Mitochondrial Macular Dystrophy—A Case Report and Mini Review of Retinal Dystrophies
Abstract
1. Introduction
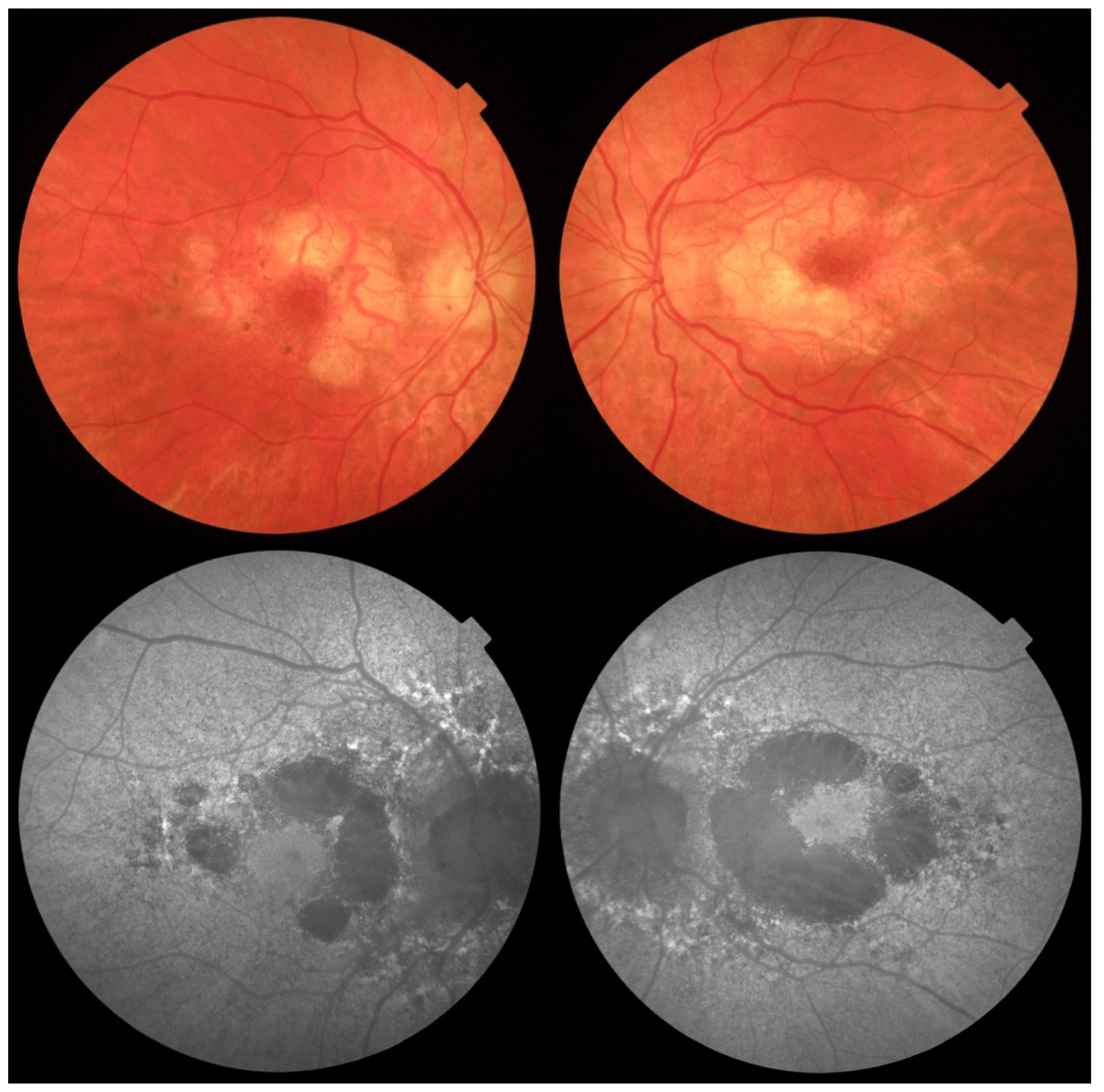
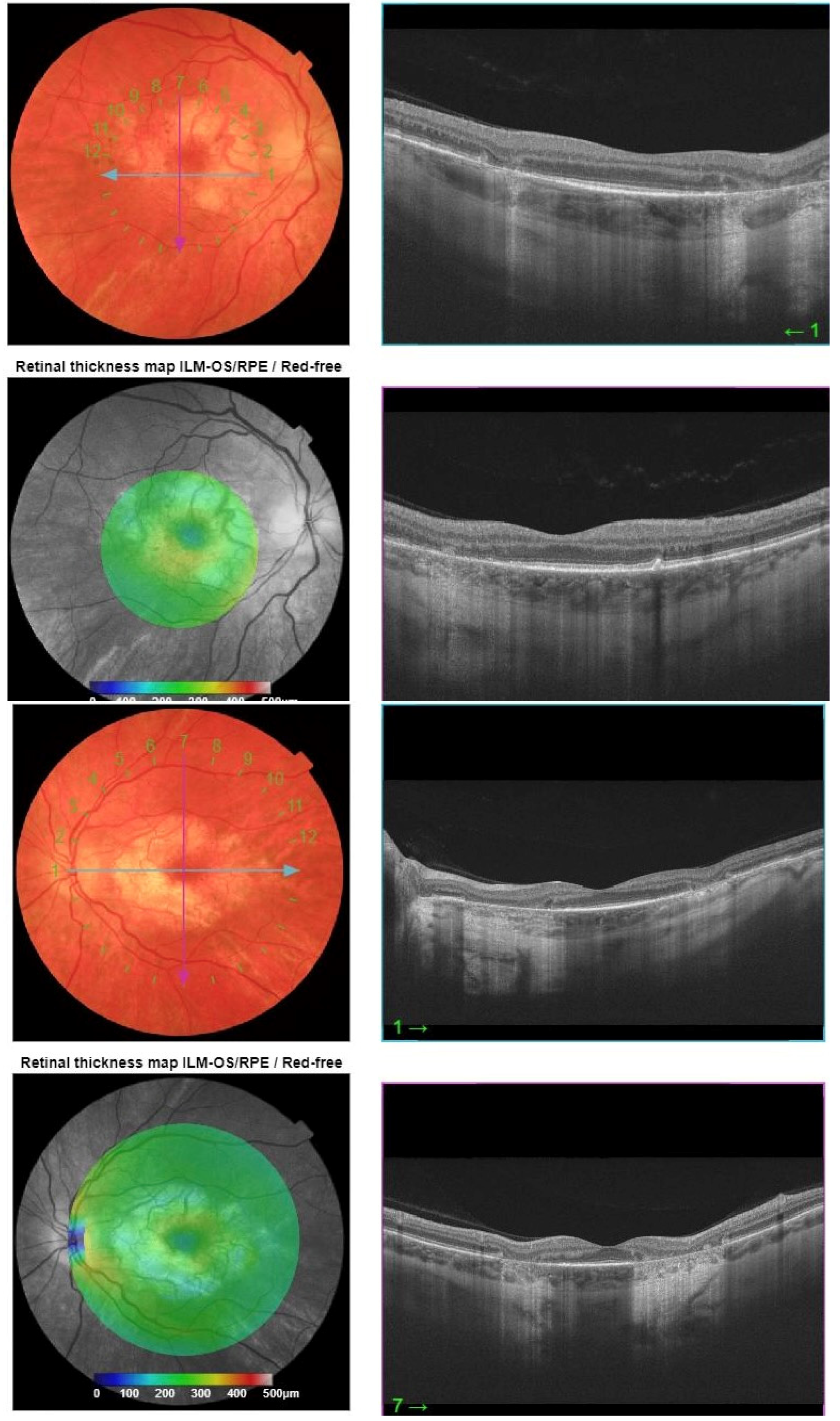
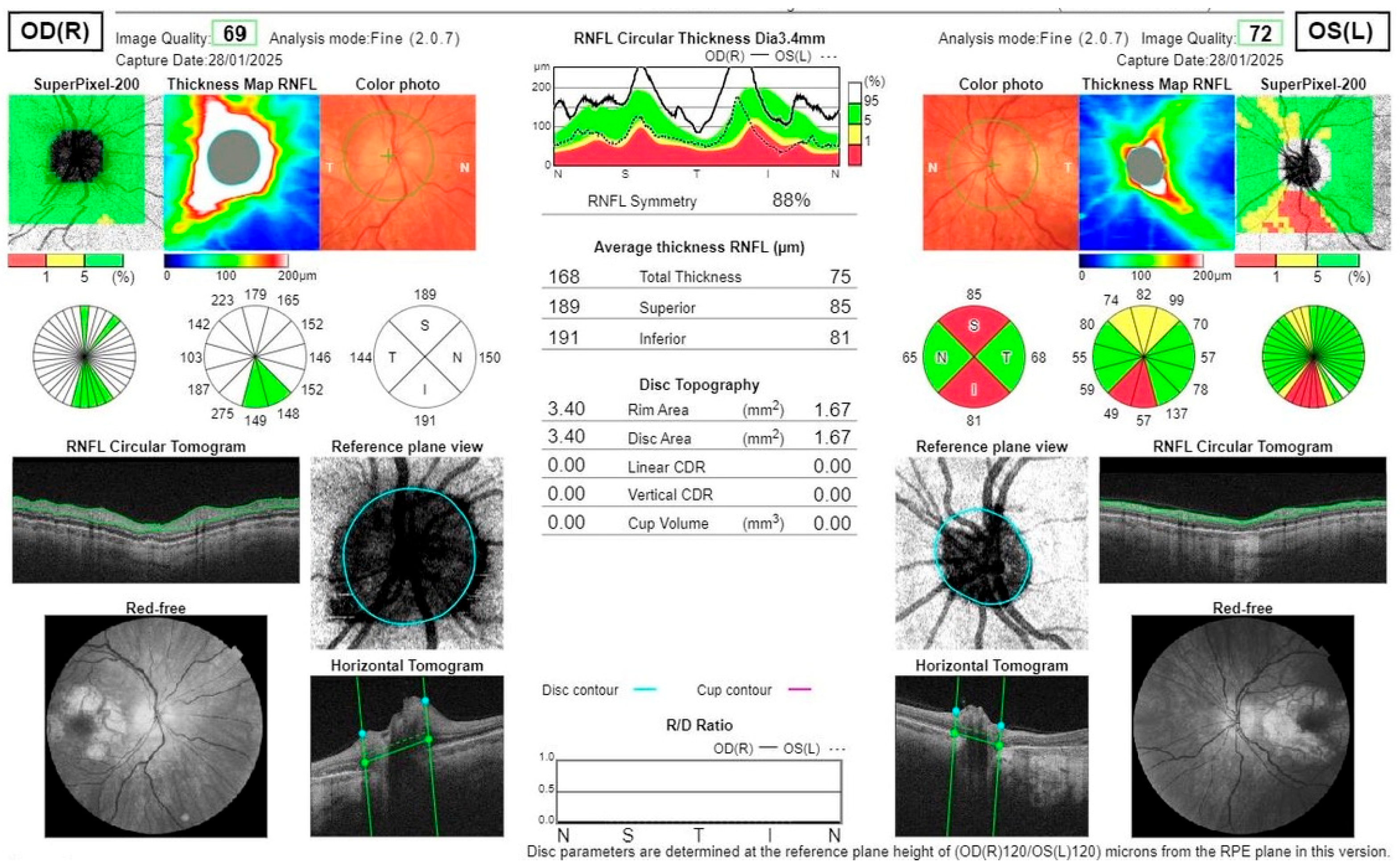
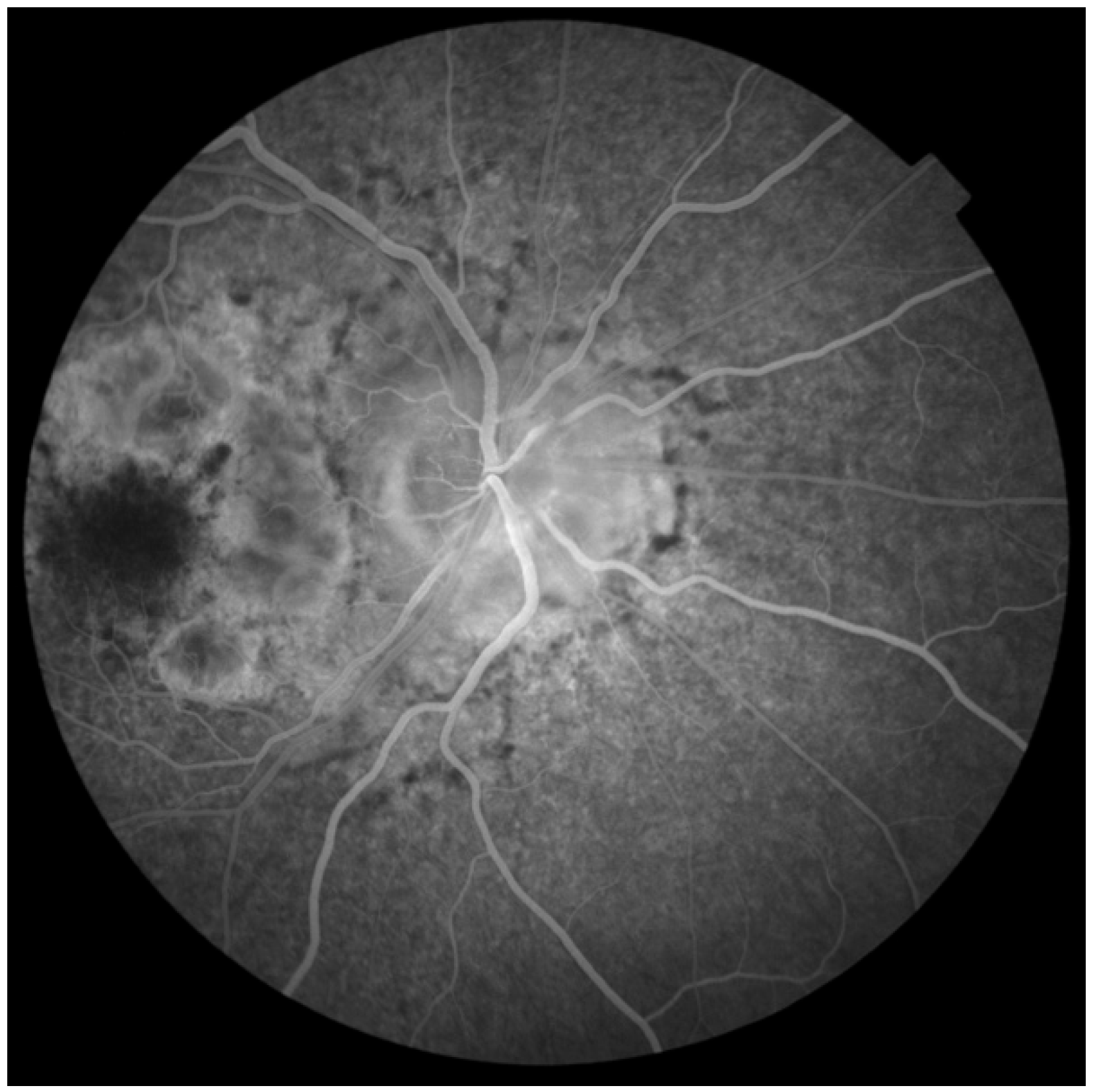
2. Case Report
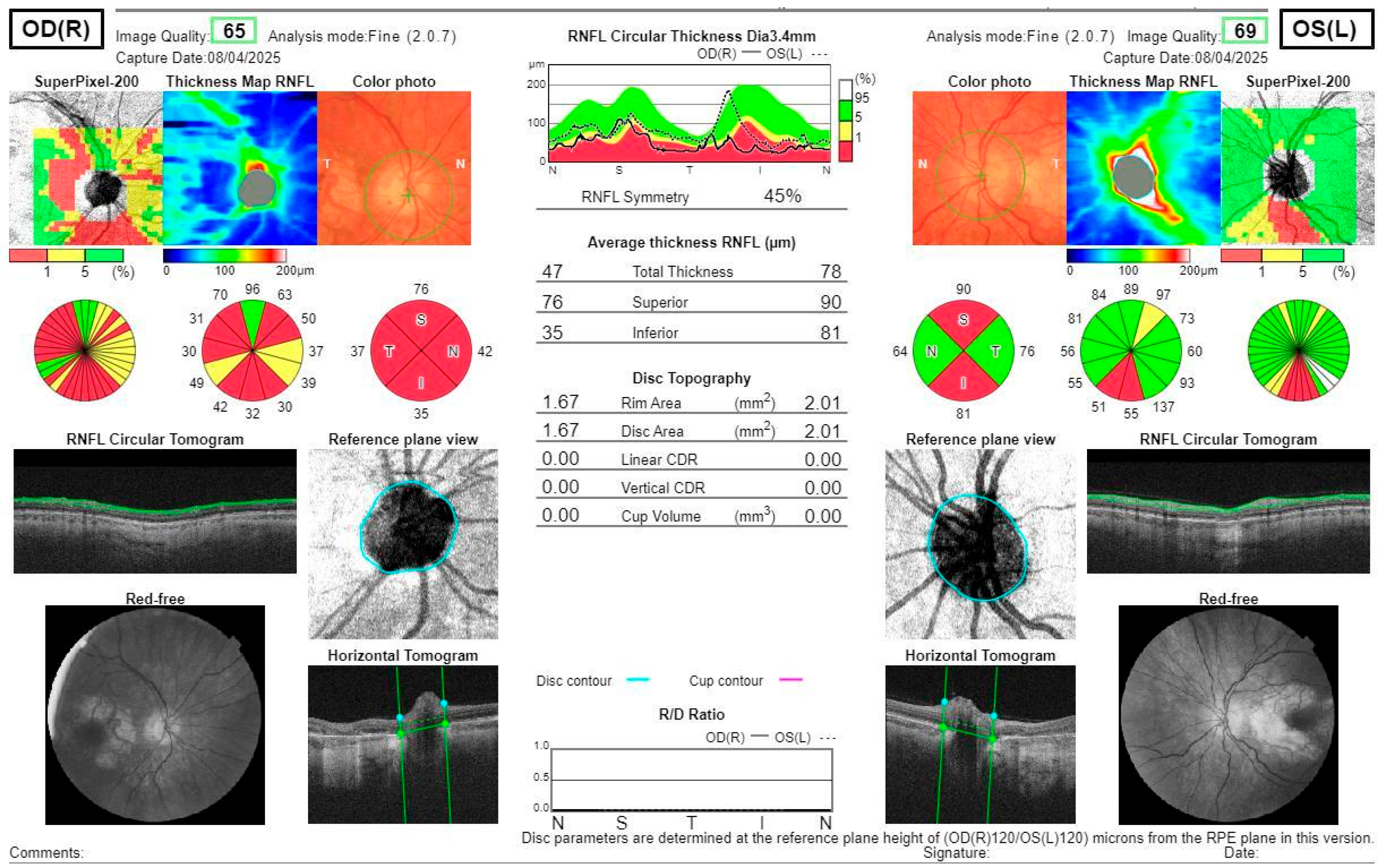
Investigations and Results
3. Discussion
Classification of Retinal Dystrophies
- PYGM—muscle glycogene phosphorylase, so they are tired soon after exercise
- DMPK—myotonic dystrophy
- ABCC6—angioid streaks (connective tissue disorders, hemoglobinopathies)
- CLN7/MFSD8—neuronal ceroid lipofuscinosis = Batten disease
- MIDD
- PRPH2—can induce various retinopathies
- BEST1—bestrophinopathies
- IMPG1/2—vitelliform macular dystrophy
- ABCA4—Stargardt disease
- CTNNA1—butterfly-shaped pigment dystrophy.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hollingsworth, T.J.; Gross, A.K. Innate and Autoimmunity in the Pathogenesis of Inherited Retinal Dystrophy. Cells 2020, 9, 630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moir, J.; Hyman, M.J.; Wang, J.; Shah, A.; Maatouk, C.; Flores, A.; Skondra, D. Associations Between Autoimmune Disease and the Development of Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2023, 64, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalogeropoulos, D.; Lotery, A.J.; Pavesio, C.; Kalogeropoulos, C.; Kanavaros, P.; Afshar, F.; Shawkat, F.; De Salvo, G. Diagnosis and treatment of autoimmune retinopathy: Review of current approaches. Int. Ophthalmol. 2025, 45, 341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nieto-Aristizábal, I.; Mera, J.J.; Giraldo, J.D.; Lopez-Arevalo, H.; Tobón, G.J. From ocular immune privilege to primary autoimmune diseases of the eye. Autoimmun. Rev. 2022, 21, 103122. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Lin, H.; Pfeiffer, N.; Grus, F.H. Age-Related Macular Degeneration and Mitochondria-Associated Autoantibodies: A Review of the Specific Pathogenesis and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 1624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farrar, G.J.; Carrigan, M.; Dockery, A.; Millington-Ward, S.; Palfi, A.; Chadderton, N.; Humphries, M.; Kiang, A.S.; Kenna, P.F.; Humphries, P. Toward an elucidation of the molecular genetics of inherited retinal degenerations. Hum. Mol. Genet. 2017, 26, R2–R11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider, E.; Märker, T.; Daser, A.; Frey-Mahn, G.; Beyer, V.; Farcas, R.; Schneider-Rätzke, B.; Kohlschmidt, N.; Grossmann, B.; Bauss, K.; et al. Homozygous disruption of PDZD7 by reciprocal translocation in a consanguineous family: A new member of the Usher syndrome protein interactome causing congenital hearing impairment. Hum. Mol. Genet. 2009, 18, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Minarovits, J.; Demcsák, A.; Banati, F.; Niller, H.H. Epigenetic Dysregulation in Virus-Associated Neoplasms. Adv. Exp. Med. Biol. 2016, 879, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Jin, S.; Wang, J.; Lv, Z.; Xin, C.; Tan, C.; Zhao, M.; Wang, L.; Liu, J. AAV vectors applied to the treatment of CNS disorders: Clinical status and challenges. J. Control Release 2023, 355, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.R.; Tsang, S.H.; Zernant, J.; Smith, R.T.; Allikmets, R. Familial discordance in Stargardt disease. Mol. Vis. 2012, 18, 227–233. [Google Scholar] [PubMed] [PubMed Central]
- Nguyen, J.; Brantley, M.A., Jr.; Schwartz, S.G. Genetics and Age-Related Macular Degeneration: A Practical Review for Clinicians. Front. Biosci. 2024, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lains, I.; Li, J.; Li, J.; Chen, Y.; Yu, B.; Qi, Q.; Boerwinkle, E.; Kaplan, R.; Thyagarajan, B.; et al. Integrating genetics and metabolomics from multi-ethnic and multi-fluid data reveals putative mechanisms for age-related macular degeneration. Cell Rep. Med. 2023, 4, 101085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kugelman, J.; Alonso-Caneiro, D.; Chen, Y.; Arunachalam, S.; Huang, D.; Vallis, N.; Collins, M.J.; Chen, F.K. Retinal Boundary Segmentation in Stargardt Disease Optical Coherence Tomography Images Using Automated Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waldstein, S.M.; Seeböck, P.; Donner, R.; Sadeghipour, A.; Bogunović, H.; Osborne, A.; Schmidt-Erfurth, U. Unbiased identification of novel subclinical imaging biomarkers using unsupervised deep learning. Sci. Rep. 2020, 10, 12954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsang, S.H.; Aycinena, A.R.P.; Sharma, T. Mitochondrial Disorder: Maternally Inherited Diabetes and Deafness. In Atlas of Inherited Retinal Diseases, 1st ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1085, pp. 163–165. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bansal, R.; Sharma, A.; Kapil, A. Macular Degeneration, Geographic Atrophy, and Inherited Retinal Disorders. In Ophthalmic Signs in Practice of Medicine; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Toto, L.; Battaglia Parodi, M.; D’Aloisio, R.; Mercuri, S.; Senatore, A.; Di Antonio, L.; Di Marzio, G.; Di Nicola, M.; Mastropasqua, R. Cone Dystrophies: An Optical Coherence Tomography Angiography Study. J. Clin. Med. 2020, 9, 1500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huryn, L.A.; Kozycki, C.T.; Serpen, J.Y.; Zein, W.M.; Ullah, E.; Iannaccone, A.; Williams, L.B.; Sobrin, L.; Brooks, B.P.; Sen, H.N.; et al. Ophthalmic Manifestations of ROSAH (Retinal Dystrophy, Optic Nerve Edema, Splenomegaly, Anhidrosis, and Headache) Syndrome, an Inherited NF κB-Mediated Autoinflammatory Disease with Retinal Dystrophy. Ophthalmology 2023, 130, 423–432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mishra, P.; Panda, B.B.; Sahu, S.K.; Bhatt, B. Bilateral disk edema with retinitis pigmentosa: Diagnostic dilemma. Indian J. Ophthalmol.-Case Rep. 2023, 3, 743–746. [Google Scholar] [CrossRef]
- Takai, Y.; Yamagami, A.; Iwasa, M.; Inoue, K.; Yasumoto, R.; Ishikawa, H.; Wakakura, M. Age-Associated Differences in Optic Disc Findings of Leber’s Hereditary Optic Neuropathy. Neuroophthalmology 2025, 49, 359–365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, F.; Tsang, S.H.; Aycinena, A.R.P.; Sharma, T. Mitochondrial Disorder: Maternally Inherited Diabetes and Deafness. In Atlas of Inherited Retinal Diseases, 2nd ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2025; Volume 1467, pp. 177–180. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, B.A.; Al-Shamrani, J.M.; El-Shehawi, A.M.; Al-Johani, I.; Al-Otaibi, B.G. Role of mitochondrial DNA in diabetes Mellitus Type I and Type II. Saudi J. Biol. Sci. 2022, 29, 103434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dabravolski, S.A.; Orekhova, V.A.; Baig, M.S.; Bezsonov, E.E.; Starodubova, A.V.; Popkova, T.V.; Orekhov, A.N. The Role of Mitochondrial Mutations and Chronic Inflammation in Diabetes. Int. J. Mol. Sci. 2021, 23, 6733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Savige, J.; Ratnaike, S.; Colville, D. Retinal abnormalities characteristic of inherited renal disease. J. Am. Soc. Nephrol. 2011, 22, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Henske, E.P.; Jóźwiak, S.; Kingswood, J.C.; Sampson, J.R.; Thiele, E.A. Tuberous sclerosis complex. Nat. Rev. Dis. Primers 2016, 2, 16035. [Google Scholar] [CrossRef] [PubMed]
- Northrup, H.; Koenig, M.K.; Pearson, D.A.; Au, K.S. Tuberous Sclerosis Complex. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1999. [Google Scholar] [PubMed]
- LeFort, K.R.; Rungratanawanich, W.; Song, B.J. Contributing roles of mitochondrial dysfunction and hepatocyte apoptosis in liver diseases through oxidative stress, post-translational modifications, inflammation, and intestinal barrier dysfunction. Cell. Mol. Life Sci. 2024, 81, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Natarelli, N.; Gahoonia, N.; Aflatooni, S.; Bhatia, S.; Sivamani, R.K. Dermatologic Manifestations of Mitochondrial Dysfunction: A Review of the Literature. Int. J. Mol. Sci. 2024, 25, 3303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wesół-Kucharska, D.; Rokicki, D.; Jezela-Stanek, A. Epilepsy in Mitochondrial Diseases—Current State of Knowledge on Aetiology and Treatment. Children 2021, 8, 532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.; Wang, Y.; Dalal, R.; Du, J.; Vollrath, D. Alternative oxidase blunts pseudohypoxia and photoreceptor degeneration due to RPE mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 2024, 121, e2402384121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lewis Luján, L.M.; McCarty, M.F.; Di Nicolantonio, J.J.; Gálvez Ruiz, J.C.; Rosas-Burgos, E.C.; Plascencia-Jatomea, M.; Iloki Assanga, S.B. Nutraceuticals/Drugs Promoting Mitophagy and Mitochondrial Biogenesis May Combat the Mitochondrial Dysfunction Driving Progression of Dry Age-Related Macular Degeneration. Nutrients 2022, 14, 1985. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wynne, M.E.; Lane, A.R.; Singleton, K.S.; Zlatic, S.A.; Gokhale, A.; Werner, E.; Duong, D.; Kwong, J.Q.; Crocker, A.J.; Faundez, V. Heterogeneous Expression of Nuclear Encoded Mitochondrial Genes Distinguishes Inhibitory and Excitatory Neurons. eNeuro 2021, 8, ENEURO.0232-21.2021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marchini, M.; Bianco, V.; Trezzi, M.; Mocka, S.; Manenti, L. Focal Segmental Glomerulosclerosis Due to A3243G Point Mutation in the mtDNA Coding for tRNALeu(UUR). G. Ital. Nefrol. 2024, 41, 2024-vol6. [Google Scholar] [CrossRef] [PubMed]
- McCormick, E.M.; Lott, M.T.; Dulik, M.C.; Shen, L.; Attimonelli, M.; Vitale, O.; Karaa, A.; Bai, R.; Pineda-Alvarez, D.E.; Singh, L.N.; et al. Specifications of the ACMG/AMP standards and guidelines for mitochondrial DNA variant interpretation. Hum. Mutat. 2020, 41, 2028–2057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yi, W.; Xu, M.; Xue, Y.; Cao, Y.; Yang, Z.; Zhou, L.; Zhou, Y.; Shi, L.; Mai, X.; Sun, Z.; et al. A spontaneous nonhuman primate model of inherited retinal degeneration. JCI Insight 2025, 6, e190807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Procaccio, V.; Neckelmann, N.; Paquis-Flucklinger, V.; Bannwarth, S.; Jimenez, R.; Davila, A.; Poole, J.C.; Wallace, D.C. Detection of low levels of the mitochondrial tRNALeu(UUR) 3243A>G mutation in blood derived from patients with diabetes. Mol. Diagn. Ther. 2006, 10, 381–389. [Google Scholar] [CrossRef] [PubMed]
- El-Hattab, A.W.; Almannai, M.; Scaglia, F. MELAS. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar] [PubMed]
- Velez-Bartolomei, F.; Lee, C.; Enns, G. MERRF. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar] [PubMed]
- Wang, L.; Zou, G.; Yan, Y.; Shi, R.; Guo, Y.; Zhang, M.; Lu, L.; Dong, K. Idebenone Protects Photoreceptors Impaired by Oxidative Phosphorylation Disorder in Retinal Detachment. Investig. Opthalmol. Vis. Sci. 2025, 66, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Emma, F.; Montini, G.; Parikh, S.M.; Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat. Rev. Nephrol. 2016, 12, 267–280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Tohari, A.M.; Marcheggiani, F.; Zhou, X.; Reilly, J.; Tiano, L.; Shu, X. Therapeutic Potential of Co-enzyme Q10 in Retinal Diseases. Curr. Med. Chem. 2017, 24, 4329–4339. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Baglivo, M.; Nasca, A.; Lamantea, E.; Vinci, S.; Spagnolo, M.; Marchet, S.; Prokisch, H.; Catania, A.; Lamperti, C.; Ghezzi, D. Evaluation of Mitochondrial Dysfunction and Idebenone Responsiveness in Fibroblasts from Leber’s Hereditary Optic Neuropathy (LHON) Subjects. Int. J. Mol. Sci. 2023, 24, 12580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, H.; Singh, D.; Mahant, A.; Sohal, S.K.; Kesavan, A.K. Development of mitochondrial replacement therapy: A review. Heliyon 2020, 6, e04643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, B.S.; Yu-Wai-Man, P.; Newman, N.J. Developments in the Treatment of Leber Hereditary Optic Neuropathy. Curr. Neurol. Neurosci. Rep. 2022, 22, 881–892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jacoby, E.; Bar-Yosef, O.; Gruber, N.; Lahav, E.; Varda-Bloom, N.; Bolkier, Y.; Bar, D.; Blumkin, M.B.; Barak, S.; Eisenstein, E.; et al. Mitochondrial augmentation of hematopoietic stem cells in children with single large-scale mitochondrial DNA deletion syndromes. Sci. Transl. Med. 2022, 14, eabo3724. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.H.; Aycinena, A.R.P.; Sharma, T. Mitochondrial Disorder: Kearns-Sayre Syndrome. Adv. Exp. Med. Biol. 2018, 1085, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Murati Calderon, R.A.; Izquierdo, N. A New Phenotypic Expression in a Patient with a Mutation in the CACNA1F Gene. Cureus 2025, 17, e82577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nash, B.M.; Wright, D.C.; Grigg, J.R.; Bennetts, B.; Jamieson, R.V. Retinal dystrophies, genomic applications in diagnosis and prospects for therapy. Transl. Pediatr. 2015, 4, 139–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahel, J.A.; Léveillard, T. Maintaining Cone Function in Rod-Cone Dystrophies. Adv. Exp. Med. Biol. 2018, 1074, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Robson, A.G.; Fujinami, K.; de Guimarães, T.A.C.; Fujinami-Yokokawa, Y.; Daich Varela, M.; Pontikos, N.; Kalitzeos, A.; Mahroo, O.A.; Webster, A.R.; et al. Phenotyping and genotyping inherited retinal diseases: Molecular genetics, clinical and imaging features, and therapeutics of macular dystrophies, cone and cone-rod dystrophies, rod-cone dystrophies, Leber congenital amaurosis, and cone dysfunction syndromes. Prog. Retin. Eye Res. 2024, 100, 101244. [Google Scholar] [CrossRef] [PubMed]
- Sarra, G.M.; Weigell-Weber, M.; Kotzot, D.; Niemeyer, G.; Messmer, E.; Hergersberg, M. Clinical description and exclusion of candidate genes in a novel autosomal recessively inherited vitreoretinal dystrophy. Arch. Ophthalmol. 2003, 121, 1109–1116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sallum, J.M.F.; Kaur, V.P.; Shaikh, J.; Banhazi, J.; Spera, C.; Aouadj, C.; Viriato, D.; Fischer, M.D. Epidemiology of Mutations in the 65-kDa Retinal Pigment Epithelium (RPE65) Gene-Mediated Inherited Retinal Dystrophies: A Systematic Literature Review. Adv. Ther. 2022, 39, 1179–1198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Birtel, J.; von Landenberg, C.; Gliem, M.; Gliem, C.; Reimann, J.; Kunz, W.S.; Herrmann, P.; Betz, C.; Caswell, R.; Nesbitt, V.; et al. Mitochondrial Retinopathy. Ophthalmol. Retina 2022, 6, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Wakili, P.; Boden, K.; Rudolph, C.N. Bull’s Eye-Makulopathie [Bull’s eye maculopathy]. Ophthalmologie 2024, 121, 605. (In German) [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Moussa, O.; Lam, B.L.; Sengillo, J.D. Fundus Autofluorescence in Inherited Retinal Disease: A Review. Cells 2025, 14, 1092. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calcagni, A.; Neveu, M.M.; Jurkute, N.; Robson, A.G. Electrodiagnostic tests of the visual pathway and applications in neuro-ophthalmology. Eye 2024, 38, 2392–2405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vyas, C.; Jolia, A.; Tsang, S.H.; Sharma, T.; Diaconita, V. Drug-Induced Retinal Toxicity. In Atlas of Inherited Retinal Diseases; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2025; Volume 1467, pp. 253–262. [Google Scholar] [CrossRef] [PubMed]
- Coussa, R.G.; Parikh, S.; Traboulsi, E.I. Mitochondrial DNA A3243G variant-associated retinopathy: Current perspectives and clinical implications. Surv. Ophthalmol. 2021, 66, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Nair, S.S.; Radhakrishnan, N.; Saraf, U.U.; Nair, M. Clinical Spectrum of Biopsy Proven Mitochondrial Myopathy. Neurol. India 2023, 71, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Koka, K.; Patel, B.C. Ptosis Correction. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Dong, L.; He, W.; Zhang, R.; Ge, Z.; Wang, Y.X.; Zhou, J.; Xu, J.; Shao, L.; Wang, Q.; Yan, Y.; et al. Artificial Intelligence for Screening of Multiple Retinal and Optic Nerve Diseases. JAMA Netw. Open 2022, 5, e229960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferraz, D.A.; Tovar-Moll, F.; Belfort, R., Jr. Artificial intelligence: From the retina to the brain. Arq. Bras. Oftalmol. 2021, 84, 197–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Date, R.C.; Jesudasen, S.J.; Weng, C.Y. Applications of Deep Learning and Artificial Intelligence in Retina. Int. Ophthalmol. Clin. 2019, 59, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, M.; de Lima Carvalho, J.R., Jr.; Oh, J.K.; Soucy, M.; Demirkol, A.; Kim, A.H.; Tsang, S.H.; Breazzano, M.P. Phenotypic Variability of Retinal Disease Among a Cohort of Patients with Variants in the CLN Genes. Investig. Ophthalmol. Vis. Sci. 2023, 64, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballios, B.G.; Mandola, A.; Tayyib, A.; Tumber, A.; Garkaby, J.; Vong, L.; Heon, E.; Roifman, C.M.; Vincent, A. Deep phenotypic characterization of the retinal dystrophy in patients with RNU4ATAC-associated Roifman syndrome. Eye 2023, 37, 3734–3742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oishi, A.; Fujinami, K.; Mawatari, G.; Naoi, N.; Ikeda, Y.; Ueno, S.; Kuniyoshi, K.; Hayashi, T.; Kondo, H.; Mizota, A.; et al. Genetic and Phenotypic Landscape of PRPH2-Associated Retinal Dystrophy in Japan. Genes 2021, 12, 1817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capra, A.P.; La Rosa, M.A.; Briguori, S.; Civa, R.; Passarelli, C.; Agolini, E.; Novelli, A.; Briuglia, S. Coexistence of Genetic Diseases Is a New Clinical Challenge: Three Unrelated Cases of Dual Diagnosis. Genes 2023, 14, 484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooley, A.M. Mitochondrial DNA Analysis. Methods Mol. Biol. 2023, 2685, 331–349. [Google Scholar] [CrossRef] [PubMed]
- González-Del Pozo, M.; Fernández-Suárez, E.; Martín-Sánchez, M.; Bravo-Gil, N.; Méndez-Vidal, C.; Rodríguez-de la Rúa, E.; Borrego, S.; Antiñolo, G. Unmasking Retinitis Pigmentosa complex cases by a whole genome sequencing algorithm based on open-access tools: Hidden recessive inheritance and potential oligogenic variants. J. Transl. Med. 2020, 18, 73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dan, H.; Huang, X.; Xing, Y.; Shen, Y. Application of targeted panel sequencing and whole exome sequencing for 76 Chinese families with retinitis pigmentosa. Mol. Genet. Genomic Med. 2020, 8, e1131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Y.; Guan, L.; Shen, T.; Zhang, J.; Xiao, X.; Jiang, H.; Li, S.; Yang, J.; Jia, X.; Yin, Y.; et al. Mutations of 60 known causative genes in 157 families with retinitis pigmentosa based on exome sequencing. Hum. Genet. 2014, 133, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- AlAbdi, L.; Shamseldin, H.E.; Khouj, E.; Helaby, R.; Aljamal, B.; Alqahtani, M.; Almulhim, A.; Hamid, H.; Hashem, M.O.; Abdulwahab, F.; et al. Beyond the exome: Utility of long-read whole genome sequencing in exome-negative autosomal recessive diseases. Genome Med. 2023, 15, 114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzàlez-Duarte, R.; de Castro-Miró, M.; Tuson, M.; Ramírez-Castañeda, V.; Gils, R.V.; Marfany, G. Scaling New Heights in the Genetic Diagnosis of Inherited Retinal Dystrophies. Adv. Exp. Med. Biol. 2019, 1185, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Martin-Merida, I.; Avila-Fernandez, A.; Del Pozo-Valero, M.; Blanco-Kelly, F.; Zurita, O.; Perez-Carro, R.; Aguilera-Garcia, D.; Riveiro-Alvarez, R.; Arteche, A.; Trujillo-Tiebas, M.J.; et al. Genomic Landscape of Sporadic Retinitis Pigmentosa: Findings from 877 Spanish Cases. Ophthalmology 2019, 126, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Harkin, J.; Peña, K.H.; Gomes, C.; Hernandez, M.; Lavekar, S.S.; So, K.; Lentsch, K.; Feder, E.M.; Morrow, S.; Huang, K.C.; et al. A highly reproducible and efficient method for retinal organoid differentiation from human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2024, 121, e2317285121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nnoromele, P.O.; Adams, M.; Pan, A.; Liu, Y.V.; Wang, J.; Singh, M.S. Cell-cell interactions between transplanted retinal organoid cells and recipient tissues. Curr. Opin. Genet. Dev. 2024, 89, 102277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giansanti, D. Advancements in Ocular Neuro-Prosthetics: Bridging Neuroscience and Information and Communication Technology for Vision Restoration. Biology 2025, 14, 134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 12, eaan4672. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, D.; Buga, A.M.; Docea, A.O.; Sarandi, E.; Mitrut, R.; Renieri, E.; Spandidos, D.A.; Rogoveanu, I.; Cercelaru, L.; Niculescu, M.; et al. Reversal of brain aging by targeting telomerase: A nutraceutical approach. Int. J. Mol. Med. 2021, 48, 199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotuski, G.; Paczwa, K.; Mędrzycka, J.; Różycki, R.; Gołębiewska, J. Mitochondrial Macular Dystrophy—A Case Report and Mini Review of Retinal Dystrophies. J. Clin. Med. 2025, 14, 8236. https://doi.org/10.3390/jcm14228236
Rotuski G, Paczwa K, Mędrzycka J, Różycki R, Gołębiewska J. Mitochondrial Macular Dystrophy—A Case Report and Mini Review of Retinal Dystrophies. Journal of Clinical Medicine. 2025; 14(22):8236. https://doi.org/10.3390/jcm14228236
Chicago/Turabian StyleRotuski, Grzegorz, Katarzyna Paczwa, Justyna Mędrzycka, Radosław Różycki, and Joanna Gołębiewska. 2025. "Mitochondrial Macular Dystrophy—A Case Report and Mini Review of Retinal Dystrophies" Journal of Clinical Medicine 14, no. 22: 8236. https://doi.org/10.3390/jcm14228236
APA StyleRotuski, G., Paczwa, K., Mędrzycka, J., Różycki, R., & Gołębiewska, J. (2025). Mitochondrial Macular Dystrophy—A Case Report and Mini Review of Retinal Dystrophies. Journal of Clinical Medicine, 14(22), 8236. https://doi.org/10.3390/jcm14228236





