Diagnostic Pitfalls of CT in Malignant Superior Cerebellar Artery Infarction: Implications for Treatment Decisions and Future Management Strategies
Abstract
1. Introduction
- (1)
- Illustrate a representative clinical case of malignant SCA infarction.
- (2)
- Provide a structured synthesis of the literature published since 2015.
- (3)
- Discuss diagnostic and therapeutic implications with a particular focus on imaging-based decision-making. By integrating clinical, radiological, and surgical perspectives, this work aims to clarify diagnostic pitfalls and highlight evidence-based strategies for improving outcomes in malignant SCA infarction.
2. Materials and Methods
2.1. Case Documentation
2.2. Literature Review Methodology
2.2.1. Search Strategy
“superior cerebellar artery infarction” OR “SCA stroke” OR “cerebellar infarction” OR “posterior fossa infarction” AND
“malignant cerebellar infarction” OR “decompressive craniectomy” OR “ventricular drainage” OR “computed tomography” OR “magnetic resonance imaging”.
2.2.2. Study Selection
- (1)
- adult patients with cerebellar infarction involving the SCA territory,
- (2)
- availability of imaging and/or clinical data relevant to diagnosis, prognosis, or surgical management, and
- (3)
- original clinical or radiological data.
2.3. Data Extraction and Synthesis
- Imaging characteristics, including CT–MRI comparisons, volumetric assessments and posterior fossa ratios;
- Prognostic markers, such as infarct volume, brainstem involvement and bilateral cerebellar lesions;
- Therapeutic strategies, covering timing of intervention, decompressive surgery, ventricular drainage and minimally invasive techniques;
- Functional outcomes, including reported recovery patterns and long-term neurological status.
3. Results
3.1. Illustrative Case
3.2. Review of the Literature
3.2.1. Study Characteristics
3.2.2. Diagnostic Imaging
3.2.3. Prognostic Factors
3.2.4. Surgical Management and Timing
3.2.5. Minimally Invasive and Endoscopic Alternatives
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Apparent Diffusion Coefficient |
| AI | Artificial Intelligence |
| AICA | Anterior Inferior Cerebellar Artery |
| CT | Computed Tomography |
| DSA | Digital Subtraction Angiography |
| DWI | Diffusion-Weighted Imaging |
| EVD | External ventricular drainage |
| FLAIR | Fluid-Attenuated Inversion Recovery |
| GCS | Glasgow Coma Scale |
| MEN | Minimally invasive endoscopic necrosectomy |
| MRI | Magnetic Resonance Imaging |
| NIHSS | National Institutes of Health Stroke Scale |
| PICA | Posterior Inferior Cerebellar Artery |
| PFO | Patent Foramen Ovale |
| PWI | Perfusion-Weighted Imaging |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| SCA | Superior Cerebellar Artery |
| TOF | Time-of-Flight (angiography) |
References
- Ortiz de Mendivil, A.; Alcalá-Galiano, A.; Ochoa, M.; Salvador, E.; Millán, J.M. Brainstem stroke: Anatomy, clinical and radiological findings. Semin. Ultrasound CT MR 2013, 34, 131–141. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Arsava, E.M.; Helenius, J.; Avery, R.; Sorgun, M.H.; Kim, G.M.; Pontes-Neto, O.M.; Park, K.Y.; Rosand, J.; Vangel, M.; Ay, H. Assessment of the Predictive Validity of Etiologic Stroke Classification. JAMA Neurol. 2017, 74, 419–426. [Google Scholar] [CrossRef]
- Wijdicks, E.F.; Sheth, K.N.; Carter, B.S.; Greer, D.M.; Kasner, S.E.; Kimberly, W.T.; Kimberly, W.T.; Schwab, S.; Smith, E.E.; Tamargo, R.J.; et al. Recommendations for the management of cerebral and cerebellar infarction with swelling. Stroke 2014, 45, 1222–1238. [Google Scholar] [CrossRef] [PubMed]
- Brazzelli, M.; Sandercock, P.A.; Chappell, F.M.; Celani, M.G.; Righetti, E.; Arestis, N.; Wardlaw, J.M.; Deeks, J.J. Magnetic Resonance Imaging versus Computed Tomography for Detection of Acute Vascular Lesions in Patients Presenting with Stroke Symptoms. Cochrane Database Syst. Rev. 2009, CD007424. [Google Scholar] [CrossRef]
- Jauss, M.; Müffelmann, B.; Krieger, D.; Zeumer, H.; Busse, O. A Computed Tomography Score for Assessment of Mass Effect in Space-Occupying Cerebellar Infarction. J. Neuroimaging 2001, 11, 268–271. [Google Scholar] [CrossRef]
- Kapapa, T.; Pala, A.; Alber, B.; Mauer, U.M.; Harth, A.; Neugebauer, H.; Sailer, L.; Kreiser, K.; Schmitz, B.; Althaus, K. Volumetry as a Criterion for Suboccipital Craniectomy after Cerebellar Infarction. J. Clin. Med. 2024, 13, 5689. [Google Scholar] [CrossRef]
- Lucia, K.; Reitz, S.; Hattingen, E.; Steinmetz, H.; Seifert, V.; Czabanka, M. Predictors of clinical outcomes in space-occupying cerebellar infarction undergoing suboccipital decompressive craniectomy. Front. Neurol. 2023, 14, 1165258. [Google Scholar] [CrossRef]
- Baki, E.; Baumgart, L.; Kehl, V.; Hess, F.; Wolff, A.W.; Wagner, A.; Petzsche, M.R.H.; Boeckh-Behrens, T.; Hemmer, B.; Meyer, B.; et al. Predictors of malignant swelling in space-occupying cerebellar infarction. Stroke Vasc. Neurol. 2025, 10, 323–329. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, S.K.; Song, J.; Oh, S.Y.; Lim, Y.C.; Sim, S.Y.; Shin, Y.S.; Chung, J. Preventive Suboccipital Decompressive Craniectomy for Cerebellar Infarction: A Retrospective-Matched Case-Control Study. Stroke 2016, 47, 2565–2573. [Google Scholar] [CrossRef]
- Won, S.-Y.; Hernández-Durán, S.; Behmanesh, B.; Bernstock, J.D.; Czabanka, M.; Dinc, N.; Dubinski, D.; Freiman, T.M.; Günther, A.; Hellmuth, K.; et al. Functional Outcomes in Conservatively vs Surgically Managed Cerebellar Infarcts. JAMA Neurol. 2024, 81, 519–528. [Google Scholar] [CrossRef]
- Hernández-Durán, S.; Walter, J.; Behmanesh, B.; Bernstock, J.D.; Czabanka, M.; Dinc, N.; Dubinski, D.; Freiman, T.M.; Konczalla, J.; Melkonian, R.; et al. Surgical infarct volume reduction and functional outcomes in patients with ischemic cerebellar stroke: Results from a multicentric retrospective study. J. Neurosurg. 2024, 141, 1681–1686. [Google Scholar] [CrossRef]
- Goulin Lippi Fernandes, E.; Ridwan, S.; Greeve, I.; Schäbitz, W.R.; Grote, A.; Simon, M. Clinical and Computerized Volumetric Analysis of Posterior Fossa Decompression for Space-Occupying Cerebellar Infarction. Front. Neurol. 2022, 13, 840212. [Google Scholar] [CrossRef]
- Villalobos-Díaz, R.; Ortiz-Llamas, L.A.; Rodríguez-Hernández, L.A.; Flores-Vázquez, J.G.; Calva-González, M.; Sangrador-Deitos, M.V.; Mondragón-Soto, M.G.; Uribe-Pacheco, R.; Castro, E.V.; A Barrera-Tello, M. Characteristics and Long-Term Outcome of Cerebellar Strokes in a Single Health Care Facility in Mexico. Cureus 2022, 14, e28993. [Google Scholar] [CrossRef] [PubMed]
- Suyama, Y.; Wakabayashi, S.; Aihara, H.; Ebiko, Y.; Kajikawa, H.; Nakahara, I. Evaluation of clinical significance of decompressive suboccipital craniectomy on the prognosis of cerebellar infarction. Fujita Med. J. 2019, 5, 21–24. [Google Scholar] [CrossRef]
- Ayling, O.G.S.; Alotaibi, N.M.; Wang, J.Z.; Fatehi, M.; Ibrahim, G.M.; Benavente, O.; Field, T.S.; Gooderham, P.A.; Macdonald, R.L. Suboccipital Decompressive Craniectomy for Cerebellar Infarction: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 110, 450–459.e5. [Google Scholar] [CrossRef]
- Mostofi, K.; Shirbache, K.; Shirbacheh, A.; Peyravi, M. Neurosurgical treatment of cerebellar infarct: Open craniectomy versus endoscopic surgery. Surg. Neurol. Int. 2024, 15, 442. [Google Scholar] [CrossRef]
- Hernández-Durán, S.; Wolfert, C.; Rohde, V.; Mielke, D. Cerebellar Necrosectomy Instead of Suboccipital Decompression: A Suitable Alternative for Patients with Space-Occupying Cerebellar Infarction. World Neurosurg. 2020, 144, e723–e733. [Google Scholar] [CrossRef]
- Baek, B.H.; Lee, Y.Y.; Kim, S.K.; Yoon, W. Superior cerebellar artery occlusion remaining after thrombectomy for acute basilar artery occlusion. Sci. Rep. 2023, 13, 22395. [Google Scholar] [CrossRef] [PubMed]
- Lindeskog, D.; Lilja-Cyron, A.; Kelsen, J.; Juhler, M. Long-Term Functional Outcome after Decompressive Suboccipital Craniectomy for Space-Occupying Cerebellar Infarction. Clin. Neurol. Neurosurg. 2019, 176, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yoh, N.; Abou-Al-Shaar, H.; Bethamcharla, R.; Beiriger, J.; Mallela, A.N.; Connolly, E.S.; Sekula, R.F. Minimally invasive surgical evacuation for spontaneous cerebellar hemorrhage: A case series and systematic review. Neurosurg. Rev. 2023, 46, 208. [Google Scholar] [CrossRef]
- Jüttler, E.; Schweickert, S.; Ringleb, P.A.; Huttner, H.B.; Köhrmann, M.; Aschoff, A. Long-Term Outcome after Surgical Treatment for Space-Occupying Cerebellar Infarction: Experience in 56 Patients. Stroke 2009, 40, 3060–3066. [Google Scholar] [CrossRef]
- Tsitsopoulos, P.P.; Tobieson, L.; Enblad, P.; Marklund, N. Surgical treatment of patients with unilateral cerebellar infarcts: Clinical outcome and prognostic factors. Acta Neurochir. 2011, 153, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Arnold, M.; Hungerbühler, H.J.; Müller, F.; Staedler, C.; Baumgartner, R.W.; Georgiadis, D.; Lyrer, P.; Mattle, H.P.; Sztajzel, R.; et al. Decompressive craniectomy for space occupying hemispheric and cerebellar ischemic strokes: Swiss recommendations. Int. J. Stroke 2009, 4, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Duran, S.; Ridwan, S.; Kranawetter, B.; Dubinski, D.; Freiman, T.M.; Rohde, V.; Gessler, F.; Won, S.Y. Surgical indications and techniques in ischemic cerebellar stroke—Results from an international survey. Brain Spine 2025, 5, 104314. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, T.; Eppinger, U.; Linn, J.; Birnbaum, T.; Herzog, J.; Straube, A.; Dichgans, M.; Grau, S. Long-term outcome after suboccipital decompressive craniectomy for malignant cerebellar infarction. Stroke 2009, 40, 3045–3050. [Google Scholar] [CrossRef]
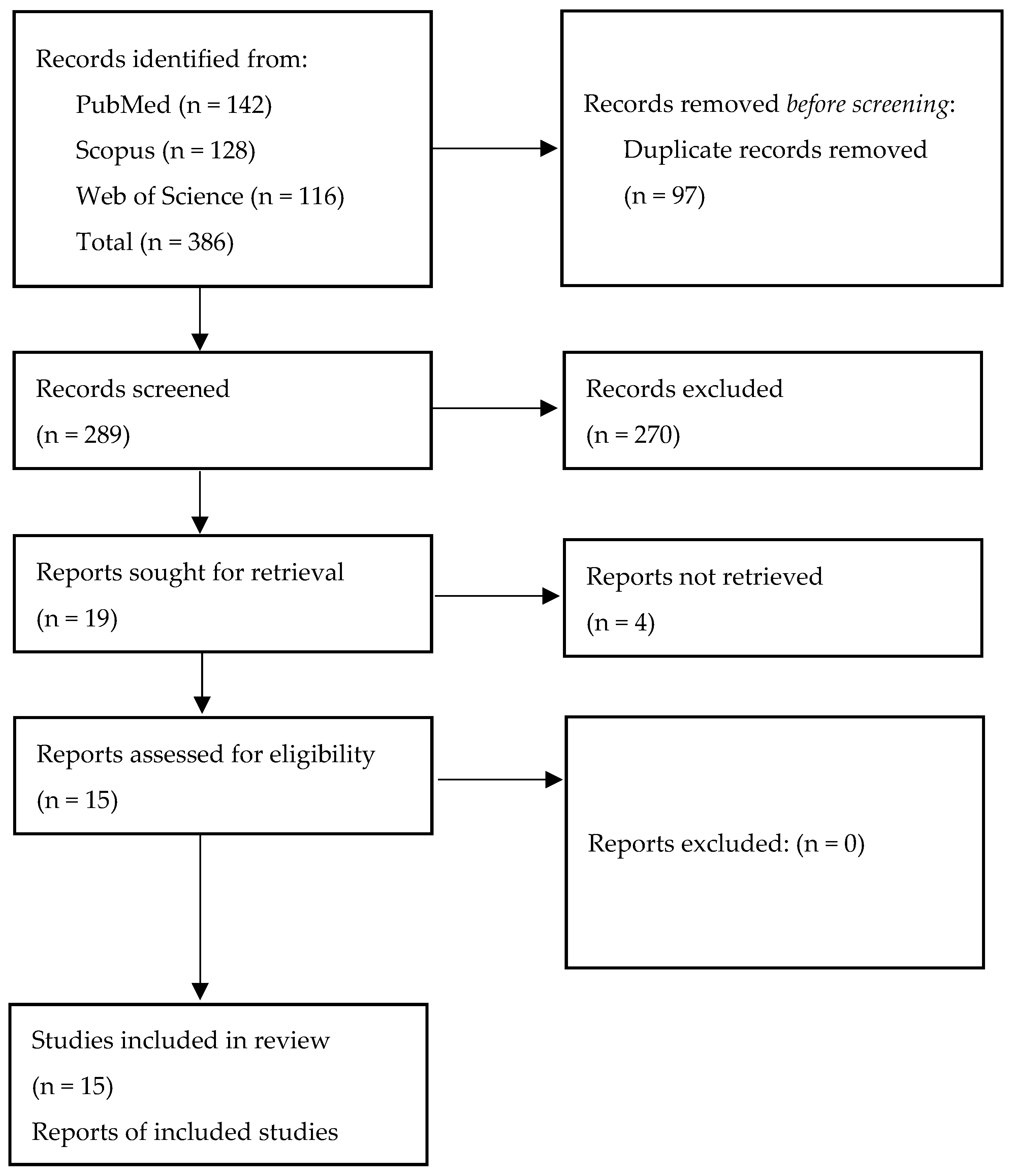
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
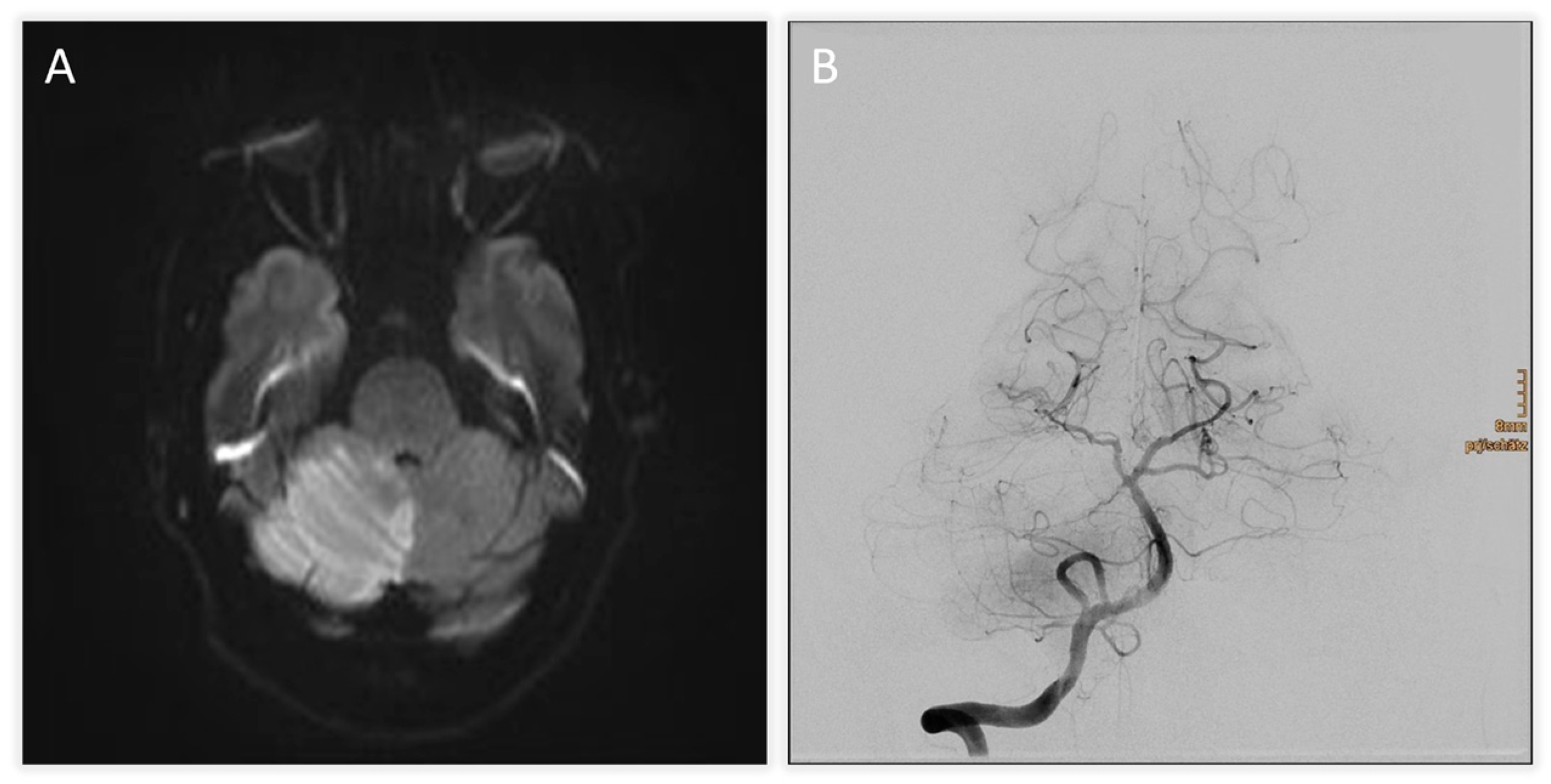
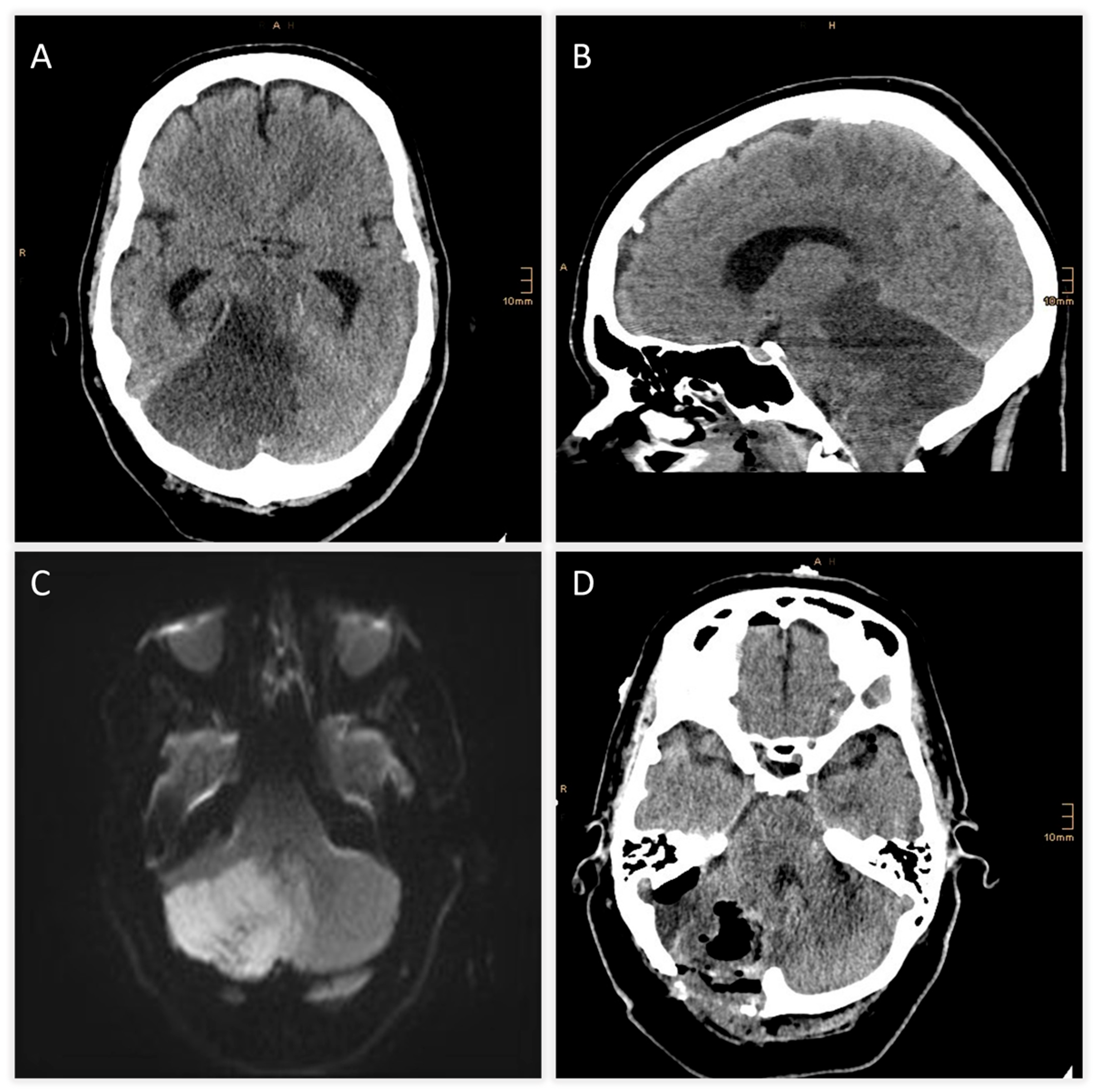
| Time from Symptom Onset | Event/Intervention | Key Findings/Outcome |
|---|---|---|
| 0 h | Symptom onset | Acute severe vertigo, dysphagia, dysarthria, diplopia, skew deviation, hemihypesthesia (NIHSS 7) |
| +2 h (admission) | Initial MRI (DWI) + TOF-angiography | Acute infarction of right cerebellum and vermis; thrombus at basilar tip; right SCA occlusion |
| +3 h | Intravenous thrombolysis | Standard-dose systemic thrombolysis initiated |
| +4 h | Digital subtraction angiography (DSA) | Basilar tip patent, right SCA occluded; mechanical thrombectomy attempted but unsuccessful |
| +6 h | CT scan | Expanding right cerebellar infarct with mass effect; apparent hypodensity in pons, midbrain, diencephalon → suspicious for brainstem infarction |
| +7 h | MRI follow-up | Brainstem infarction excluded; predominant cerebellar edema confirmed |
| +8 h | Neurosurgical intervention | Suboccipital decompression, ventricular drainage, and partial resection of necrotic tissue |
| Day 10 | Extubation | Patient successfully weaned from ventilation |
| Day 14 | Etiology work-up | Atrial septal aneurysm with patent foramen ovale (PFO) identified → referred to interventional cardiology |
| First Author | Year | Title (Short) | Study Type | Population | Key Findings |
|---|---|---|---|---|---|
| Ayling OGS [16] | 2018 | Suboccipital decompression for cerebellar infarction | Systematic review/meta-analysis | Cerebellar infarction | SDC is associated with better outcomes compared with decompressive surgery for hemispheric infarctions |
| Baki E [9] | 2025 | Predictors of malignant swelling | Retrospective cohort | Cerebellar infarction | Infarct volume >38 cm3 is associated with a swelling rate of >50% |
| Baek BH [19] | 2023 | SCA occlusion after thrombectomy | Retrospective cohort | SCA infarction | Attempts to recanalize remnant SCA occlusion may be unnecessary after basilar artery thrombectomy. |
| Goulin Lippi Fernandes E [13] | 2022 | Volumetric analysis and outcomes | Retrospective cohort | Cerebellar infarction | Surgical timing, including preventive surgery and mass effect of the infarct, in the posterior fossa is not predictive of the patients’ functional outcomes. |
| Hernández-Durán S [18] | 2020 | Cerebellar necrosectomy vs. decompression | Retrospective cohort | Malignant cerebellar infarction | No significant differences between mortality or functional outcomes |
| Hernández-Durán S [12] | 2024 | Surgical infarct volume reduction and outcomes | Retrospective multicenter cohort | Malignant cerebellar infarction | Early infarct volume reduction associated with better functional outcomes |
| Kapapa T [7] | 2024 | Volumetry as a criterion for decompression | Retrospective multicenter cohort | Cerebellar infarction | Volumetric cut-of >31 cm3 is more probable for decompression |
| Kim MJ [10] | 2016 | Preventive vs. reactive suboccipital decompression | Retrospective cohort | Cerebellar infarction | Favorable clinical outcomes including overall survival can be expected after preventive SDC in patients with a volume ratio between 0.25 and 0.33 |
| Lindeskog D [20] | 2019 | Long-term outcome after decompression | Retrospective cohort | Cerebellar infarction | After SDC, half of the patients achieved a functionally acceptable level (mRS 0–3) at 12-month follow-up |
| Lucia K [8] | 2023 | Predictors of clinical outcomes | Retrospective cohort | Cerebellar infarction | Patients with space-occupying cerebellar infarction and a preoperative GCS of 12–15 significantly benefit from early SDC |
| Mostofi K [17] | 2024 | Craniectomy vs. endoscopic surgery | Retrospective cohort | Cerebellar infarction | Endoscopic vacuation of necrotic tissue is a promising alternative to decompressive craniectomy with comparable clinical outcomes. |
| Suyama Y [15] | 2019 | Significance of decompression | Retrospective cohort | Cerebellar infarction | Early DSC should be considered for treating cerebellar infarction in patients with GCS 13 or worse |
| Villalobos-Díaz R [14] | 2022 | Long-term outcomes of cerebellar strokes | Retrospective cohort | Cerebellar infarction | GCS and hydrocephalus are crucial factors in therapeutic decision-making |
| Won SY [11] | 2024 | Surgical vs. conservative treatment | Retrospective multicenter cohort | Cerebellar infarction | Surgery beneficial for infarcts >35 mL |
| Yoh N [21] | 2023 | Minimally invasive evacuation | Systematic review and case series | Spontaneous cerebellar hemorrhage | Minimally invasive evacuation is safe and effective. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gollwitzer, M.; Atli, B.; Seiter, V.; Rossmann, T.; Horner, E.; Hauser, A.; Sardi, G.; Sölva, V.; Gruber, A.; Aufschnaiter-Hiessböck, K. Diagnostic Pitfalls of CT in Malignant Superior Cerebellar Artery Infarction: Implications for Treatment Decisions and Future Management Strategies. J. Clin. Med. 2025, 14, 8229. https://doi.org/10.3390/jcm14228229
Gollwitzer M, Atli B, Seiter V, Rossmann T, Horner E, Hauser A, Sardi G, Sölva V, Gruber A, Aufschnaiter-Hiessböck K. Diagnostic Pitfalls of CT in Malignant Superior Cerebellar Artery Infarction: Implications for Treatment Decisions and Future Management Strategies. Journal of Clinical Medicine. 2025; 14(22):8229. https://doi.org/10.3390/jcm14228229
Chicago/Turabian StyleGollwitzer, Maria, Baran Atli, Vanessa Seiter, Tobias Rossmann, Eva Horner, Anna Hauser, Gracija Sardi, Verena Sölva, Andreas Gruber, and Kathrin Aufschnaiter-Hiessböck. 2025. "Diagnostic Pitfalls of CT in Malignant Superior Cerebellar Artery Infarction: Implications for Treatment Decisions and Future Management Strategies" Journal of Clinical Medicine 14, no. 22: 8229. https://doi.org/10.3390/jcm14228229
APA StyleGollwitzer, M., Atli, B., Seiter, V., Rossmann, T., Horner, E., Hauser, A., Sardi, G., Sölva, V., Gruber, A., & Aufschnaiter-Hiessböck, K. (2025). Diagnostic Pitfalls of CT in Malignant Superior Cerebellar Artery Infarction: Implications for Treatment Decisions and Future Management Strategies. Journal of Clinical Medicine, 14(22), 8229. https://doi.org/10.3390/jcm14228229






