Abstract
Background/Objectives: Pacemaker-induced cardiomyopathy (PICM) develops in up to 30% of patients with chronic right ventricular pacing. While biventricular (BIV) upgrade is the conventional strategy, conduction system pacing (CSP) offers a physiologic alternative recently endorsed by the 2025 ESC/EHRA Consensus Statement. However, comparative evidence in PICM is limited. Therefore, we aimed to compare outcomes of PICM patients undergoing CSP versus BIV upgrade. Methods: This retrospective analysis included consecutive PICM patients who were upgraded to CSP or BIV between 2022 and 2024 at a single, experienced center. Follow-up averaged >19 months. Clinical outcomes, lead performance, echocardiographic parameters, complications, and quality of life (QoL) were evaluated. Results: Sixty-three patients were included (CSP: 26; BIV: 37). Mean age and sex distribution were similar; both groups had wide paced QRS complexes and a high ventricular pacing burden. Baseline left ventricular ejection fraction (LVEF) was lower in BIV patients (29 ± 7% vs. 35 ± 6%, p = 0.01). Procedure duration was comparable, but fluoroscopy was shorter with CSP. QRS duration narrowed significantly in both groups (CSP: 163 ± 28→132 ± 12 ms; BIV: 171 ± 23→140 ± 18 ms; both p < 0.05). During follow-up, LVEF improved (CSP: 41 ± 8%; p = 0.008; BIV: 39 ± 8%, p = 0.0001), as did NYHA class, with no significant intergroup differences. The rates of heart failure hospitalization, all-cause mortality, and QoL were similar. Notably, 34.6% of CSP patients retained their existing generator, suggesting procedural and economic benefits. Conclusions: CSP is a feasible and potentially cost-efficient alternative to BIV upgrade in PICM, with comparable improvements in ventricular function, symptoms, and clinical outcomes. Larger prospective trials are warranted.
1. Introduction
Nearly one million people worldwide develop cardiac conduction system disease each year and require permanent pacing to prevent life-threatening or symptomatic bradycardia [1]. Although right ventricular (RV) pacing is an effective therapy for bradycardia, up to 30% of patients may subsequently develop pacemaker-induced cardiomyopathy (PICM)—a condition driven by electrical dyssynchrony and progressive adverse ventricular remodeling, leading to left ventricular (LV) dysfunction, increased heart failure hospitalizations, and reduced survival [2].
Cardiac resynchronization therapy or biventricular (BIV) pacing was validated as an upgrade strategy for patients with RV pacemakers and LV dysfunction, with multicenter studies demonstrating echocardiographic and clinical improvement [3,4,5,6,7]. However, BIV pacing requires placement of a coronary sinus lead, which can be technically challenging, prolong fluoroscopy exposure, and is not always feasible due to unfavorable venous anatomy or lead instability [8,9].
Conduction system pacing (CSP), including His-bundle pacing (HBP) and left bundle branch area pacing (LBBAP), has emerged as a physiological alternative, producing a ventricular activation pattern similar to normal conduction [10]. CSP has been endorsed by the 2025 ESC/EHRA consensus statement as an upgrade option in selected patients, based primarily on evidence from single-arm observational studies showing favorable electrical, structural, and clinical outcomes [11].
Despite growing adoption, direct head-to-head comparisons of CSP and BIV in the PICM upgrade setting are scarce. To date, only two single-center studies have directly compared the two strategies, reporting greater improvements in LV function in CSP patients and similar symptomatic responses in both groups [12,13].
The present study contributes to the limited comparative evidence on CSP and BIV upgrades in PICM, representing the third cohort to date that addresses this question. Beyond procedural and echocardiographic outcomes, it uniquely integrates functional, clinical, and quality-of-life assessments, as well as cost analysis between the two strategies.
2. Materials and Methods
2.1. Study Population
This study retrospectively analyzed consecutive patients diagnosed with pacemaker-induced cardiomyopathy (PICM) from October 2022 to October 2024 who underwent cardiac implantable electronic device (CIED) upgrade to either biventricular (BIV) or conduction system pacing (CSP). PICM was defined as a decline in left ventricular ejection fraction (LVEF) of ≥10 percentage points from a baseline of ≥50%, resulting in a follow-up LVEF of <50% in patients with high right ventricular pacing (VP) burden ≥ 40% in the absence of other identifiable causes of cardiomyopathy [2].
2.2. Procedure Description
The choice between conduction system pacing and biventricular upgrade was made by the implanting physicians in accordance with the EHRA Consensus Statement [14], taking into account factors such as native QRS duration, existing lead configuration, remaining generator longevity, the need for defibrillator therapy, and patient preferences through a shared decision-making process.
Conduction system pacing upgrade procedures were performed using either Medtronic Select Secure 3830 lumenless (Medtronic plc, Minneapolis, MN, USA), active-fixation helix leads or Biotronik Solia S60 stylet-driven leads (SDL, Biotronik SE & Co. KG, Berlin, Germany). The LBBAP implantation was routinely carried out following the standard CSP implantation technique previously described in the literature [14,15,16,17,18,19]. First, the tricuspid annulus was localized by contrast injection. Next, the sheath was positioned just distal to the tricuspid valve, and pace mapping was performed at the right side of the interventricular septum. A favorable-paced QRS morphology at the penetration site was defined as a QS complex with a notched nadir in V1 resembling a “W pattern.” Discordant QRS polarity in leads II and III was accepted for fixation, as well as QS complexes or R waves in these leads. At this site, the lead was advanced across the RV septum by rapid rotations. During the screw-in process, fluoroscopy, paced QRS morphology, fixation beats, pacing threshold, and lead impedance were continuously monitored to determine optimal lead depth. After deployment, an output-dependent QRS transition was observed to confirm capture of the conduction system. The achieved pacing type was classified according to the latest EHRA consensus statement [14]. In the event of a failed LBBAP, HBP was attempted.
The biventricular pacing upgrade procedure was performed using a standard technique. Left ventricular leads were implanted via the coronary sinus, with preference for positioning in the basal lateral or posterolateral veins. Quadripolar LV leads were employed in the majority of patients (94.6%, 35 of 37) to optimize pacing configurations and minimize the risk of phrenic nerve stimulation.
2.3. Data Collection and Follow-Up
All patients underwent standardized clinical and echocardiographic assessments at baseline and during regular follow-up. At the 6-month follow-up, the quality of life was assessed with the EQ-5D-5L questionnaire which evaluates mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. Each domain is rated on five levels reflecting the severity of problems in that area. In addition, patients rated their overall health status on the visual analogue scale ranging from 0 to 100 [20,21]. The assessed outcomes included changes in left ventricular ejection fraction (LVEF), left ventricular end diastolic diameter (LVEDD), New York Heart Association (NYHA) functional class, heart failure hospitalizations, all-cause mortality, and device-related complications. Procedural, lead parameters, and pacing burden were also recorded. Procedure costs, including device-related expenses (generator, sheath, and leads) and procedural material costs, were obtained from hospital billing records and compared per patient between CSP and BIV upgrades.
2.4. Statistical Analysis
Continuous variables were expressed as mean ± standard deviation or as median with interquartile range. Categorical variables were summarized as counts and percentages. We assessed normality by the Shapiro–Wilk test and compared continuous data using independent t-tests or Mann–Whitney U tests (and paired t-tests or Wilcoxon signed-rank tests for within-group analyses). Categorical and ordinal variables were analyzed by chi-square or Fisher’s exact tests (with Kruskal–Wallis tests for overall ordinal comparisons and 2 × 2 Fisher’s exact tests for class-by-class transitions).
Patients were followed up regularly until the occurrence of the HF hospitalization, or were censored at the time of last follow-up or death.
Time-to-event outcomes were evaluated via Kaplan–Meier survival curves, and multivariable Cox regression was applied to adjust for baseline LVEF imbalance.
Absolute change in LVEF (ΔLVEF) was analyzed using analysis of covariance (ANCOVA), with treatment group as the independent variable and baseline LVEF as a covariate. Model assumptions were confirmed by visual inspection. A two-tailed p < 0.05 defined statistical significance. Given the modest sample size and event rate, propensity score matching was not performed to avoid loss of power and overfitting.
All statistical analyses were conducted in R version 4.5.0.
3. Results
3.1. Baseline Characteristics
Between January 2022 and October 2024, a total of 63 patients with pacing-induced cardiomyopathy underwent device upgrade to either biventricular pacing (n = 37) or conduction system pacing (n = 26).
Both groups were similar with respect to age (75.9 ± 6.3 vs. 75.0 ± 7.4 years), sex distribution (72.0% vs. 86.5% male), primary implant indication, ventricular pacing burden, comorbidities, baseline QRS duration, and guideline-directed heart failure therapies. Echocardiography revealed a higher baseline LVEF in the CSP group (CSP: 34.5 ± 8.0% vs. BIV: 29.7 ± 7.6%, p = 0.01), with no significant differences in chamber dimensions, right ventricular function, or tricuspid regurgitation grade (Table 1).

Table 1.
Baseline characteristics of CSP vs. BIV upgrade patients.
3.2. Procedural and Clinical Outcomes
Procedural time was similar between groups, while fluoroscopy was shorter in CSP (7.1 ± 7.3 vs. 8.6 ± 5.1 min, p = 0.03). Two BIV procedures were converted to CSP due to failure to obtain an adequate coronary sinus lead position (final cohort: 26 CSP, 37 BIV; Table 2). LBBAP was achieved in 92% of CSP cases, with a mean V6 R-wave peak time of 82.5 ± 15.9 ms (Table 3).

Table 2.
Characteristics of upgrade procedure, lead parameters, electrocardiogram and echocardiogram at the time of implant and follow-up in patients undergoing CSP vs. BIV upgrade.

Table 3.
Electrocardiographic characteristics and type of achieved conduction system pacing.
Complications were rare: one intraoperative ventricular tachycardia in the CSP arm and one coronary sinus dissection in the BIV group occurred, both without further consequences. During follow-up, one CSP patient required lead revision due to elevated thresholds of the LBBAP lead; no other procedural or device-related complications occurred.
QRS narrowing, LVEF, NYHA class improvement, and LVEDD reduction were comparable between upgrade procedures (Table 2 and Table 3, Figure 1 and Figure 2), with both groups showing significant improvement from baseline to follow-up (LVEF: CSP p = 0.008, BIV p = 0.005; NYHA CSP p = 0.01, BIV p = 0.005, LVEDD: CSP p = 0.02; BIV p = 0.03). After ANCOVA adjusting for baseline LVEF, the adjusted mean improvement (ΔLVEF) was 6.6% (95% CI 2.2–11.0) in the CSP group and 8.8% (95% CI 5.2–12.4) in the BIV group. The adjusted between-group difference was −2.2% (95% CI −7.0 to 2.6; p = 0.38), indicating no significant difference in LVEF improvement. Additionally, baseline LVEF was associated with ΔLVEF (β = −0.375, p = 0.03), indicating that patients with lower baseline EF experienced greater improvement Responder rates (≥10% LVEF increase) did not differ significantly (36.1% BIV vs. 28.0% CSP, p = 0.58).
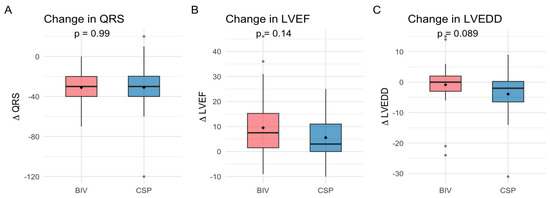
Figure 1.
Changes in QRS duration (A), left ventricular ejection fraction (LVEF) (B), and left ventricular end-diastolic diameter (LVEDD) (C) after upgrade to biventricular pacing (BIV) or conduction system pacing (CSP).
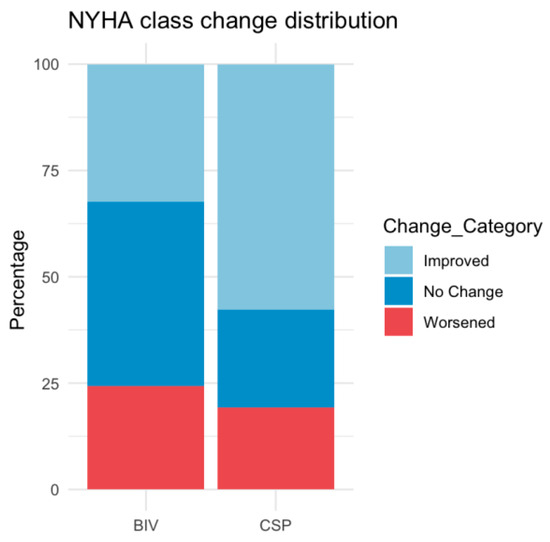
Figure 2.
Distribution of New York Heart Association (NYHA) class change after upgrade to biventricular pacing (BIV) or conduction system pacing (CSP).
3.3. Heart Failure Hospitalization and All-Cause Mortality
During a mean follow-up of 19.5 ± 8.1 months, the rates of all-cause mortality, heart failure hospitalization, and the composite of both events were similar between CSP and BIV upgrades. Adjustment for baseline LVEF, which was lower in the BIV group, did not alter these findings (Figure 3, Table 4). In this model, each 1% increase in baseline LVEF was associated with a 9% relative reduction in the risk of heart failure hospitalization (HR 0.91 per %, p = 0.18).
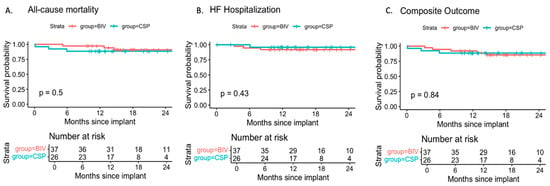
Figure 3.
Freedom from (A) All-cause mortality, (B) heart failure (HF) hospitalization, and (C) the composite of death or HF hospitalization in patients with CSP or BIV upgrade. Survival probabilities are plotted over a 24-month period since device upgrade. Risk tables below each panel indicate the number of patients at risk at 6-month intervals. Differences between groups were compared using the log-rank test. No statistically significant differences were observed.

Table 4.
Hazard ratios for hard clinical outcomes after CSP vs. BIV upgrade.
3.4. Quality of Life
Quality-of-life scores and domain responses at 6-month follow-up were similar between the CSP and BIV groups, with no significant differences observed in any measure between the groups (Table 5).

Table 5.
Quality-of-life measures after upgrade to conduction system pacing (CSP) or biventricular pacing (BIV).
3.5. Financial Cost–Benefit
In our cohort, the mean procedural cost of upgrade systems was 41% lower in CSP compared to BIV upgrades. This difference was explained mainly by device selection: the existing generator could be retained in 34.6% of CSP patients due to sufficient remaining longevity, and ICD upgrade was avoided in 76.9%.
4. Discussion
4.1. Summary of Main Findings
In this retrospective, single-center observational study, we compared conduction system pacing (CSP) with biventricular pacing (BIV) as upgrade strategies for patients with pacemaker-induced cardiomyopathy (PICM). The principal findings are that: (1) both strategies achieved significant improvements in left ventricular ejection fraction (LVEF), ventricular dimensions, and New York Heart Association (NYHA) functional class; (2) quality of life and clinical outcomes, including heart failure hospitalization (HFH) and all-cause mortality (ACM), were similar between groups; and (3) retaining of the existing generator was feasible in 34.6% of CSP patients—a novel observation not previously reported in this population.
4.2. Comparison with Prior Studies
Biventricular pacing has been the standard upgrade strategy for patients with PICM. Multicenter observational studies have consistently demonstrated symptomatic and echocardiographic improvements with BIV, and the randomized Budapest-CRT Upgrade trial provided strong evidence, showing significant reductions in HFH and ACM in addition to reverse remodeling [3,4,5,22]. The benefit of BIV upgrade is similar in patients with atrial fibrillation [23]. However, the approach can be limited by the technical challenges of coronary sinus lead placement, as anatomical variations may lead to suboptimal or impossible lead positioning [8,9].
More recently, CSP via HBP or LBBAP has emerged as a physiologic alternative for BIV [24,25]. Multiple single-arm studies and meta-analyses suggest that CSP achieves similar or even superior resynchronization and remodeling compared with BIV, while maintaining comparable rates of HFH and ACM [26]. Only two prior head-to-head studies directly compared CSP and BIV upgrades in PICM, both reporting greater improvements in LVEF and LVEDD with CSP, with no difference in HFH or ACM [12,13]. In our larger population with follow-up beyond 19 months, there was no difference in clinical outcomes, and echocardiographic improvements were similar between groups. This similarity was likely influenced by the higher baseline LVEF in the CSP arm, which was independently associated with smaller gains, whereas lower baseline LVEF predicted greater remodeling.
Responder rates (≥10% LVEF improvement) were modest—36.1% in the BIV group and 28.0% in the CSP group—lower than the 60% typically reported in de novo CRT trials [27,28]. This likely reflects the higher baseline LVEF in the CSP group, along with the greater comorbidity burden and the high prevalence of ischemic cardiomyopathy in our cohort, all of which are known to attenuate reverse remodeling [29]. Notably, both upgrade strategies demonstrated safety, as no major late complications were observed, lead thresholds remained stable, and our findings align with those of a recent meta-analysis, which reported low complication rates for CRT (3.3%) and CSP upgrades (1.8%) [30].
Quality of life has not been systematically evaluated in previous studies of PICM upgrades. In our cohort, both CSP and BIV upgrades resulted in comparable patient-reported quality of life, consistent with the parallel improvements observed in LVEF and NYHA class. However, overall QoL remained suboptimal, with low self-reported health scores (46.5 ± 34.9 for CSP vs. 41.6 ± 31.9 for BIV; p = 0.46) and frequent functional limitations in both groups (42–54%, p > 0.05). Although only 10 patients were implanted in 2022, when COVID-19-related restrictions and psychological stress may have affected perceived well-being, this factor should be considered when interpreting the QoL score. Despite these potential confounders, the similar QoL outcomes between upgrade strategies suggest that CSP offers comparable patient-centered benefits to conventional BIV. At the same time, residual symptoms likely reflect the underlying heart failure burden and comorbidities, as previously reported in device-treated populations [31,32,33,34].
Absence of appropriate ICD therapy in our study is in line with the very low rate of malignant ventricular arrhythmias in CRT upgrade populations [3,4]. Observational and randomized data suggest that routine defibrillator implantation may not be necessary in non-ischemic cardiomyopathy patients without additional arrhythmic risk factors [35,36].
4.3. Mechanistic Considerations
The comparable remodeling and functional recovery between the CSP, particularly the LBBAP and BIV groups, may be explained by both approaches’ ability to restore ventricular synchrony [6]. CSP directly recruits the His–Purkinje system, producing LV activation patterns similar to those of intrinsic conduction, whereas BIV achieves resynchronization through the fusion of LV and RV pacing, as demonstrated in studies comparing LV activation patterns in CRT and CSP [10]. Clinically, these electrical advantages have been linked to larger LVEF gains with CSP versus BIV in some upgrade PICM cohorts, although most data are from single-arm observational studies.
In PICM, where dyssynchrony is pacing-induced, either strategy could reverse the underlying pathophysiology, though comorbidities and myocardial substrate may modulate the degree of benefit. Long-term follow-up is required to determine whether the similar short- to mid-term outcomes observed here persist and whether any differences emerge in the durability of response, HFH risk, or survival [37].
4.4. Cost Implications
A novel finding of our study is that existing generators can be reused in over one-third of CSP upgrades, whereas all BIV patients require new CRT devices. This selective reuse, together with the fact that ICD upgrades were avoided in more than three-quarters, highlights a potential cost advantage of CSP. Although specialized delivery sheaths add expense, the overall balance suggests greater cost-efficiency compared with BIV upgrades.
Another practical consideration concerns the potential presence of abandoned leads after generator reuse. Although concerns may arise regarding MRI compatibility and potential complications during future transvenous lead extractions, recent evidence suggests that MRI examinations can be performed safely when appropriate institutional protocols are followed [38,39,40].
Moreover, lead extraction is typically undertaken in younger patients, with an average reported age of around 65 years, whereas the mean age in our CSP upgrade cohort was 76 years [41]. Consequently, the clinical relevance of these concerns is limited in this elderly population, and from a cost-efficiency perspective, they do not justify the implantation of a larger and more expensive CRT generator solely to avoid a retained lead.
4.5. Clinical Implications
Our findings support CSP as a physiologic alternative to BIV for PICM upgrades, offering comparable functional and clinical outcomes with added procedural efficiency and potential cost savings. CSP may be particularly beneficial in patients with unfavorable coronary sinus anatomy, venous occlusion, or when generator reuse is feasible. Until randomized data are available, device selection should be individualized, taking into account patient preferences, comorbidities, and operator expertise.
5. Limitations
As a retrospective, single-center analysis, the present study may have limited generalizability to other populations or centers with different clinical practices. The selection of upgrade strategy (CSP or BIV) was not randomized but guided by clinical judgment, introducing a potential risk of selection bias despite statistical adjustment for confounding variables, including baseline LVEF.
A standardized definition of PICM is lacking; the current study focused on patients with a persevered baseline systolic function who developed pacing-induced LV dysfunction, which may limit comparability with studies using alternative definitions.
Coronary venous anatomy was not assessed prior to CSP implantation and therefore did not influence group allocation, although two crossover cases from CRT to CSP occurred due to unfavorable sinus coronarius anatomy.
Due to the small sample size and the low number of clinical events, this study was underpowered for hard clinical outcomes. Therefore, only large between-group differences could have been detected with sufficient statistical power, and the findings related to HFH and ACM should be interpreted with caution.
Baseline QoL EQ-5D-5L assessment was not available; therefore, only intergroup comparisons at follow-up could be performed.
Larger, multicenter, randomized studies are warranted to confirm these findings and further refine patient selection criteria for CSP versus BIV upgrades.
6. Conclusions
In patients with PICM, CSP upgrade represents a feasible and potentially cost-efficient alternative to BIV. CSP demonstrated favorable procedural characteristics and was associated with comparable improvements in left ventricular function, symptomatic status, and major clinical outcomes, including HFH and ACM. These findings may support its consideration as an upgrade strategy, although confirmation in adequately powered, prospective randomized trials remains warranted.
Author Contributions
Conceptualization, G.Z.D. and P.B.; Data curation, B.M.D. and M.G.; Formal analysis, B.M.D. and M.G.; Investigation, B.P., M.B., N.V., G.Z.D. and P.B.; Methodology, B.M.D. and P.B.; Resources, P.B.; Software, B.M.D. and M.G.; Supervision, P.B.; Validation, M.B., N.V. and M.T.; Visualization, M.G.; Writing—original draft, B.M.D.; Writing—review and editing, B.P., N.V., M.T. and G.Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
Bernadett Miriam Dobai, MD was supported by the Romanian Society of Cardiology Training Scholarship (235/13 August 2024).
Institutional Review Board Statement
This study was approved by the local Institutional Ethics Committee (ÉPC-HK/236-1-2023/23 February 2023) and conducted in accordance with the principles of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data are available upon request from the corresponding author.
Acknowledgments
The authors express their gratitude to the Central Hospital of Northern Pest- Military Hospital’s Electrophysiology and Pacing team for their dedicated work and support in patient care, device implantation, and follow-up.
Conflicts of Interest
No author has any conflicts of interest that could have influenced this study. P.B. reports educational activity with Biotronik outside the submitted work. G.Z.D. reports educational activity and advisory roles with Biotronik and Medtronic, and educational activity on behalf of Boehringer Ingelheim, Novartis, Novo Nordisk, TEVA, and Lilly, all outside the submitted work. B.P. reports consulting and/or lecture fees and non-financial support from Abbott, Biosense Webster, Biotronik, Medtronic, Novartis, and Replantmed. B.M.D., M.G., M.B. and N.V. report no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACEI | angiotensin-converting enzyme inhibitor |
| ACM | all-cause mortality |
| AF | atrial fibrillation |
| ANCOVA | analysis of covariance |
| APT | antiplatelet therapy |
| ARB | angiotensin II receptor blocker |
| ARNI | angiotensin receptor–neprilysin inhibitor |
| AV | atrioventricular |
| BIV | biventricular pacing (cardiac resynchronization therapy) |
| CIED | cardiac implantable electronic device |
| CKD | chronic kidney disease |
| CSP | conduction system pacing |
| HBP | His-bundle pacing |
| HFH | heart failure hospitalization |
| ICD | implantable cardioverter defibrillator |
| LAD | left atrial diameter |
| LBBB | left bundle branch block |
| LBBAP | left bundle branch area pacing |
| LV | left ventricular |
| LVEDD | left ventricular end-diastolic diameter |
| LVEF | left ventricular ejection fraction |
| MRA | mineralocorticoid receptor antagonist |
| NIVCD | nonspecific interventricular conduction disease |
| NYHA | New York Heart Association |
| PASP | pulmonary arterial systolic pressure |
| PICM | pacemaker-induced cardiomyopathy |
| QoL | quality of life |
| RAD | right atrial diameter |
| RBBB | right bundle branch block |
| RV | right ventricular |
| SDL | stylet-driven leads |
| SGLT2i | sodium–glucose co-transporter 2 inhibitor |
| SSS | sick sinus syndrome |
| TAPSE | tricuspid annular plane systolic excursion |
| TIA | transient ischemic attack |
| TR | tricuspid regurgitation |
| V6RWPeak time | V6 R-wave peak time |
| VP | ventricular pacing |
| ΔLVEF | change in left ventricular ejection fraction |
References
- Mond, H.G.; Proclemer, A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: Calendar year 2009—A World Society of Arrhythmia’s Project. Pacing Clin. Electrophysiol. 2011, 34, 1013–1027. [Google Scholar] [CrossRef]
- Somma, V.; Ha, F.J.; Palmer, S.; Mohamed, U.; Agarwal, S. Pacing-induced cardiomyopathy: A systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm. 2023, 20, 282–290. [Google Scholar] [CrossRef]
- Merkely, B.; Hatala, R.; Wranicz, J.K.; Duray, G.; Földesi, C.; Som, Z.; Németh, M.; Goscinska-Bis, K.; Gellér, L.; Zima, E.; et al. Upgrade of right ventricular pacing to cardiac resynchronization therapy in heart failure: A randomized trial. Eur. Heart J. 2023, 44, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Vamos, M.; Erath, J.W.; Bari, Z.; Vagany, D.; Linzbach, S.P.; Burmistrava, T.; Israel, C.W.; Duray, G.Z.; Hohnloser, S.H. Effects of Upgrade Versus De Novo Cardiac Resynchronization Therapy on Clinical Response and Long-Term Survival: Results from a Multicenter Study. Circ. Arrhythmia Electrophysiol. 2017, 10, e004471. [Google Scholar] [CrossRef]
- Duray, G.Z.; Israel, C.W.; Pajitnev, D.; Hohnloser, S.H. Upgrading to biventricular pacing/defibrillation systems in right ventricular paced congestive heart failure patients: Prospective assessment of procedural parameters and response rate. EP Europace 2008, 10, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Zizek, D.; Zlahtic, T.; Mrak, M.; Stublar, J.; Ivanovski, M.; Dzananovic, D.Z.; Cvijic, M.; Meznar, A.Z. Conduction system pacing vs. biventricular pacing for cardiac resynchronization: The CSP-SYNC randomized single centre study. EP Europace 2025, 27, euaf085.605. [Google Scholar] [CrossRef]
- Sussenbek, O.; Rademakers, L.; Waldauf, P.; Jurak, P.; Smisek, R.; Stros, P.; Poviser, L.; Vesela, J.; Plesinger, F.; Halamek, J.; et al. Left bundle branch area pacing results in more physiological ventricular activation than biventricular pacing in patients with left bundle branch block heart failure. Eur. Heart J. Suppl. 2023, 25 (Suppl. E), E17–E24. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Obeng-Gyimah, E.; Supple, G.E.; Schaller, R.; Lin, D.; Owens, A.T.; Epstein, A.E.; Dixit, S.; Marchlinski, F.E.; Frankel, D.S. Reversal of Pacing-Induced Cardiomyopathy Following Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2018, 4, 168–177. [Google Scholar] [CrossRef]
- Herweg, B.; Welter-Frost, A.; Vijayaraman, P. The evolution of cardiac resynchronization therapy and an introduction to conduction system pacing: A conceptual review. Europace 2021, 23, 496–510. [Google Scholar] [CrossRef]
- Meiburg, R.; Rijks, J.H.J.; Beela, A.S.; Bressi, E.; Grieco, D.; Delhaas, T.; Luermans, J.G.L.; Prinzen, F.W.; Vernooy, K.; Lumens, J. Comparison of novel ventricular pacing strategies using an electro-mechanical simulation platform. EP Europace 2023, 25, euad144. [Google Scholar] [CrossRef]
- Glikson, M.; Burri, H.; Abdin, A.; Cano, O.; Curila, K.; De Pooter, J.; Diaz, J.C.; Drossart, I.; Huang, W.; Israel, C.W.; et al. European Society of Cardiology (ESC) clinical consensus statement on indications for conduction system pacing, with special contribution of the European Heart Rhythm Association of the ESC and endorsed by the Asia Pacific Heart Rhythm Society, the Canadian Heart Rhythm Society, the Heart Rhythm Society, and the Latin American Heart Rhythm Society. EP Europace 2025, 27, euaf050. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.-P.; Chen, Y.; Yang, Y.-H.; Li, G.-C.; Ma, C.-M.; Fa, Q.; Gao, L.-J.; Xia, Y.-L.; Dong, Y.-X. Conduction system pacing upgrade versus biventricular pacing on pacemaker-induced cardiomyopathy: A retrospective observational study. Front. Physiol. 2024, 15, 1355696. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xu, S.; Wang, C.; Li, X.; Zhu, H.; Zeng, J.; Zhang, E.; Zou, J.; Fan, X. Effectiveness of upgrading to left bundle branch area pacing compared with biventricular pacing in patients with right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2025, 22. in press. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA clinical consensus statement on conduction system pacing implantation: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). EP Europace 2023, 25, 1208–1236. [Google Scholar] [CrossRef]
- Huang, W.; Chen, X.; Su, L.; Wu, S.; Xia, X.; Vijayaraman, P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. 2019, 16, 1791–1796. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Kiełbasa, G.; Cano, O.; Curila, K.; Heckman, L.; De Pooter, J.; Chovanec, M.; Rademakers, L.; Huybrechts, W.; Grieco, D.; et al. Left bundle branch area pacing outcomes: The multicentre European MELOS study. Eur. Heart J. 2022, 43, 4161–4173. [Google Scholar] [CrossRef]
- Liu, X.; Niu, H.-X.; Gu, M.; Chen, X.; Hu, Y.; Cai, M.; Zhang, N.; Zhao, J.; Zhou, X.; Gold, M.R.; et al. Contrast-enhanced image-guided lead deployment for left bundle branch pacing. Heart Rhythm. 2021, 18, 1318–1325. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Kiełbasa, G.; Moskal, P.; Bednarek, A.; Kusiak, A.; Sondej, T.; Bednarski, A.; Rajzer, M.; Vijayaraman, P. Fixation beats: A novel marker for reaching the left bundle branch area during deep septal lead implantation. Heart Rhythm. 2021, 18, 562–569. [Google Scholar] [CrossRef]
- Jastrzębski, M. ECG and Pacing Criteria for Differentiating Conduction System Pacing from Myocardial Pacing. Arrhythmia Electrophysiol. Rev. 2021, 10, 172–180. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Janssen, M.F.; Pickard, A.S.; Golicki, D.; Gudex, C.; Niewada, M.; Scalone, L.; Swinburn, P.; Busschbach, J. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: A multi-country study. Qual. Life Res. 2013, 22, 1717–1727. [Google Scholar] [CrossRef]
- Kosztin, A.; Vamos, M.; Aradi, D.; Schwertner, W.R.; Kovacs, A.; Nagy, K.V.; Zima, E.; Geller, L.; Duray, G.Z.; Kutyifa, V.; et al. De novo implantation vs. upgrade cardiac resynchronization therapy: A systematic review and meta-analysis. Heart Fail. Rev. 2018, 23, 15–26. [Google Scholar] [CrossRef]
- Merkely, B.; Hatala, R.; Merkel, E.; Szigeti, M.; Veres, B.; Fábián, A.; Osztheimer, I.; Gellér, L.; Sasov, M.; Wranicz, J.K.; et al. Benefits of upgrading right ventricular to biventricular pacing in heart failure patients with atrial fibrillation. EP Europace 2024, 26, euae179. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Sharma, P.S.; Cano, Ó.; Ponnusamy, S.S.; Herweg, B.; Zanon, F.; Jastrzebski, M.; Zou, J.; Chelu, M.G.; Vernooy, K.; et al. Comparison of Left Bundle Branch Area Pacing and Biventricular Pacing in Candidates for Resynchronization Therapy. JACC 2023, 82, 228–241. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.E.; Ellenbogen, K.A.; Vijayaraman, P.; Chelu, M.G. Clinical outcomes of conduction system pacing versus biventricular pacing for cardiac resynchronization therapy: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2023, 34, 1718–1729. [Google Scholar] [CrossRef]
- Felix, I.F.; Collini, M.; Fonseca, R.; Guida, C.; Armaganijan, L.; Healey, J.S.; Carvalho, G. Conduction system pacing versus biventricular pacing in heart failure with reduced ejection fraction: A systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2024, 21, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Osca, J.; Francisco-Pascual, J.; Martínez-Basterra, J.; Martínez, J.G.; Reis, H.; Oliveira, M.; Campos, B.; Balaguer, J.; Rubio, J.; Pavón-Jiménez, R.; et al. Response rate in cardiac resynchronization therapy patients implanted with a left ventricular quadripolar lead and the MultiPoint™ pacing feature early activated. QUARTO III. Eur. J. Clin. Investig. 2023, 53, e13935. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Ueda, N.; Ishibashi, K.; Noda, T.; Miyazaki, Y.; Wakamiya, A.; Shimamoto, K.; Nakajima, K.; Kamakura, T.; Wada, M.; et al. Significance of effective cardiac resynchronization therapy pacing for clinical responses: An analysis based on the effective cardiac resynchronization therapy algorithm. Heart Rhythm. 2023, 20, 1289–1296. [Google Scholar] [CrossRef]
- Shanks, M.; Delgado, V.; Ng, A.C.; Auger, D.; Mooyaart, E.A.; Bertini, M.; Marsan, N.A.; van Bommel, R.J.; Holman, E.R.; Poldermans, D.; et al. Clinical and echocardiographic predictors of nonresponse to cardiac resynchronization therapy. Am. Heart J. 2011, 161, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Kaza, N.; Htun, V.; Miyazawa, A.; Simader, F.; Porter, B.; Howard, J.P.; Arnold, A.D.; Naraen, A.; Luria, D.; Glikson, M.; et al. Upgrading right ventricular pacemakers to biventricular pacing or conduction system pacing: A systematic review and meta-analysis. EP Europace 2023, 25, 1077–1086. [Google Scholar] [CrossRef]
- Dobai, B.M.; Paiu, A.; Rudzik, R.; Sus, I.; Beke, I.; Danila, M.; Dobreanu, D. Psychosocial impact of COVID-19 pandemic on QUALITY of life among heart failure patients living with an ICD. Eur. J. Heart Fail. 2021, 23 (Suppl. S2), 2–322. [Google Scholar]
- Nicmanis, M.; Chur-Hansen, A.; Oxlad, M. The psychological, social, and quality of life outcomes of people with a cardiac implantable electronic device: An umbrella review. Eur. J. Cardiovasc. Nurs. 2024, 23, 441–451. [Google Scholar] [CrossRef]
- Willy, K.; Ellermann, C.; Reinke, F.; Rath, B.; Wolfes, J.; Eckardt, L.; Doldi, F.; Wegner, F.K.; Köbe, J.; Morina, N. The Impact of Cardiac Devices on Patients’ Quality of Life—A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2022, 9, 257. [Google Scholar] [CrossRef]
- Dobai, B.M.; Iantovics, L.B.; Paiu, A.; Dobreanu, D. Exploratory factor analysis for identifying CIEDs patients’ concerns during the COVID-19 pandemic in Europe. INDECS 2022, 20, 50–56. [Google Scholar] [CrossRef]
- Kutyifa, V.; Geller, L.; Bogyi, P.; Zima, E.; Aktas, M.K.; Ozcan, E.E.; Becker, D.; Nagy, V.K.; Kosztin, A.; Szilagyi, S.; et al. Effect of cardiac resynchronization therapy with implantable cardioverter defibrillator versus cardiac resynchronization therapy with pacemaker on mortality in heart failure patients: Results of a high-volume, single-centre experience. Eur. J. Heart Fail. 2014, 16, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef]
- Karki, S.; Lakra, P.; Kumar, K.; Rao, S.J. Conduction System Pacing for Cardiac Resynchronization Therapy in Heart Failure with Reduced Ejection Fraction. J. Clin. Med. 2025, 14, 917. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Israel, C.; Eisenblätter, M.; Hoyer, A.; Stoye, F.V.; Yilmaz, A.; Gielen, S. Safety of magnetic resonance imaging in patients with cardiac implantable electronic devices and abandoned or epicardial leads: A systematic review and meta-analysis. EP Europace 2024, 26, euae165. [Google Scholar] [CrossRef]
- Schaller, R.D.; Brunker, T.; Riley, M.P.; Marchlinski, F.E.; Nazarian, S.; Litt, H. Magnetic Resonance Imaging in Patients With Cardiac Implantable Electronic Devices With Abandoned Leads. JAMA Cardiol. 2021, 6, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, M.J.; Rangan, P.; Su, W.; Weiss, J.P.; Zawaneh, M.; Unzek, S.; Tamarappoo, B.; Indik, J.; Tung, R.; Morris, M.F. MRI in Patients with Cardiovascular Implantable Electronic Devices and Fractured or Abandoned Leads. Radiol Cardiothorac Imaging 2024, 6, e230303. [Google Scholar] [CrossRef]
- Shah, K.; Pollema, T.; Birgersdotter-Green, U. Performance and outcomes of transvenous rotational lead extraction: Results from a prospective, monitored, global clinical study—“An evolution in extraction”. Heart Rhythm. O2 2021, 2, 122–123. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).