Antiepileptic Drugs for De Novo Seizure Prevention After Craniotomy: A Systematic Review and Network Meta-Analysis of Current Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Criteria for Considering Studies for This Review
2.3. Outcome Measures
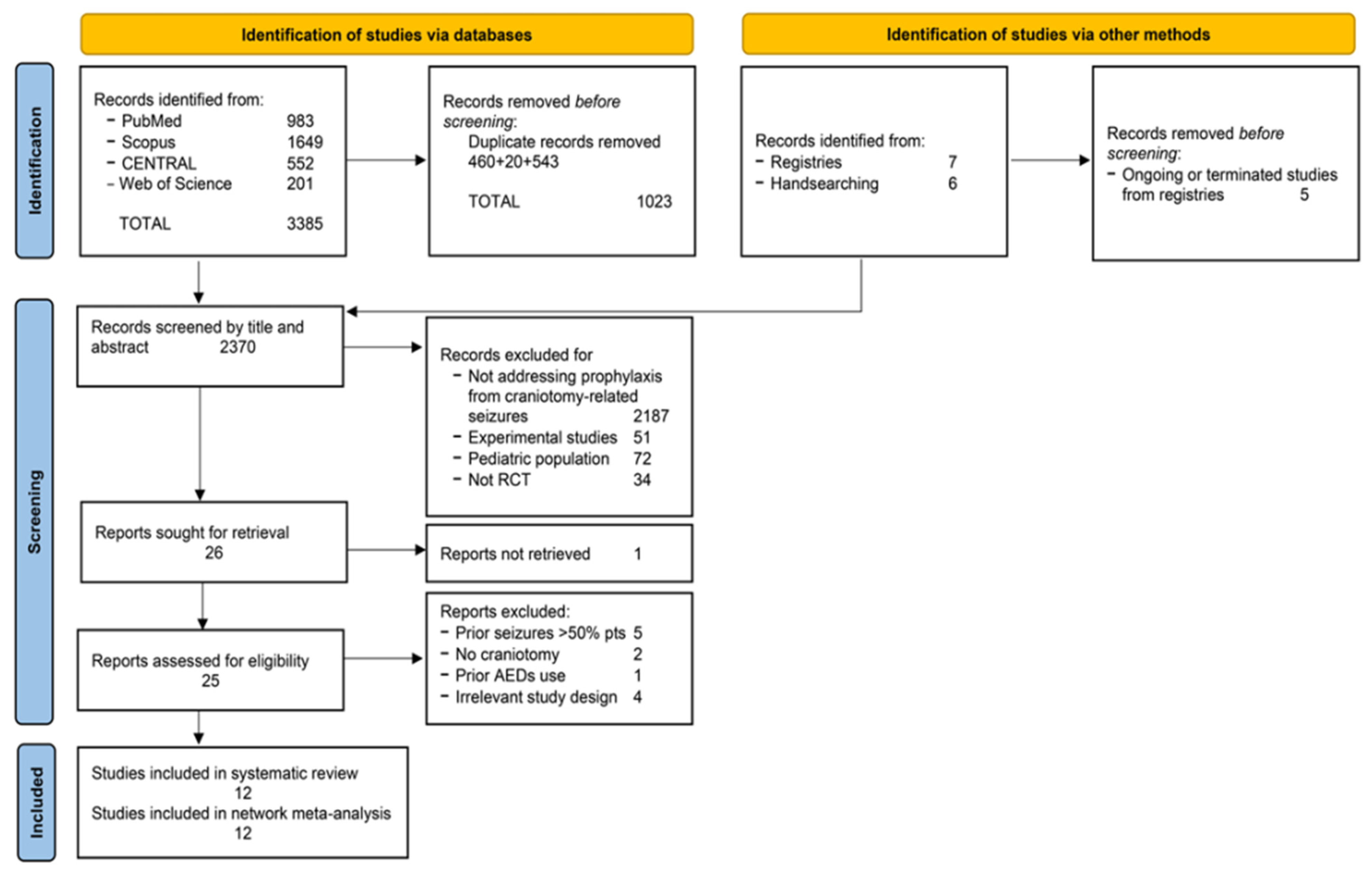
2.4. Screening and Selection Process
2.5. Data Extraction
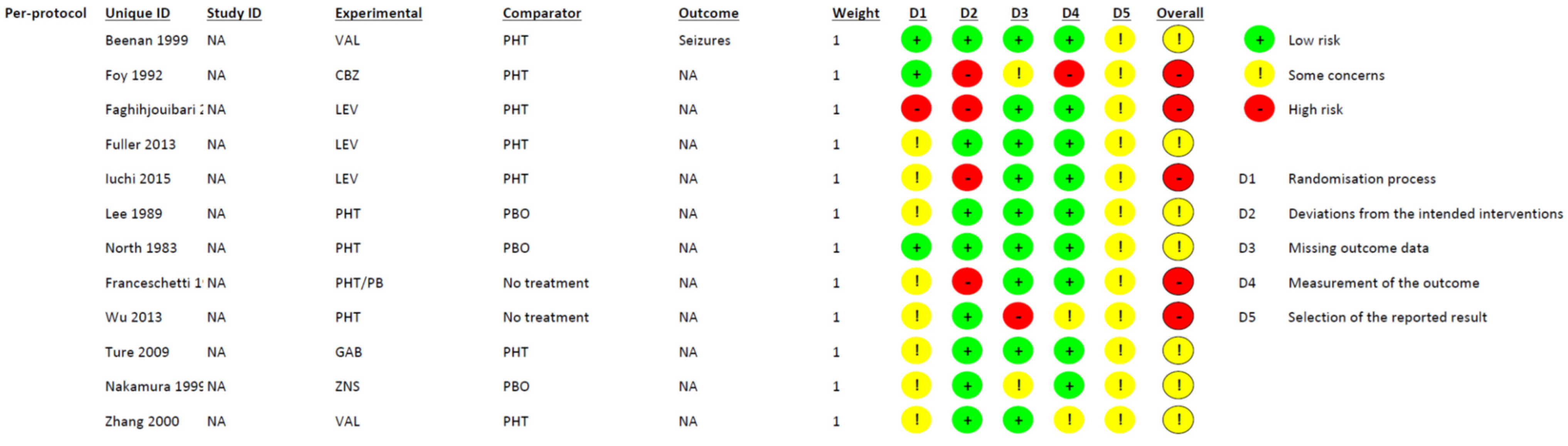
2.6. Quality Assessment
2.7. Statistical Synthesis
3. Results
3.1. Search Process
3.2. Description of Included Trials
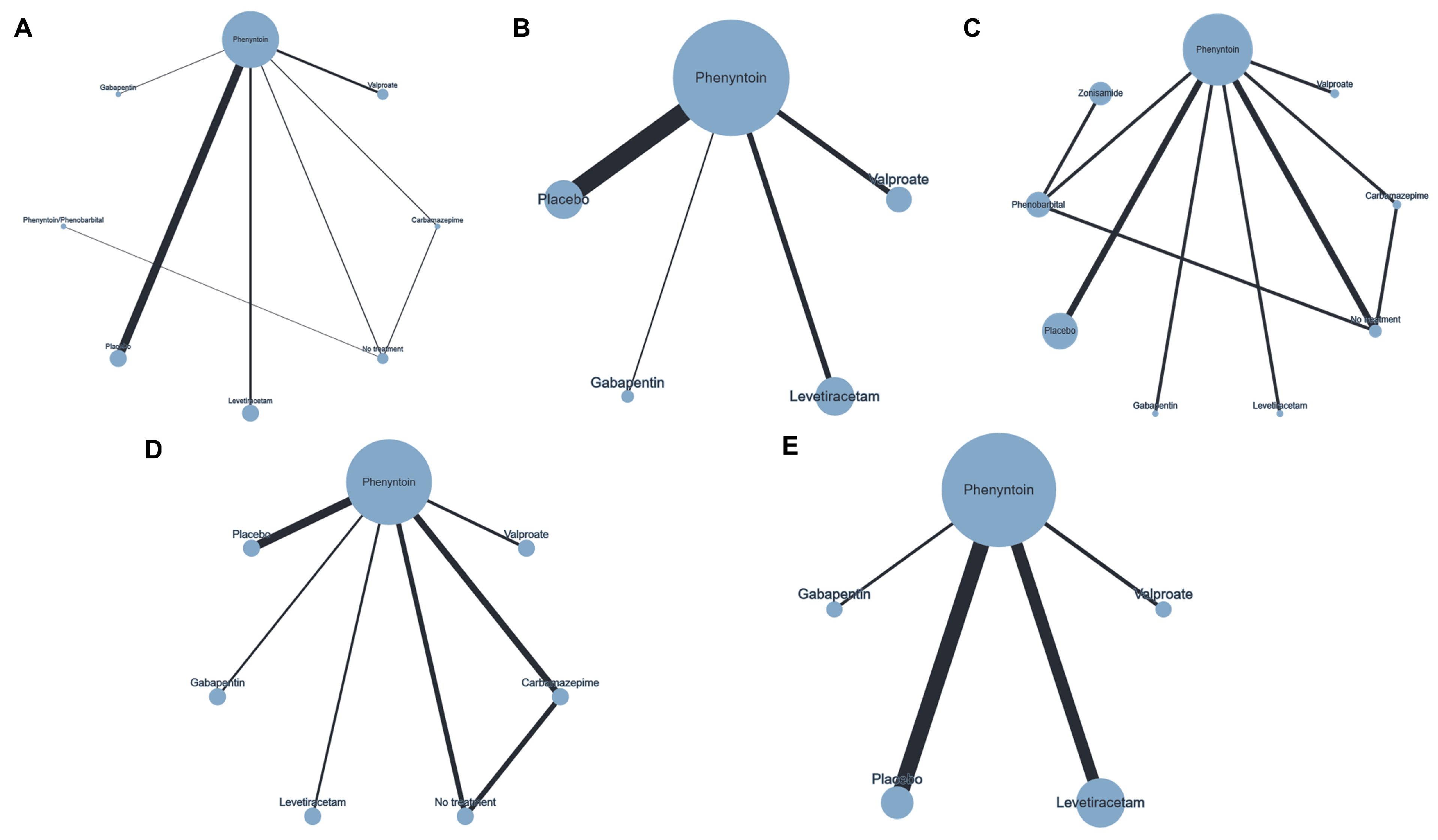
3.3. Network Plots
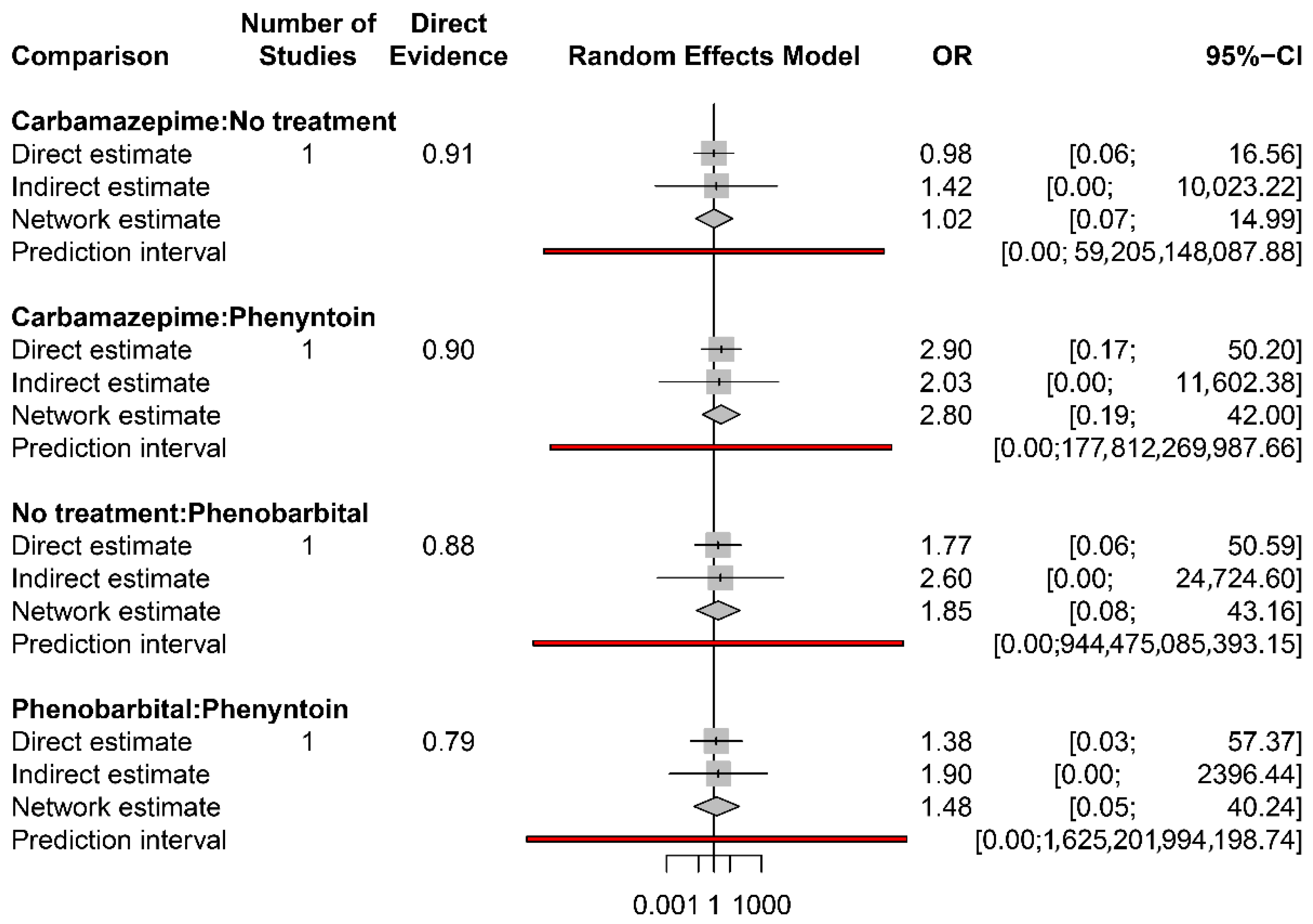
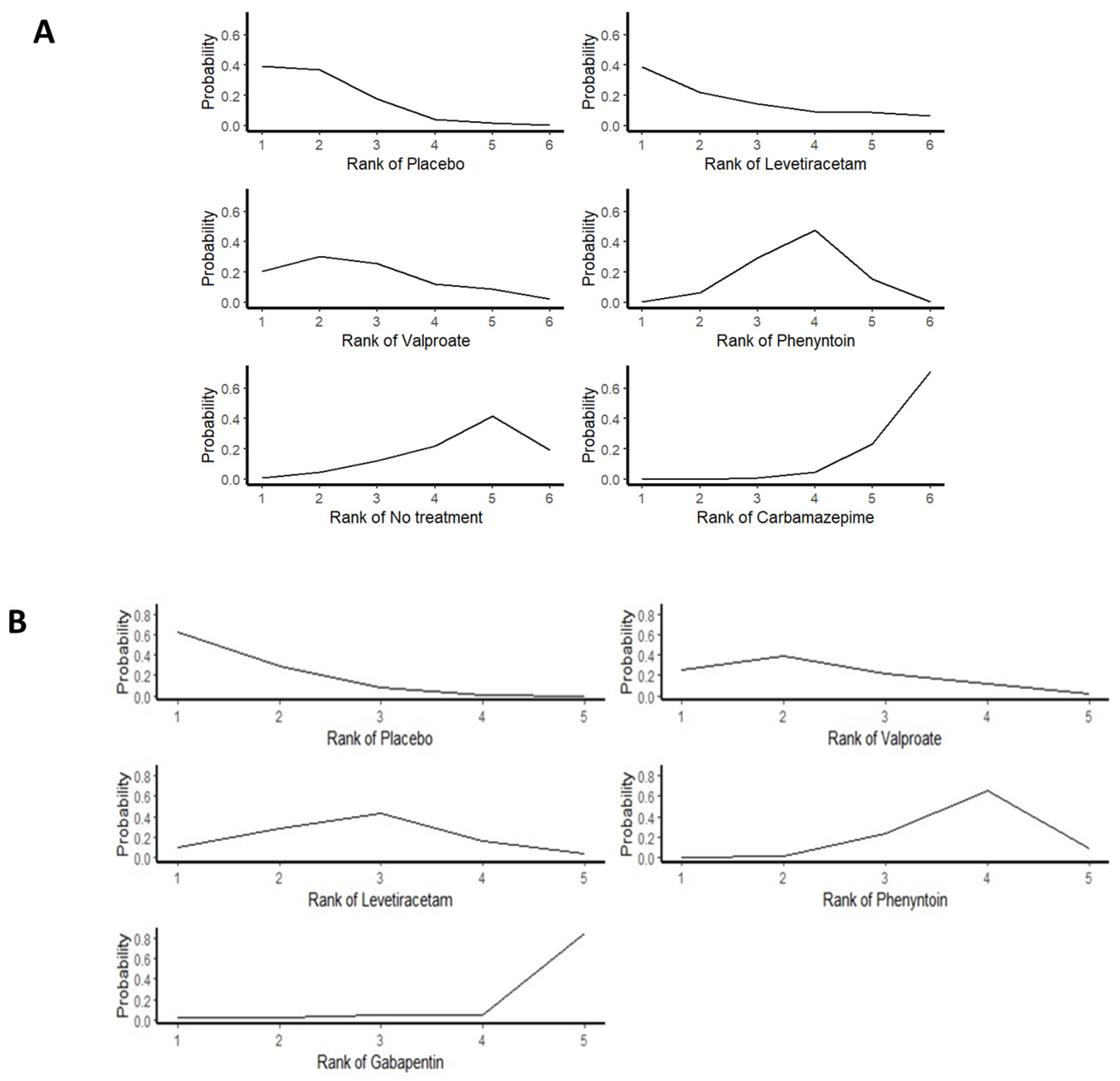
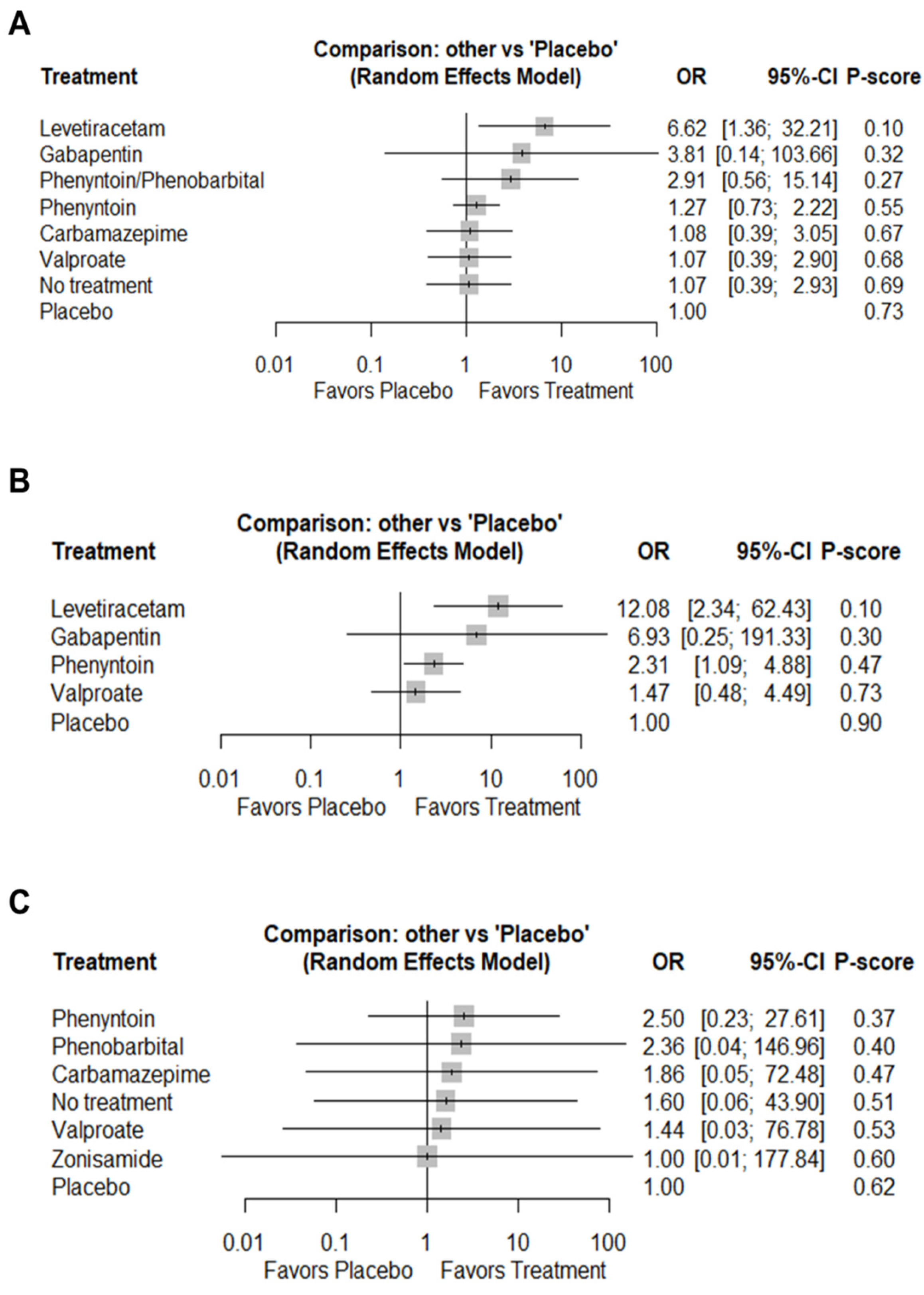
3.4. Primary Outcomes
3.4.1. Total Seizures
3.4.2. Early Seizures
3.4.3. Late Seizures
3.5. Secondary Outcomes
3.5.1. Mortality
3.5.2. Major Adverse Effects
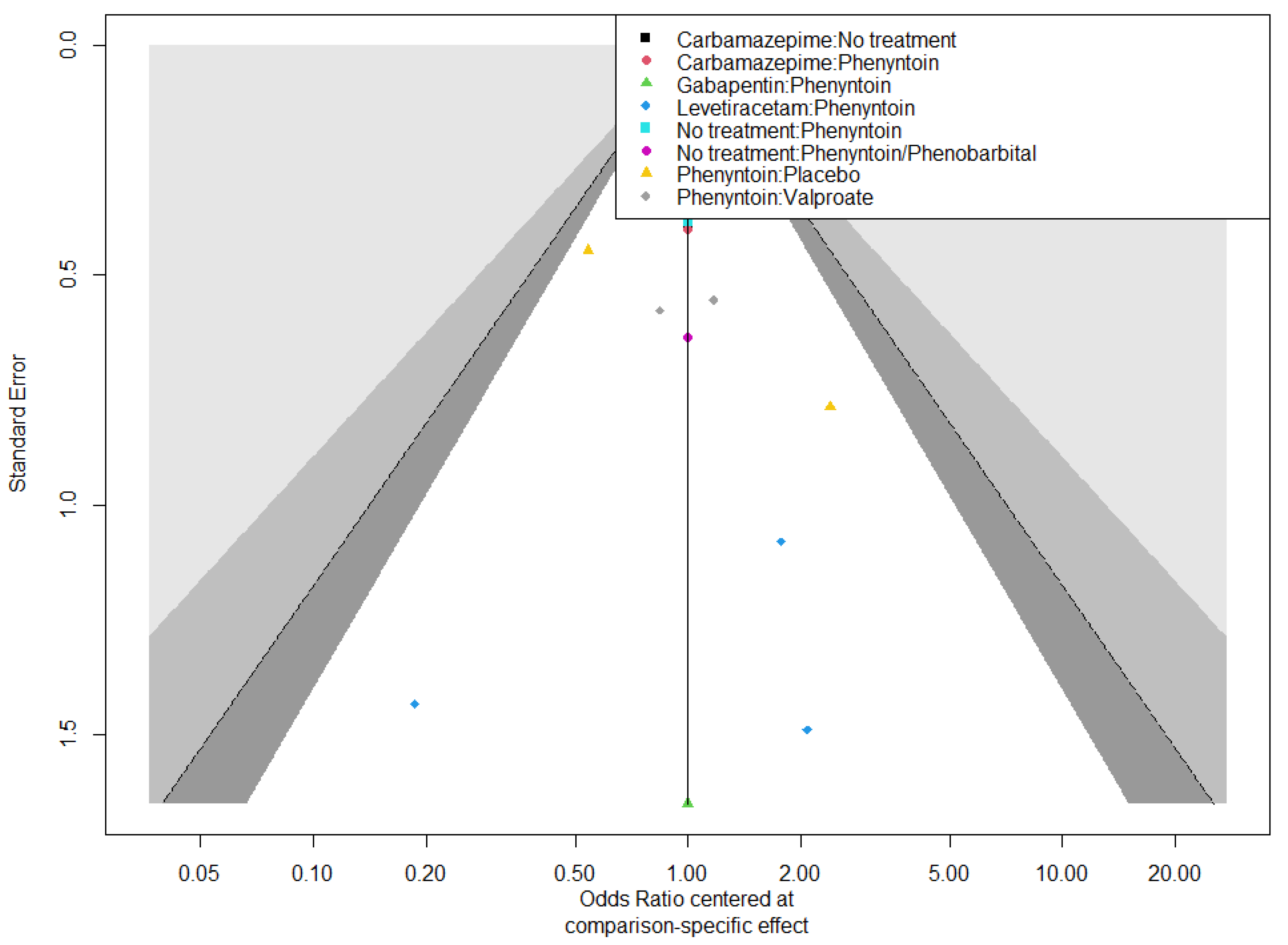
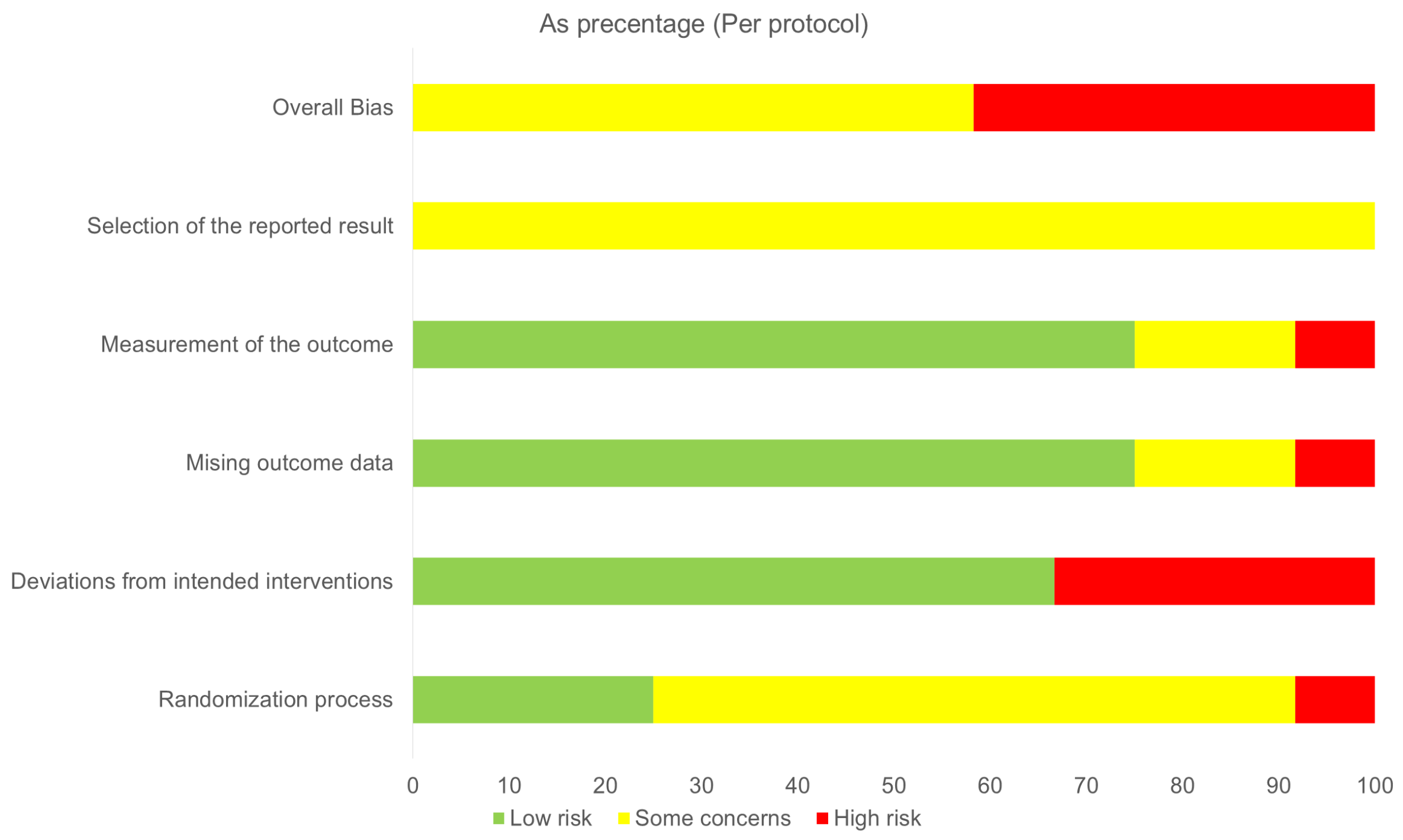
3.6. Risk of Bias Assessment and Confidence in Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCT | Randomized Controlled Trial |
| AED | Anticonvulsant Drug |
| NMA | Network Meta Analysis |
| CINeMA | Confidence in Network Meta-Analysis |
| CI | Confidence Interval |
| OR | Odds Ratio |
Appendix A
| Study ID | Study Design | No of pts | Study Arms | Brain Pathology | Treatment Duration | Follow-Up | |
|---|---|---|---|---|---|---|---|
| Early | Late | ||||||
| Beenen et al., 1999 [32] | Double-blind RCT | 100 | PHT 300 mg/d (iv) vs. VAL 1500 mg/d (iv) post-op | Vascular lesions, tumor, trauma | 12 mo | 1 wk | 2 wks–12 mo |
| Zhang et al., 2000 [33] | RCT | 152 | PHT 10 mg/kg × 3 daily (oral) for 7 d pre-op & 5 mg/kg × 3 daily (iv) for 2 d & 5 mg/kg × 3 daily (oral) for 1 mo post-op vs. VAL 30 mg/kg × 3 daily (oral) for 7 d pre-op & 20 mg/kg × 3 daily (iv) for 2 d & 20 mg/kg × 2 daily (oral) for 1 mo post-op | Vascular lesions, tumor, trauma | 1 mo | 1 wk | >3 mo |
| Foy et al., 1992 [34] | Open-label, controlled RCT | 276 | PHT (15 mg/kg for 24 h pre-op & 300 mg/d post-op) vs. CBZ (800 mg for 24 h pre-op & 600 mg/d post-op) vs. NT | Vascular lesions, abscess, tumor | 6 & 24 mo | NR | 4 yrs (median) |
| Fuller et al., 2013 [35] | Single-blind RCT | 81 | PHT 300 mg × 3/d to 1 gr/d (iv) preop & 300 mg (oral) postop vs. LEV 500 mg–1 g/d (iv or oral) Preop (3 d) & post-op (3 mo) | Vascular lesions tumor, hematoma, abscess | 3 mo | 3 d & hospital discharge | 3 mo |
| Iuchi et al., 2015 [36] | Open-cohort RCT | 146 | PHT 15–18 mg/kg & 5–7.5 mg/kg/d iv & 250 mg/d (oral) vs. LEV 500 mg iv & 1 g/d (sup or oral) intra-op & post-op | Tumors | 7 d | 1 wk | NA |
| Faghihjouibari et al., 2023 [37] | Double-blind, RCT | 80 | PHT 300 mg/d (iv or oral) vs. LEV 1 g/d (oral) preop (5 d) & post-op | Tumor | 3 mo | 1 wk | 1 mo & 3 mo |
| Türe et al., 2009 [38] | RCT | 80 | PHT 300 mg/d (oral) vs. GAB 1200 mg/d (oral) 7 d pre-op | Tumor | 6–12 mo | 1 wk | 1 mo |
| Lee et al., 1989 [39] | RCT | 374 | PHT (15 mg/kg end surgery & 5–6 mg/kg/d for 3 d post-op iv) vs. PBO | Vascular lesions tumor, hematoma, trauma | 3 d | 3 d | NA |
| North et al., 1983 [40] | Double-blind, RCT | 281 | PHT 500 mg/d (iv) & 300 mg/d (oral) post-op vs. PBO | Vascular lesions, tumor, trauma | 12 mo | 1 wk | 12 mo |
| Wu et al., 2013 [41] | RCT | 123 | PHT 15 mg/kg (iv) pre-op & 300 mg/d (iv or peros) post-op for 7 d vs. PBO | Tumor | 7 d | 1 wk | >30 d |
| Franceschetti et al., 1990 [42] | RCT | 63 | PHT 10 mg/kg (iv for 5 d) & then 5 mg/kg (oral) vs. PB 4 mg/kg (iv for 5 d) & then 2 mg/kg (oral) vs. NT | Tumor | Unclear | 1 wk | Unclear |
| Nakamura et al., 1999 [43] | Double-blind RCT | 255 | ZNS 200 mg/d (iv) vs. PB 80 mg/d (oral) for 1 mo pre-op & post-op | Vascular lesions, tumor, trauma | 12 mo | NA | 1–12 mo |
| Study ID | Primary Outcome | Secondary Outcomes | |||||
|---|---|---|---|---|---|---|---|
| All Seizures | Early Seizures | Late Seizures | Mortality | Discontinuation Due to AEs | Other Adverse Events | ||
| NT or PBO | Control AED | ||||||
| Beenen et al., 1999 [32] | - | PHT (7/50, 14%) vs. VAL (7/50, 14%) [NS] | PHT (4/50, 8%) vs. VAL (2/50, 4%) [NS] | PHT (3/50, 6%) vs. VAL (5/50, 10%) [NS] | PHT (13/50%, 26%) vs. VAL (10/50, 20%) | Skin rashes, PONV in PHT (5/50, 10%) vs. liver dysfunction, thrombopenia in VAL (2/50, 4%) | Anemia, PONV in PHT (4/50, 8%) vs. weight gain, PONV in VAL (2/50, 4%) [NS] |
| Zhang et al., 2000 [33] | - | PHT (6/72, 8%) vs. VAL (9/80, 11%) | PHT (6/72, 8%) vs. VAL (9/80, 11%) | NA | NA | NA | PHT (11/52, 15%) vs. VAL (2/80, 3%) [p < 0.05] |
| Foy et al., 1992 [34] | NT (25/59, 42%) 6-mo NT (20/59, 42%) 24 mo | PHT (21/55, 38%) vs. CBZ (21/50, 42%) 6-mo PHT (16/56, 29%) vs. CBZ (20/56, 36%) 24 mo | NA | PHT (21/55, 38%) vs. CBZ (21/50, 42%) vs. NT (25/59, 42%) 6-mo PHT (16/56, 29%) vs. CBZ (20/56, 36%) vs. NT (20/59, 42%) 24 mo | CBZ (19/106) vs. PHT (32/111) & vs. NT (13/59) [NS] | Skin rashes (28/217, 13%) Drug intoxication (6/217, 3%) | |
| Fuller et al., 2013 [35] | - | PHT (6/42, 14%) vs. LEV (0/30, 0%) up to 6 d [p = 0.01] | PHT (6/42, 14%) vs. LEV (0/30, 0%) up to 6 d [p = 0.01] | NA | PHT (5/42, 12%) vs. LEV (3/39, 8%) | Allergic reactions PHE iv (2/38, 5%) vs. skin rash LEV iv (1/36, 3%) Intoxication, rash, ataxia, nausea PHE oral (3/38, 8%) vs. delirium, pruritus, & headache LEV oral (3/36, 8%) [NS] | Thrombophlebitis PHT (3/38, 8%) Mood disturbance PHT (3/38, 8%) vs. LEV (7/36, 19%) PHT AEs (18/42, 43%) vs. LEV (22/39, 56%) |
| Iuchi et al., 2015 [36] | - | PHT (8/53, 14%) vs. LEV (1/53, 1.9%) [p = 0.034] | PHT (8/53, 14%) vs. LEV (1/53, 1.9%) [p = 0.034] | NA | NA | PHT 5/73, 6.8%) due to liver dysfunction, skin eruption & AF vs. LEV 0 [p = 0.058] | PHT (8/73, 11%) vs. LEV (3/73, 4%) [NS] |
| Faghihjouibari et al., 2023 [37] | - | PHT (1/40, 2.5%) vs. LEV (1/39, 2.6%) [NS] | PHE (1/40, 2.5%) 6 d vs. LEV (1/39, 2.6%) intra-op | None in either group | NA | None in either group | Skin rash PHT (3/40, 7.5%) vs. LEV 0% [NS] Thrombocytopenia PHT (1/40, 2.5%) vs. LEV 0% |
| Türe et al., 2009 [38] | - | PHT (1/38, 3%) vs. GAB 0% | PHT (1/38, 3%) vs. GAB 0% [NS] | PHE 0% vs. GAB 0% | PHT 0% vs. GAB 0% | Fatigue & dizziness GAB (2/37, 5%) vs. PHT (0/37, 0%) | Fatigue & dizziness GAB (10/37, 27%) vs. PHT 0% Ataxia, myalgia, PONV in GAB (12/37, 32%) vs. ataxia, PONV in PHT (18/38, 47%) |
| Lee et al., 1989 [39] | PHT (2/189, 1%) vs. PBO (9/285, 5%) | - | PHT (2/189, 1%) vs. PBO (9/285, 5%) | NA | NA | NA | NA |
| North et al., 1983 [40] | PHT (18/140, 13%) vs. PBO (26/141, 18%) | - | PHT (4/140, 3%) vs. PBO (14/141, 20%) [p < 0.05] | PHT (14/140, 10%) vs. PBO (12/141, 8.5%) | PHT (20/140, 14%) vs. PBO (12/140, 9%) [NS] | Rashes (8/140, 6%) Involuntary movements, hirsutism, headache, discomfort of the face (1 pt/effect) in PHT vs. Rash, dizziness & nausea (1 pt/effect) in PBO | |
| Wu et al., 2013 [41] | PHT (15/62, 24%) vs. PBO (11/61, 18%) | - | PHT (5/62, 8%) vs. PBO (5/61, 8%) [NS] | PHT (9/62, 14%) vs. PBO (6/61, 10%) [NS] | NA | Major AEs PHT (3/62, 5%) vs. PBO (0/61, 0%) | Minor AEs PHT (9/62, 15%) vs. PBO 0% [p < 0.01] |
| Franceschetti et al., 1990 [42] | PHT or PB (6/41, 15%) vs. NT (7/22, 32%) | Total PHT or PB (3/41, 7%) vs. NT (4/22, 18%) | PHT (1/10, 10%) vs. PB (2/15, 13%) vs. NT (3/14, 21%) [NS] | NA | NA | Neurological AEs PHT (3/16, 19%) vs. PB (1/25, 4%) within 7 d | |
| Nakamura et al., 1999 [43] | - | ZNS (13/129, 10%) vs. PB (11/126, 9%) | ZNS (6/129, 5%) vs. PB (3/126, 2%) | ZNS (7/129, 5%) vs. PB (8/126, 6%) | ZNS (8/112, 7%) vs. PB (13/107, 12%) | NA | ZNS (28/129, 22%) vs. PB (30/126, 24%) |


| CBZ | |||||
| 3.01 (0.59; 15.43) | LEV | . | |||
| 1.43 (0.68; 3.00) | 0.48 (0.09; 2.60) | No treatment | |||
| 1.85 (0.97; 3.53) | 0.62 (0.14; 2.77) | 1.29 (0.59; 2.85) | PHT | ||
| 3.30 (1.22; 8.91) | 1.10 (0.20; 5.90) | 2.30 (0.77; 6.88) | 1.78 (0.83; 3.79) | PBO | |
| 2.61 (0.84; 8.13) | 0.87 (0.15; 5.10) | 1.82 (0.53; 6.20) | 1.41 (0.55; 3.59) | 0.79 (0.24; 2.64) | VAL |
| GAB | ||||
| 10.08 (0.36; 279.10) | LEV | |||
| 5.42 (0.25; 116.90) | 0.54 (0.15; 1.90) | PHT | ||
| 25.36 (0.94; 681.34) | 2.52 (0.45; 14.20) | 4.68 (1.43; 15.27) | PBO | |
| 14.46 (0.43; 481.28) | 1.43 (0.17; 11.83) | 2.67 (0.49; 14.45) | 0.57 (0.07; 4.49) | VAL |
| Comparison | No | Within-Study Bias | Reporting Bias | Indirectness | Imprecision | Heterogeneity | Incoherence | Confidence Rating |
|---|---|---|---|---|---|---|---|---|
| Carbamazepime: No treatment | 1 | Major concerns | Some concerns | Some concerns | Some concerns | Some concerns | Major concerns | Moderate |
| Carbamazepime: Phenyntoin | 1 | Major concerns | Some concerns | Some concerns | Some concerns | Some concerns | Major concerns | Moderate |
| Gabapentin: Phenyntoin | 1 | Some concerns | Some concerns | No concerns | Major concerns | No concerns | Major concerns | Low |
| Levetiracetam: Phenyntoin | 3 | Some concerns | Some concerns | Some concerns | No concerns | Major concerns | Major concerns | Low |
| No treatment: Phenyntoin | 1 | Major concerns | Some concerns | Some concerns | Some concerns | Some concerns | Major concerns | Low |
| No treatment: Phenyntoin/Phenobarbital | 1 | Major concerns | Some concerns | Major concerns | Major concerns | Some concerns | Major concerns | Very low |
| Phenyntoin: Placebo | 3 | Some concerns | Some concerns | No concerns | Some concerns | Some concerns | Major concerns | Moderate |
| Phenyntoin: Valproate | 2 | Some concerns | Some concerns | Some concerns | Major concerns | Some concerns | Major concerns | Moderate |
| Carbamazepime: Gabapentin | 0 | Major concerns | Some concerns | Some concerns | Major concerns | Major concerns | Major concerns | Very low |
| Carbamazepime: Levetiracetam | 0 | Major concerns | Some concerns | Some concerns | Major concerns | Major concerns | Major concerns | Very low |
| Carbamazepime: Phenyntoin/Phenobarbital | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Low |
| Carbamazepime: Placebo | 0 | Major concerns | Some concerns | Some concerns | Major concerns | Some concerns | Major concerns | Very low |
| Carbamazepime: Valproate | 0 | Major concerns | Some concerns | Some concerns | Major concerns | Some concerns | Major concerns | Very low |
| Gabapentin: Levetiracetam | 0 | Some concerns | Some concerns | No concerns | Major concerns | No concerns | Major concerns | Moderate |
| Gabapentin: No treatment | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Low |
| Gabapentin: Phenyntoin/Phenobarbital | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Low |
| Gabapentin: Placebo | 0 | Some concerns | Some concerns | No concerns | Major concerns | No concerns | Major concerns | Low |
| Gabapentin: Valporate | 0 | Some concerns | Some concerns | No concerns | Major concerns | No concerns | Major concerns | Very low |
| Levetiracetam: No treatment | 0 | Major concerns | Some concerns | Some concerns | No concerns | Major concerns | Major concerns | Very low |
| Levetiracetam: Phenyntoin/Phenobarbital | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Low |
| Levetiracetam: Placebo | 0 | Major concerns | Some concerns | No concerns | No concerns | No concerns | Major concerns | Moderate |
| Levetiracetam: Valproate | 0 | Some concerns | Some concerns | Some concerns | No concerns | No concerns | Major concerns | Low |
| No treatment: Placebo | 0 | Major concerns | Some concerns | Some concerns | Major concerns | Major concerns | Major concerns | Very low |
| No treatment: Valproate | 0 | Major concerns | Some concerns | Some concerns | Major concerns | Major concerns | Major concerns | Very low |
| Phenyntoin: : Phenyntoin/Phenobarbital | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Low |
| Phenyntoin/Phenobarbital: Placebo | 0 | Major concerns | Some concerns | Some concerns | Some concerns | No concerns | Major concerns | Moderate |
| Phenyntoin/Phenobarbital: Valproate | 0 | Major concerns | Some concerns | Some concerns | Some concerns | No concerns | Major concerns | Moderate |
| Placebo: Valproate | 0 | Some concerns | Some concerns | No concerns | Some concerns | No concerns | Major concerns | Moderate |
| Comparison | No | Within-Study bias | Reporting Bias | Indirectness | Imprecision | Heterogeneity | Incoherence | Confidence Rating |
|---|---|---|---|---|---|---|---|---|
| GAB:PHT | 1 | Some concerns | Some concerns | No concerns | Major concerns | No concerns | Major concerns | Low |
| LEV:PHT | 3 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| NT:PHE | 1 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| NT:PHT | 1 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| PBO:PHT | 2 | Some concerns | Some concerns | No concerns | No concerns | Major concerns | Major concerns | Very low |
| PHE:PHT | 1 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| PHT:VAL | 2 | Some concerns | Some concerns | No concerns | Major concerns | No concerns | Major concerns | Low |
| GAB:LEV | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| GAB:NT | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| GAB:PBO | 0 | Some concerns | Some concerns | No concerns | Major concerns | No concerns | Major concerns | Very low |
| GAB:PHE | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| GAB:VAL | 0 | Some concerns | Some concerns | No concerns | Major concerns | No concerns | Major concerns | Low |
| LEV:NT | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| LEV:PBO | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| LEV:PHE | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| LEV:VAL | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| NT:PBO | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| NT:VAL | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| PBO:PHE | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
| PBO:VAL | 0 | Some concerns | Some concerns | No concerns | Major concerns | No concerns | Major concerns | Low |
| PHE:VAL | 0 | Major concerns | Some concerns | Some concerns | Major concerns | No concerns | Major concerns | Very low |
References
- Slegers, R.J.; Blumcke, I. Low-grade developmental and epilepsy associated brain tumors: A critical update 2020. Acta Neuropathol. Commun. 2020, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.S.; Grant, R.; Gilbert, M.R.; Lee, J.W.; Norden, A.D. Epilepsy in glioma patients: Mechanisms, management, and impact of anticonvulsant therapy. Neuro Oncol. 2016, 18, 779–789. [Google Scholar] [CrossRef]
- Goldstein, E.D.; Feyissa, A.M. Brain tumor related-epilepsy. Neurol. Neurochir. Pol. 2018, 52, 436–447. [Google Scholar] [CrossRef]
- Aronica, E.; Ciusani, E.; Coppola, A.; Costa, C.; Russo, E.; Salmaggi, A.; Perversi, F.; Maschio, M. Epilepsy and brain tumors: Two sides of the same coin. J. Neurol. Sci. 2023, 446, 120584. [Google Scholar] [CrossRef]
- Peart, R.; Melnick, K.; Cibula, J.; Walbert, T.; Gerstner, E.R.; Rahman, M.; Peters, K.B.; Mrugala, M.; Ghiaseddin, A. Clinical management of seizures in patients with meningiomas: Efficacy of surgical resection for seizure control and patient-tailored postoperative anti-epileptic drug management. Neuro-Oncol. Adv. 2023, 5 (Suppl. S1), i58–i66. [Google Scholar] [CrossRef]
- Xue, H.; Sveinsson, O.; Tomson, T.; Mathiesen, T. Intracranial meningiomas and seizures: A review of the literature. Acta Neurochir. 2018, 157, 1541–1548. [Google Scholar] [CrossRef]
- Turnbull, D.; Singatullina, N.; Reilly, C. A Systematic appraisal of neurosurgical seizure prophylaxis: Guidance for critical care management. J. Neurosurg. Anesthesiol. 2016, 28, 233–249. [Google Scholar] [CrossRef]
- Youngerman, B.E.; Joiner, E.F.; Wang, X.; Yang, J.; Welch, M.R.; McKhann, G.M., 2nd; Wright, J.D.; Hershman, D.L.; Neugut, A.I.; Bruce, J.N. Patterns of seizure prophylaxis after oncologic neurosurgery. J. Neuro-Oncol. 2020, 146, 171–180. [Google Scholar] [CrossRef]
- Van Breemen, M.S.; Wilms, E.B.; Vecht, C.J. Seizure control in brain tumors. Handb. Clin. Neurol. 2012, 104, 381–389. [Google Scholar] [CrossRef]
- Islim, A.I.; Ali, A.; Bagchi, A.; Ahmad, M.U.; Mills, S.J.; Chavredakis, E.; Brodbelt, A.R.; Jenkinson, M.D. Postoperative seizures in meningioma patients: Improving patient selection for antiepileptic drug therapy. J. Neuro-Oncol. 2018, 140, 123–134. [Google Scholar] [CrossRef]
- Greenhalgh, J.; Weston, J.; Dundar, Y.; Nevitt, S.J.; Marson, A.G. Antiepileptic drugs as prophylaxis for postcraniotomy seizures. Cochrane Database Syst. Rev. 2020, 4, CD007286. [Google Scholar] [CrossRef] [PubMed]
- Joiner, E.F.; Youngerman, B.E.; Hudson, T.S.; Yang, J.; Welch, M.R.; McKhann, G.M.; Neugut, A.I.; Bruce, J.N. Effectiveness of perioperative antiepileptic drug prophylaxis for early and late seizures following oncologic neurosurgery: A meta-analysis. J. Neurosurg. 2018, 130, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Mirian, C.; Møller Pedersen, M.; Sabers, A.; Mathiesen, T. Antiepileptic drugs as prophylaxis for de novo brain tumour-related epilepsy after craniotomy: A systematic review and meta-analysis of harm and benefits. J. Neurol. Neurosurg. Psychiatry 2019, 90, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Islim, A.I.; McKeever, S.; Kusu-Orkar, T.E.; Jenkinson, M.D. The role of prophylactic antiepileptic drugs for seizure prophylaxis in meningioma surgery: A systematic review. J. Clin. Neurosci. 2017, 43, 47–53. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, X.; Hu, S.; Xing, A.; Wang, Z.; Song, Y.; Chen, J.; Tian, S.; Mao, Y.; Chi, X. Efficacy of perioperative anticonvulsant prophylaxis in seizure-naïve glioma patients: A meta-analysis. Clin. Neurol. Neurosurg. 2019, 186, 105529. [Google Scholar] [CrossRef]
- Jackson, C.; Choi, J.; Khalafallah, A.M.; Price, C.; Bettegowda, C.; Lim, M.; Gallia, G.; Weingart, J.; Brem, H.; Mukherjee, D. A systematic review and meta-analysis of supratotal versus gross total resection for glioblastoma. J. Neuro-Oncol. 2020, 148, 419–431. [Google Scholar] [CrossRef]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef]
- Pallud, J.; Huberfeld, G. Time to dispense with antiepileptic drug prophylaxis in brain tumor surgery? Neurochirurgie 2022, 68, 148–149. [Google Scholar] [CrossRef]
- Chen, C.C.; Rennert, R.C.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the role of prophylactic anticonvulsants in the treatment of adults with metastatic brain tumors. Neurosurgery 2019, 84, E195–E197. [Google Scholar] [CrossRef]
- Kim, S.K.; Moon, J.; Cho, J.M.; Kim, K.H.; Kim, S.H.; Kim, Y.I.; Kim, Y.Z.; Kim, H.S.; Dho, Y.S.; Park, J.S.; et al. A National Consensus Survey for current practice in brain tumor management I: Antiepileptic drug and steroid usage. Brain Tumor Res. Treat. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Marson, A.; Burnside, G.; Appleton, R.; Smith, D.; Leach, J.P.; Sills, G.; Tudur-Smith, C.; Plumpton, C.; Hughes, D.A.; Williamson, P.; et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: An open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet 2021, 397, 1375–1386. [Google Scholar] [CrossRef]
- Cardona, A.F.; Rojas, L.; Wills, B.; Bernal, L.; Ruiz-Patiño, A.; Arrieta, O.; Hakim, E.J.; Hakim, F.; Mejía, J.A.; Useche, N.; et al. Efficacy and safety of Levetiracetam vs. other antiepileptic drugs in Hispanic patients with glioblastoma. J. Neuro-Oncol. 2018, 136, 363–371. [Google Scholar] [CrossRef]
- Chandra, V.; Rock, A.K.; Opalak, C.; Stary, J.M.; Sima, A.P.; Carr, M.; Vega, R.A.; Broaddus, W.C. A systematic review of perioperative seizure prophylaxis during brain tumor resection: The case for a multicenter randomized clinical trial. Neurosurg. Focus. 2017, 43, E18. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane. 2019. Available online: www.training.cochrane.org/handbook (accessed on 3 June 2020).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2020, 18, e1003583. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.; Higgins, J.P.T.; Papakonstantinou, T.; Chaimani, A.; Del Giovane, C.; Egger, M.; Salanti, G. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020, 17, e1003082. [Google Scholar] [CrossRef]
- Ryan, R. Cochrane Consumers and Communication Review Group. Heterogeneity and Subgroup Analyses in Cochrane Consumers and Communication Group Reviews: Planning the Analysis at Protocol Stage. December 2016. Available online: http://cccrg.cochrane.org (accessed on 1 June 2021).
- Rücker, G. Network meta-analysis, electrical networks and graph theory. Res. Synth. Methods 2012, 3, 312–324. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef]
- Beenen, L.F.M.; Lindeboom, J.; Trenité, D.G.A.K.-N.; Heimans, J.J.; Snoek, F.J.; Touw, D.J.; Ader, H.J.; Alphen, H.A.M.v. Comparative double blind clinical trial of phenytoin and sodium valproate as anticonvulsant prophylaxis after craniotomy: Efficacy, tolerability, and cognitive effects. J. Neurol. Neurosurg. Psychiatry 1999, 67, 474–480. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.F.; Du, G.H.; Gao, L.; Xu, B.; Xu, J.; Gu, Y.X. Phenytoin or sodium valproate for prophylaxis of postoperative epilepsy: A randomised comparison. Chin. J. Nerv. Ment. Dis. 2000, 26, 231–233. [Google Scholar]
- Foy, P.M.; Chadwick, D.W.; Rajgopalan, N.; Johnson, A.L.; Shaw, M.D. Do prophylactic anticonvulsant drugs alter the pattern of seizures after craniotomy? J. Neurol. Neurosurg. Psychiatry 1992, 55, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.L.; Wang, Y.Y.; Cook, M.J.; Murphy, M.A.; D’Souza, W.J. Tolerability, safety, and side effects of levetiracetam versus phenytoin in intravenous and total prophylactic regimen among craniotomy patients: A prospective randomized study. Epilepsia 2013, 54, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, T.; Kuwabara, K.; Matsumoto, M.; Kawasaki, K.; Hasegawa, Y.; Sakaida, T. Levetiracetam versus phenytoin for seizure prophylaxis during and early after craniotomy for brain tumours: A phase II prospective, randomised study. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1158–1162. [Google Scholar] [CrossRef]
- Faghihjouibari, M.; Khadivi, M.; Rouhani, R.; Toroudi, H.P.; Nazari, M.; Sadeghian, M.; Abolfazli, M. Comparison of efficacy and safety of levetiracetam versus phenytoin for post-craniotomy seizure prophylaxis. Med. J. Islam. Repub. Iran 2023, 37, 49–53. [Google Scholar] [CrossRef]
- Türe, H.; Sayin, M.; Karlikaya, G.; Bingol, C.A.; Aykac, B.; Türe, U. The analgesic effect of gabapentin as a prophylactic anticonvulsant drug on postcraniotomy pain: A prospective randomized study. Anesth. Analg. 2009, 109, 1625–1631. [Google Scholar] [CrossRef]
- Lee, S.-T.; Lui, T.-N.; Chang, C.-N.; Cheng, W.-C.; Wang, D.-J.; Heimburger, R.F.; Lin, C.-G. Prophylactic anticonvulsants for prevention of immediate and early postcraniotomy seizures. Surg. Neurol. 1989, 31, 361–364. [Google Scholar] [CrossRef]
- North, J.B.; Penhall, R.K.; Hanieh, A.; Frewin, D.B.; Taylor, W.B. Phenytoin and postoperative epilepsy. A double-blind study. J. Neurosurg. 1983, 58, 672–677. [Google Scholar] [CrossRef]
- Wu, A.S.; Trinh, V.T.; Suki, D.; Graham, S.; Forman, A.; Weinberg, J.S.; McCutcheon, I.E.; Prabhu, S.S.; Heimberger, A.B.; Sawaya, R.; et al. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumors. J. Neurosurg. 2013, 118, 873–883. [Google Scholar] [CrossRef]
- Franceschetti, S.; Binelli, S.; Casazza, M.; Lodrini, S.; Panzica, F.; Pluchino, F.; Solero, C.L.; Avanzini, G. Influence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumours. Acta Neurochir. 1990, 103, 47–51. [Google Scholar] [CrossRef]
- Nakamura, N.; Ishijima, B.; Mayanagi, Y.; Manaka, S. A randomized controlled trial of zonisamide in postoperative epilepsy: A report of the Cooperative Group Study. Jpn. J. Neurosurg. 1999, 8, 647–656. [Google Scholar] [CrossRef]
- Li, L.; Fang, S.; Li, G.; Zhang, K.; Huang, R.; Wang, Y.; Zhang, C.; Li, Y.; Zhang, W.; Zhang, Z.; et al. Glioma-related epilepsy in patients with diffuse high-grade glioma after the 2016 WHO update: Seizure characteristics, risk factors, and clinical outcomes. J. Neurosurg. 2021, 136, 67–75. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, P.B.; Dirven, L.; Bent, M.J.v.D.; Preusser, M.; Taphoorn, M.J.B.; Rudá, R.; Koekkoek, J.A.F. Prescription preferences of antiepileptic drugs in brain tumor patients: An international survey among EANO members. Neuro-Oncol. Pract. 2021, 9, 105–113. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, M.E.; van der Meer, P.B.; Dirven, L.; Taphoorn, M.J.B.; Koekkoek, J.A.F. Efficacy of antiepileptic drugs in glioma patients with epilepsy: A systematic review. Neuro-Oncol. Pract. 2021, 8, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Koo, H.W.; Han, S.R.; Choi, C.Y.; Sohn, M.J.; Lee, C.H. Phenytoin versus levetiracetam as prophylaxis for postcraniotomy seizure in patients with no history of seizures: Systematic review and meta-analysis. J. Neurosurg. 2019, 130, 2063–2070. [Google Scholar] [CrossRef]
- Walbert, T.; A Harrison, R.; Schiff, D.; Avila, E.K.; Chen, M.; Kandula, P.; Lee, J.W.; Le Rhun, E.; Stevens, G.H.J.; A Vogelbaum, M.; et al. SNO and EANO practice guideline update: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro-Oncology 2021, 23, 1835–1844. [Google Scholar] [CrossRef]
- Dewan, M.C.; Thompson, R.C.; Kalkanis, S.N.; Barker, F.G., 2nd; Hadjipanayis, C.G. Prophylactic antiepileptic drug administration following brain tumor resection: Results of a recent AANS/CNS Section on Tumors survey. J. Neurosurg. 2017, 126, 1772–1778. [Google Scholar] [CrossRef]
- Siomin, V.; Angelov, L.; Li, L.; Vogelbaum, M.A. Results of a survey of neurosurgical practice patterns regarding the prophylactic use of anti-epilepsy drugs in patients with brain tumors. J. Neuro-Oncol. 2005, 74, 211–215. [Google Scholar] [CrossRef]
- Wu, A.; Weingart, J.D.; Gallia, G.L.; Lim, M.; Brem, H.; Bettegowda, C.; Chaichana, K.L. Risk factors for preoperative seizures and loss of seizure control in patients undergoing surgery for metastatic brain tumors. World Neurosurg. 2017, 104, 120–128. [Google Scholar] [CrossRef]
- Sayegh, E.T.; Fakurnejad, S.; Oh, T.; Bloch, O.; Parsa, A.T. Anticonvulsant prophylaxis for brain tumor surgery: Determining the current best available evidence. J. Neurosurg. 2014, 121, 1139–1147. [Google Scholar] [CrossRef]
- Afshari, F.T.; Michael, S.; Ughratdar, I.; Samarasekera, S. A practical guide to the use of anti-epileptic drugs by neurosurgeons. Br. J. Neurosurg. 2017, 31, 551–556. [Google Scholar] [CrossRef]
| CBZ | |||||||
| 0.28 (0.01; 8.27) | GAB | ||||||
| 0.16 (0.03; 0.91) | 0.58 (0.02; 20.58) | LEV | |||||
| 1.02 (0.43; 2.39) | 3.57 (0.12; 103.11) | 6.20 (1.13; 34.09) | No treatment | ||||
| 0.85 (0.36; 2.04) | 3.00 (0.12; 77.79) | 5.21 (1.19; 22.91) | 0.84 (0.36; 1.95) | PHT | |||
| 0.37 (0.08; 1.78) | 1.31 (0.04; 48.36) | 2.28 (0.27; 19.49) | 0.37 (0.10; 1.35) | 0.44 (0.09; 2.07) | PHT/PB | ||
| 1.08 (0.39; 3.05) | 3.81 (0.14; 103.66) | 6.62 (1.36; 32.21) | 1.07 (0.39; 2.93) | 1.27 (0.73; 2.22) | 2.91 (0.56; 15.14) | PBO | |
| 1.01 (0.30; 3.37) | 3.56 (0.12; 102.51) | 6.19 (1.13; 33.77) | 1.00 (0.31; 3.26) | 1.19 (0.52; 2.72) | 2.72 (0.47; 15.81) | 0.93 (0.34; 2.54) | VAL |
| GAB | ||||
| 0.57 (0.02; 19.93) | LEV | |||
| 3.00 (0.12; 76.03) | 5.23 (1.21; 22.57) | PHT | ||
| 6.93 (0.25; 191.33) | 12.08 (2.34; 62.43) | 2.31 (1.09; 4.88) | PBO | |
| 4.72 (0.17; 133.01) | 8.23 (1.53; 44.29) | 1.57 (0.68; 3.62) | 0.68 (0.22; 2.09) | VAL |
| CBZ | ||||||
| 1.02 (0.07; 14.99) | No treatment | |||||
| 1.89 (0.04; 92.78) | 1.85 (0.08; 43.16) | PB | ||||
| 2.80 (0.19; 42.00) | 2.75 (0.29; 25.85) | 1.48 (0.05; 40.24) | PHT | |||
| 1.14 (0.03; 41.19) | 1.12 (0.04; 28.84) | 0.60 (0.01; 34.78) | 0.41 (0.04; 4.28) | PBO | ||
| 1.61 (0.03; 98.49) | 1.58 (0.03; 72.28) | 0.85 (0.01; 78.78) | 0.57 (0.03; 12.73) | 1.41 (0.03; 68.75) | VAL | |
| 0.80 (0.01; 112.65) | 0.79 (0.01; 62.92) | 0.43 (0.02; 8.94) | 0.29 (0.00; 25.64) | 0.70 (0.00; 111.9) | 0.50 (0.00; 117.12) | ZNS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsaousi, G.; Nikolakopoulou, A.; Tsitsopoulos, P.P.; Pourzitaki, C.; Mavridis, D.; Haidich, A.B. Antiepileptic Drugs for De Novo Seizure Prevention After Craniotomy: A Systematic Review and Network Meta-Analysis of Current Evidence. J. Clin. Med. 2025, 14, 7854. https://doi.org/10.3390/jcm14217854
Tsaousi G, Nikolakopoulou A, Tsitsopoulos PP, Pourzitaki C, Mavridis D, Haidich AB. Antiepileptic Drugs for De Novo Seizure Prevention After Craniotomy: A Systematic Review and Network Meta-Analysis of Current Evidence. Journal of Clinical Medicine. 2025; 14(21):7854. https://doi.org/10.3390/jcm14217854
Chicago/Turabian StyleTsaousi, Georgia, Adriani Nikolakopoulou, Parmenion P. Tsitsopoulos, Chryssa Pourzitaki, Dimitrios Mavridis, and Anna Bettina Haidich. 2025. "Antiepileptic Drugs for De Novo Seizure Prevention After Craniotomy: A Systematic Review and Network Meta-Analysis of Current Evidence" Journal of Clinical Medicine 14, no. 21: 7854. https://doi.org/10.3390/jcm14217854
APA StyleTsaousi, G., Nikolakopoulou, A., Tsitsopoulos, P. P., Pourzitaki, C., Mavridis, D., & Haidich, A. B. (2025). Antiepileptic Drugs for De Novo Seizure Prevention After Craniotomy: A Systematic Review and Network Meta-Analysis of Current Evidence. Journal of Clinical Medicine, 14(21), 7854. https://doi.org/10.3390/jcm14217854






