Abstract
Since the early 1990s, Gaucher Disease has been a pioneering condition for home-based enzyme replacement therapy (ERT), marking a significant shift in patient care. Since then, many countries have adopted this approach. However, home ERT is not possible in all countries. Objectives: The aim of this article is to explore the implementation of home ERT for Gaucher disease, focusing on patient expectations, safety, compliance, economic benefits, and practical considerations. Methods: The PRISMA reporting protocol was followed, focusing on articles about home ERT for Gaucher Disease. Results: Twenty articles were analyzed in the review, revealing promising outcomes. Home ERT has consistently been shown to be safe, to improve patients’ quality of life, to reduce the utilization of hospital resources, and to pose no compliance issues. Conclusions: We believe it is essential to expand the availability of home ERT for Gaucher Disease to all countries where ERT is accessible. Based on the literature review, we present the conditions that must be met before starting home ERT programs.
1. Introduction
Enzyme replacement therapy (ERT) has been available for patients with Gaucher disease (GD) since the early 1990s [1]. ERT can be administered in hospitals or at home based on patient needs, resource availability, and safety considerations. Hospital administration ensures medical supervision, which is vital for managing potential adverse reactions but is costlier and less convenient. Home therapy, while more comfortable and cost-effective, demands well-trained patients or caregivers and robust support systems, potentially reducing hospital resource strain but increasing the need for monitoring infrastructure, impacting the National Healthcare System through resource reallocation and potential cost efficiencies. In some countries, home infusion, including self-infusion, was advocated from the outset. Currently, in many countries, patients have the option to receive ERT at home. However, there are still countries or regions where home ERT is not approved or not reimbursed [2].
The COVID-19 pandemic, which impacted the delivery of ERT in hospitals worldwide, prompted the International Gaucher Alliance (IGA), an organization bringing together all organizations of patients with GD, to investigate with its global community the current provision of home ERT and how it is organized in each country. Their project aimed to facilitate a proper home ERT provision, especially in countries where home ERT is unavailable, and to develop guidelines and potentially other informative resources. The International Working Group on Gaucher Disease (IWGGD) aims to promote clinical and basic research into GD and strives to improve the quality of life for patients. Bringing together GD experts, healthcare professionals, and patients in an open forum for discussion, the IWGGD members are working on consensus guidelines for treating patients with GD (https://www.iwggd.com). The IGA and IWGGD have joined forces to expand the use of home ERT in regions where it is not yet available. In this article, we present a literature review following PRISMA guidelines [3] on home ERT for GD and provide recommendations for the necessary steps and conditions to be met before implementing such a program.
2. Materials and Methods
A search of the literature was conducted in Pubmed/Medline database, Cochrane Review and Google Scholar with Windows version 0.24121.37.0, No entry date was set, and the search was revised in November 2024. The keywords used were as follows: ((“Gaucher Disease”[Mesh]) OR Gaucher Disease, OR (“lysosomal storage”[Mesh])) OR lysosomal storage AND (“enzyme replacement therapy”[Mesh]) OR Enzyme replacement Therapy AND “Home therapy” [Mesh] OR Home therapy to extract original studies, expert opinions, guidelines, and reports published in international journals. This investigation was conducted by two assessors. One hundred twenty results were retrieved. Extracted publications were screened for relevance and duplication. Studies discussing patients with LSDs other than type 1 GD, non-enzymatic home therapy, or non-English articles were excluded. Twenty relevant articles were then analyzed (Figure 1).
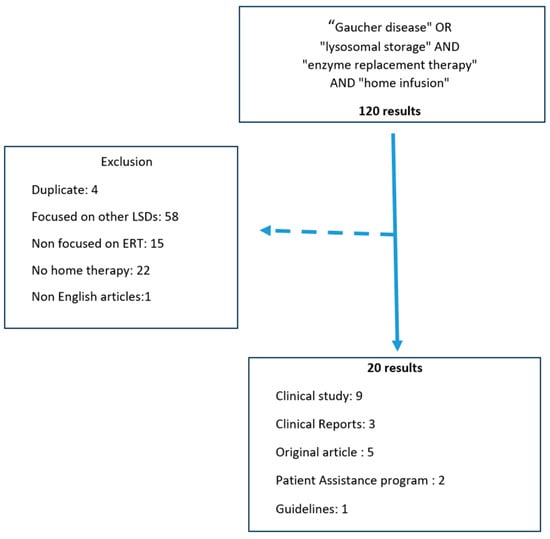
Figure 1.
Flow chart of literature search [2,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22].
3. Results
3.1. Historical Overview
GD was the first lysosomal storage disease (LSD) to be treated with ERT [1]. ERT has been shown to be highly effective in treating hematological, visceral, and bone manifestations of GD [23]. It is a lifelong treatment administered via intravenous infusion every two weeks. GD was also the first LSD to be treated with home ERT [16]. In the first international collaborative study, the authors reported that home ERT for GD was safe, feasible, and well accepted by the patients and their families [16]. The primary motivation for opting for home ERT was to reduce the burden related to recurrent hospital visits. Since then, clinical trials, including those involving children, have confirmed the high safety profile of home ERT [4,5,6,7].
3.2. Patients Perspectives
In a survey published by Milligan et al. [9] comparing hospital therapy versus home ERT in the United Kingdom (UK), the authors found that 21 out of 25 adult patients with type 1 GD preferred home ERT. These patients initially received ERT in the hospital for at least a year before transitioning to home administration. Interestingly, although most patients preferred home ERT, half of the patients reported that being treated in a hospital was not an added burden. The main stressors associated with hospital-based ERT were the need to travel to the hospital, the hospital environment itself, waiting for the treatment to be administered, and missing work or school days. However, some patients expressed concerns about the quality of health monitoring while undergoing home ERT. Patients who preferred in-hospital treatment cited a perceived increased safety, as well as the opportunity for interaction and exchange with other patients, as the greatest benefits. Conversely, most patients reported that home ERT did not negatively impact their family life. According to the survey results, most patients with type 1 GD felt that home ERT was “more effective” and easier to accept. The authors emphasized that patients adjusted rapidly to receiving therapy at home. Only 2 out of 25 patients opted to continue receiving in-hospital ERT, finding home ERT more stressful than hospital therapy.
3.3. Safety of Home ERT
While home ERT significantly improves patient comfort and quality of life, the question of safety is crucial. Although ERT for GD is generally well tolerated, ensuring safety in a home setting is essential.
The first report on home ERT for GD was an international collaborative study published in 1993, focusing on alglucerase home infusion [16]. The authors reported the safety and feasibility of low-dose/high-frequency home ERT in 33 patients with GD. They also highlighted the feasibility of a venous access device implanted into 24 patients to address vein access issues and the necessity of regular visits by the nurse.
Subsequent publications showed that home ERT was safe for both adults and children with type 1 GD, based on both clinical trials and real-life experience [6]. In another study, 18 out of 35 patients reported no problems with home ERT [10]. Those who did experience issues reported only minor adverse events (AEs) such as fever while still feeling more in control of their treatment and condition.
Home ERT was also offered as an option for velaglucerase alfa in the four open-label clinical studies [11] to eligible patients who received their initial ERT in the hospital (Table 1). A total of 104 patients receiving at least one infusion of velaglucerase alfa at home were included in these four trials [7]. No safety concerns related to the location of the treatment (home versus hospital) were observed. All patients underwent their initial infusions in the hospital before transitioning to home care.

Table 1.
Summary of the studies dedicated to home enzyme replacement therapy for Gaucher disease.
In another study, Zimran et al. evaluated the safety of progressively decreased infusion time of velaglucerase alfa [17], allowing for home ERT in the final phase. Fifteen patients were included, and the infusion was accelerated gradually from 60 min to 10 min without any adverse events (AEs), and the return to home settings was also uneventful. This possibility was corroborated by two additional studies from the same team [5,22] (Table 1).
A safety analysis that included all taliglucerase alfa AEs reported in a global safety database retrieved data for 163 cases [4]; among them, 33 were associated with home use (19 definite, 14 possible). None were fatal, 14 were serious, and 19 were non-serious. No specific or increased risk associated with home administration was noted (Table 1).
3.4. Experience During the COVID-19 Pandemic
In recent years, the SARS-CoV-2 pandemic has highlighted some limitations of the global healthcare system. It necessitated changes in the organization of work, shifting certain procedures outside of hospital settings. New challenges arose in managing care for patients with chronic diseases. In a 2021 report, 44.6% of 92 healthcare professionals treating patients with LSD, including GD, mentioned that access to ERT was a critical issue during the COVID-19 pandemic [24]. Access was not only impacted by hospital ERT protocols but also by self-isolation or care restrictions due to COVID-19 infection or fear of infection [25]. A panel of experts published recommendations related to the COVID-19 pandemic and GD treatment, emphasizing that ERT must be continued without prolonged interruption. While missing one or two infusions is generally not harmful for most patients, a prolonged interruption must be avoided. Home ERT was proposed as a solution to avoid prolonged interruptions, particularly during crisis situations [19,20].
3.5. Home ERT and Compliance
While home ERT provides greater independence for patients, treatment compliance can be a concern for healthcare providers. In a study by Hughes et al. involving patients with type 1 GD, more than half have received home imiglucerase for over 6 years. Among these, 21 out of 35 reported that they had never missed a dose [10]. For those who did miss doses, it was typically due to vacations lasting longer than two weeks. Additionally, nearly all patients considered themselves to be highly cooperative with their healthcare team. Clinical studies have shown that home ERT can improve patient compliance. In a clinical trial with velaglucerase alfa, the challenge of long distances traveled for hospital-based infusions was highlighted [18]. Home ERT was introduced to address this issue, and its effectiveness was reflected by the inclusion of home treatment in the protocol after the extension study began. Recently, Revel-Vilk et al. reported excellent (≥95%) annual compliance with taliglucerase alfa at home [4].
3.6. Medical Requirements/Resources to Start Home ERT
Home ERT must be implemented with a focus on safety. According to Hughes et al. [10], one hospital-based infusion is generally sufficient for adult patients with type 1 GD. However, in practice, patients often receive three infusions in a hospital setting before transitioning to home ERT [4,21]. In some countries, like the Netherlands, the first infusion can take place at home under the supervision of a trained nurse (Carla Hollak’s personal communication). Home ERT requires a well-organized and regulated community infrastructure, individual assessments of patient suitability, and protocols for the management of possible infusion-related reactions. A small number of patients may still prefer in-hospital ERT, often due to hospital proximity, challenges with venous access, or the need for other specialist procedures and consultations that cannot be performed at home [10]. Respecting patient choice is essential.
Although self-infusion is sometimes practiced in some countries, we did not find any articles evaluating this practice in GD.
3.7. Economic Advantages
Home-based ERT offers additional financial benefits for the healthcare system, including reduced use of hospital resources such as treatment rooms and nursing staff. The significant financial savings have made home ERT a favorable option for third-party payers (funding agencies depending on the country: Ministry of Health, insurance companies, etc.) [26].
A recent budget impact assessment from the perspective of the United States (US) payers found that home ERT costs 25% to 50% less than ERT administered in outpatient infusion clinics or hospitals [26].
4. Discussion
According to the literature, all ERTs registered for GD (imiglucerase, velaglucerase alfa, and taliglucérase according to FDA/EMA approvals) can be administered at home [4,7,27]. This review found that home ERT in GD was safe and well-accepted by the patients, who found it to be more comfortable and less stressful, with improvements in their quality of life. It also reduced hospitalization costs, and there was no issue with compliance. Enzyme replacement therapy for patients with GD was the first to have been administered at home. Thanks to this initial successful experience, patients with Fabry disease (FD) were also home treated [28,29]. As with GD, home ERT was associated with an improvement in the quality of life, enhancing patients’ ability to manage their own care and increasing their independence, particularly for those who learned to self-cannulate. Receiving home ERT and, possibly, learning how to administer the ERT independently could mitigate the negative impact caused by frequent hospital visits and associated travel [29]. Due to the positive and encouraging results, the implementation of ERT at home was then started for other LSDs such as mucopolysaccharidosis (MPS) type I, MPS type II and VI, and Pompe disease [30,31,32,33]. To date, more than 15 ERTs are home-administered.
Most of the patients with GD prefer to be treated at home. This preference is also reflected in surveys of LSDs in general. In two surveys conducted in Italy, not specifically focused on GD, the authors reported the negative impact on the quality of life when ERT is administered in hospitals [8]. The first survey was a nationwide study involving reference centers. It revealed that only a small percentage of patients (2.6%) received ERT at home; the majority were treated in a local hospital or at reference centers. The procedures of the Italian health service do not permit home ERT in every region. The second survey was a regional study in Lombardy, where 12% of patients received ERT at home. The results clearly indicated that in-hospital ERT significantly affects the quality of life of these patients.
Furthermore, pandemics highlight the crucial role of home therapy for LSD, including GD. In one Italian experience during the COVID-19 pandemic, half of the patients receiving ERT in hospitals experienced disruptions while only a few of those treated at home were affected. The primary reasons were fear of infection and the re-organization of the infusion centers [34]. According to patients, in-hospital ERT is disruptive, leading to lost days at school or work, stress, and family problems, whereas patients treated at home did not encounter these issues. Additionally, studies on home ERT emphasize increased treatment compliance and an improvement in quality of life for both patients and their families [35]. Home therapy can restore a degree of independence, allowing patients to schedule their own treatment at times that do not interfere with work commitments and family life. Moreover, clinical trials for new therapies in LSDs are conducted with a limited number of patients, and patients may refuse to participate if participation requires hospital treatment. Any participant dropping out of a trial can significantly impact its results. Therefore, the use of home ERT can enhance patients’ compliance and facilitate the collection of reliable and clinically valuable data. These various studies underscore the importance of involving the patient in the choice of treatment modalities, particularly during chronic diseases.
According to the literature, ERT for GD is usually very well tolerated, with most side effects occurring in the first three months of treatment and usually being non-severe. Although antibodies against the enzyme may develop, they are not always associated with side effects [27,36]. Most of the AEs associated with ERT in GD are type B side effects, i.e., idiosyncratic, bizarre, or novel responses that cannot be predicted by the pharmacology and are often the result of an immune reaction (allergy) to the drug [37]. In cases of an allergic reaction to an ERT, switching to a different ERT should ideally be performed in a hospital setting, with the first infusion administered under medical supervision. At home, certain precautions should be taken: patients must be trained to recognize AEs and know how to stop the infusion, possibly taking an antihistamine and/or paracetamol and/or corticosteroids. They must be able to contact their healthcare professional. It is recommended, though not mandatory, that a third person be present during the infusion. All AEs should be reported to the reference center and documented in the patient’s records. If prophylactic medication is used to prevent allergic reactions, these drugs should be included in this patient’s administration protocol before ERT administration and reviewed regularly.
Based on the literature review, we developed recommendations for home ERT in GD (Table 2). Written materials and informational leaflets should be provided to the patient regarding the organization of the home infusion.

Table 2.
Conditions that must be met before starting home enzyme replacement therapy for Gaucher Disease.
By analogy with FD, home ERT for GD can also offer economic benefits. An interesting analysis was carried out in a study from Norway [38], modeling the resource implications of managing adults with FD. The authors noted that in an average year, patients receiving ERT for FD are expected to make 586 visits to their family practitioner’s office for ERT, which equates to 128 eight-hour days dedicated to ERT. The authors concluded that increasing the proportion of adults with FD receiving home-based ERT could free-up community-based resources, thereby improving the efficiency of medical care for other patients in the public healthcare system in Norway. Additional economic advantages include time savings, less time off work due to illness, or more time available for family responsibilities.
In some countries, ERT can be administered by self-infusion or infusion by a third party. This decision, of course, requires a discussion between the patient and the treating physician, as well as training in the technique. However, no literature on self-infusion was found for GD.
5. Conclusions
Home ERT offers several advantages over hospital-based therapy: it eliminates the need for frequent and regular hospital visits, provides the patient with greater independence and a sense of control over the disease, reduces the risk of exposure to potential hospital-acquired infections, and, from an economic standpoint, reduces the use of hospital resources. Home ERT can be integrated into the patient’s daily life, reducing the impact of treatment on work and family life, and thereby significantly improving the quality of life for the patient and his/her relatives. The authors recommend that, with patient agreement and after verifying the eligibility criteria, home ERT should be offered to all patients with GD, which is not yet possible in all countries where ERT is available. The IGA and the IWGGD are continuing their work together to develop guidelines for the proper, safe, cost-effective, and satisfactory implementation of home ERT for GD.
Author Contributions
B.K.-W., P.G. and C.S. were involved in the conceptualization and design of the study and have drafted this work. B.K.-W., P.G., C.S., M.D., S.R.-V. and M.A. provided critical revisions, final approval, and are accountable for the accuracy and integrity of the manuscript. Board members of IWGGD careful review and provided constructive suggestions. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data extracted from the included studies are publicly available in PubMed (https://pubmed.ncbi.nlm.nih.gov/), in Google Scholar (https://scholar.google.com/), and Cochrane Library (https://www.cochranelibrary.com/advanced-search). The accessed date was on 15 November 2024.
Acknowledgments
We would like to thank the IWGGD board members and Tanya Collin-Histed for their careful review and their constructive suggestions.
Conflicts of Interest
C.S. reports receiving honoraria and advisory fees from Sanofi/Genzyme and grant/research support, as well as honoraria and advisory fees from Takeda. S.R.-V. reports receiving grant/research support, honoraria, and advisory fees from Takeda, Pfizer, and Sanofi/Genzyme. B.K.-W., M.D., P.G. and M.A. have no conflicts of interest to declare. IGA has received funding for its annual work program from Avrobio, Freeline, Gain, Lily, M6P, Pfizer, Sanofi, and Takeda.
References
- Brady, R.O.; Murray, G.J.; Barton, N.W. Modifying exogenous glucocerebrosidase for effective replacement therapy in Gaucher disease. J. Inherit. Metab. Dis. 1994, 17, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Znidar, I.; Collin-Histed, T.; Niemeyer, P.; Parkkinen, J.; Lauridsen, A.G.; Zarina, S.; Cohen, Y.; Manuel, J. The European Gaucher Alliance: A survey of member patient organisations’ activities, healthcare environments and concerns. Orphanet J. Rare Dis. 2014, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Revel-Vilk, S.; Mansfield, R.; Feder-Krengel, N.; Machtiger-Azoulay, N.; Kuter, D.; Szer, J.; Rosenbaum, H.; Ferreira, D.C.; Ruhrman-Shahar, N.; Wajnrajch, M.; et al. Real-World Experiences with Taliglucerase Alfa Home Infusions for Patients with Gaucher Disease: A Global Cohort Study. J. Clin. Med. 2023, 12, 5913. [Google Scholar] [CrossRef]
- Becker-Cohen, M.; Revel-Vilk, S.; Frydman, D.; Dinur, T.; Tiomkin, M.; Istaiti, M.; Arbel, N.; Bauer, P.; Cozma, C.; Rolfs, A.; et al. Rapid home therapy infusion of velaglucerase alfa in naïve patients with Gaucher disease. Intern. Med. J. 2023, 54, 398–403. [Google Scholar] [CrossRef]
- Elstein, D.; Abrahamov, A.; Oz, A.; Arbel, N.; Baris, H.; Zimran, A. 13,845 home therapy infusions with velaglucerase alfa exemplify safety of velaglucerase alfa and increased compliance to every-other-week intravenous enzyme replacement therapy for Gaucher disease. Blood Cells Mol. Dis. 2015, 55, 415–418. [Google Scholar] [CrossRef]
- Elstein, D.; Burrow, T.A.; Charrow, J.; Giraldo, P.; Mehta, A.; Pastores, G.M.; Lee, H.M.; Mellgard, B.; Zimran, A. Home infusion of intravenous velaglucerase alfa: Experience from pooled clinical studies in 104 patients with type 1 Gaucher disease. Mol. Genet. Metab. 2017, 120, 111–115. [Google Scholar] [CrossRef]
- Parini, R.; Pozzi, K.; Di Mauro, S.; Furlan, F.; Rigoldi, M. Intravenous enzyme replacement therapy: Hospital vs home. Br. J. Nurs. 2010, 19, 892–894; 896–898. [Google Scholar] [CrossRef]
- Milligan, A.; Hughes, D.; Goodwin, S.; Richfield, L.; Mehta, A. Intravenous enzyme replacement therapy: Better in home or hospital? Br. J. Nurs. 2006, 15, 330–333. [Google Scholar] [CrossRef]
- Hughes, D.A.; Mlilligan, A.; Mehta, A. Home therapy for lysosomal storage disorders. Br. J. Nurs. 2007, 16, 1384; 1386–1389. [Google Scholar] [CrossRef]
- Zimran, A.; Altarescu, G.; Philips, M.; Attias, D.; Jmoudiak, M.; Deeb, M.; Wang, N.; Bhirangi, K.; Cohn, G.M.; Elstein, D. Phase 1/2 and extension study of velaglucerase alfa replacement therapy in adults with type 1 Gaucher disease: 48-month experience. Blood 2010, 115, 4651–4656. [Google Scholar] [CrossRef] [PubMed]
- Deegan, P.; Hughes, D.; Mehta, A.; Cox, T. UK National Guideline for Adult Gaucher Disease, April 2005. Available online: https://www.researchgate.net/publication/265282933 (accessed on 1 January 2024).
- McLoughlin, M.; Stepien, K.M.; McNelly, B.; Thompson, L.; Gorton, J.; Hendriksz, C.J. The use of port-a-caths in adult patients with Lysosomal Storage Disorders receiving Enzyme Replacement Therapy-one centre experience. Mol. Genet. Metab. Rep. 2017, 13, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Muranjan, M.; Karande, S.; Rajoria, S. Impact of COVID-19 pandemic on healthcare delivery for lysosomal storage disorders at a tertiary care public hospital in Mumbai. J. Postgrad. Med. 2024, 70, 23–28. [Google Scholar] [CrossRef]
- Goldwater, R.S. Gaucher disease, enzyme replacement therapy, and the Patient Assistance Program. J. Intraven. Nurs. 1996, 19, 83–88. [Google Scholar]
- Zimran, A.; Hollak, C.E.; Abrahamov, A.; van Oers, M.H.; Kelly, M.; Beutler, E. Home treatment with intravenous enzyme replacement therapy for Gaucher disease: An international collaborative study of 33 patients. Blood 1993, 82, 1107–1109. [Google Scholar] [CrossRef]
- Zimran, A.; Revel-Vilk, S.; Becker-Cohen, M.; Chicco, G.; Arbel, N.; Rolfs, A.; Szer, J. Rapid intravenous infusion of velaglucerase-alfa in adults with type 1 Gaucher disease. Am. J. Hematol. 2018, 93, E246–E248. [Google Scholar] [CrossRef]
- Zimran, A.; Wang, N.; Ogg, C.; Crombez, E.; Cohn, G.M.; Elstein, D. Seven-year safety and efficacy with velaglucerase alfa for treatment-naive adult patients with type 1 Gaucher disease. Am. J. Hematol. 2015, 90, 577–583. [Google Scholar] [CrossRef]
- Andrade-Campos, M.; Escuder-Azuara, B.; de Frutos, L.L.; Serrano-Gonzalo, I.; Giraldo, P. Direct and indirect effects of the SARS-CoV-2 pandemic on Gaucher Disease patients in Spain: Time to reconsider home-based therapies? Blood Cells Mol. Dis. 2020, 85, 102478. [Google Scholar] [CrossRef]
- Heinrich, R.; Claus, F.; Schoenfelder, T. The patients’ perspective on home-based infusion: A longitudinal observational study in the German healthcare setting for patients with lysosomal storage disorders treated with enzyme replacement therapy. Mol. Genet. Metab. Rep. 2023, 35, 100971. [Google Scholar] [CrossRef]
- van Dussen, L.; Cox, T.M.; Hendriks, E.J.; Morris, E.; Akkerman, E.M.; Maas, M.; Groener, J.E.; Aerts, J.M.; Deegan, P.B.; Hollak, C.E. Effects of switching from a reduced dose imiglucerase to velaglucerase in type 1 Gaucher disease: Clinical and biochemical outcomes. Haematologica 2012, 97, 1850–1854. [Google Scholar] [CrossRef][Green Version]
- Becker-Cohen, M.; Szer, J.; Arbel, N.; Frydman, D.; Dinur, T.; Istaiti, M.; Revel-Vilk, S.; Zimran, A. Rapid velaglucerase alfa infusion for Gaucher disease: 5-year data. Intern. Med. J. 2022, 52, 1645–1646. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, E.; Deroma, L.; Bembi, B.; Deegan, P.; Hollak, C.; Weinreb, N.J.; Cox, T.M. Enzyme replacement and substrate reduction therapy for Gaucher disease. Cochrane Database Syst. Rev. 2015, CD010324. [Google Scholar] [CrossRef] [PubMed]
- Elstein, D.; Giugliani, R.; Muenzer, J.; Schenk, J.; Schwartz, I.V.D.; Anagnostopoulou, C. Impact of the COVID-19 pandemic on the standard of care for patients with lysosomal storage diseases: A survey of healthcare professionals in the Fabry, Gaucher, and Hunter Outcome Survey registries. Mol. Genet. Metab. Rep. 2021, 28, 100788. [Google Scholar] [CrossRef] [PubMed]
- Tobor-Swietek, E.; Sykut-Cegielska, J.; Bik-Multanowski, M.; Walczak, M.; Rokicki, D.; Kaluzny, L.; Wierzba, J.; Pac, M.; Jahnz-Rozyk, K.; Wiesik-Szewczyk, E.; et al. COVID-19 Pandemic and Patients with Rare Inherited Metabolic Disorders and Rare Autoinflammatory Diseases-Organizational Challenges from the Point of View of Healthcare Providers. J. Clin. Med. 2021, 10, 4862. [Google Scholar] [CrossRef] [PubMed]
- Nalysnyk, L.; Sugarman, R.; Cele, C.; Uyei, J.; Ward, A. Budget Impact Analysis of Eliglustat for the Treatment of Gaucher Disease Type 1 in the United States. J. Manag. Care Spec. Pharm. 2018, 24, 1002–1008. [Google Scholar] [CrossRef]
- Serratrice, C.; Carballo, S.; Serratrice, J.; Stirnemann, J. Imiglucerase in the management of Gaucher disease type 1: An evidence-based review of its place in therapy. Core Evid. 2016, 11, 37–47. [Google Scholar] [CrossRef]
- Schiffmann, R.; Ries, M.; Timmons, M.; Flaherty, J.T.; Brady, R.O. Long-term therapy with agalsidase alfa for Fabry disease: Safety and effects on renal function in a home infusion setting. Nephrol. Dial. Transpl. 2006, 21, 345–354. [Google Scholar] [CrossRef]
- Cousins, A.; Lee, P.; Rorman, D.; Raas-Rothschild, A.; Banikazemi, M.; Waldek, S.; Thompson, L. Home-based infusion therapy for patients with Fabry disease. Br. J. Nurs. 2008, 17, 653–657. [Google Scholar] [CrossRef]
- Cox-Brinkman, J.; Timmermans, R.G.; Wijburg, F.A.; Donker, W.E.; van de Ploeg, A.T.; Aerts, J.M.; Hollak, C.E. Home treatment with enzyme replacement therapy for mucopolysaccharidosis type I is feasible and safe. J. Inherit. Metab. Dis. 2007, 30, 984. [Google Scholar] [CrossRef]
- Bagewadi, S.; Roberts, J.; Mercer, J.; Jones, S.; Stephenson, J.; Wraith, J.E. Home treatment with Elaprase and Naglazyme is safe in patients with mucopolysaccharidoses types II and VI, respectively. J. Inherit. Metab. Dis. 2008, 31, 733–737. [Google Scholar] [CrossRef]
- Ditters, I.A.M.; van der Beek, N.; Brusse, E.; van der Ploeg, A.T.; van den Hout, J.M.P.; Huidekoper, H.H. Home-based enzyme replacement therapy in children and adults with Pompe disease; a prospective study. Orphanet J. Rare Dis. 2023, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Toscano, A.; Musumeci, O.; Sacchini, M.; Ravaglia, S.; Siciliano, G.; Fiumara, A.; Verrecchia, E.; Maione, M.; Gentile, J.; Fischetto, R.; et al. Safety outcomes and patients’ preferences for home-based intravenous enzyme replacement therapy (ERT) in pompe disease and mucopolysaccharidosis type I (MPS I) disorder: COVID-19 and beyond. Orphanet J. Rare Dis. 2023, 18, 338. [Google Scholar] [CrossRef] [PubMed]
- Sechi, A.; Macor, D.; Valent, S.; Da Riol, R.M.; Zanatta, M.; Spinelli, A.; Bianchi, K.; Bertossi, N.; Dardis, A.; Valent, F.; et al. Impact of COVID-19 related healthcare crisis on treatments for patients with lysosomal storage disorders, the first Italian experience. Mol. Genet. Metab. 2020, 130, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Ceravolo, F.; Mascaro, I.; Sestito, S.; Pascale, E.; Lauricella, A.; Dizione, E.; Concolino, D. Home treatment in paediatric patients with Hunter syndrome: The first Italian experience. Ital. J. Pediatr. 2013, 39, 53. [Google Scholar] [CrossRef][Green Version]
- Revel-Vilk, S.; Szer, J.; Mehta, A.; Zimran, A. How we manage Gaucher Disease in the era of choices. Br. J. Haematol. 2018, 182, 467–480. [Google Scholar] [CrossRef]
- Iasella, C.J.; Johnson, H.J.; Dunn, M.A. Adverse Drug Reactions: Type A (Intrinsic) or Type B (Idiosyncratic). Clin. Liver Dis. 2017, 21, 73–87. [Google Scholar] [CrossRef]
- Guest, J.F.; Jenssen, T.; Houge, G.; Aaseboe, W.; Tondel, C.; Svarstad, E. Modelling the resource implications of managing adults with Fabry disease in Norway favours home infusion. Eur. J. Clin. Investig. 2010, 40, 1104–1112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).