Therapeutic Drug Monitoring in Special Circumstances in Inflammatory Bowel Disease
Abstract
1. Introduction
2. Methods
3. Disease Severity and Phenotype
3.1. Acute Severe Ulcerative Colitis
3.2. Albumin
3.3. Perianal Fistulising Crohn’s Disease
4. Body Composition
4.1. Obesity
4.2. Pregnancy
5. Age
5.1. Elderly
5.2. Paediatrics
6. Administration
6.1. Thiopurines: Monotherapy or Combination
6.2. Route: Subcutaneous or Intravenous
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADM | Adalimumab |
| ASUC | Acute severe ulcerative colitis |
| CRP | C-reactive protein |
| CYP | Cytochrome P450 |
| fCal | Faecal calprotectin |
| GLP-1 | Glucagon-like peptide-1 |
| IV | Intravenous |
| mAb | Monoclonal antibody |
| PK | Pharmacokinetic |
| RBC | Red blood cell |
| SC | Subcutaneous |
| TDM | Therapeutic drug monitoring |
| TNFα | Tumour necrosis factor alpha |
| UST | Ustekinumab |
| VDZ | Vedolizumab |
| 6-MMP | 6-Methylmercaptopurine |
| 6-TGN | 6-Thioguanine |
References
- Berre, C.L.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Dolinger, M.; Torres, J.; Vermeire, S. Crohn’s disease. Lancet 2024, 403, 1177–1191. [Google Scholar] [CrossRef]
- Zhao, M.; Sall Jensen, M.; Knudsen, T.; Kelsen, J.; Coskun, M.; Kjellberg, J.; Burisch, J. Trends in the use of biologicals and their treatment outcomes among patients with inflammatory bowel diseases—A Danish nationwide cohort study. Aliment. Pharmacol. Ther. 2022, 55, 541–557. [Google Scholar] [CrossRef]
- Papamichael, K.; Cheifetz, A.S.; Melmed, G.Y.; Irving, P.M.; Vande Casteele, N.; Kozuch, P.L.; Raffals, L.E.; Baidoo, L.; Bressler, B.; Devlin, S.M.; et al. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 1655–1668.e3. [Google Scholar] [CrossRef]
- Cheifetz, A.S.; Abreu, M.T.; Afif, W.; Cross, R.K.; Dubinsky, M.C.; Loftus, E.V.; Osterman, M.T.; Saroufim, A.; Siegel, C.A.; Yarur, A.J.; et al. A Comprehensive Literature Review and Expert Consensus Statement on Therapeutic Drug Monitoring of Biologics in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2021, 116, 2014–2025. [Google Scholar] [CrossRef] [PubMed]
- Assa, A.; Matar, M.; Turner, D.; Broide, E.; Weiss, B.; Ledder, O.; Guz-Mark, A.; Rinawi, F.; Cohen, S.; Topf-Olivestone, C.; et al. Proactive Monitoring of Adalimumab Trough Concentration Associated with Increased Clinical Remission in Children with Crohn’s Disease Compared with Reactive Monitoring. Gastroenterology 2019, 157, 985–996.e2. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Juncadella, A.; Wong, D.; Rakowsky, S.; Sattler, L.A.; Campbell, J.P.; Vaughn, B.P.; Cheifetz, A.S. Proactive Therapeutic Drug Monitoring of Adalimumab Is Associated with Better Long-term Outcomes Compared with Standard of Care in Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2019, 13, 976–981. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Solitano, V.; Vuyyuru, S.K.; MacDonald, J.K.; Syversen, S.W.; Jørgensen, K.K.; Crowley, E.; Ma, C.; Jairath, V.; Singh, S. Proactive Therapeutic Drug Monitoring Versus Conventional Management for Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 163, 937–949.e2. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Dias, S.; Kumar, A.; Blackwell, J.; Brookes, M.J.; Segal, J.P. Meta-analysis: The efficacy of therapeutic drug monitoring of anti-TNF-therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2023, 57, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Irving, P.M.; Gecse, K.B. Optimizing Therapies Using Therapeutic Drug Monitoring: Current Strategies and Future Perspectives. Gastroenterology 2022, 162, 1512–1524. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Heap, G.A.; Green, H.D.; Hamilton, B.; Bewshea, C.; Walker, G.J.; Thomas, A.; Nice, R.; Perry, M.H.; Bouri, S.; et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: A prospective, multicentre, cohort study. Lancet Gastroenterol. Hepatol. 2019, 4, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn’s Colitis 2021, 15, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.; Minozzi, S.; Kopylov, U.; Verstockt, B.; Chaparro, M.; Buskens, C.; Warusavitarne, J.; Agrawal, M.; Allocca, M.; Atreya, R.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2024, 18, 1531–1555. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Loftus, E.V.; Afzali, A.; Long, M.D.; Barnes, E.L.; Isaacs, K.L.; Ha, C.Y. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2025, 120, 1225–1264. [Google Scholar] [CrossRef]
- Moran, G.W.; Gordon, M.; Sinopolou, V.; Radford, S.J.; Darie, A.-M.; Vuyyuru, S.K.; Alrubaiy, L.; Arebi, N.; Blackwell, J.; Butler, T.D.; et al. British Society of Gastroenterology guidelines on inflammatory bowel disease in adults: 2025. Gut 2025, 74, s1–s101. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Papamichael, K.; Afif, W.; Drobne, D.; Dubinsky, M.C.; Ferrante, M.; Irving, P.M.; Kamperidis, N.; Kobayashi, T.; Kotze, P.G.; Lambert, J.; et al. Therapeutic drug monitoring of biologics in inflammatory bowel disease: Unmet needs and future perspectives. Lancet Gastroenterol. Hepatol. 2022, 7, 171–185. [Google Scholar] [CrossRef]
- Barrau, M.; Roblin, X.; Andromaque, L.; Rozieres, A.; Faure, M.; Paul, S.; Nancey, S. What Should We Know about Drug Levels and Therapeutic Drug Monitoring during Pregnancy and Breastfeeding in Inflammatory Bowel Disease under Biologic Therapy? J. Clin. Med. 2023, 12, 7495. [Google Scholar] [CrossRef]
- Honap, S.; Jairath, V.; Sands, B.E.; Dulai, P.S.; Danese, S.; Peyrin-Biroulet, L. Acute severe ulcerative colitis trials: The past, the present and the future. Gut 2024, 73, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Festa, S.; Scribano, M.L.; Pugliese, D.; Bezzio, C.; Principi, M.; Ribaldone, D.G.; Allocca, M.; Mocci, G.; Bodini, G.; Spagnuolo, R.; et al. Long-term outcomes of acute severe ulcerative colitis in the rescue therapy era: A multicentre cohort study. UEG J. 2021, 9, 507–516. [Google Scholar] [CrossRef]
- Sebastian, S.; Walker, G.J.; Kennedy, N.A.; Conley, T.E.; Patel, K.V.; Subramanian, S.; Kent, A.J.; Segal, J.P.; Brookes, M.J.; Bhala, N.; et al. Assessment, endoscopy, and treatment in patients with acute severe ulcerative colitis during the COVID-19 pandemic (PROTECT-ASUC): A multicentre, observational, case-control study. Lancet Gastroenterol. Hepatol. 2021, 6, 271–281. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Xu, Z.; Marano, C.W.; Johanns, J.; Zhou, H.; Davis, H.M.; Cornillie, F.; et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014, 147, 1296–1307.e5. [Google Scholar] [CrossRef]
- Battat, R.; Hemperly, A.; Truong, S.; Whitmire, N.; Boland, B.S.; Dulai, P.S.; Holmer, A.K.; Nguyen, N.H.; Singh, S.; Vande Casteele, N.; et al. Baseline Clearance of Infliximab Is Associated with Requirement for Colectomy in Patients with Acute Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 511–518.e6. [Google Scholar] [CrossRef]
- Hindryckx, P.; Novak, G.; Vande Casteele, N.; Laukens, D.; Parker, C.; Shackelton, L.M.; Narula, N.; Khanna, R.; Dulai, P.; Levesque, B.G.; et al. Review article: Dose optimisation of infliximab for acute severe ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 45, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Yarur, A.J.; Jain, A.; Sussman, D.A.; Barkin, J.S.; Quintero, M.A.; Princen, F.; Kirkland, R.; Deshpande, A.R.; Singh, S.; Abreu, M.T. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: The ATLAS study. Gut 2016, 65, 249–255. [Google Scholar] [CrossRef]
- Brandse, J.F.; van den Brink, G.R.; Wildenberg, M.E.; van der Kleij, D.; Rispens, T.; Jansen, J.M.; Mathôt, R.A.; Ponsioen, C.Y.; Löwenberg, M.; D’Haens, G.R.A.M. Loss of Infliximab into Feces Is Associated with Lack of Response to Therapy in Patients with Severe Ulcerative Colitis. Gastroenterology 2015, 149, 350–355.e2. [Google Scholar] [CrossRef]
- Ordás, I.; Mould, D.R.; Feagan, B.G.; Sandborn, W.J. Anti-TNF monoclonal antibodies in inflammatory bowel disease: Pharmacokinetics-based dosing paradigms. Clin. Pharmacol. Ther. 2012, 91, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Biancheri, P.; Brezski, R.J.; Di Sabatino, A.; Greenplate, A.R.; Soring, K.L.; Corazza, G.R.; Kok, K.B.; Rovedatti, L.; Vossenkämper, A.; Ahmad, N.; et al. Proteolytic cleavage and loss of function of biologic agents that neutralize tumor necrosis factor in the mucosa of patients with inflammatory bowel disease. Gastroenterology 2015, 149, 1564–1574.e3. [Google Scholar] [CrossRef]
- Brandse, J.F.; Mathôt, R.A.; van der Kleij, D.; Rispens, T.; Ashruf, Y.; Jansen, J.M.; Rietdijk, S.; Löwenberg, M.; Ponsioen, C.Y.; Singh, S.; et al. Pharmacokinetic Features and Presence of Antidrug Antibodies Associate with Response to Infliximab Induction Therapy in Patients with Moderate to Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2016, 14, 251–258.e2. [Google Scholar] [CrossRef] [PubMed]
- Choy, M.C.; Li Wai Suen, C.F.D.; Con, D.; Boyd, K.; Pena, R.; Burrell, K.; Rosella, O.; Proud, D.; Brouwer, R.; Gorelik, A.; et al. Intensified versus standard dose infliximab induction therapy for steroid-refractory acute severe ulcerative colitis (PREDICT-UC): An open-label, multicentre, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2024, 9, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Li Wai Suen, C.F.D.; Choy, M.C.; Con, D.; Cheng, K.; Nigro, J.; Breheney, K.; Boyd, K.; Pena, R.; Burrell, K.; Rosella, O.; et al. Early infliximab levels and clearance predict outcomes after infliximab rescue in acute severe ulcerative colitis: Results from PREDICT-UC. Gastroenterology 2025, in press. [CrossRef]
- Gecse, K.; Van Oostrom, J.; Rietdijk, S.; Frigstad, S.O.; Doherty, G.; Irving, P.; Laharie, D.; De Voogd, F.; Hammersboen Bjorlykke, K.; Mould, D.R.; et al. DOP056 TDM-Based Dose-Intensification of Infliximab is not Superior to Standard Dosing in Patients with Acute Severe Ulcerative Colitis: Results from the TITRATE Study. J. Crohn’s Colitis 2025, 19, i194–i195. [Google Scholar] [CrossRef]
- Niyigena, E.; Hoffert, Y.; Peyrin-Biroulet, L.; Afif, W.; Roblin, X.; Hanžel, J.; Papamichael, K.; Kobayashi, T.; Wang, Z.; Verstockt, B.; et al. P0643 Development of a personalized infliximab dosing algorithm for Acute Severe Ulcerative Colitis: Results of a multi-center pharmacometrics analysis. J. Crohn’s Colitis 2025, 19, i1263–i1265. [Google Scholar] [CrossRef]
- Braamskamp, M.J.A.M.; Dolman, K.M.; Tabbers, M.M. Clinical practice: Protein-losing enteropathy in children. Eur. J. Pediatr. 2010, 169, 1179–1185. [Google Scholar] [CrossRef]
- Cameron, K.; Nguyen, A.L.; Gibson, D.J.; Ward, M.G.; Sparrow, M.P.; Gibson, P.R. Review Article: Albumin and Its Role in Inflammatory Bowel Disease: The Old, the New, and the Future. J. Gastro Hepatol. 2025, 40, 808–820. [Google Scholar] [CrossRef]
- Dotan, I.; Ron, Y.; Yanai, H.; Becker, S.; Fishman, S.; Yahav, L.; Ben Yehoyada, M.; Mould, D.R. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: A population pharmacokinetic study. Inflamm. Bowel Dis. 2014, 20, 2247–2259. [Google Scholar] [CrossRef]
- Yarur, A.J.; Kanagala, V.; Stein, D.J.; Czul, F.; Quintero, M.A.; Agrawal, D.; Patel, A.; Best, K.; Fox, C.; Idstein, K.; et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2017, 45, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.S.; Malietzis, G.; Lung, P.F.C.; Penez, L.; Yip, W.M.; Gabe, S.; Jenkins, J.T.; Hart, A. The body composition profile is associated with response to anti-TNF therapy in Crohn’s disease and may offer an alternative dosing paradigm. Aliment. Pharmacol. Ther. 2017, 46, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Megias, S.; Nalda-Molina, R.; Más-Serrano, P.; Ramon-Lopez, A. Population Pharmacokinetic Model of Adalimumab Based on Prior Information Using Real World Data. Biomedicines 2023, 11, 2822. [Google Scholar] [CrossRef] [PubMed]
- Rosario, M.; Dirks, N.L.; Gastonguay, M.R.; Fasanmade, A.A.; Wyant, T.; Parikh, A.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Fox, I. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 42, 188–202. [Google Scholar] [CrossRef]
- Alric, H.; Amiot, A.; Kirchgesner, J.; Tréton, X.; Allez, M.; Bouhnik, Y.; Beaugerie, L.; Carbonnel, F.; Meyer, A. Vedolizumab Clinical Decision Support Tool Predicts Efficacy of Vedolizumab but Not Ustekinumab in Refractory Crohn’s Disease. Inflamm. Bowel Dis. 2022, 28, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Samaan, M.A.; Cunningham, G.; Lim, S.H.; Dawson, P.; Kottoor, S.H.; Bheekhun, Z.; Lee, E.; Anderson, S.H.; Mawdsley, J.; Ray, S.; et al. Faecal loss of vedolizumab is associated with UC severity, lower serum vedolizumab levels and rates of clinical response: Results from the FAVOUR study. J. Crohns Colitis 2025, 19, jjaf159. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, O.J.; Xu, Z.; Marano, C.; O’Brien, C.; Szapary, P.; Zhang, H.; Johanns, J.; Leong, R.W.; Hisamatsu, T.; Van Assche, G.; et al. Ustekinumab Pharmacokinetics and Exposure Response in a Phase 3 Randomized Trial of Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2244–2255.e9. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Xu, Z.; Gasink, C.; Kowalski, K.; Sandborn, W.J.; Feagan, B. Population Pharmacokinetics and Exposure–Response Analyses of Ustekinumab in Patients with Moderately to Severely Active Crohn’s Disease. Clin. Ther. 2022, 44, 1336–1355. [Google Scholar] [CrossRef]
- Chua, L.; Otani, Y.; Lin, Z.; Friedrich, S.; Durand, F.; Zhang, X.C. Mirikizumab Pharmacokinetics and Exposure-Response in Patients with Moderately-To-Severely Active Crohn’s Disease: Results from Two Randomized Studies. Clin. Transl. Sci. 2025, 18, e70320. [Google Scholar] [CrossRef]
- Suleiman, A.A.; Goebel, A.; Bhatnagar, S.; D’Cunha, R.; Liu, W.; Pang, Y. Population Pharmacokinetic and Exposure-Response Analyses for Efficacy and Safety of Risankizumab in Patients with Active Crohn’s Disease. Clin. Pharmacol. Ther. 2023, 113, 839–850. [Google Scholar] [CrossRef]
- Deyhim, T.; Cheifetz, A.S.; Papamichael, K. Drug Clearance in Patients with Inflammatory Bowel Disease Treated with Biologics. J. Clin. Med. JCM 2023, 12, 7132. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Schlachter, L.; Eckert, D.; Stodtmann, S.; Liu, W.; Lacerda, A.P.; Mohamed, M.-E.F. Pharmacokinetics and Exposure-Response Analyses to Support Dose Selection of Upadacitinib in Crohn’s Disease. Clin. Pharmacol. Ther. 2024, 116, 1240–1251. [Google Scholar] [CrossRef]
- Ponce-Bobadilla, A.V.; Stodtmann, S.; Eckert, D.; Zhou, W.; Liu, W.; Mohamed, M.-E.F. Upadacitinib Population Pharmacokinetics and Exposure-Response Relationships in Ulcerative Colitis Patients. Clin. Pharmacokinet. 2023, 62, 101–112. [Google Scholar] [CrossRef]
- Tsai, L.; McCurdy, J.D.; Ma, C.; Jairath, V.; Singh, S. Epidemiology and Natural History of Perianal Crohn’s Disease: A Systematic Review and Meta-Analysis of Population-Based Cohorts. Inflamm. Bowel Dis. 2022, 28, 1477–1484. [Google Scholar] [CrossRef]
- Sands, B.E.; Anderson, F.H.; Bernstein, C.N.; Chey, W.Y.; Feagan, B.G.; Fedorak, R.N.; Kamm, M.A.; Korzenik, J.R.; Lashner, B.A.; Onken, J.E.; et al. Infliximab Maintenance Therapy for Fistulizing Crohn’s Disease. N. Engl. J. Med. 2004, 350, 876–885. [Google Scholar] [CrossRef]
- Papamichael, K.; Vande Casteele, N.; Jeyarajah, J.; Jairath, V.; Osterman, M.T.; Cheifetz, A.S. Higher Postinduction Infliximab Concentrations Are Associated with Improved Clinical Outcomes in Fistulizing Crohn’s Disease: An ACCENT-II Post Hoc Analysis. Am. J. Gastroenterol. 2021, 116, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, T. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn’s disease. Gut 2004, 53, 701–709. [Google Scholar] [CrossRef]
- Papamichael, K.; Centritto, A.; Guillo, L.; Hier, J.; Sherman, Z.; Cheifetz, A.S.; International Consortium of Therapeutic Drug Monitoring (Spectrum). Higher Adalimumab Concentration Is Associated with Complete Fistula Healing in Patients with Perianal Fistulizing Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2024, 22, 2134–2136.e2. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, H.; Su, T.; Peng, X.; Zhao, J.; Liu, T.; Wang, W.; Hu, P.; Zhi, M.; Zhang, M. Ustekinumab Promotes Radiological Fistula Healing in Perianal Fistulizing Crohn’s Disease: A Retrospective Real-World Analysis. J. Clin. Med. 2023, 12, 939. [Google Scholar] [CrossRef]
- Straatmijer, T.; Biemans, V.B.C.; Moes, D.J.A.R.; Hoentjen, F.; Ter Heine, R.; Maljaars, P.W.J.; Theeuwen, R.; Pierik, M.; Duijvestein, M.; van der Meulen-de Jong, A.E.; et al. Ustekinumab Trough Concentrations Are Associated with Biochemical Outcomes in Patients with Crohn’s Disease. Dig. Dis. Sci. 2023, 68, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, H.; Morita, Y.; Imai, T.; Takahashi, K.; Yoshida, A.; Bamba, S.; Inatomi, O.; Andoh, A. Ustekinumab trough levels predicting laboratory and endoscopic remission in patients with Crohn’s disease. BMC Gastroenterol. 2022, 22, 195. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef]
- Colwill, M.; Povlsen, S.; Pollok, R.; Patel, K.; Goodhand, J.; Ahmad, T.; Honap, S. Glucagon-Like Peptide (GLP-1) Receptor Agonists in Inflammatory Bowel Disease: Mechanisms, Clinical Implications, and Therapeutic Potential. J. Crohns Colitis 2025, 19, jjaf167. [Google Scholar] [CrossRef]
- Flores, A.; Burstein, E.; Cipher, D.J.; Feagins, L.A. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig. Dis. Sci. 2015, 60, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Sinanan, M.N.; Zisman, T.L. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2118–2124. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Proudfoot, J.; Xu, R.; Sandborn, W.J. Obesity and Response to Infliximab in Patients with Inflammatory Bowel Diseases: Pooled Analysis of Individual Participant Data from Clinical Trials. Am. J. Gastroenterol. 2018, 113, 883–889. [Google Scholar] [CrossRef]
- Bond, A.; Asher, R.; Jackson, R.; Sager, K.; Martin, K.; Kneebone, A.; Philips, S.; Taylor, W.; Subramanian, S. Comparative analysis of the influence of clinical factors including BMI on adalimumab and infliximab trough levels. Eur. J. Gastroenterol. Hepatol. 2016, 28, 271–276. [Google Scholar] [CrossRef]
- Fasanmade, A.A.; Adedokun, O.J.; Ford, J.; Hernandez, D.; Johanns, J.; Hu, C.; Davis, H.M.; Zhou, H. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur. J. Clin. Pharmacol. 2009, 65, 1211–1228. [Google Scholar] [CrossRef]
- Ebach, D.R.; Jester, T.W.; Galanko, J.A.; Firestine, A.M.; Ammoury, R.; Cabrera, J.; Bass, J.; Minar, P.; Olano, K.; Margolis, P.; et al. High Body Mass Index and Response to Anti-Tumor Necrosis Factor Therapy in Pediatric Crohn’s Disease. Am. J. Gastroenterol. 2024, 119, 1110–1116. [Google Scholar] [CrossRef]
- Ponce-Bobadilla, A.V.; Stodtmann, S.; Chen, M.-J.; Winzenborg, I.; Mensing, S.; Blaes, J.; Haslberger, T.; Laplanche, L.; Dreher, I.; Mostafa, N.M. Assessing the Impact of Immunogenicity and Improving Prediction of Trough Concentrations: Population Pharmacokinetic Modeling of Adalimumab in Patients with Crohn’s Disease and Ulcerative Colitis. Clin. Pharmacokinet. 2023, 62, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Kurnool, S.; Nguyen, N.H.; Proudfoot, J.; Dulai, P.S.; Boland, B.S.; Vande Casteele, N.; Evans, E.; Grunvald, E.L.; Zarrinpar, A.; Sandborn, W.J.; et al. High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 1472–1479. [Google Scholar] [CrossRef]
- Jensen, M.D.; Haymond, M.W. Protein metabolism in obesity: Effects of body fat distribution and hyperinsulinemia on leucine turnover. Am. J. Clin. Nutr. 1991, 53, 172–176. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Desreumaux, P.; Ernst, O.; Geboes, K.; Gambiez, L.; Berrebi, D.; Müller-Alouf, H.; Hafraoui, S.; Emilie, D.; Ectors, N.; Peuchmaur, M.; et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology 1999, 117, 73–81. [Google Scholar] [CrossRef]
- Sheehan, A.L.; Warren, B.F.; Gear, M.W.; Shepherd, N.A. Fat-wrapping in Crohn’s disease: Pathological basis and relevance to surgical practice. Br. J. Surg. 1992, 79, 955–958. [Google Scholar] [CrossRef]
- Althoff, P.; Schmiegel, W.; Lang, G.; Nicolas, V.; Brechmann, T. Creeping Fat Assessed by Small Bowel MRI Is Linked to Bowel Damage and Abdominal Surgery in Crohn’s Disease. Dig. Dis. Sci. 2019, 64, 204–212. [Google Scholar] [CrossRef]
- Gu, P.; Chhabra, A.; Chittajallu, P.; Chang, C.; Mendez, D.; Gilman, A.; Fudman, D.I.; Xi, Y.; Feagins, L.A. Visceral Adipose Tissue Volumetrics Inform Odds of Treatment Response and Risk of Subsequent Surgery in IBD Patients Starting Antitumor Necrosis Factor Therapy. Inflamm. Bowel Dis. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Liu, S.; Ding, X.; Maggiore, G.; Pietrobattista, A.; Satapathy, S.K.; Tian, Z.; Jing, X. Sarcopenia is associated with poor clinical outcomes in patients with inflammatory bowel disease: A prospective cohort study. Ann. Transl. Med. 2022, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Yarur, A.J.; Abreu, M.T.; Deepak, P.; Beniwal-Patel, P.; Papamichael, K.; Vaughn, B.; Bruss, A.; Sekhri, S.; Moosreiner, A.; Gu, P.; et al. Patients with Inflammatory Bowel Diseases and Higher Visceral Adipose Tissue Burden May Benefit from Higher Infliximab Concentrations to Achieve Remission. Am. J. Gastroenterol. 2023, 118, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.C.L.; Marshall, J.K.; Reinisch, W.; Narula, N. Body Mass Index Does Not Impact Clinical Efficacy of Ustekinumab in Crohn’s Disease: A Post Hoc Analysis of the IM-UNITI Trial. Inflamm. Bowel Dis. 2021, 27, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.Q.; Strauss, B.J.; Moore, G.T. Weight and Body Composition Compartments Do Not Predict Therapeutic Thiopurine Metabolite Levels in Inflammatory Bowel Disease. Clin. Transl. Gastroenterol. 2016, 7, e199. [Google Scholar] [CrossRef]
- Shen, J.; Tatosian, D.; Sid-Otmane, L.; Teuscher, N.; Chen, L.; Zhang, P.; Tirucherai, G.; Chitkara, D.; Marta, C. Population Pharmacokinetics of Ozanimod and Active Metabolite Cc112273 in Patients with Ulcerative Colitis. Gastroenterology 2022, 162, S17–S18. [Google Scholar] [CrossRef]
- Teasdale, S.; Morton, A. Changes in biochemical tests in pregnancy and their clinical significance. Obstet. Med. 2018, 11, 160–170. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Gubatan, J.M.; Kolho, K.-L.; Streett, S.E.; Maxwell, C. Updates on the management of inflammatory bowel disease from periconception to pregnancy and lactation. Lancet 2024, 403, 1291–1303. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Fridman Lev, S.; Rotem, R.; Mishael, T.; Grisaru Granovsky, S.; Koslowsky, B.; Goldin, E.; Bar-Gil Shitrit, A. Disease flare at prior pregnancy and disease activity at conception are important determinants of disease relapse at subsequent pregnancy in women with inflammatory bowel diseases. Arch. Gynecol. Obstet. 2020, 301, 1449–1454. [Google Scholar] [CrossRef]
- Seow, C.H.; Leung, Y.; Vande Casteele, N.; Ehteshami Afshar, E.; Tanyingoh, D.; Bindra, G.; Stewart, M.J.; Beck, P.L.; Kaplan, G.G.; Ghosh, S.; et al. The effects of pregnancy on the pharmacokinetics of infliximab and adalimumab in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 1329–1338. [Google Scholar] [CrossRef]
- Flanagan, E.; Gibson, P.R.; Wright, E.K.; Moore, G.T.; Sparrow, M.P.; Connell, W.; Kamm, M.A.; Begun, J.; Christensen, B.; De Cruz, P.; et al. Infliximab, adalimumab and vedolizumab concentrations across pregnancy and vedolizumab concentrations in infants following intrauterine exposure. Aliment. Pharmacol. Ther. 2020, 52, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Lozano, N.A.; Lozano, A.; Marini, V.; Saranz, R.J.; Blumberg, R.S.; Baker, K.; Agresta, M.F.; Ponzio, M.F. Expression of FcRn receptor in placental tissue and its relationship with IgG levels in term and preterm newborns. Am. J. Rep. Immunol. 2018, 80, e12972. [Google Scholar] [CrossRef]
- Flanagan, E.; Wright, E.K.; Hardikar, W.; Sparrow, M.P.; Connell, W.R.; Kamm, M.A.; De Cruz, P.; Brown, S.J.; Thompson, A.; Greenway, A.; et al. Maternal thiopurine metabolism during pregnancy in inflammatory bowel disease and clearance of thiopurine metabolites and outcomes in exposed neonates. Aliment. Pharmacol. Ther. 2021, 53, 810–820. [Google Scholar] [CrossRef]
- Prentice, R.; Flanagan, E.; Wright, E.K.; Gibson, P.R.; Rosella, S.; Rosella, O.; Begun, J.; An, Y.-K.; Lawrance, I.C.; Kamm, M.A.; et al. Vedolizumab and Ustekinumab Levels in Pregnant Women with Inflammatory Bowel Disease and Infants Exposed In Utero. Clin. Gastroenterol. Hepatol. 2025, 23, 124–133.e7. [Google Scholar] [CrossRef]
- Rowan, C.R.; Cullen, G.; Mulcahy, H.E.; Keegan, D.; Byrne, K.; Murphy, D.J.; Sheridan, J.; Doherty, G.A. Ustekinumab Drug Levels in Maternal and Cord Blood in a Woman with Crohn’s Disease Treated Until 33 Weeks of Gestation. J. Crohn’s Colitis 2018, 12, 376–378. [Google Scholar] [CrossRef]
- Torres, J.; Chaparro, M.; Julsgaard, M.; Katsanos, K.; Zelinkova, Z.; Agrawal, M.; Ardizzone, S.; Campmans-Kuijpers, M.; Dragoni, G.; Ferrante, M.; et al. European Crohn’s and Colitis Guidelines on Sexuality, Fertility, Pregnancy, and Lactation. J. Crohn’s Colitis 2023, 17, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Chugh, R.; Long, M.D.; Jiang, Y.; Weaver, K.N.; Beaulieu, D.B.; Scherl, E.J.; Mahadevan, U. Maternal and Neonatal Outcomes in Vedolizumab- and Ustekinumab-Exposed Pregnancies: Results from the PIANO Registry. Am. J. Gastroenterol. 2024, 119, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, U.; Long, M.D.; Kane, S.V.; Roy, A.; Dubinsky, M.C.; Sands, B.E.; Cohen, R.D.; Chambers, C.D.; Sandborn, W.J. Pregnancy and Neonatal Outcomes After Fetal Exposure to Biologics and Thiopurines Among Women with Inflammatory Bowel Disease. Gastroenterology 2021, 160, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Kane, S.; Beaulieu, D.; Abraham, B.; Zhang, X.; Mahadevan, U. Use of Biosimilars to Infliximab During Pregnancy in Women with Inflammatory Bowel Disease: Results from the Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes Study. Clin. Transl. Gastroenterol. 2024, 15, e00795. [Google Scholar] [CrossRef]
- Mahadevan, U.; Long, M. Low Placental Transfer Rates of Risankizumab Among Pregnant Women with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2024, 30, 2240–2241. [Google Scholar] [CrossRef]
- Luu, M.; Benzenine, E.; Doret, M.; Michiels, C.; Barkun, A.; Degand, T.; Quantin, C.; Bardou, M. Continuous Anti-TNFα Use Throughout Pregnancy: Possible Complications for the Mother but Not for the Fetus. A Retrospective Cohort on the French National Health Insurance Database (EVASION). Am. J. Gastroenterol. 2018, 113, 1669–1677. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Hracs, L.; Windsor, J.W.; Gorospe, J.; Cummings, M.; Coward, S.; Buie, M.J.; Quan, J.; Goddard, Q.; Caplan, L.; Markovinović, A.; et al. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature 2025, 642, 458–466. [Google Scholar] [CrossRef]
- Honap, S.; Agorogianni, A.; Colwill, M.J.; Mehta, S.K.; Donovan, F.; Pollok, R.; Poullis, A.; Patel, K. JAK inhibitors for inflammatory bowel disease: Recent advances. Frontline Gastroenterol. 2024, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Calafat, M.; Mañosa, M.; Cañete, F.; Ricart, E.; Iglesias, E.; Calvo, M.; Rodríguez-Moranta, F.; Taxonera, C.; Nos, P.; Mesonero, F.; et al. Increased risk of thiopurine-related adverse events in elderly patients with IBD. Aliment. Pharmacol. Ther. 2019, 50, 780–788. [Google Scholar] [CrossRef]
- Ruiter, R.; Burggraaf, J.; Rissmann, R. Under-representation of elderly in clinical trials: An analysis of the initial approval documents in the Food and Drug Administration database. Br. J. Clin. Pharmacol. 2019, 85, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Barnes, E.L.; Zhang, X.; Long, M.D. Trends and Characteristics of Clinical Trials Participation for Inflammatory Bowel Disease in the United States: A Report from IBD Partners. Crohn’s Colitis 360 2020, 2, otaa023. [Google Scholar] [CrossRef]
- Kantasiripitak, W.; Verstockt, B.; Alsoud, D.; Lobatón, T.; Thomas, D.; Gils, A.; Vermeire, S.; Ferrante, M.; Dreesen, E. The effect of aging on infliximab exposure and response in patients with inflammatory bowel diseases. Br. J. Clin. Pharmacol. 2021, 87, 3776–3789. [Google Scholar] [CrossRef]
- Harnik, S.; Ungar, B.; Loebstein, R.; Ben-Horin, S. A Gastroenterologist’s guide to drug interactions of small molecules for inflammatory bowel disease. United Eur. Gastroenterol. J. 2024, 12, 627–637. [Google Scholar] [CrossRef]
- Sands, B.E.; Schreiber, S.; Blumenstein, I.; Chiorean, M.V.; Ungaro, R.C.; Rubin, D.T. Clinician’s Guide to Using Ozanimod for the Treatment of Ulcerative Colitis. J. Crohns Colitis 2023, 17, 2012–2025. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; De Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care—An Evidence-based Guideline From European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 257–291. [Google Scholar] [CrossRef] [PubMed]
- El-Matary, W.; Walters, T.D.; Huynh, H.Q.; deBruyn, J.; Mack, D.R.; Jacobson, K.; Sherlock, M.E.; Church, P.; Wine, E.; Carroll, M.W.; et al. Higher Postinduction Infliximab Serum Trough Levels Are Associated with Healing of Fistulizing Perianal Crohn’s Disease in Children. Inflamm. Bowel Dis. 2019, 25, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, M.M.E.; Winter, D.A.; Huynh, H.Q.; Norsa, L.; Hussey, S.; Kolho, K.-L.; Bronsky, J.; Assa, A.; Cohen, S.; Lev-Tzion, R.; et al. Infliximab in young paediatric IBD patients: It is all about the dosing. Eur. J. Pediatr. 2020, 179, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Rosh, J.R.; Turner, D.; Griffiths, A.; Cohen, S.A.; Jacobstein, D.; Adedokun, O.J.; Padgett, L.; Terry, N.A.; O’Brien, C.; Hyams, J.S. Ustekinumab in Paediatric Patients with Moderately to Severely Active Crohn’s Disease: Pharmacokinetics, Safety, and Efficacy Results from UniStar, a Phase 1 Study. J. Crohns Colitis 2021, 15, 1931–1942. [Google Scholar] [CrossRef]
- Whaley, K.G.; Xiong, Y.; Karns, R.; Hyams, J.S.; Kugathasan, S.; Boyle, B.M.; Walters, T.D.; Kelsen, J.; LeLeiko, N.; Shapiro, J.; et al. Multicenter Cohort Study of Infliximab Pharmacokinetics and Therapy Response in Pediatric Acute Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2023, 21, 1338–1347. [Google Scholar] [CrossRef]
- Turner, D.; Mack, D.; Leleiko, N.; Walters, T.D.; Uusoue, K.; Leach, S.T.; Day, A.S.; Crandall, W.; Silverberg, M.S.; Markowitz, J.; et al. Severe Pediatric Ulcerative Colitis: A Prospective Multicenter Study of Outcomes and Predictors of Response. Gastroenterology 2010, 138, 2282–2291. [Google Scholar] [CrossRef]
- Chung, A.; Carroll, M.; Almeida, P.; Petrova, A.; Isaac, D.; Mould, D.; Wine, E.; Huynh, H. Early Infliximab Clearance Predicts Remission in Children with Crohn’s Disease. Dig. Dis. Sci. 2023, 68, 1995–2005. [Google Scholar] [CrossRef]
- Colman, R.J.; Xiong, Y.; Mizuno, T.; Hyams, J.S.; Noe, J.D.; Boyle, B.; D’Haens, G.R.; van Limbergen, J.; Chun, K.; Yang, J.; et al. Antibodies-to-infliximab accelerate clearance while dose intensification reverses immunogenicity and recaptures clinical response in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2022, 55, 593–603. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Rabizadeh, S.; Panetta, J.C.; Spencer, E.A.; Everts-van Der Wind, A.; Dervieux, T. The Combination of Predictive Factors of Pharmacokinetic Origin Associates with Enhanced Disease Control during Treatment of Pediatric Crohn’s Disease with Infliximab. Pharmaceutics 2023, 15, 2408. [Google Scholar] [CrossRef] [PubMed]
- Kantasiripitak, W.; Wicha, S.G.; Thomas, D.; Hoffman, I.; Ferrante, M.; Vermeire, S.; Van Hoeve, K.; Dreesen, E. A Model-Based Tool for Guiding Infliximab Induction Dosing to Maximize Long-term Deep Remission in Children with Inflammatory Bowel Diseases. J. Crohn’s Colitis 2023, 17, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Minar, P.P.; Colman, R.J.; Zhang, N.; Mizuno, T.; Vinks, A.A. Precise infliximab exposure and pharmacodynamic control to achieve deep remission in paediatric Crohn’s disease (REMODEL-CD): Study protocol for a multicentre, open-label, pragmatic clinical trial in the USA. BMJ Open 2024, 14, e077193. [Google Scholar] [CrossRef]
- Yarur, A.J.; Kubiliun, M.J.; Czul, F.; Sussman, D.A.; Quintero, M.A.; Jain, A.; Drake, K.A.; Hauenstein, S.I.; Lockton, S.; Deshpande, A.R.; et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin. Gastroenterol. Hepatol. 2015, 13, 1118–1124.e3. [Google Scholar] [CrossRef]
- Mogensen, D.V.; Brynskov, J.; Ainsworth, M.A.; Nersting, J.; Schmiegelow, K.; Steenholdt, C. A Role for Thiopurine Metabolites in the Synergism Between Thiopurines and Infliximab in Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, 298–305. [Google Scholar] [CrossRef]
- Luber, R.P.; Honap, S.; Cunningham, G.; Irving, P.M. Can We Predict the Toxicity and Response to Thiopurines in Inflammatory Bowel Diseases? Front. Med. 2019, 6, 279. [Google Scholar] [CrossRef]
- Phillips, J.; Leary, S.; Tyrrell-Price, J. Association between 6-thioguanine nucleotide levels and preventing production of antibodies to infliximab in patients with inflammatory bowel disease. BMJ Open Gastroenterol. 2023, 10, e001149. [Google Scholar] [CrossRef] [PubMed]
- Chanchlani, N.; Lin, S.; Bewshea, C.; Hamilton, B.; Thomas, A.; Smith, R.; Roberts, C.; Bishara, M.; Nice, R.; Lees, C.W.; et al. Mechanisms and management of loss of response to anti-TNF therapy for patients with Crohn’s disease: 3-year data from the prospective, multicentre PANTS cohort study. Lancet Gastroenterol. Hepatol. 2024, 9, 521–538. [Google Scholar] [CrossRef]
- Roblin, X.; Boschetti, G.; Williet, N.; Nancey, S.; Marotte, H.; Berger, A.; Phelip, J.M.; Peyrin-Biroulet, L.; Colombel, J.F.; Del Tedesco, E.; et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: An open-label, prospective and randomised clinical trial. Aliment. Pharmacol. Ther. 2017, 46, 142–149. [Google Scholar] [CrossRef]
- Ungar, B.; Engel, T.; Yablecovitch, D.; Lahat, A.; Lang, A.; Avidan, B.; Har-Noy, O.; Carter, D.; Levhar, N.; Selinger, L.; et al. Prospective Observational Evaluation of Time-Dependency of Adalimumab Immunogenicity and Drug Concentrations: The POETIC Study. Am. J. Gastroenterol. 2018, 113, 890–898. [Google Scholar] [CrossRef]
- Smith, P.J.; Critchley, L.; Storey, D.; Gregg, B.; Stenson, J.; Kneebone, A.; Rimmer, T.; Burke, S.; Hussain, S.; Yi Teoh, W.; et al. Efficacy and Safety of Elective Switching from Intravenous to Subcutaneous Infliximab [CT-P13]: A Multicentre Cohort Study. J. Crohns Colitis 2022, 16, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Harno-Tasihin, J.; Siregar, L.; Paajanen, M.; Arkkila, P.; Punkkinen, J. Switching from intravenous to subcutaneous infliximab and vedolizumab in patients with inflammatory bowel disease: Impact on trough levels, day hospital visits, and medical expenses. Scand. J. Gastroenterol. 2024, 59, 280–287. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.; Reinisch, W.; Schreiber, S.; Cummings, F.; Irving, P.M.; Ye, B.D.; Kim, D.-H.; Yoon, S.; Ben-Horin, S. Subcutaneous Infliximab Monotherapy Versus Combination Therapy with Immunosuppressants in Inflammatory Bowel Disease: A Post Hoc Analysis of a Randomised Clinical Trial. Clin. Drug Investig. 2023, 43, 277–288. [Google Scholar] [CrossRef]
- Irving, P.; Irving, P.; Rahman, A. Investigating the Need to Continue Taking Immunomodulator Tablets for Patients with Inflammatory Bowel Disease, When Switching from Treatment with Intravenous Infliximab Infusions (Infliximab Given Directly into a Vein) to Subcutaneous Infliximab (Infliximab Given by an Injection Under the Skin). Available online: https://www.isrctn.com/ISRCTN95420128 (accessed on 5 November 2025).
- Hanauer, S.B.; Sands, B.E.; Schreiber, S.; Danese, S.; Kłopocka, M.; Kierkuś, J.; Kulynych, R.; Gonciarz, M.; Sołtysiak, A.; Smoliński, P.; et al. Subcutaneous Infliximab (CT-P13 SC) as Maintenance Therapy for Inflammatory Bowel Disease: Two Randomized Phase 3 Trials (LIBERTY). Gastroenterology 2024, 167, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Verstockt, B.; Sabino, J.; Ferrante, M.; Vermeire, S.; Dreesen, E. Therapeutic Drug Monitoring Can Guide the Intravenous-to-Subcutaneous Switch of Infliximab and Vedolizumab: A Simulation Study. Clin. Gastroenterol. Hepatol. 2023, 21, 3188–3190.e2. [Google Scholar] [CrossRef]
- Chetwood, J.D.; Tran, Y.; Subramanian, S.; Smith, P.J.; Iborra, M.; Buisson, A.; Paramsothy, S.; Leong, R.W. Intravenous Versus Subcutaneous Infliximab in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2024, 18, 1440–1449. [Google Scholar] [CrossRef]
- Iborra, M.; Caballol, B.; Garrido, A.; Huguet, J.M.; Mesonero, F.; Ponferrada, Á.; Arias García, L.; Boscá Watts, M.M.; Fernández Prada, S.J.; Brunet Mas, E.; et al. Subcutaneous Infliximab Cutoff Points in Patients with Inflammatory Bowel Disease: Data from the ENEIDA Registry. J. Crohn’s Colitis 2025, 19, jjae127. [Google Scholar] [CrossRef]
- Roblin, X.; Boschetti, G.; Duru, G.; Williet, N.; Deltedesco, E.; Phelip, J.M.; Peyrin-Biroulet, L.; Nancey, S.; Flourié, B.; Paul, S. Distinct Thresholds of Infliximab Trough Level Are Associated with Different Therapeutic Outcomes in Patients with Inflammatory Bowel Disease: A Prospective Observational Study. Inflamm. Bowel Dis. 2017, 23, 2048–2053. [Google Scholar] [CrossRef]
- Ungar, B.; Kopylov, U.; Yavzori, M.; Fudim, E.; Picard, O.; Lahat, A.; Coscas, D.; Waterman, M.; Haj-Natour, O.; Orbach-Zingboim, N.; et al. Association of Vedolizumab Level, Anti-Drug Antibodies, and α4β7 Occupancy with Response in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 697–705.e7. [Google Scholar] [CrossRef] [PubMed]
- Steenholdt, C.; Lorentsen, R.D.; Petersen, P.N.; Widigson, E.S.; Kloft, C.; Klaasen, R.A.; Brynskov, J. Therapeutic drug monitoring of vedolizumab therapy in inflammatory bowel disease. J. Gastro Hepatol. 2024, 39, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Seow, C.H.; Marshall, J.K.; Stewart, E.; Pettengell, C.; Ward, R.; Afif, W. The relationship among vedolizumab drug concentrations, biomarkers of inflammation, and clinical outcomes in a Canadian real-world study. J. Can. Assoc. Gastroenterol. 2024, 7, 290–298. [Google Scholar] [CrossRef]
- Yacoub, W.; Williet, N.; Pouillon, L.; Di-Bernado, T.; De Carvalho Bittencourt, M.; Nancey, S.; Lopez, A.; Paul, S.; Zallot, C.; Roblin, X.; et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: A multicentre prospective observational study. Aliment. Pharmacol. Ther. 2018, 47, 906–912. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Xu, Z.; Gasink, C.; Jacobstein, D.; Szapary, P.; Johanns, J.; Gao, L.-L.; Davis, H.M.; Hanauer, S.B.; Feagan, B.G.; et al. Pharmacokinetics and Exposure Response Relationships of Ustekinumab in Patients with Crohn’s Disease. Gastroenterology 2018, 154, 1660–1671. [Google Scholar] [CrossRef]
- Bossuyt, P.; Rahier, J.F.; Baert, F.; Louis, E.; Macken, E.; Lobaton, T.; Busschaert, J.; Peeters, H.; Dewint, P.; Franchimont, D.; et al. OP35 Low remission recapture after ustekinumab dose optimization in Crohn’s disease: Results of the randomized placebo-controlled double-blind REScUE study. J. Crohn’s Colitis 2025, 19, i69–i70. [Google Scholar] [CrossRef]
- Feagan, B.G.; Danese, S.; Loftus, E.V.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempiński, R.; et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Hazra, A.; Smith, M.K.; Martin, S.W.; Mould, D.R.; Su, C.; Niezychowski, W. Exposure-response characterization of tofacitinib efficacy in moderate to severe ulcerative colitis: Results from a dose-ranging phase 2 trial. Br. J. Clin. Pharmacol. 2018, 84, 1136–1145. [Google Scholar] [CrossRef]

| Scenario | Drug | Factors Affecting Pharmacokinetics | |
|---|---|---|---|
| ASUC | Negative factors | ||
| IFX | Increased faecal drug loss † TNFα sink a,† Increased proteolysis † Decreased neonatal Fc receptor-mediated drug recycling † ADA formation with low drug levels † | ||
| Hypoalbuminaemia | Negative factors | ||
| IFX and ADM | Indirect marker of high inflammatory burden Increased faecal drug loss † TNFα sink a,† Increased proteolysis † Decreased neonatal Fc receptor-mediated drug recycling † ADA formation with low drug levels † | ||
| VDZ | Indirect marker of high inflammatory burden Increased faecal drug loss † | ||
| Perianal fistulising CD | Negative factors | ||
| IFX and ADM | Increased local fistula expression of MMPs, which degrade mAbs | ||
| Obesity | Negative factors | Positive factors | |
| IFX and ADM | TNFα sink a,† Increased proteolysis | Decreased peripheral volume of distribution at higher bodyweights ‡ | |
| VDZ | Volume of distribution at extremes of bodyweight b,† | ||
| Pregnancy | Negative factors | Positive Factors | |
| IFX | Increased neonatal Fc receptor recycling ‡ | ||
| Thiopurine | Increased 6-MMP, decreased 6-TGN | ||
| VDZ | Increased volume of distribution with increasing bodyweight † | ||
| Elderly | No specific differences | ||
| Paediatrics | Negative factors | ||
| IFX and ADM | Children <10 more prone to subtherapeutic dosing Increased peripheral volume of distribution in lower bodyweights † | ||
| Route: SC or IV | Positive factors | ||
| IFX | Slower absorption, more stable exposure profile Potentially reduced immunogenicity ‡ | ||
| VDZ | Slower absorption, more stable exposure profile | ||
| Co-administration with anti-TNFα | Positive factors | ||
| Thiopurine | Reduced anti-TNFα immunogenicity ‡ Increased anti-TNFα drug levels | ||
| Scenario | Infliximab | Adalimumab | Thiopurine |
|---|---|---|---|
| General: post-induction | Week 14: 7–10 μg/mL | Week 16: 8–12 μg/mL CD ≥ 12 μg/mL | 6-TGN 235–450 pmol/8 × 108 RBCs |
| General: maintenance | 5–10 μg/mL | 8–12 μg/mL CD ≥ 12 μg/mL | 6-TGN 235–450 pmol/8 × 108 RBCs |
| ASUC | Consider 10 mg/kg dosing +/− accelerating induction in select patients, particularly in hypoalbuminaemia | - | - |
| Hypoalbuminaemia | Consider 10 mg/kg dosing or early TDM to direct dose escalation | Consider 40 mg weekly dosing or early TDM to direct dose escalation | No evidence for different targets |
| Perianal fistulising CD | Consider ≥10 μg/mL | ≥12 μg/mL | No evidence for different targets |
| Obesity | High visceral adipose tissue: consider higher target | High visceral adipose tissue: consider higher target | No evidence for different targets |
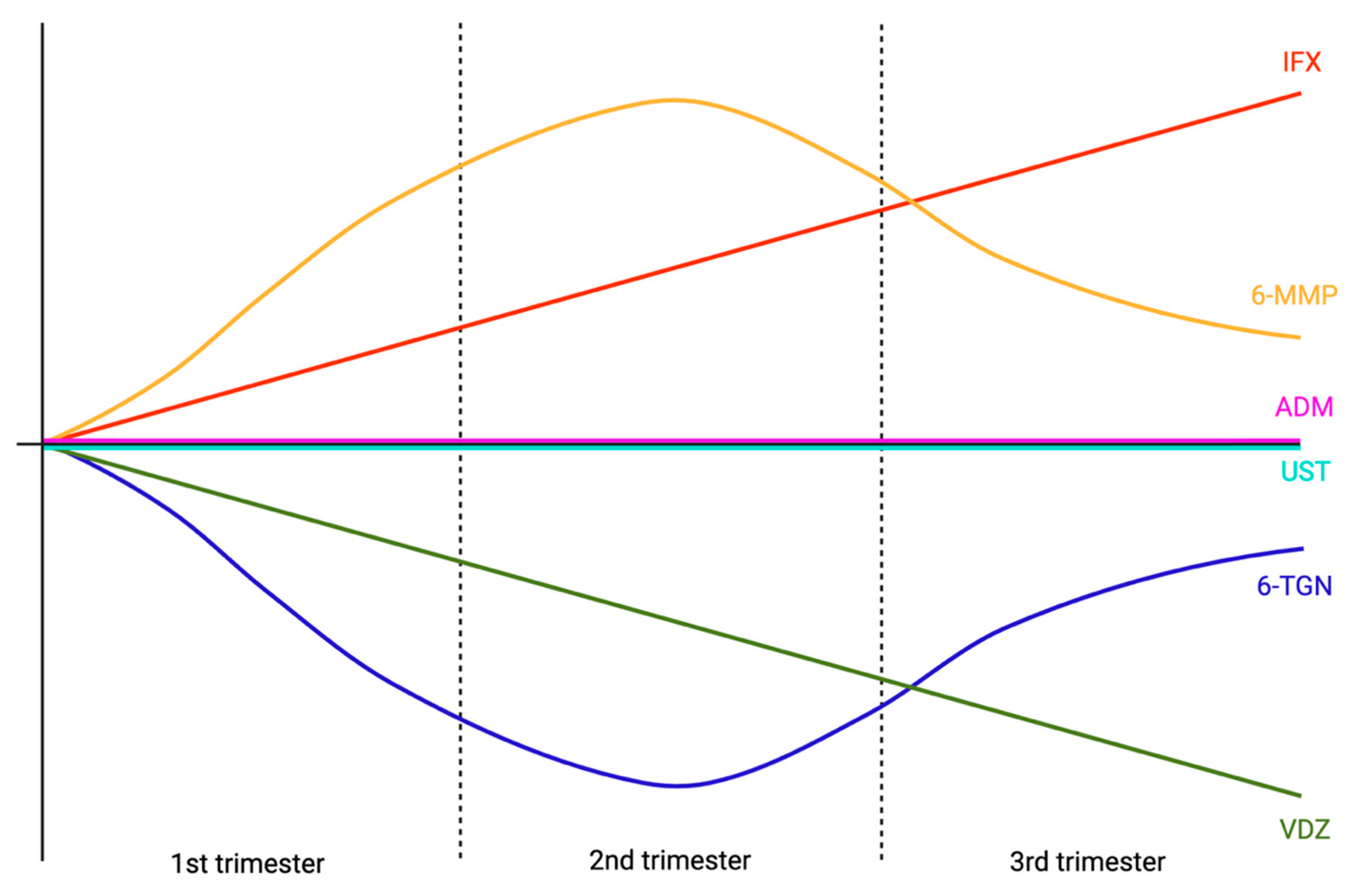
| Pregnancy | Levels rise throughout pregnancy No evidence for different targets | Levels stable throughout pregnancy No evidence for different targets | 2nd trimester: 6-TGNs drop, 6-MMPs rise 3rd trimester: return towards baseline |
| Elderly | No evidence for different targets | No evidence for different targets | No evidence for different targets |
| Paediatrics | Proactive TDM—no evidence for different targets Age <10 more likely to have sub-optimal levels, consider intensified dosing and early proactive TDM | Proactive TDM—no evidence for different targets Age <10 more likely to have sub-optimal levels, consider intensified dosing and early proactive TDM | No evidence for different targets Adherence is main factor affecting levels |
| Route: SC or IV | SC switch from typical IV regimens may raise drug levels by 6–10 μg/mL Consider ≥12–20 μg/mL | - | - |
| Co-administration with anti-TNFα | - | - | 6-TGN >125 pmol/8 × 108 RBCs may be sufficient |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Povlsen, S.; Patel, K.; Roblin, X.; Papamichael, K.; Honap, S. Therapeutic Drug Monitoring in Special Circumstances in Inflammatory Bowel Disease. J. Clin. Med. 2025, 14, 7956. https://doi.org/10.3390/jcm14227956
Povlsen S, Patel K, Roblin X, Papamichael K, Honap S. Therapeutic Drug Monitoring in Special Circumstances in Inflammatory Bowel Disease. Journal of Clinical Medicine. 2025; 14(22):7956. https://doi.org/10.3390/jcm14227956
Chicago/Turabian StylePovlsen, Sebastian, Kamal Patel, Xavier Roblin, Konstantinos Papamichael, and Sailish Honap. 2025. "Therapeutic Drug Monitoring in Special Circumstances in Inflammatory Bowel Disease" Journal of Clinical Medicine 14, no. 22: 7956. https://doi.org/10.3390/jcm14227956
APA StylePovlsen, S., Patel, K., Roblin, X., Papamichael, K., & Honap, S. (2025). Therapeutic Drug Monitoring in Special Circumstances in Inflammatory Bowel Disease. Journal of Clinical Medicine, 14(22), 7956. https://doi.org/10.3390/jcm14227956








