Abstract
Background/Objectives: Task-oriented training (TOT) is a functional, goal-directed rehabilitation approach that promotes motor recovery after stroke through repetitive, task-specific practice; however, its overall effects on gait and balance in stroke survivors remain unclear. This systematic review- and meta-analysis-based study aims to evaluate the effects of TOT on gait and balance in patients with stroke. Methods: Comprehensive searches of PubMed, Embase, Web of Science, the Cochrane Library, and Scopus were performed. Eligible studies involving TOT interventions in patients with stroke were included, and methodological quality was assessed using the PEDro scale. A random-effects meta-analysis was then performed to estimate pooled effect sizes. Results: In total, 17 randomized controlled trials involving 888 participants were included. Compared with control interventions, TOT significantly improved gait speed (standardized mean difference [SMD] = 0.48, 95% confidence interval [CI]: 0.27–0.69, p < 0.0001), gait endurance (SMD = 0.49, 95% CI: 0.27–0.71, p < 0.0001), Berg Balance Scale (BBS) (SMD = 0.45, 95% CI: 0.08–0.82, p = 0.02), and timed up and go test performance (SMD = −0.28, 95% CI: −0.47 to −0.09, p = 0.003). Subgroup analysis of the BBS revealed differences based on stroke phase. Conclusions: Task-oriented training effectively improves gait and balance in stroke survivors and should be considered a key component of post-stroke rehabilitation. Future studies should explore its long-term effects and determine optimal training parameters according to stroke phase and patient characteristics.
1. Introduction
Stroke remains a leading cause of death and disability worldwide, with its incidence continuing to rise as populations age [1]. It is classified as either ischemic or hemorrhagic, with symptoms and prognosis varying depending on lesion type and location [2]. Most stroke survivors experience motor and cognitive impairments that limit walking, balance, and daily activities [3].
Gait and balance deficits increase the risk of falls, reduce independence, and diminish quality of life [4]. Since gait speed and distance are core rehabilitation goals, and balance is essential for gait stability, both are considered critical outcomes in post-stroke recovery [5].
Conventional gait training focuses on repetitive walking exercise, muscle strengthening, and orthotic use, whereas balance training emphasizes movement strategies to maintain posture and control the center of gravity of the body [6]. However, these approaches have limitations because they lack task specificity and adaptability to real-world environments [7]. Studies also report that conventional gait training alone is insufficient for improving overall balance [8].
In contrast, task-oriented training (TOT) promotes sensorimotor integration and adaptation to environmental variability through repetitive, goal-directed functional tasks. It enhances patient engagement, thereby strengthening motor learning and functional recovery [9,10].
Growing evidence suggests that task-oriented activities such as gait and balance training provide greater benefits for functional recovery than simple repetitive exercises. In addition to conventional task-oriented approaches, recent studies have highlighted the role of robotic-assisted task-oriented rehabilitation in stroke recovery. Robotic systems can provide high-intensity, repetitive, and task-specific training while reducing physical burden on therapists and increasing patient engagement. These robotic-assisted programs have demonstrated potential benefits for improving gait and balance outcomes [11,12]. A systematic review comparing intervention methods in patients with stroke reports that virtual reality-based gait training significantly improves specific balance outcomes [13]. Virtual reality (VR)–based rehabilitation provides enriched multisensory input, real-time feedback, and task variability that facilitate motor learning and neural plasticity, thereby enhancing functional mobility and balance recovery in stroke survivors [14]. Similarly, a meta-analysis of exercise interventions in patients with chronic stroke reports positive effects on mobility, balance, and gait [15]. However, exercise-based interventions also have limitations. For example, treadmill-based gait training improves walking distance, but some studies report no significant improvement in gait speed or balance [16].
A meta-analysis of rehabilitation strategies for improving balance reports that core stability training, balance exercises, and combined interventions are effective [17]. However, many studies vary considerably in intervention methods and task specificity, complicating effect comparisons. Furthermore, some studies focus on a single outcome (either gait or balance), limiting the assessment of overall effectiveness.
Therefore, this study aims to quantitatively evaluate the effects of task-directed training on gait outcomes and balance in patients with stroke, focusing on randomized controlled trials (RCTs). By exploring how intervention characteristics (e.g., session frequency, intervention duration) and disease stage (acute/subacute/chronic) influence effect size, this study seeks to provide clearer evidence to guide rehabilitation practice.
2. Materials and Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 guidelines, and the completed PRISMA checklist is provided in the Supplementary Material [18,19].
2.1. Search Strategy and Data Resource
A comprehensive literature search was conducted between May and June 2025 across EMBASE, Ovid-LWW, Scopus, PubMed, and CINAHL databases, including only articles published in English.
The primary search terms were “stroke” and “task-oriented training,” combined with the Boolean operators “AND” and “OR.”
2.2. Eligibility and Exclusion Criteria
Eligibility criteria were defined using the patient, intervention, comparison, outcome (PICO) framework as follows:
- Patients (P): Patients with a stroke (except where a person without disability acts as a person with disabilities);
- Intervention (I): TOT;
- Comparison (C): Conventional therapy or other rehabilitation interventions;
- Outcome (O): Gait and balance outcomes.
Studies were excluded if they were reviews, meta-analyses, letters, or conference proceedings, or if they did not involve stroke survivors. Articles lacking details on interventions, results, or full-text availability were also excluded.
2.3. Screening, Selection, and Extraction Process
This review followed the PRISMA 2020 guidelines for study selection. Two researchers independently screened and extracted data using electronic databases, applying predefined inclusion and exclusion criteria. Duplicate and irrelevant records were removed during the identification phase. Titles and abstracts were first reviewed to identify eligible studies, followed by full-text assessment to confirm final inclusion based on the study objectives.
2.4. Data Extraction
The data extraction form was developed following the Cochrane Handbook for Systematic Reviews of Interventions [20]. Two independent reviewers (M.-H.L. and D.-Y.L.) pilot-tested the form on five randomly selected studies to verify extraction accuracy [21]. All extracted data were recorded and managed using Microsoft Excel 365. The data extracted included authors, publication year, study design, stroke type, sample size, sex, mean age, stroke stage, intervention method, control group, gait- and balance-related outcomes, measurement type, unit, intention-to-treat (ITT) analysis, missing data handling, and dropout rate.
2.5. Risk of Bias Assessment
The methodological quality of the selected studies was assessed using the Physiotherapy Evidence Database (PEDro) scale [22]. The PEDro scale is an established tool for evaluating the quality of clinical interventions and comprises 11 criteria: clearly defined eligibility, random allocation, concealed allocation, baseline group comparability, blinding of participants, therapists, and assessors, dropout rate below 15%, ITT analysis, between-group statistical comparisons, and reporting of point estimates and variability.
2.6. Reporting Bias Assessment
A meta-analysis was conducted when ten or more studies examined the same outcome, and funnel plots were used to assess small-study effects and publication bias [23]. Publication bias was further evaluated using the Egger regression test in R-Studio, with a p-value < 0.05 indicating statistical significance [24].
2.7. Statistical Analysis (Synthesis Methods)
A meta-analysis was conducted using Review Manager v5.4 to synthesize the results.
Heterogeneity and significance were visually interpreted using forest plots. The inverse variance method was used to calculate the standardized mean difference (SMD) for continuous outcomes [25]. The overall effect of TOT on lower limb function was evaluated using the Z-statistic at a significance level of p < 0.05. Effect sizes were expressed as the SMD or Cohen’s d, with Hedge’s g also calculated as an adjusted estimate [26]. Since Cohen’s d can overestimate effect sizes in small samples [27], Hedge’s g was calculated to provide corrected values, categorized as small (<0.3), moderate (0.3–0.8), large (0.8–1.3), or very large (>1.3) [28].
Heterogeneity was assessed using the I2 statistic and chi-square (χ2) test and classified as low (≤40%), moderate (30–60%), substantial (50–90%), or very high (75–100%) [25]. A p-value < 0.10 for χ2 indicated statistically significant heterogeneity.
3. Results
3.1. Study Selection
A database search initially identified 1112 articles. After excluding 529 duplicates, 583 articles remained for title and abstract screening, which excluded 537 studies and retained 46 for full-text review. Thirteen studies lacked full-text access, leaving 33 eligible studies. Of these, five were abstracts only, four used inappropriate interventions, four employed different outcome measures, two lacked control groups, and one was a case study. Overall, 16 studies were excluded after full-text review, and 17 were finally included in the review. Figure 1 shows the PRISMA flow diagram.

Figure 1.
Flow diagram.
3.2. General Characteristics of the Studies
Table 1 summarizes the study characteristics. Overall, 888 participants aged 47–68 years (mean ± standard deviation: 56.36 ± 9.68) were included, comprising 554 men and 334 women. Regarding the stroke phase, one study involved patients in the acute phase, eight in the subacute phase, and eight in the chronic phase.

Table 1.
The characteristics of the studies included in the meta-analysis.
3.3. Outcomes Characteristics
Eight studies assessed gait speed, three of which measured it under two conditions: comfortable and maximal walking pace. Nine studies evaluated walking endurance using the 6 min walk test.
Balance was assessed using the BBS in nine studies and the timed up and go (TUG) test in ten studies.
3.4. Quality Assessment and Risk of Bias
The quality assessment results based on the PEDro scale are summarized as follows: 17 studies were included, with an average PEDro score of 6.17. Five studies report a dropout rate of 15% or higher, and 10 applied the ITT principle. Only one study was rated low quality (score ≤ 3), five were rated moderate quality (score 4–5), and 11 were rated high quality (score 6–10). All assessments were independently verified by the research team and supervised by two PhD-level physical therapists. Table 2 presents the results.

Table 2.
An assessment of methodological quality using the PEDro scale.
3.5. Meta-Analysis Results
3.5.1. Gait Speed
Gait speed was assessed in eight studies comprising 654 participants. The homogeneity test revealed low heterogeneity (χ2 = 15.71, p = 0.15, I2 = 30%), warranting the use of a random-effects model to account for interstudy variability. The meta-analysis revealed a statistically significant improvement in the experimental group compared to the control group (Z = 4.43, p < 0.0001; SMD = 0.48, 95% CI: 0.27, 0.69), indicating a moderate effect size. Owing to the limited number of included studies, publication bias was not assessed, and no funnel plot analysis was performed. Figure 2 and Figure 3 show the forest and funnel plots, respectively.
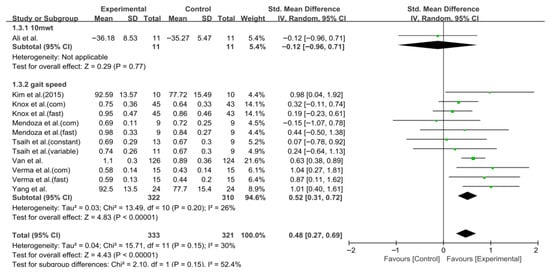
Figure 2.
Forest plot of gait speed. Studies included: Ali et al. [30], Kim et al. (2015) [40], Knox et al. [36], Mendoza et al. [43], Tsaih et al. [35], Van et al. [44], Verma et al. [37], Yang et al. [38].

Figure 3.
Funnel plot of gait speed.
3.5.2. Six-Minute Walk Test
The 6 Min Walk Test (6MWT) was assessed in nine studies comprising 623 participants. The homogeneity test revealed low heterogeneity (χ2 = 14.43, p = 0.15, I2 = 31%), warranting the use of a random-effects model to account for interstudy variability. The meta-analysis showed a statistically significant improvement in the experimental group compared to the control group (Z = 4.43, p < 0.0001; SMD: 0.49, 95% CI: 0.27, 0.71), reflecting a moderate effect size. Owing to the limited number of included studies, publication bias was not assessed, and no funnel plot analysis was performed. Forest and funnel plots are presented in Figure 4 and Figure 5, respectively.
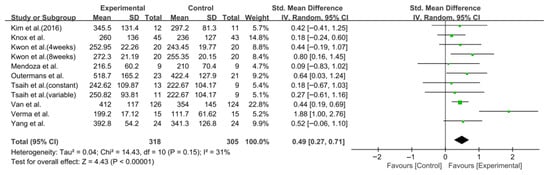
Figure 4.
A forest plot of the 6-Minute Walk Test. Studies included: Kim et al. (2016) [31], Knox et al. [36], Kwon et al. [34], Mendoza et al. [43], Outermans et al. [45], Tsaih et al. [35], Van et al. [44], Verma et al. [37], Yang et al. [38].

Figure 5.
A funnel plot of the 6-Minute Walk Test.
3.5.3. Berg Balance Scale
Balance was assessed using the BBS in nine studies comprising 395 participants. The homogeneity test indicated high heterogeneity (χ2 = 22.79, p = 0.004, I2 = 65%), warranting the use of a random-effects model to account for interstudy variability. Because heterogeneity remained moderate (I2 = 65%), subgroup analyses were performed, and detailed results are presented in Table 3. The meta-analysis showed a statistically significant improvement in the experimental group compared with the control group (Z = 2.36, p = 0.02; SMD: 0.45, 95% CI: 0.08, 0.82), reflecting a moderate effect size. Owing to the limited number of included studies, publication bias was not assessed, and no funnel plot analysis was performed. Figure 6 and Figure 7 show the forest and funnel plots.

Table 3.
A subgroup analyses of task-oriented training on the Berg Balance Scale in nine trials.
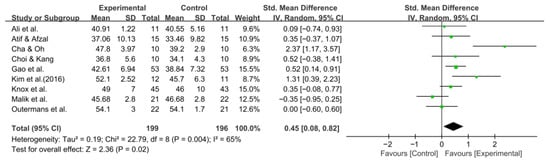
Figure 6.
A forest plot of the Berg Balance Scale. Studies included: Ali et al. [30], Atif and Afzal [41], Cha and Oh [32], Choi and Kang [42], Gao et al. [29], Kim et al. (2016) [31], Knox et al. [36], Malik et al. [39], Outermans et al. [45].
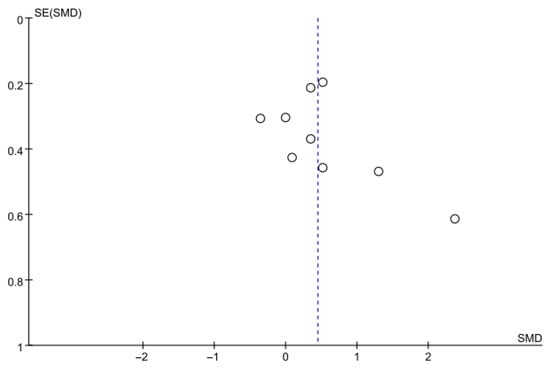
Figure 7.
A funnel plot of the Berg Balance Scale.
3.5.4. Timed up and Go Test
Balance was also evaluated using the TUG test in 10 studies comprising 642 participants. The homogeneity test indicated low heterogeneity (χ2 = 13.33, p = 0.27, I2 = 17%), warranting the use of a random-effects model to account for interstudy variability. The meta-analysis revealed a statistically significant reduction in the experimental group compared with the control group (Z = 2.92, p = 0.003; SMD: −0.28, 95% CI: −0.47, −0.09), indicating a small effect size. Egger’s regression test (t = −0.81, p = 0.44) and the Trim and Fill method identified no additional studies, suggesting no publication bias. Figure 8 and Figure 9 show the forest and funnel plots.
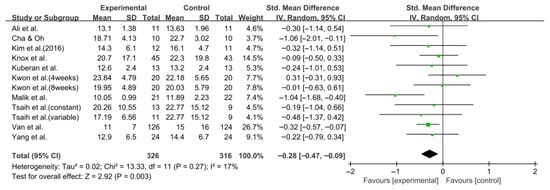
Figure 8.
A forest plot of the Timed Up and Go test. Studies included: Ali et al. [30], Cha and Oh [32], Kim et al. (2016) [31], Knox et al. [36], Kuberan et al. [33], Kwon et al. [34], Malik et al. [39], Tsaih et al. [35], Van et al. [44], Yang et al. [38].
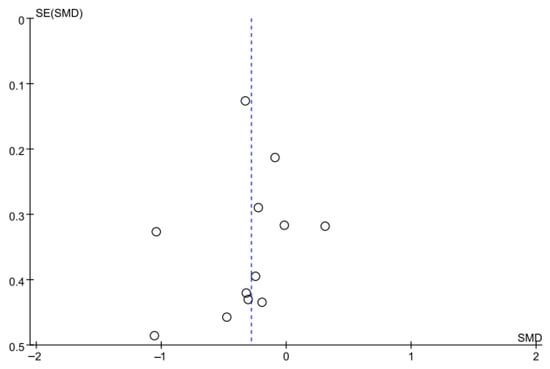
Figure 9.
A funnel plot of the Timed Up and Go test.
4. Discussion
This systematic review and meta-analysis examined the effects of TOT on gait and balance in patients with stroke, incorporating 17 RCTs. TOT significantly improved gait speed and 6MWT performance, as well as balance outcomes measured via the BBS and the TUG test.
For gait speed, a random-effects model analysis of eight studies indicated a moderate effect. Similarly, a meta-analysis of nine studies on the 6MWT showed a moderate improvement. These findings align with those of previous research demonstrating that TOT and circuit-based gait training significantly improve gait speed and endurance. Jeon et al. (2015) report that TOT improves overall functional recovery and gait-related outcomes [46], while Schröder et al. (2019) indicate that early repetitive gait training improves gait performance during initial rehabilitation [47]. Similarly, the results of this study regarding gait speed and the 6MWT suggest that intervention timing and intensity may affect the magnitude of improvement. Furthermore, Bonini-Rocha et al. (2018) report that circuit-based gait training was more effective than conventional gait rehabilitation in improving gait speed and endurance [48]. A network meta-analysis by Lyu et al. (2023) also reports that TOT significantly improves gait function and outperforms conventional gait training in enhancing functional mobility [8].
These consistent findings suggest that task-oriented approaches improve gait-related fitness, mobility, and environmental adaptability. This indicates that such training may enhance walking endurance beyond mere speed improvements. These findings support the mechanism that gait rehabilitation promotes motor learning and neuromuscular adaptation through repeated, task-directed movements [49]. However, because the included patients were at different stages of stroke recovery, variations in intervention timing and intensity (e.g., number of sessions, total treatment time) may have contributed to outcome differences. Therefore, future research should systematically examine these moderating effects through subgroup analyses or meta-regression.
This study assessed balance using the BBS and TUG test. The BBS showed a moderate effect, while the TUG showed a significant reduction in completion time across 10 studies involving 642 participants. Egger’s regression test and the Trim and Fill analysis revealed no evidence of publication bias. These findings align with those of previous studies. Zhou et al. (2024) report significant improvements in BBS and TUG in a meta-analysis of 29 RCTs examining the effects of exercise on balance in patients with stroke [50]. Similarly, Li et al. (2019) report that various balance interventions significantly enhance postural control and mobility during stroke recovery [51]. Conversely, Li et al. (2025) report significant improvements in BBS but no notable changes in TUG in their meta-analysis of home-based exercise interventions [52]. Compared with previous findings, the BBS and TUG effects in this study are directionally consistent but differ in magnitude and significance. These differences likely result from variations in participant characteristics, heterogeneity in intervention components, and differences in assessment timing across studies. In particular, balance and mobility improvements may be more limited in chronic-phase patients than in acute-phase patients owing to reduced neuroplasticity, which could explain the variability in TUG outcomes.
However, the BBS showed high heterogeneity. Subgroup analysis revealed significant differences based on stroke phase. This variation likely stemmed from differences in patient status (acute vs. chronic phase) and the timing of initial intervention across studies. Specifically, while some studies emphasized static balance tasks, others included dynamic tasks such as weight shifting and maintaining balance during walking, indicating notable differences in training intensity and stimuli. These factors likely contribute to the variability in effect size and overall heterogeneity. Furthermore, since the effectiveness of balance training varies with intervention frequency and duration, these variables may also have influenced the BBS outcomes.
In this study, interventions lasting 60 min or less and more than three sessions per week were more effective in improving BBS scores. This suggests that higher intervention intensity and frequency may play a key role in enhancing balance recovery. Similarly, Li et al. (2025) report the greatest balance improvements in programs lasting at least 8 weeks with three or more sessions weekly [52]. Conversely, some studies may have underestimated effects due to short intervention durations or evaluations conducted only immediately after intervention. Nonetheless, these findings should be interpreted cautiously, given the limited number of studies included in this meta-analysis.
Balance recovery after stroke depends on the integration of visual, somatosensory, and vestibular inputs, and because stroke often disrupts vestibular processing or sensory reweighting, task-oriented activities involving head movements, gait transitions, and dynamic postural adjustments may help stimulate vestibular pathways [53]. This enhanced multisensory integration may partly explain the improvements observed in BBS and TUG outcomes [54].
In summary, this study demonstrates that the interventions improve balance and mobility, as reflected in the BBS and TUG outcomes. Clinically, TOT that incorporates both static and dynamic balance components may be more effective, and ensuring adequate intervention duration and intensity is crucial. Additionally, tailoring the timing of balance training to the recovery stage of the patient may be a key determinant of its effectiveness.
These findings suggest that interventions targeting gait and balance substantially enhance motor function recovery in patients with stroke. First, incorporating balance-challenging tasks into gait training or combining both approaches may maximize therapeutic benefits. Second, response patterns may vary across recovery phases (acute, subacute, chronic), highlighting the importance of appropriate intervention timing and participant selection. Third, factors such as training intensity, session frequency, and program duration may influence outcomes, underscoring the need for standardized and evidence-based intervention protocols.
Participants in the included studies were randomly assigned to experimental or control groups, with a mean PEDro score of 6.17, indicating high methodological quality (PEDro scale: 6–10). The PEDro score assesses the internal validity and methodological rigor of clinical trials, and the average score in this review exceeded the mean in the PEDro database of 5 [55]. These findings suggest that the internal validity of this review is well established. However, this study has several limitations. First, the limited number of RCTs reduces the robustness of the findings, warranting cautious interpretation. Second, the high heterogeneity observed in the BBS analysis may reflect insufficient control of confounding variables or the influence of unreported factors. Third, most studies report only immediate post-intervention outcomes, providing limited evidence of long-term effects after treatment cessation. Fourth, the small number of available studies limited meta-regression analysis, restricting subgroup analyses to exploratory interpretation. Fifth, the coexistence of gait-focused, balance-focused, and combined interventions complicates direct comparisons of their effects. Therefore, future research should incorporate more high-quality RCTs to examine the influence of intervention components (intensity, frequency, modality), integrated gait and balance strategies, long-term retention effects, and differences in response across patient characteristics.
5. Conclusions
This systematic review and meta-analysis, synthesizing 17 RCTs involving patients with stroke, demonstrated that TOT significantly improves gait speed, walking endurance, and balance. Moderate effect sizes were observed for gait outcomes, including gait speed and the 6MWT, while balance measures such as the BBS and the TUG test also showed significant improvements. These findings suggest that a TOT is more effective than simple repetitive exercise in promoting functional recovery and enhancing environmental adaptability. Furthermore, intervention intensity (number of sessions and weekly frequency) and stroke phase (acute vs. chronic) were identified as key factors influencing balance outcomes. Therefore, in clinical practice, task-oriented programs should integrate gait and balance components tailored to the recovery phase and target functions of the patients. Future research should focus on long-term follow-up outcomes, the effects of specific intervention components, and the development of standardized training protocols. These efforts will help enhance functional independence and quality of life in patients with stroke.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14248766/s1, Table S1: PRISMA 2020 Checklist.
Author Contributions
Conceptualization, methodology, M.-H.L. and D.-Y.L.; Software, M.-H.L.; validation, formal analysis, M.-H.L.; investigation, M.-H.L. and D.-Y.L.; resources and data curation, M.-H.L.; writing—original draft preparation, M.-H.L. and D.-Y.L.; writing—review and editing and visualization, M.-H.L.; Supervision, project administration and funding acquisition, D.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a research grant from the Korean Vestibular Rehabilitation Therapy Association (2025).
Institutional Review Board Statement
PROSPERO 2025 CRD420251166430. Available from https://www.crd.york.ac.uk/PROSPERO/view/CRD420251166430 (accessed on 12 October 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current research are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Capirossi, C.; Laiso, A.; Renieri, L.; Capasso, F.; Limbucci, N. Epidemiology, organization, diagnosis and treatment of acute ischemic stroke. Eur. J. Radiol. Open 2023, 11, 100527. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World stroke organization: Global stroke fact sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Van De Port, I.G.; Kwakkel, G.; Van Wijk, I.; Lindeman, E. Susceptibility to deterioration of mobility long-term after stroke: A prospective cohort study. Stroke 2006, 37, 167–171. [Google Scholar] [CrossRef]
- Gandolfi, M.; Valè, N.; Dimitrova, E.; Zanolin, M.E.; Mattiuz, N.; Battistuzzi, E.; Beccari, M.; Geroin, C.; Picelli, A.; Waldner, A. Robot-assisted stair climbing training on postural control and sensory integration processes in chronic post-stroke patients: A randomized controlled clinical trial. Front. Neurosci. 2019, 13, 1143. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research into Clinical Practice; Lippincott Williams & Wilkins: Ambler, PA, USA, 2007. [Google Scholar]
- Salem, Y.; Ciani, G.; Elokda, A.; Hegazy, F.; Aboelnasr, E.; Li, H. Immediate Effects of TaskOriented Training on Walking and Balance in Patients with Stroke: A Preliminary Study. Ageing Sci. Ment. Health Stud. 2022, 6, 1–5. [Google Scholar]
- Lyu, T.; Yan, K.; Lyu, J.; Zhao, X.; Wang, R.; Zhang, C.; Liu, M.; Xiong, C.; Liu, C.; Wei, Y. Comparative efficacy of gait training for balance outcomes in patients with stroke: A systematic review and network meta-analysis. Front. Neurol. 2023, 14, 1093779. [Google Scholar] [CrossRef]
- Alashram, A.R. Task-oriented training for gait rehabilitation in people with multiple sclerosis: A systematic review. J. Bodyw. Mov. Ther. 2024, 39, 87–96. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Giovannini, S.; Iacovelli, C.; Brau, F.; Loreti, C.; Fusco, A.; Caliandro, P.; Biscotti, L.; Padua, L.; Bernabei, R.; Castelli, L. RObotic-Assisted Rehabilitation for balance and gait in Stroke patients (ROAR-S): Study protocol for a preliminary randomized controlled trial. Trials 2022, 23, 872. [Google Scholar] [CrossRef]
- Kawamoto, H.; Kamibayashi, K.; Nakata, Y.; Yamawaki, K.; Ariyasu, R.; Sankai, Y.; Sakane, M.; Eguchi, K.; Ochiai, N. Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol. 2013, 13, 141. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, Z.; Li, Y.; Meng, J.; Jiang, X.; Xu, B.; Li, H.; Liu, T. The effect of balance and gait training on specific balance abilities of survivors with stroke: A systematic review and network meta-analysis. Front. Neurol. 2023, 14, 1234017. [Google Scholar] [CrossRef]
- Potcovaru, C.-G.; Cinteză, D.; Săndulescu, M.I.; Poenaru, D.; Chiriac, O.; Lambru, C.; Moldoveanu, A.; Anghel, A.M.; Berteanu, M. The Impact of Virtual Reality as a Rehabilitation Method Using TRAVEE System on Functional Outcomes and Disability in Stroke Patients: A Pilot Study. Biomedicines 2024, 12, 2450. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, M.; Pan, X.; Geng, J. Effects of exercise on mobility, balance and gait in patients with the chronic stroke: A systematic review and meta-analysis. Sci. Rep. 2025, 15, 24158. [Google Scholar] [CrossRef] [PubMed]
- Gelaw, A.Y.; Janakiraman, B.; Teshome, A.; Ravichandran, H. Effectiveness of treadmill assisted gait training in stroke survivors: A systematic review and meta-analysis. Glob. Epidemiol. 2019, 1, 100012. [Google Scholar] [CrossRef]
- Liu, H.; Yin, H.; Yi, Y.; Liu, C.; Li, C. Effects of different rehabilitation training on balance function in stroke patients: A systematic review and network meta-analysis. Arch. Med. Sci. AMS 2023, 19, 1671. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Jpt, H. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2008; Available online: http://www.cochrane.org/handbook (accessed on 15 July 2025).
- Higgins, J.; Li, T.; Deeks, J.J. Choosing effect measures and computing estimates of effect. In Cochrane Handbook of Systematic Reviews of Interventions; Cochrane: London, UK, 2019. [Google Scholar]
- Bhogal, S.K.; Teasell, R.W.; Foley, N.C.; Speechley, M.R. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad scale in stroke rehabilitation literature. J. Clin. Epidemiol. 2005, 58, 668–673. [Google Scholar] [CrossRef]
- Mavridis, D.; Salanti, G. How to assess publication bias: Funnel plot, trim-and-fill method and selection models. Èvid. Based Ment. Health 2014, 17, 30. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D. Doing Meta-Analysis with R: A Hands-on Guide; Chapman and Hall/CRC: Boca Raton, FL, USA, 2021. [Google Scholar]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Group, C.S.M. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2019; pp. 241–284. [Google Scholar]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Durlak, J.A. How to select, calculate, and interpret effect sizes. J. Pediatr. Psychol. 2009, 34, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using effect size—Or why the P value is not enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Jin, Q.; Zhou, C.; Huang, Y.; Liu, J.; Chen, Y.; Hu, H.; Dai, Y.; Jin, R. Effect of Task-Oriented Biomechanical Perception-Balance Training on Motor Gait in Stroke Patients With Hemiplegia. J. Altern. Complement. Med. 2024, 30, 290–294. [Google Scholar]
- Ali, M.; Khan, S.U.; Asim, H.A.B. Effects of individual task specific training verses group circuit training on balance and ambulation in sub-acute stroke. Rawal Med. J. 2020, 45, 233–235. [Google Scholar]
- Kim, B.; Park, Y.; Seo, Y.; Park, S.; Cho, H.; Moon, H.; Lee, H.; Kim, M.; Yu, J. Effects of individualized versus group task-oriented circuit training on balance ability and gait endurance in chronic stroke inpatients. J. Phys. Ther. Sci. 2016, 28, 1872–1875. [Google Scholar] [CrossRef][Green Version]
- Cha, H.-G.; Oh, D.-W. Effects of mirror therapy integrated with task-oriented exercise on the balance function of patients with poststroke hemiparesis: A randomized-controlled pilot trial. Int. J. Rehabil. Res. 2016, 39, 70–76. [Google Scholar] [CrossRef]
- Kuberan, P.; Kumar, V.; Joshua, A.M.; Misri, Z.; Chakrapani, M. Effects of task oriented exercises with altered sensory input on balance and functional mobility in chronic stroke: A pilot randomized controlled trial. Bangladesh J. Med. Sci. 2017, 16, 307–313. [Google Scholar] [CrossRef]
- Kwon, O.-h.; Woo, Y.; Lee, J.-s.; Kim, K.-h. Effects of task-oriented treadmill-walking training on walking ability of stoke patients. Top. Stroke Rehabil. 2015, 22, 444–452. [Google Scholar] [CrossRef]
- Tsaih, P.-L.; Chiu, M.-J.; Luh, J.-J.; Yang, Y.-R.; Lin, J.-J.; Hu, M.-H. Practice variability combined with task-oriented electromyographic biofeedback enhances strength and balance in people with chronic stroke. Behav. Neurol. 2018, 2018, 7080218. [Google Scholar] [CrossRef]
- Knox, M.; Stewart, A.; Richards, C.L. Six hours of task-oriented training optimizes walking competency post stroke: A randomized controlled trial in the public health-care system of South Africa. Clin. Rehabil. 2018, 32, 1057–1068. [Google Scholar] [CrossRef]
- Verma, R.; Narayan Arya, K.; Garg, R.; Singh, T. Task-oriented circuit class training program with motor imagery for gait rehabilitation in poststroke patients: A randomized controlled trial. Top. Stroke Rehabil. 2011, 18, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-R.; Wang, R.-Y.; Lin, K.-H.; Chu, M.-Y.; Chan, R.-C. Task-oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clin. Rehabil. 2006, 20, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Masood, T. Task-oriented training and exer-gaming for improving mobility after stroke: A randomized trial. J. Pak. Med. Assoc. 2021, 71, 186–190. [Google Scholar] [PubMed]
- Kim, C.-Y.; Lee, J.-S.; Kim, H.-D.; Kim, J.-S. The effect of progressive task-oriented training on a supplementary tilt table on lower extremity muscle strength and gait recovery in patients with hemiplegic stroke. Gait Posture 2015, 41, 425–430. [Google Scholar] [CrossRef]
- Atif, M.M.; Afzal, F. The effects of a task-oriented walking intervention on improving balance self-efficacy in post-stroke patients. Adv. Neurol. 2023, 2, 388. [Google Scholar] [CrossRef]
- Choi, J.-U.; Kang, S.-h. The effects of patient-centered task-oriented training on balance activities of daily living and self-efficacy following stroke. J. Phys. Ther. Sci. 2015, 27, 2985–2988. [Google Scholar] [CrossRef]
- Mendoza, K.G.; Aguila, M.E.R.; Alfonso, F.C.S.; Alfonso, M.G.T.; Elmi, K.D.; Gorgon, E.J.R. Comparison of two circuit class therapy programs on walking capacity, gait velocity and stair ambulation among patients with chronic stroke: A parallel pretest-posttest pilot study. Acta Medica Philipp. 2021, 55, 379–386. [Google Scholar] [CrossRef]
- Van de Port, I.G.; Wevers, L.E.; Lindeman, E.; Kwakkel, G. Effects of circuit training as alternative to usual physiotherapy after stroke: Randomised controlled trial. Br. Med. J. 2012, 344, e2672. [Google Scholar] [CrossRef]
- Outermans, J.C.; van Peppen, R.P.; Wittink, H.; Takken, T.; Kwakkel, G. Effects of a high-intensity task-oriented training on gait performance early after stroke: A pilot study. Clin. Rehabil. 2010, 24, 979–987. [Google Scholar] [CrossRef]
- Jeon, B.-J.; Kim, W.-H.; Park, E.-Y. Effect of task-oriented training for people with stroke: A meta-analysis focused on repetitive or circuit training. Top. Stroke Rehabil. 2015, 22, 34–43. [Google Scholar] [CrossRef]
- Schröder, J.; Truijen, S.; Van Criekinge, T.; Saeys, W. Feasibility and effectiveness of repetitive gait training early after stroke: A systematic review and meta-analysis. J. Rehabil. Med. 2019, 51, 78–88. [Google Scholar] [CrossRef]
- Bonini-Rocha, A.C.; de Andrade, A.L.S.; Moraes, A.M.; Matheus, L.B.G.; Diniz, L.R.; Martins, W.R. Effectiveness of circuit-based exercises on gait speed, balance, and functional mobility in people affected by stroke: A meta-analysis. Phys. Med. Rehabil. 2018, 10, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.; Lewthwaite, R.; Blanton, S.R.; Wolf, L.B.; Wishart, L. Infusing motor learning research into neurorehabilitation practice: A historical perspective with case exemplar from the accelerated skill acquisition program. J. Neurol. Phys. Ther. 2014, 38, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, H.; Hou, X.; Dong, X.; Zhang, S.; Lv, Y.; Li, C.; Yu, L. The effect of exercise on balance function in stroke patients: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2024, 271, 4751–4768. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, D.; Ye, J.; He, M.; Liu, X.; Zheng, H.; Jin, R.; Zhang, S.-l. Rehabilitation for balance impairment in patients after stroke: A protocol of a systematic review and network meta-analysis. BMJ Open 2019, 9, e026844. [Google Scholar] [CrossRef]
- Li, X.; Wu, C.; Zhang, J.; Zeng, Q.; Wang, Y. Home-Based Exercise for Improving Balance Ability in Post-Stroke Patients: A Systematic Review and Meta-Analysis. Worldviews Evid.-Based Nurs. 2025, 22, e70057. [Google Scholar] [CrossRef]
- Awosika, O.O.; Garver, A.; Drury, C.; Sucharew, H.J.; Boyne, P.; Schwab, S.M.; Wasik, E.; Earnest, M.; Dunning, K.; Bhattacharya, A. Insufficiencies in sensory systems reweighting is associated with walking impairment severity in chronic stroke: An observational cohort study. Front. Neurol. 2023, 14, 1244657. [Google Scholar] [CrossRef]
- Meng, L.; Liang, Q.; Yuan, J.; Li, S.; Ge, Y.; Yang, J.; Tsang, R.C.; Wei, Q. Vestibular rehabilitation therapy on balance and gait in patients after stroke: A systematic review and meta-analysis. BMC Med. 2023, 21, 322. [Google Scholar] [CrossRef]
- Yamato, T.P.; Arora, M.; Stevens, M.L.; Elkins, M.R.; Moseley, A.M. Quality, language, subdiscipline and promotion were associated with article accesses on Physiotherapy Evidence Database (PEDro). Physiotherapy 2018, 104, 122–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).