Abstract
Background: The prevalence and the prognostic impact of chronic obstructive pulmonary disease (COPD) and asthma in patients with myeloproliferative neoplasms (MPNs) are unknown. Methods: This retrospective multicenter cohort analyzed the prevalence and prognostic implications of COPD and asthma in 246 patients with essential thrombocythemia (ET) and polycythemia vera (PV). Results: A total of 6.5% and 1.6% patients had COPD or asthma, respectively, without statistically significant differences with respect to disease phenotype. The presence of COPD/asthma was more frequently associated with active/prior smoking (p = 0.021) and constitutional symptoms (p = 0.001). After the median follow-up of 47.5 months, the presence of COPD/asthma was univariately associated with an inferior time to thrombosis (TTT; median 135 vs. 190 months, 95% confidence interval (CI) 1.8–29.5, hazard ratio-HR 7.75, p = 0.005), mainly driven by venous (HR 37.3, 95% CI 3.2–43.6, p = 0.003) and not arterial events (HR 1.77, 95% CI 0.40–7.78, p = 0.445, p = 0.445). Statistically significant interactions existed between COPD/asthma, female sex (HR 3.94, 95% CI 1.01–11.02), ET phenotype (HR 7.1, 95% CI 15.3–16.7), JAK2 positive status (HR 4.17, 95% CI 1.04–6.9), hydroxyurea use (HR 4.67, 95% CI 1.10–7.43), and the presence of other cardiovascular risk factors (HR 8.1, 95% CI 1.55–10.72) with overall thrombotic risk (interaction p < 0.050 for all analyses). Finally, the negative effect of COPD/asthma on TTT persisted in the multivariate analysis (HR 6.54, p = 0.010) independently of older (>60 years) age (p = 0.030) when being adjusted for other clinically meaningful variables. There was no effect of COPD/asthma on overall survival. Conclusions: These results provide an important signal regarding the potentially inferior outcomes in ET/PV patients presenting with these common respiratory disorders and may help to further personalize MPN management.
1. Introduction
Polycythemia vera (PV) and essential thrombocythemia (ET) are BCR::ABL1 negative myeloproliferative neoplasms (MPNs) characterized by overproduction of one or more mature myeloid cell lineages, presence of Janus Kinase 2 (JAK2), calreticulin (CALR) or myeloproliferative leukemia virus (MPL) gene mutations, chronic inflammatory state, debilitating constitutional symptoms, and high thrombotic risk []. Therapeutically, all PV patients receive low-dose aspirin and are phlebotomized with an aim to maintain the hematocrit < 45% as two randomized clinical trials have shown that these interventions may lower cardiovascular risk, whereas ET patients usually receive aspirin if they have previously experienced thrombosis or are JAK2 positive. High-risk ET and PV patients (those having >60 years or with prior thrombosis) are additionally treated with cytoreduction (hydroxyurea or interferons) [].
Cardiovascular comorbidities, such as arterial hypertension, hyperlipidemia, and smoking, are frequent in PV and ET patients and may additionally contribute to already high thrombotic risk [,]. Therefore, stringent control of cardiovascular risk factors is recommended for all MPN patients. In fact, inadequately controlled cardiovascular risk factors in MPNs may also represent an indication for cytoreductive treatment (i.e., interferons or hydroxyurea) and even twice-daily aspirin in otherwise low-risk patients (those without prior thrombosis and younger than 60 years of age) [,,].
Chronic obstructive pulmonary disease (COPD) and asthma are major public health concerns, and their prevalences are on the rise [,,,]. These common respiratory disorders significantly affect patients’ quality of life and are associated with an increased cardiovascular risk [,]. Besides the traditional cardiovascular risk factors (i.e., smoking) frequently present in patients with COPD and asthma, other pathophysiological mechanisms for the increased thrombotic risk in these patients also exist, such as increased blood hypercoagulability, chronic hypoxemia, pulmonary hypertension, hyperinflation, oxidative stress, and chronic inflammation [,].
The current prevalence of COPD and asthma and their prognostic implications in MPN patients are unknown. Additionally, both disorders primarily occur in the elderly, present with chronic inflammation, and are burdened with thrombotic complications. Therefore, the aims of this study were: (1) to analyze the prevalences of COPD andasthma in MPNs, (2) to analyze whether the presence of COPD/asthma in MPNs may be associated with distinct MPN disease features, and (3) to analyze the association of COPD/asthma with thrombotic risk and survival in MPNs.
2. Patients and Methods
2.1. Study Design and Patient Inclusion Criteria
This multicenter study was conducted at three hospitals in Croatia (General Hospital of Sibenik-Knin County, General Hospital Zadar, and dr. Josip Bencevic Slavonski Brod) and one center in Serbia (Clinical Center Serbia Belgrade) in the period between January 1997 and January 2023 and included PV and ET patients whose disease diagnosis was reassessed for all patients according to 2016 World Health Organization criteria []. Clinical and laboratory variables were recorded through electronic medical chart review at the time of MPN disease diagnosis. The presence of COPD/asthma was coded at baseline, that is, if diagnosed by a pulmonologist and recorded as such in the medical documentation at the time of MPN diagnosis. Other cardiovascular risk factors considered were arterial hypertension, hyperlipidemia, and smoking (defined as active/prior vs. never). High-risk PV and ET patients were classified as those with age > 60 years and/or prior (arterial or venous) thrombosis documented as such in the medical records []. Constitutional symptoms were defined as the presence of any of the usual MPN-related symptoms: fatigue, night sweats, itching, weight loss (>10% in the preceding 6 months), and fever. Excluded from participation were patients younger than 18 years of age, pregnant women, and those lost to follow-up.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committees from all participating centers; the details are presented at the end of the manuscript.
2.2. Statistics
The distribution of data was checked using the Shapiro–Wilk test. One proportion test was used to compare the prevalence of COPD and asthma in ET and PV patients with that in the general population. Categorical variables between the two patient groups were compared with the chi-square or Fisher’s exact test as appropriate, whereas differences in continuous variables were assessed with the Mann–Whitney U test. Univariate and multivariate survival analyses were performed with the Kaplan–Meier method, log-rank test, and Cox regression analysis. Time to thrombosis (TTT) was calculated from the time of diagnosis until the first arterial (myocardial infarction, transitory ischemic attack, or ischemic cerebrovascular stroke, or peripheral arterial occlusion) or venous (hand/leg thrombosis and/or pulmonary embolism). Overall survival (OS) was measured from the time of diagnosis until death or the last follow-up visit. Statistical calculations were performed with MedCalc Statistical software (Ostend, Belgium, version 23.2.7), and a significant p-value was set at <0.050 for all presented analyses.
3. Results
3.1. Patient Characteristics and the Prevalence of COPD and Asthma
A total of 246 patients were included (PV = 154, ET = 92); median age was 68 years (range 20–91), 137 (55.7%) were females, 16 (6.5%) had COPD and 4 (1.6%) had asthma. There were no statistically significant differences in the prevalence of COPD and asthma with respect to disease phenotype (p > 0.050 for both analyses). On the other hand, prevalences of COPD (6.5% vs. 10.3%; p = 0.051) and asthma (1.6% vs. 6.6%; p = 0.001) seemed to be somewhat lower in ET and PV patients than in the general population [,]. None of the COPD/asthma patients required long-term oxygen therapy.
Considering the relatively small number of COPD and asthma patients, they were grouped together for the purpose of statistical analyses. Overall patient characteristics and stratified according to the presence of COPD/asthma are shown in Table 1. As presented, patients with COPD/asthma were more frequently active or prior smokers (p = 0.021) with constitutional symptoms (p = 0.001), whereas there were no differences with respect to other tested clinical and laboratory variables at baseline (p > 0.050 for all analyses).

Table 1.
Overall patient characteristics and stratified according to COPD/asthma presence.
3.2. Survival Analyses
The median follow-up time was 47.5 months (range 1–307), and a total of 40 (16.3%) thrombotic events (arterial = 30, venous = 10) occurred during this time. Thrombotic events during follow-up were numerically more frequent in COPD/asthma patients (n = 6/20, 30% vs. 34/226, 15%; p = 0.083) and were mainly driven by venous events (n = 3/20, 15% vs. 7/226, 3.1%; p = 0.009) whereas there was no difference in the frequency of arterial events (n = 3/20, 15% vs. 27/226, 11.9%; p = 0.689).
In univariate analyses, the presence of COPD/asthma was statistically significantly associated with an inferior TTT in the overall cohort (median 135 vs. 190 months, 95% confidence interval (CI) 1.8–29.5, hazard ratio-HR 7.75 p = 0.005) which was also mainly driven by venous (HR 37.3, 95% CI 3.2–43.6, p = 0.003) and not arterial events (HR 1.77, 95% CI 0.40–7.78, p = 0.445), as shown in Figure 1A–C.
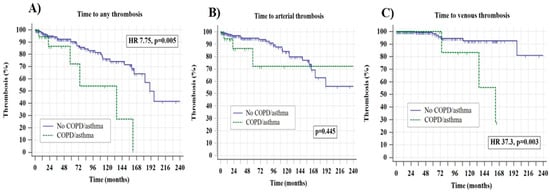
Figure 1.
Time to any thrombosis panel (A), arterial panel (B), and venous thrombosis (C) stratified according to the presence of chronic obstructive pulmonary disorder (COPD)/asthma.
Table 2 summarizes all univariate associations of the clinically most relevant variables with overall, arterial, and venous thrombosis during the follow-up, as well as interactions between these variables and COPD/asthma with overall thrombotic risk. As shown, besides the association of COPD/asthma with overall and venous thrombotic events, other statistically significant associations were found between older (>60 years) age and overall and arterial thrombotic risk, as well as an association between JAK2 positive status and arterial thrombosis (p < 0.050 for all analyses), whereas no other statistically significant associations were recorded. Statistically significant interactions existed between COPD/asthma, female sex, ET phenotype, JAK2 positive status, hydroxyurea use, and the presence of additional cardiovascular risk factors with thrombotic risk (interaction p < 0.050 for all analyses), with a near-significant p value for high-risk disease (interaction p = 0.063). Targeted analysis of these subgroups with respect to separate arterial vs. venous events was not attempted due to the small number of venous events in specific subsets and the subsequent lack of statistical power.

Table 2.
Associations of the most clinically relevant variables with overall, arterial, and venous thrombosis and the interaction analysis of these variables with chronic obstructive pulmonary disease (COPD/asthma) and overall thrombotic risk.
Finally, in the multivariate Cox regression model, presence of COPD/asthma (HR 6.54, p = 0.010) and older (>60 years) age (HR 4.65, p = 0.030) were independently of each other associated with an inferior TTT when being additionally adjusted for sex, disease phenotype, prior thrombosis, presence of other cardiovascular risk factors, and the use of aspirin and cytoreduction (p > 0.050 for all analyses).
The presence of COPD/asthma did not have an impact on OS in the entire cohort (p = 0.310) nor when ET (p = 0.412) and PV patients (p = 0.575) were analyzed separately.
4. Discussion
To our knowledge, this is the first study to investigate the prevalence and the potential prognostic impact of COPD/asthma on major outcomes in ET and PV patients. Several important observations arise from this study. First, patients with COPD/asthma and polycythemia are frequently referred to hematologists for diagnostic exclusion of PV, as these two disorders have been shown to sometimes co-exist []. In addition, more recent reports have demonstrated that secondary polycythemia may not be as benign as previously thought, as it may be associated with cardiovascular risk higher than that in the general population and similar to that of low-risk PV [,,]. The presented results suggest that the prevalence of COPD and asthma in MPNs may not be higher than in the general population and was consistent in both time periods analyzed (1997–2009 and 2010–2023). The reasons for this observation remain unknown. Due to a retrospective study design, the potential of selection bias may exist. For example, medical information regarding the presence of these respiratory disorders may not have been captured despite the fact that all documentation was manually reviewed. Also, due to a lack of prospective follow-up, some asymptomatic patients or those with few respiratory symptoms may have been undiagnosed. In addition, some COPD/asthma patients with more severe disease and early deaths before study entry may not have been documented.
On the other hand, some pathophysiological explanations may also exist regarding the lower prevalence of COPD/asthma in MPN patients than in the general population. Besides the bone marrow, thrombocytopoiesis also occurs in the lungs, and platelets are an important source of many cytokines, which possess important immunomodulatory properties and may actually support lung regeneration []. This effect has also been shown in patients with MPNs and coronavirus disease 2019 (COVID-19), where patients with higher platelet count actually had improved outcomes []. Therefore, it is possible that increased lung thrombocytopoiesis in ET/PV patients may, in fact, help to modulate the inflammatory response in the lungs and thus alleviate respiratory symptomatology, potentially leading to a lower frequency of COPD/asthma. Conversely, one observational cohort study has reported that thrombocytosis in patients with COPD exacerbations may be associated with an inferior one-year survival and reduced in-hospital mortality; cardiovascular hospitalizations were not associated with thrombocytosis, and antiplatelet use improved survival []. Therefore, further multinational collaborative studies (preferably prospective), including a larger number of MPN patients, are needed to confirm our findings and to elucidate the exact pathophysiological mechanisms underlying this interesting observation.
Second, it seems that the presence of COPD/asthma does significantly affect MPN disease phenotype, as both patient groups had similar baseline clinical and laboratory characteristics. More specifically, only constitutional symptoms were more frequent in patients with COPD/asthma. The increased frequency of the latter could be, at least partly, attributable to the negative effect of these respiratory disorders on patients’ symptoms and the quality of life. Also, prior or active smoking was more frequent in patients with COPD/asthma. Considering the known widespread detrimental health effects of smoking and the fact that it may also impair treatment responses and survival in MPNs [], all patients should be strongly advised to quit smoking. Due to a retrospective study design, we were unable to analyze differences in specific symptoms and cytokine profiles between MPN patients with and without COPD/asthma, and further studies should also focus on this topic.
Third, the presence of COPD/asthma was both in univariate and multivariate analyses associated with an inferior TTT, which was mainly driven by venous events. As both COPD and asthma are associated with a hypercoagulable state [,,,] and an increased risk for both arterial and venous thrombosis in the general population [,,,,,,,], a significant prothrombotic synergism between COPD/asthma and MPN may indeed exist. However, in the current study, the presence of COPD/asthma was associated with an increased risk of venous, but not arterial, thrombosis. A similar effect has also been shown in MPN patients with COVID-19, where a significant risk of venous thromboembolism has been noted []. In addition, a significant proportion of study patients used aspirin as thromboprophylaxis per the current MPN guidelines, and hydroxyurea, which are not that potent in the protection of the venous district []. Also, patients with COPD/asthma are often less physically active and more sedentary, leading to venous stasis, which predisposes them more to venous than arterial thrombosis. Moreover, there were statistically significant interactions between female sex, ET phenotype, hydroxyurea use, JAK2 positivity, high-risk disease, and the presence of other cardiovascular risk factors with overall thrombotic risk, suggesting that special consideration and clinical surveillance should be targeted at this specific MPN patient subpopulation having these high-risk features [,]. It should also be pointed out that both hydroxyurea use and high-risk may present with overlapping prognostic properties, as both may represent the same patient subpopulation. Finally, it should be acknowledged that due to the aforementioned competing-risk caveats, a small number of study patients and events leading to overfitting and wide CI, some of our analyses may lack sufficient power. Therefore, additional studies on a larger number of MPN patients are needed to confirm our observations.
It should also be noted that both COPD/asthma and MPN patients during the disease course may also eventually develop pulmonary hypertension, which is associated with inferior outcomes in MPNs [,]. Collectively, the presented results suggest that careful clinical surveillance may be needed for MPN patients with COPD and asthma, with stringent control of other cardiovascular risk factors. Prospective cohort studies on a larger number of MPN patients with COPD/asthma are needed to unravel optimal treatment approaches for this specific patient population.
Limitations of this study are its retrospective design, the small number of COPD and asthma MPN patients included, and the small number of events of interest during follow-up. Additionally, due to the retrospective design of the study, we could not assess other important variables of interest in MPN patients with COPD/asthma, such as the effect of pulmonary function tests, pulmonary arterial pressure, and other echocardiographic findings on different clinical outcomes. Also, the potential effect of the number and the severity of COPD/asthma exacerbations on ET/PV disease outcomes was not assessed—the negative effect of COPD exacerbations on cardiovascular risk has been demonstrated in the general population [,].
Nevertheless, the presented study provides an important signal regarding the potentially inferior clinical outcomes of ET and PV patients with COPD/asthma and may help to further personalize MPN management. Additional prospective cohort studies on a larger number of MPN patients with COPD/asthma using standardized respiratory phenotyping are needed, additionally including patients with primary and secondary myelofibrosis, to validate these results and to elucidate optimal treatment approaches for this MPN patient population.
Author Contributions
Conceptualization, I.K., D.L., A.B., M.S. and M.L.; methodology, I.K., D.L., A.B. and M.L.; software, I.K.; formal analysis, I.K.; investigation, I.K., D.L., I.A., N.D., I.I., H.H., I.Z., M.M.P. and A.A.M.; data curation, I.K., D.L., I.I., N.D., H.H., I.Z. and M.M.P.; writing—original draft preparation, I.K., D.L., M.S. and M.L.; writing—review and editing, I.K., D.L., I.I., N.D., I.A., H.H., I.Z., M.M.P., A.A.M., A.B., M.S. and M.L.; visualization, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Ethics Committees from all participating centers; the General Hospital of Šibenik-Knin County, Croatia (reference number 01-3618/1-20, dated 26 February 2020); General Hospital Zadar, Croatia (reference number 02-2025/20-6/20, dated 10 April 2020); “Dr. Josip Benčević” General Hospital, Croatia (reference number 04000000/20-37, dated 10 June 2020); and University Clinical Center of Serbia, Serbia (reference number 499/05, dated 30 November 2021).
Informed Consent Statement
Due to the retrospective study design, informed consent was waived by the relevant Ethics Committees.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (Ivan Krecak) due to privacy reasons.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krecak, I.; Lucijanic, M.; Verstovsek, S. Advances in Risk Stratification and Treatment of Polycythemia Vera and Essential Thrombocythemia. Curr. Hematol. Malig. Rep. 2022, 17, 155–169. [Google Scholar] [CrossRef]
- Krecak, I.; Verstovsek, S.; Lucijanic, M. Optimization of cardiovascular risk factor management in patients with BCR::ABL1 negative chronic myeloproliferative neoplasms, current knowledge, and perspectives. Ann. Hematol. 2024, 103, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Krecak, I.; Verstovsek, S.; Lucijanic, M. Reappraisal of cardiovascular risk factors in patients with chronic myeloproliferative neoplasms. Clin. Adv. Hematol. Oncol. 2023, 21, 541–548. [Google Scholar]
- Barbui, T.; Tefferi, A.; Vannucchi, A.M.; Passamonti, F.; Silver, R.T.; Hoffman, R.; Verstovsek, S.; Mesa, R.; Kiladjian, J.-J.; Hehlmann, R.; et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: Revised management recommendations from European LeukemiaNet. Leukemia 2018, 32, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Vannucchi, A.M.; Griesshammer, M.; Harrison, C.; Koschmieder, S.; Gisslinger, H.; Álvarez-Larrán, A.; De Stefano, V.; Guglielmelli, P.; Palandri, F.; et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 2022, 9, e301–e311. [Google Scholar] [CrossRef]
- Tefferi, A.; Barbui, T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 1599–1613. [Google Scholar] [CrossRef]
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open 2023, 6, e2346598. [Google Scholar] [CrossRef]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. NIHR RESPIRE Global Respiratory Health Unit. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef]
- GBD 2021 Asthma and Allergic Diseases Collaborators. Global, regional, and national burden of asthma and atopic dermatitis, 1990-2021, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Respir. Med. 2025, 13, 425–446. [Google Scholar] [CrossRef]
- Chakaya, J.; Aït-Khaled, N. The Global Asthma Report 2022. Int. J. Tuberc. Lung Dis. 2022, 26 (Suppl. S1), 1–104. [Google Scholar] [PubMed]
- Polman, R.; Hurst, J.R.; Uysal, O.F.; Mandal, S.; Linz, D.; Simons, S. Cardiovascular disease and risk in COPD: A state of the art review. Expert Rev. Cardiovasc. Ther. 2024, 22, 177–191. [Google Scholar] [CrossRef]
- Pollevick, M.E.; Xu, K.Y.; Mhango, G.; Federmann, E.G.; Vedanthan, R.; Busse, P.; Holguin, F.; Federman, A.D.; Wisnivesky, J.P. The Relationship Between Asthma and Cardiovascular Disease: An Examination of the Framingham Offspring Study. Chest 2021, 159, 1338–1345. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Vicari, A.M.; Ponzoni, M.; Alberetto, M.; Martani, C.; Pontiroli, A.E.; Folli, F. Erythrocytosis in a patient with chronic obstructive pulmonary disease. Haematologica 1998, 83, 183–186. [Google Scholar] [PubMed]
- Nguyen, E.; Harnois, M.; Busque, L.; Sirhan, S.; Assouline, S.; Chamaki, I.; Olney, H.; Mollica, L.; Szuber, N. Phenotypical differences and thrombosis rates in secondary erythrocytosis versus polycythemia vera. Blood Cancer J. 2021, 11, 75. [Google Scholar] [CrossRef]
- Wouters, H.J.C.M.; Mulder, R.; van Zeventer, I.A.; Schuringa, J.J.; van der Klauw, M.M.; van der Harst, P.; Diepstra, A.; Mulder, A.B.; Huls, G. Erythrocytosis in the general population: Clinical characteristics and association with clonal hematopoiesis. Blood Adv. 2020, 4, 6353–6363. [Google Scholar] [CrossRef] [PubMed]
- Krečak, I.; Holik, H.; Zekanović, I.; Morić Perić, M.; Marketin, T.; Coha, B.; Gverić-Krečak, V.; Vodanović, M.; Lucijanić, M. Thrombotic risk in secondary polycythemia resembles low-risk polycythemia vera and increases in specific subsets of patients. Thromb. Res. 2022, 209, 47–50. [Google Scholar] [CrossRef]
- Yeung, A.K.; Villacorta-Martin, C.; Hon, S.; Rock, J.R.; Murphy, G.J. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 2020, 4, 6204–6217. [Google Scholar] [CrossRef] [PubMed]
- Lucijanic, M.; Krecak, I.; Soric, E.; Sedinic, M.; Sabljic, A.; Derek, L.; Jaksic, O.; Kusec, R. Thrombocytosis in COVID-19 patients without myeloproliferative neoplasms is associated with better prognosis but higher rate of venous thromboembolism. Blood Cancer J. 2021, 11, 189. [Google Scholar] [CrossRef]
- Harrison, M.T.; Short, P.; Williamson, P.A.; Singanayagam, A.; Chalmers, J.D.; Schembri, S. Thrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: A role for antiplatelet therapy? Thorax 2014, 69, 609–615. [Google Scholar] [CrossRef]
- Sørensen, A.L.; Knudsen, T.A.; Skov, V.; Kjaer, L.; Holm, N.; Ellervik, C.; Hasselbalch, H.C. Smoking impairs molecular response, and reduces overall survival in patients with chronic myeloproliferative neoplasms: A retrospective cohort study. Br. J. Haematol. 2021, 193, 83–92. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.; Chronis, C.; Papapetrou, E.; Tatsioni, A.; Gartzonika, K.; Tsaousi, C.; Gogali, A.; Katsanos, C.; Vaggeli, A.; Tselepi, C.; et al. Prothrombotic state in patients with stable COPD: An observational study. ERJ Open Res. 2021, 7, 00297-2021. [Google Scholar] [CrossRef] [PubMed]
- Tuleta, I.; Skowasch, D.; Aurich, F.; Eckstein, N.; Schueler, R.; Pizarro, C.; Schahab, N.; Nickenig, G.; Schaefer, C.; Pingel, S. Asthma is associated with atherosclerotic artery changes. PLoS ONE 2017, 12, e0186820. [Google Scholar] [CrossRef]
- Meng, K.; Zhang, X.; Dai, H. Obstructive Airway Disease is Associated with Increased Cardiovascular Disease Risk Independent of Phenotype: Evidence from Two Nationwide Population-Based Studies. Int. J. Chronic Obstr. Pulm. Dis. 2025, 20, 1435–1446. [Google Scholar] [CrossRef]
- Morgan, A.D.; Herrett, E.; De Stavola, B.L.; Smeeth, L.; Quint, J.K. COPD disease severity and the risk of venous thromboembolic events: A matched case-control study. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Portegies, M.L.; Lahousse, L.; Joos, G.F.; Hofman, A.; Koudstaal, P.J.; Stricker, B.H.; Brusselle, G.G.; Ikram, M.A. Chronic Obstructive Pulmonary Disease and the Risk of Stroke. The Rotterdam Study. Am. J. Respir. Crit. Care Med. 2016, 193, 251–258. [Google Scholar] [CrossRef]
- Ambrosetti, M.; Ageno, W.; Spanevello, A.; Salerno, M.; Pedretti, R.F. Prevalence and prevention of venous thromboembolism in patients with acute exacerbations of COPD. Thromb. Res. 2003, 112, 203–207. [Google Scholar] [CrossRef]
- Kim, V.; Goel, N.; Gangar, J.; Zhao, H.; Ciccolella, D.E.; Silverman, E.K.; Crapo, J.D.; Criner, G.J.; the COPD Gene Investigators. Risk Factors for Venous Thromboembolism in Chronic Obstructive Pulmonary Disease. Chronic Obstr. Pulm. Dis. 2014, 1, 239–249. [Google Scholar] [CrossRef]
- Chung, W.S.; Lin, C.L.; Ho, F.M.; Li, R.Y.; Sung, F.C.; Kao, C.H.; Yeh, J.J. Asthma increases pulmonary thromboembolism risk: A nationwide population cohort study. Eur. Respir. J. 2014, 43, 801–807. [Google Scholar] [CrossRef]
- Barbui, T.; De Stefano, V.; Ghirardi, A.; Masciulli, A.; Finazzi, G.; Vannucchi, A.M. Different effect of hydroxyurea and phlebotomy on prevention of arterial and venous thrombosis in Polycythemia Vera. Blood Cancer J. 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Thoré, P.; Mignard, X.; Jaïs, X.; Boucly, A.; Jevnikar, M.; Seferian, A.; Jutant, E.-M.; Cottin, V.; Fadel, E.; et al. Clinical Phenotype and Outcomes of Pulmonary Hypertension Associated with Myeloproliferative Neoplasms: A Population-based Study. Am. J. Respir. Crit. Care Med. 2023, 208, 600–612. [Google Scholar] [CrossRef]
- Leiva, O.; Ren, S.; Neuberg, D.; Bhatt, A.; Jenkins, A.; Rosovsky, R.; Leaf, R.K.; Goodarzi, K.; Hobbs, G. Pulmonary hypertension is associated with poor cardiovascular and hematologic outcomes in patients with myeloproliferative neoplasms and cardiovascular disease. Int. J. Hematol. 2023, 117, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, M.T.; Criner, G.J.; Halpin, D.M.G.; Han, M.K.; Hartley, B.; Kalhan, R.; Lange, P.; Lipson, D.A.; Martinez, F.J.; Midwinter, D.; et al. Time-Dependent Risk of Cardiovascular Events Following an Exacerbation in Patients With Chronic Obstructive Pulmonary Disease: Post Hoc Analysis from the IMPACT Trial. J. Am. Heart Assoc. 2022, 11, e024350. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M.; Dransfield, M.T.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Crim, C.; Hartley, B.F.; Martinez, F.J.; Newby, D.E.; et al. Exacerbations of Chronic Obstructive Pulmonary Disease and Cardiac Events. A Post Hoc Cohort Analysis from the SUMMIT Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 51–57. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).