.J. Down Syndrome. N. Engl. J. Med. 2020, 382, 2344–2352. [Google Scholar] [CrossRef]
- Donnelly, L.F.; Shott, S.R.; LaRose, C.R.; Chini, B.A.; Amin, R.S. Causes of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome as depicted on static and dynamic cine MRI. AJR Am. J. Roentgenol. 2004, 183, 175–181. [Google Scholar] [CrossRef]
- Watts, R.; Vyas, H. An overview of respiratory problems in children with Down’s syndrome. Arch. Dis. Child. 2013, 98, 812–817. [Google Scholar] [CrossRef]
- Hamilton, J.; Yaneza, M.M.; Clement, W.A.; Kubba, H. The prevalence of airway problems in children with Down’s syndrome. Int. J. Pediatr. Otorhinolaryngol. 2016, 81, 1–4. [Google Scholar] [CrossRef]
- Miller, R.; Gray, S.D.; Cotton, R.T.; Myer, C.M., 3rd; Netterville, J. Subglottic stenosis and Down syndrome. Am. J. Otolaryngol. 1990, 11, 274–277. [Google Scholar] [CrossRef]
- Martinez-Espinosa, R.M.; Molina Vila, M.D.; Reig Garcia-Galbis, M. Evidences from Clinical Trials in Down Syndrome: Diet, Exercise and Body Composition. Int. J. Environ. Res. Public Health 2020, 17, 4294. [Google Scholar] [CrossRef]
- Bertapelli, F.; Pitetti, K.; Agiovlasitis, S.; Guerra-Junior, G. Overweight and obesity in children and adolescents with Down syndrome-prevalence, determinants, consequences, and interventions: A literature review. Res. Dev. Disabil. 2016, 57, 181–192. [Google Scholar] [CrossRef]
- Jacobs, I.N.; Gray, R.F.; Todd, N.W. Upper airway obstruction in children with Down syndrome. Arch. Otolaryngol. Head. Neck Surg. 1996, 122, 945–950. [Google Scholar] [CrossRef]
- Pandit, C.; Fitzgerald, D.A. Respiratory problems in children with Down syndrome. J. Paediatr. Child. Health 2012, 48, E147–E152. [Google Scholar] [CrossRef]
- Lewanda, A.F.; Matisoff, A.; Revenis, M.; Harahsheh, A.; Futterman, C.; Nino, G.; Greenberg, J.; Myseros, J.S.; Rosenbaum, K.N.; Summar, M. Preoperative evaluation and comprehensive risk assessment for children with Down syndrome. Paediatr. Anaesth. 2016, 26, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; Dott, B.; Alembik, Y.; Roth, M.P. Associated congenital anomalies among cases with Down syndrome. Eur. J. Med. Genet. 2015, 58, 674–680. [Google Scholar] [CrossRef]
- Irving, C.A.; Chaudhari, M.P. Cardiovascular abnormalities in Down’s syndrome: Spectrum, management and survival over 22 years. Arch. Dis. Child. 2012, 97, 326–330. [Google Scholar] [CrossRef]
- Dimopoulos, K.; Constantine, A.; Clift, P.; Condliffe, R.; Moledina, S.; Jansen, K.; Inuzuka, R.; Veldtman, G.R.; Cua, C.L.; Tay, E.L.W.; et al. Cardiovascular Complications of Down Syndrome: Scoping Review and Expert Consensus. Circulation 2023, 147, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Voepel-Lewis, T.; Malviya, S. Hemodynamic changes in children with Down syndrome during and following inhalation induction of anesthesia with sevoflurane. J. Clin. Anesth. 2010, 22, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, F.W.; Stricker, P.A.; Gurnaney, H.G.; McClung, H.; Meador, M.R.; Sussman, E.; Burgess, B.J.; Ciampa, B.; Mendelsohn, J.; Rehman, M.A.; et al. Bradycardia during induction of anesthesia with sevoflurane in children with Down syndrome. Anesth. Analg. 2010, 111, 1259–1263. [Google Scholar] [CrossRef]
- Lagan, N.; Huggard, D.; Mc Grane, F.; Leahy, T.R.; Franklin, O.; Roche, E.; Webb, D.; O’Marcaigh, A.; Cox, D.; El-Khuffash, A.; et al. Multiorgan involvement and management in children with Down syndrome. Acta Paediatr. 2020, 109, 1096–1111. [Google Scholar] [CrossRef]
- McDowell, K.M.; Craven, D.I. Pulmonary complications of Down syndrome during childhood. J. Pediatr. 2011, 158, 319–325. [Google Scholar] [CrossRef]
- Ramba, M.; Bogunovic, D. The immune system in Down Syndrome: Autoimmunity and severe infections. Immunol. Rev. 2024, 322, 300–310. [Google Scholar] [CrossRef]
- Egbuta, C.; Mason, K.P. Recognizing Risks and Optimizing Perioperative Care to Reduce Respiratory Complications in the Pediatric Patient. J. Clin. Med. 2020, 9, 1942. [Google Scholar] [CrossRef]
- Habre, W.; Disma, N.; Virag, K.; Becke, K.; Hansen, T.G.; Johr, M.; Leva, B.; Morton, N.S.; Vermeulen, P.M.; Zielinska, M.; et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): A prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir. Med. 2017, 5, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Mamie, C.; Habre, W.; Delhumeau, C.; Argiroffo, C.B.; Morabia, A. Incidence and risk factors of perioperative respiratory adverse events in children undergoing elective surgery. Paediatr. Anaesth. 2004, 14, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bhananker, S.M.; Ramamoorthy, C.; Geiduschek, J.M.; Posner, K.L.; Domino, K.B.; Haberkern, C.M.; Campos, J.S.; Morray, J.P. Anesthesia-related cardiac arrest in children: Update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth. Analg. 2007, 105, 344–350. [Google Scholar] [CrossRef]
- Disma, N.; Veyckemans, F.; Virag, K.; Hansen, T.G.; Becke, K.; Harlet, P.; Vutskits, L.; Walker, S.M.; de Graaff, J.C.; Zielinska, M.; et al. Morbidity and mortality after anaesthesia in early life: Results of the European prospective multicentre observational study, neonate and children audit of anaesthesia practice in Europe (NECTARINE). Br. J. Anaesth. 2021, 126, 1157–1172. [Google Scholar] [CrossRef]
- Borland, L.M.; Colligan, J.; Brandom, B.W. Frequency of anesthesia-related complications in children with Down syndrome under general anesthesia for noncardiac procedures. Paediatr. Anaesth. 2004, 14, 733–738. [Google Scholar] [CrossRef]
- Cairo, S.B.; Zeinali, L.I.; Berkelhamer, S.K.; Harmon, C.M.; Rao, S.O.; Rothstein, D.H. Down Syndrome and Postoperative Complications in Children Undergoing Intestinal Operations. J. Pediatr. Surg. 2019, 54, 1832–1837. [Google Scholar] [CrossRef]
- Evans, J.M.; Dharmar, M.; Meierhenry, E.; Marcin, J.P.; Raff, G.W. Association between Down syndrome and in-hospital death among children undergoing surgery for congenital heart disease: A US population-based study. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 445–452. [Google Scholar] [CrossRef]
- Graber, T.J.; Baskin, P.L.; Soria, C.; Greenberg, M.; Gabriel, R.A.; Brzenski, A. An assessment of perioperative respiratory adverse events and difficult intubation in pediatric patients with Trisomy 21. Paediatr. Anaesth. 2021, 31, 410–418. [Google Scholar] [CrossRef]
- Yumusakhuylu, A.C.; Binnetoglu, A.; Demir, B.; Baglam, T.; Sari, M. Is it safe to perform adenotonsillectomy in children with Down syndrome? Eur. Arch. Otorhinolaryngol. 2016, 273, 2819–2823. [Google Scholar] [CrossRef]
- Sha, S.; Abdelsabour, H.; Vijimohan, S.J.; Board, T.; Alshryda, S. Total hip arthroplasty in patients with Trisomy 21: Systematic review and exploratory patient level analysis. Surgeon 2019, 17, 52–57. [Google Scholar] [CrossRef]
- Bartels, D.D.; McCann, M.E.; Davidson, A.J.; Polaner, D.M.; Whitlock, E.L.; Bateman, B.T. Estimating pediatric general anesthesia exposure: Quantifying duration and risk. Paediatr. Anaesth. 2018, 28, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Gloff, M.S.; Robinson, R.; Correll, L.R.; Lander, H.; Pyne, S.; Webber, A. Preoperative optimization in the pediatric patient. Int. Anesthesiol. Clin. 2022, 60, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lerman, J. Pediatric ambulatory anesthesia: An update. Curr. Opin. Anaesthesiol. 2019, 32, 708–713. [Google Scholar] [CrossRef]
- Construction Financial Management Association. National Physician Fee Schedule Relative Value File January Release. Available online: https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files (accessed on 26 September 2023).
- Dyas, A.R.; Meguid, R.A.; Bronsert, M.R.; Madsen, H.J.; Colborn, K.L.; Lambert-Kerzner, A.; Henderson, W.G. Does Work Relative Value Unit Measure Surgical Complexity for Risk Adjustment of Surgical Outcomes? J. Surg. Res. 2023, 287, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Petri, H.; Ding, Y.; Wandel, C.; Khwaja, O.; Foskett, N. Morbidity and medication in a large population of individuals with Down syndrome compared to the general population. Dev. Med. Child. Neurol. 2016, 58, 246–254. [Google Scholar] [CrossRef]
- Roizen, N.J.; Magyar, C.I.; Kuschner, E.S.; Sulkes, S.B.; Druschel, C.; van Wijngaarden, E.; Rodgers, L.; Diehl, A.; Lowry, R.; Hyman, S.L. A community cross-sectional survey of medical problems in 440 children with Down syndrome in New York State. J. Pediatr. 2014, 164, 871–875. [Google Scholar] [CrossRef]
- von Ungern-Sternberg, B.S.; Boda, K.; Chambers, N.A.; Rebmann, C.; Johnson, C.; Sly, P.D.; Habre, W. Risk assessment for respiratory complications in paediatric anaesthesia: A prospective cohort study. Lancet 2010, 376, 773–783. [Google Scholar] [CrossRef]
- von Ungern-Sternberg, B.S.; Sommerfield, D.; Slevin, L.; Drake-Brockman, T.F.E.; Zhang, G.; Hall, G.L. Effect of Albuterol Premedication vs Placebo on the Occurrence of Respiratory Adverse Events in Children Undergoing Tonsillectomies: The REACT Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 527–533. [Google Scholar] [CrossRef]
- Engelhardt, T.; Ayansina, D.; Bell, G.T.; Oshan, V.; Rutherford, J.S.; Morton, N.S.; APRICOT Group of the European Society of Anaesthesiology Clinical Trial Network. Incidence of severe critical events in paediatric anaesthesia in the United Kingdom: Secondary analysis of the anaesthesia practice in children observational trial (APRICOT study). Anaesthesia 2019, 74, 300–311. [Google Scholar] [CrossRef]
- Hansen, T.G.; Borke, W.B.; Isohanni, M.H.; Castellheim, A.; APRICOT Study Group of the European Society of Anaesthesiology Clinical Trial Network. Incidence of severe critical events in paediatric anaesthesia in Scandinavia: Secondary analysis of Anaesthesia PRactice in Children Observational Trial (APRICOT). Acta Anaesthesiol. Scand. 2019, 63, 601–609. [Google Scholar] [CrossRef]
- Toth, R.; Szanto, P.; Prodan, Z.; Lex, D.J.; Sapi, E.; Szatmari, A.; Gal, J.; Szanto, T.; Szekely, A. Down syndrome and postoperative complications after paediatric cardiac surgery: A propensity-matched analysis. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Hoashi, T.; Hirahara, N.; Murakami, A.; Hirata, Y.; Ichikawa, H.; Kobayashi, J.; Takamoto, S. Current Surgical Outcomes of Congenital Heart Surgery for Patients with Down Syndrome in Japan. Circ. J. 2018, 82, 403–408. [Google Scholar] [CrossRef] [PubMed]
- St Louis, J.D.; Jodhka, U.; Jacobs, J.P.; He, X.; Hill, K.D.; Pasquali, S.K.; Jacobs, M.L. Contemporary outcomes of complete atrioventricular septal defect repair: Analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J. Thorac. Cardiovasc. Surg. 2014, 148, 2526–2531. [Google Scholar] [CrossRef]
- Bartz-Kurycki, M.A.; Anderson, K.T.; Austin, M.T.; Kao, L.S.; Tsao, K.; Lally, K.P.; Kawaguchi, A.L. Increased complications in pediatric surgery are associated with comorbidities and not with Down syndrome itself. J. Surg. Res. 2018, 230, 125–130. [Google Scholar] [CrossRef]
- Crute, W.; Wofford, A.; Powers, J.; Smith, D.P. Comprehensive review of a large cohort of outpatient versus inpatient open renal and bladder surgery in children. J. Pediatr. Urol. 2023, 19, 432.e1–432.e8. [Google Scholar] [CrossRef]
- Jardaly, A.; Torrez, T.W.; McGwin, G.; Gilbert, S.R. Comparing complications of outpatient management of slipped capital femoral epiphysis and Blount’s disease: A database study. World J. Orthop. 2022, 13, 373–380. [Google Scholar] [CrossRef]
- Miketic, R.M.; Uffman, J.; Tumin, D.; Tobias, J.D.; Raman, V.T. Experience with Combining Pediatric Procedures into a Single Anesthetic. Pediatr. Qual. Saf. 2019, 4, e207. [Google Scholar] [CrossRef]
- Cheon, E.C.; Palac, H.L.; Paik, K.H.; Hajduk, J.; De Oliveira, G.S.; Jagannathan, N.; Suresh, S. Unplanned, Postoperative Intubation in Pediatric Surgical Patients: Development and Validation of a Multivariable Prediction Model. Anesthesiology 2016, 125, 914–928. [Google Scholar] [CrossRef]
- van der Griend, B.F.; Lister, N.A.; McKenzie, I.M.; Martin, N.; Ragg, P.G.; Sheppard, S.J.; Davidson, A.J. Postoperative mortality in children after 101,885 anesthetics at a tertiary pediatric hospital. Anesth. Analg. 2011, 112, 1440–1447. [Google Scholar] [CrossRef]
- Murat, I.; Constant, I.; Maud’huy, H. Perioperative anaesthetic morbidity in children: A database of 24,165 anaesthetics over a 30-month period. Paediatr. Anaesth. 2004, 14, 158–166. [Google Scholar] [CrossRef]
- Simpao, A.F.; Pruitt, E.Y.; Cook-Sather, S.D.; Gurnaney, H.G.; Rehman, M.A. The reliability of manual reporting of clinical events in an anesthesia information management system (AIMS). J. Clin. Monit. Comput. 2012, 26, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Zgleszewski, S.E.; Graham, D.A.; Hickey, P.R.; Brustowicz, R.M.; Odegard, K.C.; Koka, R.; Seefelder, C.; Navedo, A.T.; Randolph, A.G. Anesthesiologist- and System-Related Risk Factors for Risk-Adjusted Pediatric Anesthesia-Related Cardiac Arrest. Anesth. Analg. 2016, 122, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.P.; Webber, A.M.; Patel, C.V.; Chin, W.A.; Butz, S.F.; Rajan, N. Care of the Pediatric Patient for Ambulatory Tonsillectomy with or Without Adenoidectomy: The Society for Ambulatory Anesthesia Position Statement. Anesth. Analg. 2024, 139, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Templeton, T.W.; Sommerfield, D.; Hii, J.; Sommerfield, A.; Matava, C.T.; von Ungern-Sternberg, B.S. Risk assessment and optimization strategies to reduce perioperative respiratory adverse events in Pediatric Anesthesia-Part 2: Anesthesia-related risk and treatment options. Paediatr. Anaesth. 2022, 32, 217–227. [Google Scholar] [CrossRef]
- Nasr, V.G.; DiNardo, J.A.; Faraoni, D. Development of a Pediatric Risk Assessment Score to Predict Perioperative Mortality in Children Undergoing Noncardiac Surgery. Anesth. Analg. 2017, 124, 1514–1519. [Google Scholar] [CrossRef]
- Tait, A.R.; Voepel-Lewis, T.; Christensen, R.; O’Brien, L.M. The STBUR questionnaire for predicting perioperative respiratory adverse events in children at risk for sleep-disordered breathing. Paediatr. Anaesth. 2013, 23, 510–516. [Google Scholar] [CrossRef]
- Subramanyam, R.; Yeramaneni, S.; Hossain, M.M.; Anneken, A.M.; Varughese, A.M. Perioperative Respiratory Adverse Events in Pediatric Ambulatory Anesthesia: Development and Validation of a Risk Prediction Tool. Anesth. Analg. 2016, 122, 1578–1585. [Google Scholar] [CrossRef]
- Lee, L.K.; Bernardo, M.K.L.; Grogan, T.R.; Elashoff, D.A.; Ren, W.H.P. Perioperative respiratory adverse event risk assessment in children with upper respiratory tract infection: Validation of the COLDS score. Paediatr. Anaesth. 2018, 28, 1007–1014. [Google Scholar] [CrossRef]
- Hii, J.; Templeton, T.W.; Sommerfield, D.; Sommerfield, A.; Matava, C.T.; von Ungern-Sternberg, B.S. Risk assessment and optimization strategies to reduce perioperative respiratory adverse events in pediatric anesthesia-Part 1 patient and surgical factors. Paediatr. Anaesth. 2022, 32, 209–216. [Google Scholar] [CrossRef]
- August, D.A.; Everett, L.L. Pediatric ambulatory anesthesia. Anesthesiol. Clin. 2014, 32, 411–429. [Google Scholar] [CrossRef]
- Drake-Brockman, T.F.; Ramgolam, A.; Zhang, G.; Hall, G.L.; von Ungern-Sternberg, B.S. The effect of endotracheal tubes versus laryngeal mask airways on perioperative respiratory adverse events in infants: A randomised controlled trial. Lancet 2017, 389, 701–708. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).