Abstract
Background: Systemic inflammation and nutritional status have emerged as promising prognostic indicators across various malignancies; however, their clinical relevance in advanced laryngeal cancer remains underexplored. This study aimed to evaluate the prognostic significance of inflammation- and nutrition-based indices on the overall survival (OS) and progression-free survival (PFS) in patients with locally advanced or metastatic laryngeal cancer. Methods: A total of 147 patients treated at Pamukkale University between 2013 and 2022 were retrospectively analyzed. Baseline hematologic and biochemical parameters were used to calculate the Naples Prognostic Score (NPS), the Controlling Nutritional Status (CONUT) score, the Systemic Immune–Inflammation Index (SII), the Systemic Inflammation Response Index (SIRI), the C-reactive Protein/Albumin Ratio (CAR), and the Prognostic Nutritional Index (PNI). Survival outcomes were estimated using the Kaplan–Meier method, and independent prognostic factors were identified by Cox regression analyses. Results: The median OS and PFS were 55.5 and 48.8 months, respectively. In univariate analyses, high NPS, CONUT, SIRI, SII, and CAR values were significantly associated with inferior OS and PFS (p < 0.05). Multivariate analyses identified advanced stage, disease progression during chemotherapy, and high NPS as independent predictors of both the OS and PFS, whereas surgery conferred a survival advantage. Conclusions: Inflammation- and nutrition-based indices, particularly NPS, are strong prognostic markers for survival in patients with advanced laryngeal cancer. Routine integration of these parameters may enhance individualized risk stratification and guide treatment decisions in clinical practice.
1. Introduction
Globally, laryngeal cancer accounts for more than 189,000 new cases and over 103,000 deaths annually [1]. By anatomical subsite, glottic tumors comprise approximately two-thirds of cases, supraglottic tumors account for one-third, and subglottic tumors represent 1–2%. Owing to their early symptom presentation, glottic tumors are more frequently detected at earlier stages. Early diagnosis plays a critical role in treatment opportunities and prognosis. In contrast, supraglottic and subglottic tumors generally present with symptoms later on and are often diagnosed at locally advanced or metastatic stages [2,3].
The evaluation of advanced-stage laryngeal cancer patients by multidisciplinary head and neck tumor boards leads to favorable outcomes in terms of prognosis and survival [4,5]. Even systemic treatment decisions benefit from multidisciplinary discussion, primarily because survival rates remain low in locally advanced and advanced disease. Therefore, identifying prognostic factors that are inexpensive, easily accessible, and applicable in daily practice is of great importance. Biomarkers derived from complete blood count (CBC) parameters represent a cost-effective and feasible approach to characterize the tumor microenvironment [6]. A meta-analysis including 49 studies that investigated CBC-derived ratios in head and neck cancer patients demonstrated significant associations with poorer overall survival (OS) [7].
In recent years, multiple studies have shown that composite scores integrating patients’ nutritional status, immune system parameters, and serum protein levels are associated with progression-free survival (PFS) and OS. The Prognostic Nutritional Index (PNI), Controlling Nutritional Status (CONUT), Systemic Immune-Inflammation Index (SII), Systemic Inflammation Response Index (SIRI), Naples Prognostic Score (NPS), and C-reactive Protein/Albumin Ratio (CAR) have all been defined as prognostic parameters reflecting nutritional and immune status [8,9,10,11,12].
Although several inflammation- and nutrition-based biomarkers have been studied in head and neck cancers, evidence specific to laryngeal squamous cell carcinoma (LSCC) remains limited. The current literature indicates that prognostic indices in LSCC are not well established, and available studies are heterogeneous, often combining multiple head and neck subsites, which prevents LSCC-specific conclusions being made [13]. Moreover, recent investigations suggest that systemic inflammatory markers such as NLR and SIRI may have prognostic potential in LSCC, but their predictive performance varies widely across cohorts, indicating inconsistency and limited validation [14]. Importantly, studies evaluating composite inflammation–nutrition scores such as SIRI, SII, NPS, and CONUT simultaneously in LSCC populations are extremely scarce, and no consensus exists regarding their relative prognostic strength [15]. These gaps in the literature highlight the need for focused research investigating accessible, cost-effective biomarkers that may support clinical risk stratification specifically in LSCC.
In this study, we aimed to evaluate the prognostic impact of inflammation- and nutrition-based parameters, which can be derived from routine blood tests, on OS and PFS in patients with locally advanced and metastatic laryngeal cancer.
2. Materials and Methods
2.1. Patient Data
Between March 2013 and January 2022, pretreatment clinical and laboratory data of patients diagnosed with locally advanced or metastatic laryngeal squamous cell carcinoma (LSCC) who were admitted to the Department of Medical Oncology at Pamukkale University were retrospectively reviewed. A total of 147 consecutive patients met the eligibility criteria and were included in the study. Tumor staging was performed according to the American Joint Committee on Cancer (AJCC) 8th edition. T-stage distribution, including both early-stage (T1–T2) and advanced-stage (T3–T4) disease, was as follows: T1 in 5 patients, T2 in 30 patients, T3 in 58 patients, and T4 in 54 patients. Nodal involvement included N0 in 41 patients, N1 in 71, N2 in 29, and N3 in 6. Metastatic evaluation revealed M0 in 118 patients and M1 in 29 patients. Based on overall AJCC stage grouping, all patients had advanced-stage disease, including 59 patients with Stage III, 56 with Stage IVA, 5 with Stage IVB, and 27 with Stage IVC. No Stage I or Stage II cases were present in the cohort, reflecting a predominantly advanced-stage population typically referred to tertiary oncology centers.
Inclusion Criteria
- Histologically confirmed laryngeal squamous cell carcinoma (LSCC).
- Age ≥ 18 years.
- Availability of complete pretreatment laboratory parameters including full blood count, albumin, CRP, and cholesterol.
- Documented TNM staging (AJCC 8th edition).
- Received definitive or systemic oncologic treatment at the participating center.
- Blood samples obtained within 1–7 days before treatment initiation.
- Complete clinical follow-up information available for survival analysis.
Exclusion Criteria
- Active local or systemic infections at the time of blood sampling (acute tonsillitis, sinusitis, dental/periodontal infection, pneumonia, urinary infection).
- Chronic inflammatory or autoimmune diseases (RA, IBD, SLE, sarcoidosis).
- Hematologic malignancy or concurrent active cancer other than LSCC.
- Chronic liver disease, cirrhosis, chronic kidney disease, or severe heart failure.
- Use of corticosteroids, immunosuppressants, or NSAIDs within 2 weeks before blood sampling.
- Laboratory tests not obtained within the standardized pretreatment window.
- Incomplete clinical or biochemical data.
2.2. Laboratory Measurements
A total of 147 patients were included. The following laboratory and clinical variables were retrieved from their medical records: age; sex; ECOG performance status (PS); smoking and alcohol history; previous treatments (surgery, radiotherapy, and chemotherapy); disease stage; body mass index (BMI); neutrophil, lymphocyte, monocyte, and platelet counts; mean platelet volume (MPV); serum albumin; C-reactive protein (CRP); and total cholesterol levels. All inflammatory and nutritional biomarkers (NLR, PLR, LMR, SIRI, SII, CONUT, CAR, PNI, and NPS) were calculated using venous blood samples obtained strictly within 1–7 days before initiation of oncologic treatment. CBC parameters were analyzed using the Mindray CAL 8000 (Shanghai, China) autoanalyzer, employing electrical impedance and optical density measurement methods. Serum CRP and albumin levels were measured using the Cobas 702 analyzer (Roche Diagnostics, Mannheim, Germany) based on the electrochemiluminescence method. To ensure analytical consistency, all measurements were performed using standardized automated systems within a single center.
2.3. Calculated Parameters
The following prognostic scores were calculated using laboratory values:
- PNI = [Serum albumin (g/dL) × 10] + [Lymphocyte count (/nL) × 0.005].
- SII = (Platelet count × Neutrophil count)/Lymphocyte count.
- SIRI = (Neutrophil count × Monocyte count)/Lymphocyte count.
- NLR = Neutrophil/Lymphocyte.
- PLR = Platelet/Lymphocyte.
- LMR = Lymphocyte/Monocyte.
- CAR = CRP/Albumin.
- BMI = weight (kg)/height2 (m2).
2.4. Nutritional Status Score (CONUT)
The Controlling Nutritional Status (CONUT) score was calculated using three laboratory parameters—serum albumin concentration, total lymphocyte count, and total cholesterol level—reflecting both the nutritional and immunological status of patients. The total CONUT score was obtained by summing these three components, and patients were subsequently classified as follows: normal nutritional status (0–1 points), mildly malnourished (2–4 points), moderately malnourished (5–8 points), or severely malnourished (9–12 points) (Table 1) [16].

Table 1.
Calculation of the CONUT Score.
2.5. Naples Prognostic Score (NPS) Classification
Patients were stratified according to the Naples Prognostic Score (NPS), which integrates serum albumin, total cholesterol, neutrophil-to-lymphocyte ratio (NLR), and lymphocyte-to-monocyte ratio (LMR). Based on the total NPS, participants were divided into three groups for analysis: Group 1 (score = 0), Group 2 (score = 1–2), and Group 3 (score = 3–4) (Table 2) [17].

Table 2.
Calculation of the NPS.
Ethical approval for the study was obtained from the Non-Interventional Ethics Committee of Pamukkale University (Approval No: E-60116787-020-374745, Date: 1 June 2023).
2.6. Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows version 25.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA). Descriptive statistics were expressed as mean ± standard deviation (SD) or median (range: minimum–maximum) for continuous variables, and as frequency (percentages) for categorical variables. The Kolmogorov–Smirnov test was applied to assess the normality of data distribution. Categorical variables were compared using the Chi-square test or Fisher’s exact test, while continuous variables were analyzed with Student’s t-test or the Mann–Whitney U test, depending on the normality of the data.
Receiver operating characteristic (ROC) curve analyses were carried out to determine optimal cut-off points for continuous inflammatory and nutritional indices, including SIRI, SII, CAR, and PNI. The area under the curve (AUC) with 95% confidence intervals (CIs) was calculated to evaluate their discriminatory ability for mortality prediction. Optimal thresholds were identified using the maximum Youden index (sensitivity + specificity −1). The identified cut-off values (SIRI ≥ 1890.62, SII ≥ 909,583.93, CAR ≥ 2.86, and PNI ≤ 48.77) were subsequently used in all survival analyses.
To evaluate the robustness of the ROC-derived thresholds, a sensitivity analysis was performed. For each biomarker, the optimal cut-off value was recalculated with a ±10% variation, and the resulting changes in sensitivity, specificity, and AUC were examined. SIRI, SII, CAR, and PNI demonstrated stable performance across the alternative thresholds, whereas BSA exhibited marked variability. Detailed sensitivity analysis results are provided in Supplementary Table S1.
OS and PFS were estimated using the Kaplan–Meier method, and survival differences between groups were compared by the Log-rank test. Median survival times, together with 2- and 5-year survival rates, were calculated for each subgroup according to clinical and inflammatory–nutritional parameters. Variables showing statistical significance in univariate analyses (p < 0.05) were input into a multivariate Cox proportional hazards regression model to identify independent prognostic factors for OS and PFS. The results were expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). The proportional hazards assumption was verified by Schoenfeld residuals. All statistical tests were two-tailed, and a p-value < 0.05 was considered statistically significant.
Overall Survival (OS): Time from the diagnosis of locally advanced or metastatic disease to death from any cause.
Progression-Free Survival (PFS): Time from diagnosis to disease progression.
3. Results
Our study included 147 patients, comprising 138 men (93.9%) and 9 women (6.1%), with a median age of 61 years (range, 33–86 years). The median follow-up period was 34.6 months (range, 2.9–258.9 months). Patients were classified according to pathological stage, with 40.1% in Stage III and 59.9% in Stage IV disease. The most common primary tumor site was glottic (53.1%), followed by supraglottic (30.6%) and subglottic (16.3%) locations. Surgical treatment was performed in 65.3% of the cohort; among these, 74.4% underwent total laryngectomy, while 25.6% underwent subtotal procedures. Regarding treatment modalities, radiotherapy (RT) (39.5%) was the most frequently applied initial approach, followed by chemotherapy (CT) (33.3%) and concurrent chemoradiotherapy (CRT) (19.0%). Adjuvant therapy was administered to 12.2% of the patients, and induction chemotherapy to 8.8%. In terms of viral biomarkers, HPV positivity was identified in 5.4%, and p16 positivity in 22.4% of evaluable cases. Based on nutritional and inflammatory indices, high-risk features were common: NPS grade 2 (44.2%), CONUT ≥ 3 (34.7%), SIRI ≥ 1890.62 (51.7%), SII ≥ 909,583.93 (52.4%), and CAR ≥ 2.86 (51.7%). During follow-up, disease progression occurred in 62.6% of the patients, and 57.8% had died by the end of the observation period. Detailed demographic and clinical characteristics are summarized in Supplementary Table S2.
The clinical variables presented in Table 3. Specifically, factors associated with more advanced disease burden—such as older age, smoking, alcohol use, advanced stage, and lack of surgery—were similarly linked to shorter OS and PFS. These patterns also paralleled the distribution of inflammatory and nutritional markers: patients with higher NPS, CONUT, SIRI, SII, and CAR values tended to have a more advanced tumor stage and display poorer treatment responses. This alignment between clinical characteristics and biomarker profiles suggests that systemic inflammation and nutritional impairment may partially reflect underlying tumor aggressiveness.

Table 3.
Distribution of Sociodemographic and Clinical Variables.
The descriptive statistics of the inflammatory and nutritional indices revealed wide variability. For example, the mean body surface area (BSA) was 1.78 ± 0.18 m2 (median 1.77; range, 1.30–2.38); the mean SIRI and SII were 3124.51 ± 3631.02 and 1,540,696.10 ± 1,494,576.91, respectively; the median SIRI was 1965.05 (range 150.38–29,458.33) and median SII 994,170.98 (range 96,075.95–76,977,330.77); and the mean CAR was 9.74 ± 25.67 (median 3.06; range 0.07–290.00), and mean PNI 48.93 ± 7.10 (median 48.15; range 31.10–64.15). These findings reflect the heterogeneous clinical and metabolic profiles of patients with laryngeal cancer.
The median overall survival (OS) was 55.53 months (95% CI: 27.90–82.15); 2- and 5-year OS rates were 71.5% and 48.7%, respectively. OS differed significantly according to age (p = 0.005), smoking status (p = 0.042), alcohol use (p = 0.046), surgery (p = 0.007), stage (p = 0.002), adjuvant therapy (p = 0.048), and chemotherapy response (p < 0.001). Patients aged ≤ 65 years, those undergoing surgery, and those receiving adjuvant therapy lived longer, whereas Stage IV disease and poor chemotherapy response were associated with shorter OS. Among the inflammation–nutrition indices, higher NPS (p < 0.001), CONUT (p = 0.001), SIRI (p < 0.001), SII (p < 0.001), and CAR (p = 0.001) were linked to reduced OS, while a higher PNI showed a non-significant trend with better outcomes. The OS-related findings are summarized in Table 4.

Table 4.
Univariate analysis of clinicopathological and systemic inflammation- and nutrition-based parameters for OS.
Across the cohort, elevated systemic inflammatory and nutritional markers were more frequently observed in patients with advanced-stage disease, those who did not undergo surgery, and those who demonstrated progressive disease after chemotherapy. Patients requiring second-line treatment also exhibited markedly higher SIRI, SII, NPS, CONUT, and CAR levels. These patterns suggest that systemic inflammation is influenced not only by tumor biology but also by disease burden and treatment responsiveness, providing important context for interpreting the prognostic performance of these indices.
Figure 1A–E Kaplan–Meier overall survival curves according to inflammatory and nutritional indices. Figure 1A NPS: Higher NPS grades were associated with significantly reduced OS (p < 0.001). Figure 1B CONUT: Patients with CONUT ≥ 3 demonstrated worse OS (p = 0.001). Figure 1C SIRI: Elevated SIRI (≥1890.62) was associated with poorer OS (p < 0.001). Figure 1D SII: Higher SII (≥909,583.93) predicted significantly shorter OS (p < 0.001). Figure 1E CAR: CAR ≥2.86 was linked to worse OS (p = 0.001).
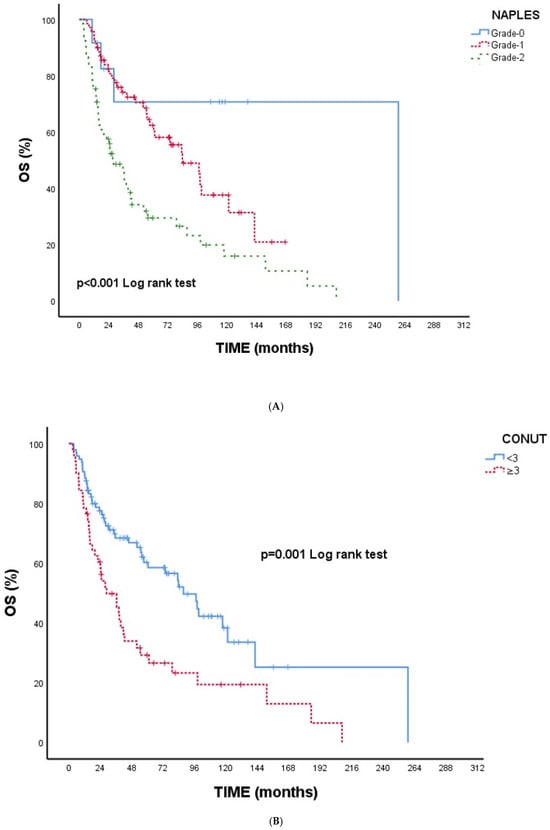
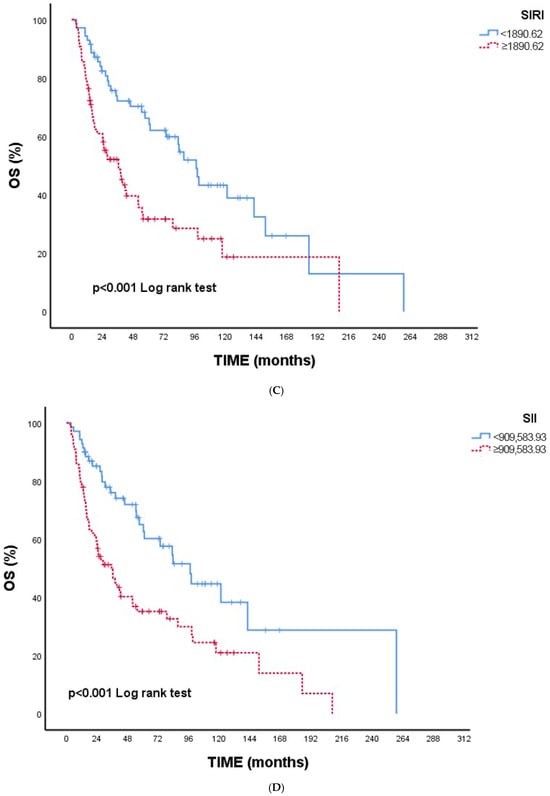
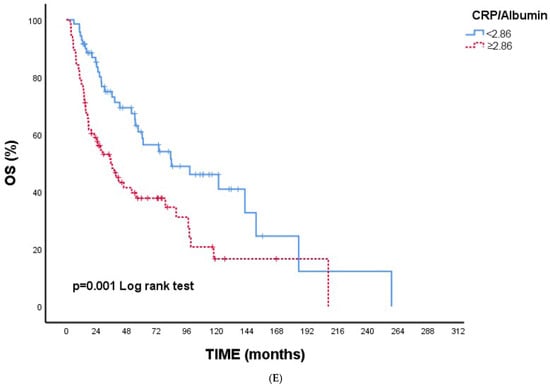
Figure 1.
(A) Overall survival according to NPS. (B) Overall survival according to CONUT. (C) Overall survival according to SIRI. (D) Overall survival according to SII. (E) Overall survival according to CAR. Abbreviations: NPS—Naples Prognostic Score; CONUT—Controlling Nutritional Status; SIRI—Systemic Inflammation Response Index; SII—Systemic Immune–Inflammation Index; CAR—C-reactive protein/albumin ratio.
Overall, all indices were consistently indicative of poorer survival with higher inflammatory or nutritional impairment, supporting the univariate findings (Table 4).
The median PFS was 48.83 months (95% CI: 30.15–67.50), with 2- and 5-year survival rates of 63.4% and 43.3%, respectively. Significant differences in PFS were observed according to age (p = 0.026), smoking status (p = 0.001), alcohol consumption (p = 0.032), surgery (p = 0.008), disease stage (p = 0.003), chemotherapy response (p < 0.001), second-line chemotherapy (p < 0.001), and inflammation–nutrition indices including NPS (p = 0.001), CONUT (p = 0.003), SIRI (p = 0.001), SII (p = 0.003), and CAR (p < 0.001). Patients aged ≤65 years, smokers, and those undergoing surgery demonstrated significantly longer PFS. Similarly, patients with Stage III disease, who displayed stable or complete chemotherapy response, and had no need for second-line chemotherapy had superior outcomes. Conversely, patients with higher NPS, CONUT, SIRI, SII, and CAR values showed shorter PFS durations, underscoring the strong prognostic value of systemic inflammation and nutritional status in predicting disease progression among laryngeal cancer patients. The findings related to PFS are summarized in Table 5.

Table 5.
Univariate analysis of clinicopathological and systemic inflammation- and nutrition-based parameters for PFS.
The results of the multivariate Cox regression for mortality risk are summarized in Table 6. Independent predictors of increased mortality were Stage IV disease (HR = 3.71; 95% CI: 1.76–7.84; p = 0.001), disease progression in response to chemotherapy (HR = 3.21; 95% CI: 1.19–8.65; p = 0.021), and higher NPS grades—Grade 1 (HR = 7.66; 95% CI: 1.56–37.63; p = 0.012) and Grade 2 (HR = 12.74; 95% CI: 1.94–83.52; p = 0.008). Also, surgery reduced mortality risk (HR = 0.48; 95% CI: 0.25–0.91; p = 0.025).

Table 6.
Multivariate Cox regression results of mortality risk according to various clinical variables.
The results of the multivariate Cox regression analysis for progression risk are presented in Table 7. Independent predictors of shorter PFS included Stage IV disease (HR = 2.19; 95% CI: 1.11–4.34; p = 0.024), disease progression in response to chemotherapy (HR = 3.25; 95% CI: 1.31–8.08; p = 0.011), and higher NPS grades, specifically Grade 1 (HR = 3.79; 95% CI: 1.16–12.40; p = 0.027) and Grade 2 (HR = 8.05; 95% CI: 1.75–37.05; p = 0.007). In contrast, surgery (HR 0.33; 95% CI 0.17–0.64; p = 0.001) and the absence of second-line chemotherapy (HR = 0.37; 95% CI: 0.18–0.78; p = 0.009) were independently associated with a reduced risk of progression. These findings highlight that disease stage, treatment response, surgical management, and the NPS index are significant prognostic factors influencing PFS in patients with laryngeal cancer.

Table 7.
Multivariate Cox regression results of progression risk according to various clinical variables.
4. Discussion
The present study provides a comprehensive evaluation of multiple inflammation- and nutrition-based prognostic indices in patients with laryngeal cancer, demonstrating clear and consistent associations between these biomarkers and survival outcomes. Among all of the evaluated indices, NPS showed the strongest prognostic performance, maintaining independent significance for both OS and PFS after adjustment for clinical variables. CONUT, SIRI, SII, and CAR also exhibited meaningful stratification in univariate analyses, further supporting their relevance as markers of host inflammatory and nutritional status. These patterns closely parallel the Kaplan–Meier estimations and the multivariate Cox models presented in Section 3, reinforcing the concept that elevated systemic inflammation and impaired nutrition contribute to poorer prognosis. By integrating our findings with the existing literature, this discussion contextualizes the prognostic capacity of these indices and underscores their potential clinical implications.
Consistent with previous literature, our study demonstrated that higher CONUT scores were significantly associated with poorer OS and PFS, reflecting the adverse prognostic impact of impaired nutritional and immunological status in laryngeal cancer. Although CONUT showed strong discrimination in the univariate analyses, it did not remain an independent predictor in the multivariate model, suggesting that its prognostic value may be partially mediated through factors such as tumor stage, systemic inflammatory burden, and comorbidity-related nutritional decline. This pattern has also been observed in prior meta-analyses evaluating CONUT and GNRI across multiple malignancies, in which these indices correlated strongly with survival outcomes but occasionally lost statistical independence when adjusted for major clinicopathological variables [18].
Recent studies specifically examining laryngeal carcinoma further support our findings: elevated CONUT scores have been associated with advanced stage, increased tumor burden, and inferior OS and DFS following curative surgery [19]. Similarly, both CONUT and PNI have been identified as reliable prognostic indicators in head and neck cancers, including laryngeal tumors [20]. Taken together, these results highlight that nutritional depletion—captured by CONUT—plays a meaningful role in shaping prognosis, even when overshadowed by more dominant factors in multivariable models.
In contrast, the Prognostic Nutritional Index (PNI) did not reach statistical significance in our cohort, possibly due to the predominance of advanced-stage disease, in which systemic inflammatory burden may outweigh isolated nutritional parameters. Additionally, because PNI is mainly based on serum albumin and lymphocyte counts, it may be influenced by acute-phase responses and treatment-related factors, limiting its prognostic discrimination in real-world populations. This reinforces the multidimensional nature of prognostic assessment in laryngeal cancer and underscores the complementary value of composite indices such as NPS, which integrate both nutritional and inflammatory domains into a single prognostic framework.
Additional evidence from recent head and neck cancer research further strengthens the prognostic relevance of the CONUT system. A large meta-analysis including patients with various head and neck subsites demonstrated that higher CONUT scores were consistently associated with poorer OS and DFS, as well as with adverse pathological characteristics such as advanced T/N stage and greater tumor aggressiveness [21]. Moreover, it has recently been demonstrated in a large cohort of surgically treated head and neck squamous cell carcinoma that CONUT effectively stratified survival outcomes and significantly improved prognostic accuracy when incorporated into clinicopathological nomograms, outperforming AJCC staging alone [22]. Furthermore, a dedicated cohort study focusing exclusively on laryngeal squamous cell carcinoma demonstrated that CONUT, together with PNI and GPS, provided strong prognostic stratification for overall and disease-free survival, and significantly enhanced prognostic accuracy when evaluated alongside traditional clinicopathological variables [23]. Collectively, these findings highlight that CONUT represents a robust and reproducible biomarker across head and neck malignancies, aligning with the patterns observed in our cohort.
The NPS, which integrates both nutritional and inflammatory components, provides a multidimensional reflection of the host’s physiological status. Its prognostic value has been validated in population-based analyses [11], and several studies in oral cavity and hypopharyngeal squamous cell carcinoma have reported NPS as an independent predictor of overall and disease-free survival, with higher scores consistently indicating poorer outcomes [24,25]. Furthermore, prognostic models incorporating NPS alongside clinicopathological variables have demonstrated superior predictive accuracy compared with the AJCC staging system alone, effectively stratifying patients into distinct risk groups [26]. In accordance with these observations, our findings showed that higher NPS was independently associated with both reduced OS and PFS in laryngeal cancer. The strong and persistent prognostic effect of NPS in our multivariate analysis suggests that it may better capture the interplay between systemic inflammation, nutritional decline, and tumor biology, underscoring its potential utility as a comprehensive biomarker for individualized risk assessment and treatment planning in head and neck malignancies.
Systemic inflammation-based biomarkers have emerged as important prognostic indicators in head and neck cancers. In our study, elevated SII and SIRI values were strongly associated with shorter OS and PFS, underscoring their ability to reflect tumor aggressiveness. These findings are consistent with recent evidence showing that higher SII levels predict poorer survival outcomes in head and neck cancer patients [27], and this association is further supported by meta-analytic data confirming the adverse prognostic impact of SII [28]. Similarly, the prognostic relevance of SIRI has been demonstrated in oral cavity squamous cell carcinoma, where propensity score-based analyses have identified elevated SIRI as an independent predictor of mortality, and this has been validated in large head and neck squamous cell carcinoma cohorts, showing its capacity to capture systemic inflammatory burden associated with worse outcomes [29,30]. In our cohort, higher SII and SIRI values paralleled adverse clinical features—including advanced disease stage, lack of surgery, progressive chemotherapy response, and the need for second-line treatment—suggesting that systemic inflammation reflects not only tumor biology but also disease burden and treatment responsiveness. Collectively, these findings support the integration of inflammation-based indices such as SII and SIRI into routine prognostic evaluation for patients with laryngeal cancer.
Consistent with our findings, previous studies have shown that inflammation-based markers reflecting both systemic inflammatory activity and nutritional status hold prognostic relevance in head and neck cancers. Among these, the CAR has emerged as a robust indicator of adverse outcomes. Studies in laryngeal and hypopharyngeal squamous cell carcinoma have demonstrated that elevated CAR is significantly associated with advanced tumor burden, higher recurrence rates, and poorer survival [31,32]. A recent meta-analysis further confirmed that elevated CRP levels independently predict worse overall survival across head and neck squamous cell carcinomas, underscoring the strong link between systemic inflammation and tumor aggressiveness [33]. In line with this evidence, our cohort showed that a higher CAR was significantly associated with reduced OS and PFS, supporting its potential role as an accessible and clinically meaningful prognostic biomarker in laryngeal cancer.
The biological heterogeneity of head and neck cancers should also be considered when interpreting inflammation-based biomarkers. HPV-driven carcinogenesis is predominantly associated with oropharyngeal tumors, whereas it is considerably less common in laryngeal cancer. Previous studies have shown that systemic inflammatory ratios such as NLR, PLR, and SII may exhibit stronger prognostic relevance in HPV-positive head and neck cancers [34]. However, HPV/p16 testing was not routinely performed in our cohort; therefore, the potential modifying effect of HPV status on the inflammatory biomarkers’ prognostic performance could not be assessed. This limitation should be taken into account when interpreting our findings.
This study possesses several notable strengths. Specifically, it provides one of the most comprehensive evaluations of multiple inflammation- and nutrition-based biomarkers in laryngeal cancer, integrating NPS, CONUT, SII, SIRI, and CAR within a single, uniformly analyzed cohort. The combined use of Kaplan–Meier survival estimations and multivariate Cox regression strengthened the robustness of the prognostic assessments. Furthermore, the real-world nature of the dataset, inclusion of clinically meaningful outcomes—such as chemotherapy response and second-line treatment requirements—and the long follow-up duration enhance the applicability of the findings. By examining the interplay between systemic inflammation, nutritional status, and tumor aggressiveness, this study offers clinically relevant insights that extend beyond traditional prognostic factors.
However, several limitations should be acknowledged. The retrospective, single-center design may have introduced selection bias and restricted generalizability. The cut-off values for the inflammatory and nutritional indices were derived from this cohort and may not be universally applicable, emphasizing the need for external validation. The biomarkers were assessed only at baseline, preventing an analysis of dynamic changes in systemic inflammation or nutritional status over time. Additionally, HPV/p16 status was not routinely available, limiting the ability to evaluate its potential modifying effect on biomarker performance. Unmeasured confounders such as comorbidities and treatment heterogeneity may have also influenced the results. Finally, molecular or genomic markers were not incorporated, which may have further refined risk stratification. Prospective multicenter studies are needed to validate these findings and establish the clinical utility of these indices in laryngeal cancer.
5. Conclusions
This study demonstrates that inflammation- and nutrition-based biomarkers provide meaningful prognostic information in laryngeal cancer, complementing traditional clinicopathological factors. Among all of the indices evaluated, NPS showed the strongest independent prognostic performance, while CONUT, SII, SIRI, and CAR also exhibited clear survival stratification and mirrored adverse clinical features such as an advanced stage, poor treatment responses, and the need for second-line therapy. These findings suggest that systemic inflammation and nutritional impairment reflect underlying tumor aggressiveness and may assist in identifying high-risk patients who require closer monitoring or more intensive treatment strategies. Incorporating these biomarkers into routine assessments could enhance risk stratification in laryngeal cancer. Prospective multicenter studies with longitudinal biomarker evaluation are warranted to validate these results and define their potential role in guiding individualized treatment planning.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14248924/s1, Table S1: Sensitivity Analysis for Prognostic Scores; Table S2: Detailed Treatment Characteristics.
Author Contributions
Conceptualization, B.Ç.D., T.D., A.G.D., B.Y.T., S.D., G.G.D., and A.Y.; methodology, B.Ç.D., M.Ö., and C.K.; software, B.Ç.D., T.G.K., S.T., A.B., and M.Ö.; validation, B.Ç.D., A.Y., and G.G.D.; formal analysis, B.Ç.D., and A.Y.; investigation, B.Ç.D.; resources, B.Ç.D., and A.B.; data curation, B.Ç.D., T.D., B.Y.T., S.T., T.G.K., and A.G.D.; initial manuscript was drafted by B.Ç.D., and A.B.; B.Ç.D. was responsible for manuscript revision and editing; data visualization was performed by B.Ç.D.; supervision was led by A.B.; B.Ç.D., and A.Y. managed project coordination and oversight. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Pamukkale University Local Ethics Committee (approved date: 1 June 2023 and approved number: E-60116787-020-374745) and designed in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data in this study are available from the corresponding author upon reasonable request.
Acknowledgments
No individuals or organizations contributed to this study beyond the listed authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Global Cancer Observatory. International Agency for Research on Cancer. World Health Organization. Available online: https://gco.iarc.fr/ (accessed on 8 February 2024).
- National Comprehensive Cancer Network (NCCN). Head and Neck Cancers; Version 2.2025; NCCN Clinical Practice Guidelines in Oncology; NCCN: Plymouth Meeting, PA, USA, 2025; Available online: https://www.nccn.org/ (accessed on 26 October 2025).
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef]
- Kelly, S.L.; Jackson, J.E.; Hickey, B.E.; Szallasi, F.G.; Bond, C.A. Multidisciplinary clinic care improves adherence to best practice in head and neck cancer. Am. J. Otolaryngol. 2013, 34, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Wheless, S.A.; McKinney, K.A.; Zanation, A.M. A prospective study of the clinical impact of a multidisciplinary head and neck tumor board. Otolaryngol. Head Neck Surg. 2010, 143, 650–654. [Google Scholar] [CrossRef]
- Song, M.; Graubard, B.I.; Rabkin, C.S.; Engels, E.A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 2021, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, C.; Tiwary, V.; Sunil, K.; Suresh, D.; Shetty, S.; Muthukaliannan, G.K.; Baxi, S.; Jayaraj, R. Prognostic Utility of Platelet–Lymphocyte Ratio, Neutrophil–Lymphocyte Ratio and Monocyte–Lymphocyte Ratio in Head and Neck Cancers: A Detailed PRISMA Compliant Systematic Review and Meta-Analysis. Cancers 2021, 13, 4166. [Google Scholar] [CrossRef]
- Sun, K.; Chen, S.; Xu, J.; Li, G.; He, Y. The prognostic significance of the prognostic nutritional index in cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1537–1549. [Google Scholar] [CrossRef]
- Kheirouri, S.; Alizadeh, M. Prognostic potential of the preoperative controlling nutritional status (CONUT) score in predicting survival of patients with cancer: A systematic review. Adv. Nutr. 2021, 12, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Nøst, T.H.; Alcala, K.; Urbarova, I.; Byrne, K.S.; Guida, F.; Sandanger, T.M.; Johansson, M. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur. J. Epidemiol. 2021, 36, 841–848. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, C.; Song, J.; Yan, L.; Xiao, Y.; Cheng, N.; Wu, H.; Chen, X.; Yang, J. The Naples prognostic score serves as a predictor and prognostic indicator for cancer survivors in the community. BMC Cancer 2024, 24, 696. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, Z.; Zhang, X.; Hang, D.; Yin, R.; Feng, J.; Xu, L.; Shen, H. C-reactive protein and cancer risk: A pan-cancer study of prospective cohort and Mendelian randomization analysis. BMC Med. 2022, 20, 301. [Google Scholar] [CrossRef]
- Woodley, N.; Rogers, A.D.G.; Turnbull, K.; Slim, M.A.M.; Ton, T.; Montgomery, J.; Douglas, C. Prognostic scores in laryngeal cancer. Eur. Arch. Otorhinolaryngol. 2022, 279, 3705–3715. [Google Scholar] [CrossRef]
- Murad, L.D.; Silva, T.Q.; Schilithz, A.O.C.; Monteiro, M.C.; Murad, L.B.; Fialho, E. Body mass index alters the predictive value of the neutrophil-to-lymphocyte ratio and systemic inflammation response index in laryngeal squamous cell carcinoma patients. Nutr. Cancer 2022, 74, 1261–1269. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Tang, D.; Heng, Y.; Lu, L.M.; Tao, L. The role of systemic inflammatory response index (SIRI) and tumor-infiltrating lymphocytes (TILs) in the prognosis of patients with laryngeal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 5627–5636. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Galizia, G.; Lieto, E.; Auricchio, A.; Cardella, F.; Mabilia, A.; Podzemny, V.; Castellano, P.; Orditura, M.; Napolitano, V. Naples Prognostic Score, Based on Nutritional and Inflammatory Status, is an Independent Predictor of Long-term Outcome in Patients Undergoing Surgery for Colorectal Cancer. Dis. Colon Rectum 2017, 60, 1273–1284. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.-W.; Chiang, Y.-C. Prognostic value of GNRI and CONUT in head and neck cancer: A systematic review and meta-analysis. BMC Cancer 2025, 25, 242. [Google Scholar] [CrossRef]
- Lin, Q.; Lin, S.; Chen, W.; Chen, X.; Yi, X.; Lu, S.; Li, H.; Li, C.; Wang, D. Controlling Nutritional Status (CONUT) score is a prognostic marker for laryngeal cancer patients with curative resection. Head Neck 2022, 44, 2834–2841. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Fernandes, T.; Carvalho, M.d.C.; Loureiro, H.S. The Prognostic Role of Prognostic Nutritional Index and Controlling Nutritional Status in Predicting Survival in Older Adults with Oncological Disease: A Systematic Review. Onco 2024, 4, 101–115. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, C. Prognostic and clinicopathological value of the controlling nutritional status (CONUT) score in patients with head and neck cancer: A meta-analysis. World J. Surg. Oncol. 2024, 22, 223. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Yang, C.-H.; Tsai, Y.-T.; Lin, Y.-T.; Chao, C.-Y.; Chuang, H.-C.; Huang, T.-L.; Lu, H.; Tsai, W.-L.; Chien, C.-Y.; et al. Controlling nutritional status score as a survival prognosticator in patients with head and neck cancer. J. Formos. Med. Assoc. 2025. S0929-6646(25)00186-X Advance online publication. [Google Scholar] [CrossRef]
- Yi, H.; Chen, C.; Zhou, S.; Wang, Y.; Zhou, Y.; Chen, J.; Liang, Q. Comparison of three nutritional assessment methods associated with the prognostic impact of laryngeal cancer. Support. Care Cancer 2023, 31, 737. [Google Scholar] [CrossRef]
- Xu, X.L.; Wu, C.C.; Cheng, H. Prognostic significance of preoperative Naples prognostic score for disease-free and overall survival in oral cavity squamous cell carcinoma post-surgery. BMC Cancer 2025, 25, 757. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Cheng, H. A novel survival prediction nomogram based on the Naples prognostic score and clinicopathological factors for postoperative hypopharyngeal squamous cell carcinoma patients. J. Inflamm. Res. 2025, 18, 7245–7257. [Google Scholar] [CrossRef]
- Wang, Y.S.; Niu, L.; Shi, W.X.; Li, X.Y.; Shen, L. Naples prognostic score as a predictor of outcomes in lung cancer: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8144–8153. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.-C.; Chang, C.-M.; Wu, C.-Y.; Hsieh, C.-H.; Shueng, P.-W.; Cheng, P.-W.; Liao, L.-J. A predictive model for advanced oropharyngeal cancer patients treated with chemoradiation. BMC Cancer 2022, 22, 615. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Kuo, L.-T.; Weng, H.-H.; Hsu, C.-M.; Tsai, M.-S.; Chang, G.-H.; Lee, Y.-C.; Huang, E.I.; Tsai, Y.-T. Systemic immune–inflammation index as a predictor for head and neck cancer prognosis: A meta-analysis. Front. Oncol. 2022, 12, 899518. [Google Scholar] [CrossRef]
- Lin, J.; Chen, L.; Chen, Q.; Zhuang, Z.; Bao, X.; Qian, J.; Hong, Y.; Yan, L.; Lin, L.; Shi, B.; et al. Prognostic value of preoperative systemic inflammation response index in patients with oral squamous cell carcinoma: Propensity score-based analysis. Head Neck 2020, 42, 3263–3274. [Google Scholar] [CrossRef]
- Valero, C.; Pardo, L.; Sansa, A.; Garcia Lorenzo, J.; López, M.; Quer, M.; León, X. Prognostic capacity of Systemic Inflammation Response Index (SIRI) in patients with head and neck squamous cell carcinoma. Head Neck 2020, 42, 336–343. [Google Scholar] [CrossRef]
- Yu, S.-T.; Zhou, Z.; Cai, Q.; Liang, F.; Han, P.; Chen, R.; Huang, X.-M. Prognostic value of the C-reactive protein/albumin ratio in patients with laryngeal squamous cell carcinoma: A retrospective study. Oncol. Targets Ther. 2017, 10, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, A.; Kanaya, H.; Nakayama, T.; Konno, W.; Goto, K.; Nakajima, I.; Kashiwagi, T.; Hirabayashi, H.; Haruna, S.I. Prognostic value of C-reactive protein/albumin ratio for patients with hypopharyngeal and laryngeal cancer undergoing invasive surgery involving laryngectomy. Head Neck 2019, 41, 1342–1350. [Google Scholar] [CrossRef]
- Chen, Y.; Cong, R.; Ji, C.; Ruan, W. The prognostic role of C-reactive protein in patients with head and neck squamous cell carcinoma: A systematic review and meta-analysis. Cancer Med. 2020, 9, 3493–3505. [Google Scholar] [CrossRef] [PubMed]
- Noormohammadpour, P.; Hueniken, K.; Pienkowski, M.; Huang, S.H.; Yuan, B.; Grant, B.; Yao, C.; Hope, A.; Tsai, J.; McPartlin, A.; et al. Differential prognostic association of systemic inflammatory biomarkers on survival outcomes in head and neck squamous cell carcinoma patients by human papillomavirus status. Neoplasia 2025, 66, 101178. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).