Temporal Trends and Prognostic Impact of Pacemaker-Associated Heart Failure: Insights from a Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
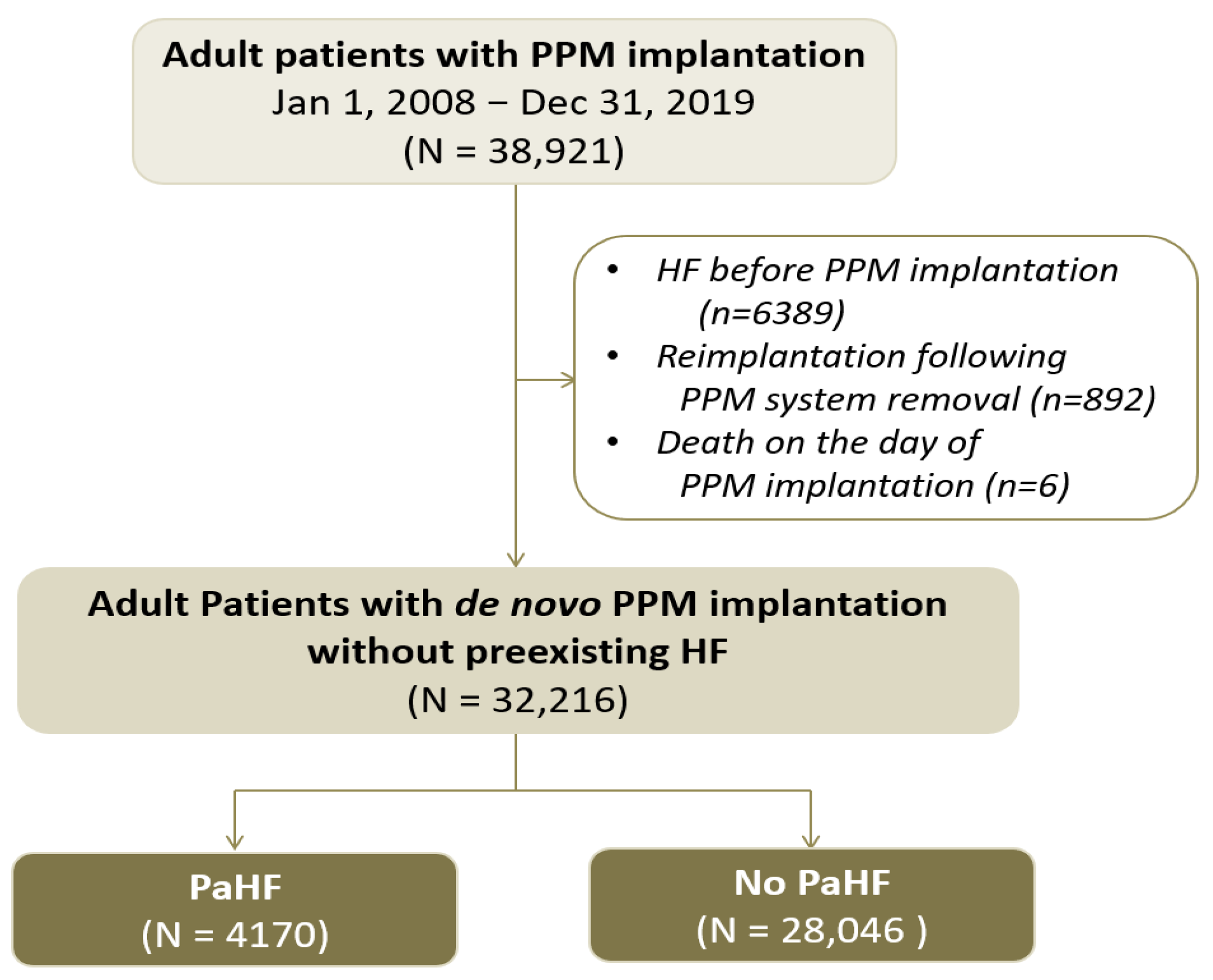
2.2. Study Design and Population
2.3. Data Acquisition
2.4. Study Outcomes and Follow-Up
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics and Incidence of PaHF
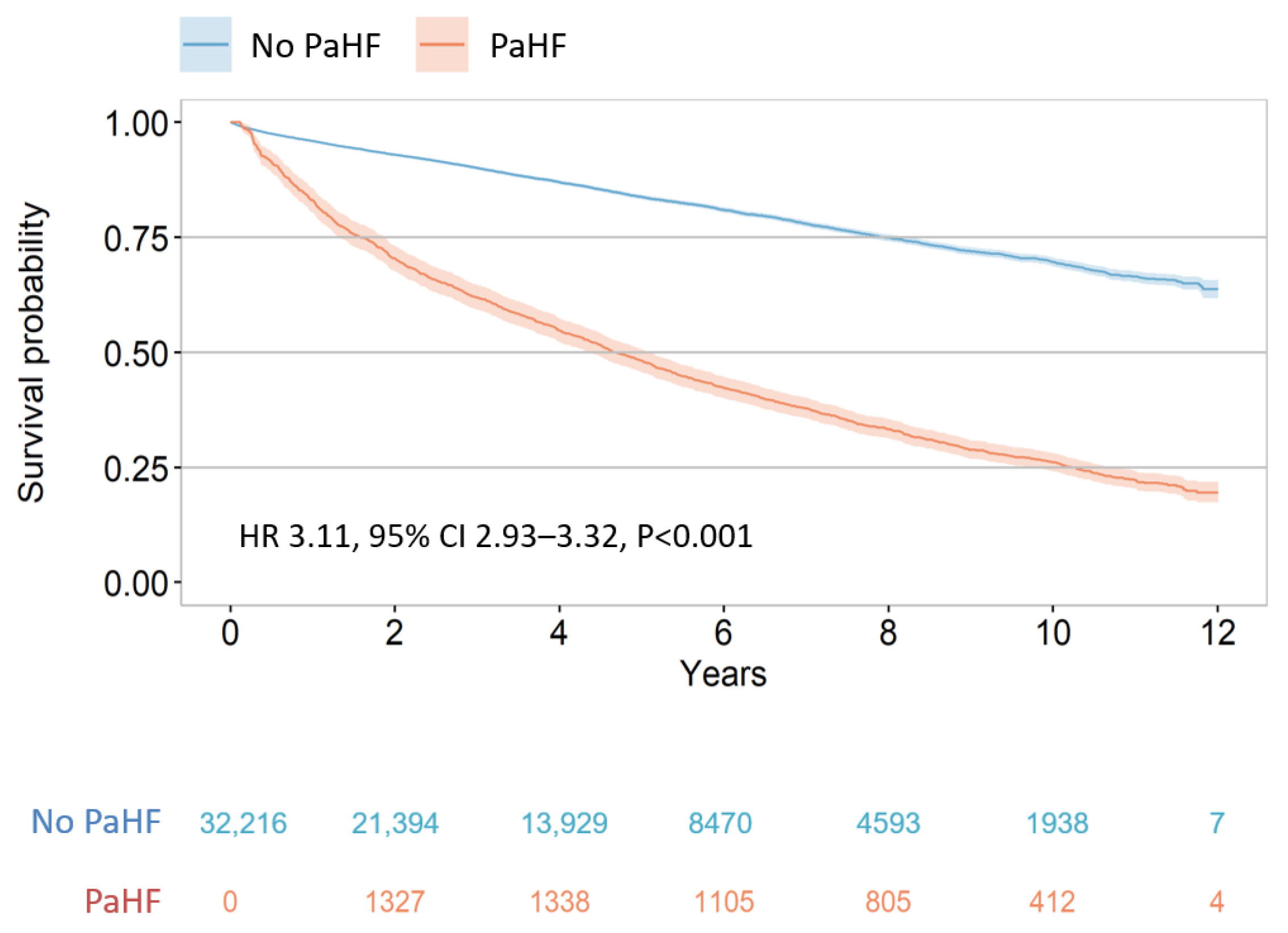
3.2. Risk of All-Cause Mortality According to the Development of PaHF
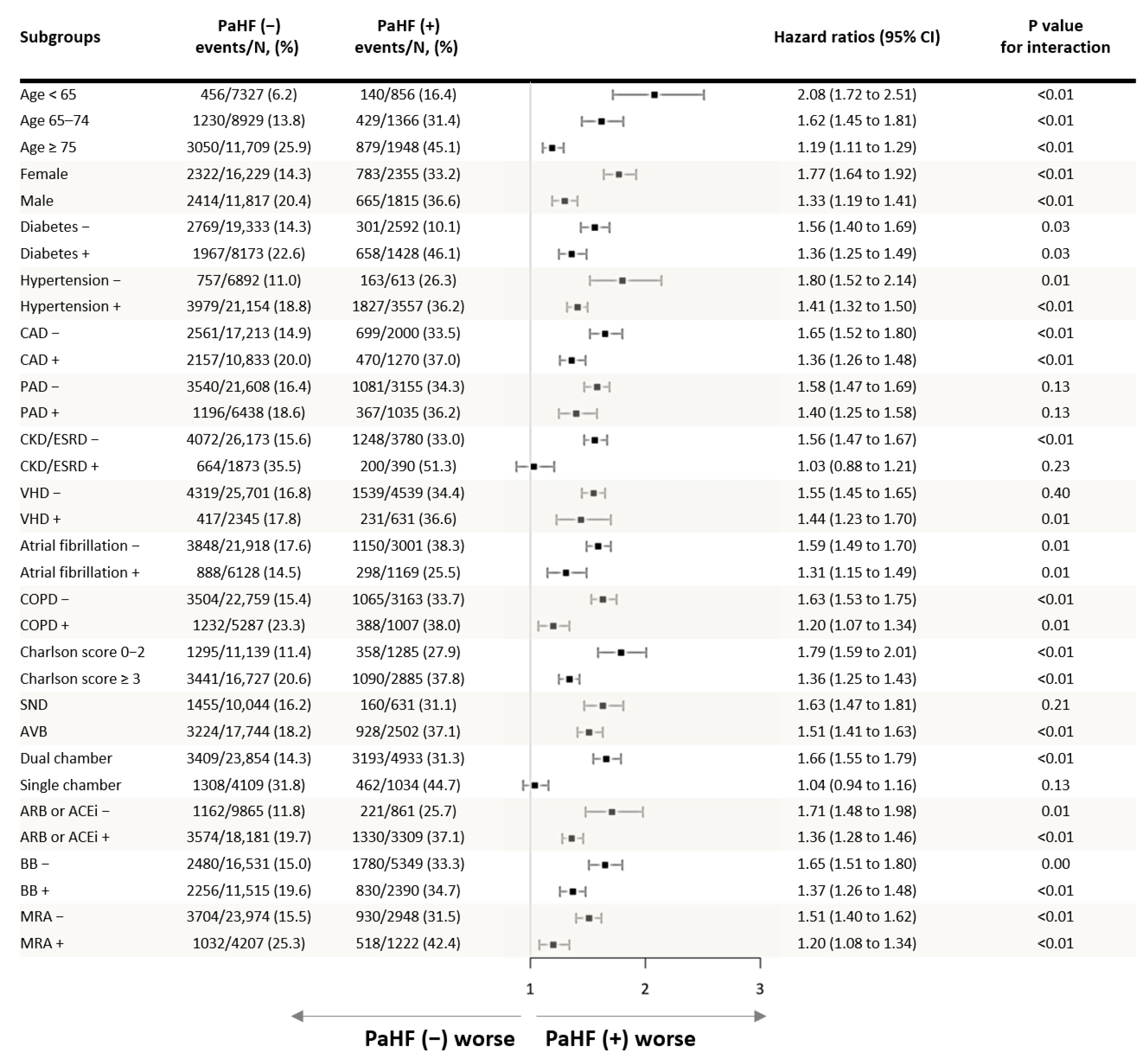
3.3. Predictors of PaHF
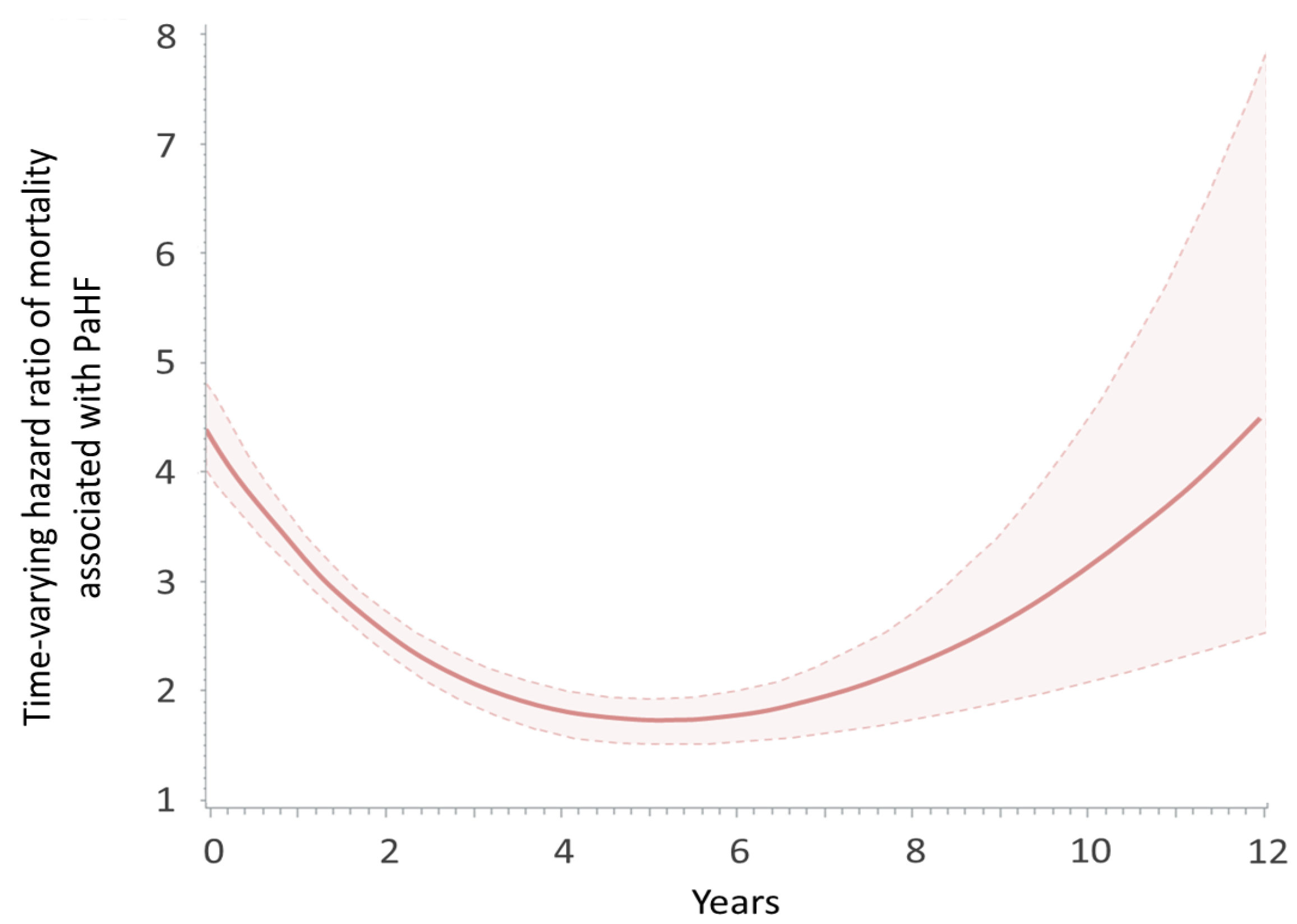
3.4. Sensitivity Analyses
4. Discussion
4.1. Main Findings
4.2. Incidence and Monitoring of PaHF
4.3. Prognostic Impact of PaHF on Mortality
4.4. Risk Factors of PaHF
4.5. Prevention Strategies and Subgroup Considerations
4.6. Implications in the Era of New Conduction System and Leadless Pacing
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEI | angiotensin-converting enzyme inhibitor |
| AF | atrial fibrillation |
| ARB | angiotensin receptor blocker |
| AVB | atrioventricular block |
| CAD | coronary artery disease |
| CCI | Charlson comorbidity index |
| COPD | chronic obstructive pulmonary disease |
| CKD | chronic kidney disease |
| CRT | cardiac resynchronization therapy. |
| ESRD | end-stage renal disease |
| MRA | mineralocorticoid receptor antagonist |
| PAD | peripheral artery disease |
| PaHF | pacemaker-associated heart failure |
| PPM | permanent pacemaker |
| SND | sinus node dysfunction |
| VHD | valvular heart disease |
References
- Cho, S.W.; Gwag, H.B.; Hwang, J.K.; Chun, K.J.; Park, K.M.; On, Y.K.; Kim, J.S.; Park, S.J. Clinical features, predictors, and long-term prognosis of pacing-induced cardiomyopathy. Eur. J. Heart Fail. 2019, 21, 643–651. [Google Scholar] [CrossRef]
- Kiehl, E.L.; Makki, T.; Kumar, R.; Gumber, D.; Kwon, D.H.; Rickard, J.W.; Kanj, M.; Wazni, O.M.; Saliba, W.I.; Varma, N.; et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016, 13, 2272–2278. [Google Scholar] [CrossRef]
- Cho, E.J.; Park, S.J.; Park, K.M.; On, Y.K.; Kim, J.S. Paced QT interval as a risk factor for new-onset left ventricular systolic dysfunction and cardiac death after permanent pacemaker implantation. Int. J. Cardiol. 2016, 203, 158–163. [Google Scholar] [CrossRef]
- Ebert, M.; Jander, N.; Minners, J.; Blum, T.; Doering, M.; Bollmann, A.; Hindricks, G.; Arentz, T.; Kalusche, D.; Richter, S. Long-Term Impact of Right Ventricular Pacing on Left Ventricular Systolic Function in Pacemaker Recipients With Preserved Ejection Fraction: Results From a Large Single-Center Registry. J. Am. Heart Assoc. 2016, 5, 7. [Google Scholar] [CrossRef]
- Dor, O.; Haim, M.; Barrett, O.; Novack, V.; Konstantino, Y. Incidence and Clinical Outcomes of Pacing Induced Cardiomyopathy in Patients With Normal Left Ventricular Systolic Function and Atrioventricular Block. Am. J. Cardiol. 2020, 128, 174–180. [Google Scholar] [CrossRef]
- Khurshid, S.; Epstein, A.E.; Verdino, R.J.; Lin, D.; Goldberg, L.R.; Marchlinski, F.E.; Frankel, D.S. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014, 11, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Merchant, F.M.; Mittal, S. Pacing induced cardiomyopathy. J. Cardiovasc. Electrophysiol. 2020, 31, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K. Cardiovascular Research Using the Korean National Health Information Database. Korean Circ. J. 2020, 50, 754–772. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Yoon, S.; Kim, L.Y.; Kim, D.S. Towards Actualizing the Value Potential of Korea Health Insurance Review and Assessment (HIRA) Data as a Resource for Health Research: Strengths, Limitations, Applications, and Strategies for Optimal Use of HIRA Data. J. Korean Med. Sci. 2017, 32, 718–728. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.H.; Myong, J.P.; Kim, Y.R.; Kim, T.S.; Kim, J.H.; Jang, S.W.; Oh, Y.S.; Lee, M.Y.; Rho, T.H. Outcomes of Direct Oral Anticoagulants in Patients with Mitral Stenosis. J. Am. Coll. Cardiol. 2019, 73, 1123–1131. [Google Scholar] [CrossRef]
- Park, J.; Kwon, S.; Choi, E.-K.; Choi, Y.-j.; Lee, E.; Choe, W.; Lee, S.-R.; Cha, M.-J.; Lim, W.-H.; Oh, S. Validation of diagnostic codes of major clinical outcomes in a National Health Insurance database. Int. J. Arrhythm. 2019, 20, 5. [Google Scholar] [CrossRef]
- Rebora, P.; Salim, A.; Reilly, M. Bshazard: A flexible tool for nonparametric smoothing of the hazard function. R. J. 2014, 6, 114–122. [Google Scholar] [CrossRef]
- Axtell, A.L.; Bhambhani, V.; Moonsamy, P.; Healy, E.W.; Picard, M.H.; Sundt, T.M., 3rd; Wasfy, J.H. Surgery Does Not Improve Survival in Patients With Isolated Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 715–725. [Google Scholar] [CrossRef]
- Snapinn, S.M.; Jiang, Q.; Iglewicz, B. Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am. Stat. 2005, 59, 301–307. [Google Scholar] [CrossRef]
- Heinzl, H.; Kaider, A.; Zlabinger, G. Assessing interactions of binary time-dependent covariates with time in cox proportional hazards regression models using cubic spline functions. Stat. Med. 1996, 15, 2589–2601. [Google Scholar] [CrossRef]
- Merchant, F.M.; Hoskins, M.H.; Musat, D.L.; Prillinger, J.B.; Roberts, G.J.; Nabutovsky, Y.; Mittal, S. Incidence and Time Course for Developing Heart Failure With High-Burden Right Ventricular Pacing. Cardiovasc. Qual. Outcomes 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Tayal, B.; Fruelund, P.; Sogaard, P.; Riahi, S.; Polcwiartek, C.; Atwater, B.D.; Gislason, G.; Risum, N.; Torp-Pedersen, C.; Kober, L.; et al. Incidence of heart failure after pacemaker implantation: A nationwide Danish Registry-based follow-up study. Eur. Heart J. 2019, 40, 3641–3648. [Google Scholar] [CrossRef]
- Sweeney, M.O.; Hellkamp, A.S.; Ellenbogen, K.A.; Greenspon, A.J.; Freedman, R.A.; Lee, K.L.; Lamas, G.A.; Investigators, M.O.S.T. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003, 107, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L.; Cook, J.R.; Epstein, A.E.; Greene, H.L.; Hallstrom, A.P.; Hsia, H.; Kutalek, S.P.; Sharma, A.; Dual, C.; Investigators, V.V.I.I.D.T. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002, 288, 3115–3123. [Google Scholar] [CrossRef]
- Sweeney, M.O.; Bank, A.J.; Nsah, E.; Koullick, M.; Zeng, Q.C.; Hettrick, D.; Sheldon, T.; Lamas, G.A.; Search, A.V.E. Managed Ventricular Pacing for Promoting Atrioventricular Conduction, T. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N. Engl. J. Med. 2007, 357, 1000–1008. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Thomsen, P.E.; Hojberg, S.; Moller, M.; Vesterlund, T.; Dalsgaard, D.; Mortensen, L.S.; Nielsen, T.; Asklund, M.; Friis, E.V.; et al. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur. Heart J. 2011, 32, 686–696. [Google Scholar] [CrossRef]
- Stockburger, M.; Boveda, S.; Moreno, J.; Da Costa, A.; Hatala, R.; Brachmann, J.; Butter, C.; Garcia Seara, J.; Rolando, M.; Defaye, P. Long-term clinical effects of ventricular pacing reduction with a changeover mode to minimize ventricular pacing in a general pacemaker population. Eur. Heart J. 2015, 36, 151–157. [Google Scholar] [CrossRef]
- Mei, D.A.; Imberti, J.F.; Vitolo, M.; Bonini, N.; Serafini, K.; Mantovani, M.; Tartaglia, E.; Birtolo, C.; Zuin, M.; Bertini, M.; et al. Systematic review and meta-analysis on the impact on outcomes of device algorithms for minimizing right ventricular pacing. Europace 2024, 26, euae212. [Google Scholar] [CrossRef]
- Chen, H.C.; Liu, W.H.; Tseng, C.H.; Chen, Y.L.; Lee, W.C.; Fang, Y.N.; Chong, S.Z.; Chen, M.C. Diabetes Increases Risk of Cardiovascular Events in Patients Receiving Permanent Pacemaker: A Propensity Score-Matched Cohort Study. J. Diabetes Res. 2022, 2022, 6758297. [Google Scholar] [CrossRef] [PubMed]
- Riahi, S.; Nielsen, J.C.; Hjortshøj, S.; Thomsen, P.E.; Højberg, S.; Møller, M.; Dalsgaard, D.; Nielsen, T.; Asklund, M.; Friis, E.V.; et al. Heart failure in patients with sick sinus syndrome treated with single lead atrial or dual-chamber pacing: No association with pacing mode or right ventricular pacing site. Europace 2012, 14, 1475–1482. [Google Scholar] [CrossRef]
- Murakami, Y.; Tsuboi, N.; Inden, Y.; Yoshida, Y.; Murohara, T.; Ihara, Z.; Takami, M. Difference in percentage of ventricular pacing between two algorithms for minimizing ventricular pacing: Results of the IDEAL RVP (Identify the Best Algorithm for Reducing Unnecessary Right Ventricular Pacing) study. Europace 2010, 12, 96–102. [Google Scholar] [CrossRef]
- Tokavanich, N.; Prasitlumkum, N.; Mongkonsritragoon, W.; Cheungpasitporn, W.; Thongprayoon, C.; Vallabhajosyula, S.; Chokesuwattanaskul, R. A network meta-analysis and systematic review of change in QRS duration after left bundle branch pacing, His bundle pacing, biventricular pacing, or right ventricular pacing in patients requiring permanent pacemaker. Sci. Rep. 2021, 11, 12200. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Patton, K.K.; Lau, C.P.; Dal Forno, A.R.J.; Al-Khatib, S.M.; Arora, V.; Birgersdotter-Green, U.M.; Cha, Y.M.; Chung, E.H.; Cronin, E.M.; et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm. 2023, 20, e17–e91. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, T.; Nagashima, M.; Kono, H.; Sadohara, Y.; Hirokami, J.; Kuji, R.; Korai, K.; Fukunaga, M.; Hiroshima, K.; Ando, K. Clinical outcome for heart failure hospitalizations in patients with leadless pacemaker. J. Arrhythm. 2022, 38, 730–735. [Google Scholar] [CrossRef]
- Saeed Al-Asad, K.; Martinez, A.; Prasad, R.M.; Ukponmwan, E.U.; Baloch, Z.Q.; Ali, A.; Ip, J. Pacing-Induced Cardiomyopathy in Leadless and Traditional Pacemakers: A Single-Center Retrospective Analysis. Cureus 2023, 15, e41393. [Google Scholar] [CrossRef]

| Variables | Overall (n = 32,216) | PaHF (n = 4170) | No PaHF (n = 28,046) | aSMD | p Value |
|---|---|---|---|---|---|
| Demographics and medical history | |||||
| Age, years | 70.6 ± 12.1 | 71.9 ± 11.2 | 70.4 ± 12.2 | 0.128 | <0.001 |
| Age ≥ 65 years | 24,033 (74.6) | 3314 (79.5) | 20,719 (73.9) | 0.133 | <0.001 |
| Male | 13,632 (42.3) | 1815 (43.5) | 11,817 (42.1) | 0.028 | 0.093 |
| Diabetes | 10,291 (31.9) | 1578 (37.8) | 8713 (31.1) | 0.143 | <0.001 |
| Hypertension | 24,711 (76.7) | 3557 (85.3) | 21,154 (75.4) | 0.250 | <0.001 |
| Coronary artery disease | 13,003 (40.4) | 2170 (52.0) | 10,833 (38.6) | 0.272 | <0.001 |
| Peripheral artery disease | 7453 (23.1) | 1015 (24.3) | 6438 (23.0) | 0.033 | 0.050 |
| CKD/ESRD | 2263 (7.0) | 390 (9.4) | 1873 (6.7) | 0.099 | <0.001 |
| Valvular heart disease | 2976 (9.2) | 631 (15.1) | 2345 (8.4) | 0.211 | <0.001 |
| Atrial fibrillation | 7297 (22.7) | 1169 (28.0) | 6128 (21.8) | 0.143 | <0.001 |
| COPD | 6294 (19.5) | 1007 (24.1) | 5287 (18.9) | 0.129 | <0.001 |
| CCI ≥ 3 unit | 19,612 (60.9) | 2885 (69.2) | 16,727 (59.6) | 0.200 | <0.001 |
| Pacemaker-related variables | |||||
| AV block | 20,246 (63.4) | 2502 (60.0) | 17,744 (63.3) | 0.068 | <0.001 |
| Sinus node dysfunction | 11,675 (36.6) | 1631 (39.1) | 10,044 (35.8) | ||
| Dual chamber | 27,073 (84.0) | 3136 (75.2) | 23,937 (85.3) | 0.262 | <0.001 |
| Medications | |||||
| ACEIs or ARBs | 21,490 (66.7) | 3309 (79.4) | 18,181 (64.8) | 0.328 | <0.001 |
| Beta blockers | 13,905 (43.2) | 2390 (57.3) | 11,515 (41.1) | 0.330 | <0.001 |
| MRAs | 5274 (16.4) | 1222 (29.3) | 4072 (14.5) | 0.363 | <0.001 |
| Loop diuretics | 12,611 (39.1) | 2389 (57.3) | 10,222 (36.4) | 0.427 | <0.001 |
| Thiazide | 6200 (19.2) | 1038 (24.9) | 5162 (18.4) | 0.158 | <0.001 |
| Antiplatelet agents | 19,301 (59.9) | 2990 (71.7) | 16,311 (58.2) | 0.287 | <0.001 |
| Anticoagulants | 6382 (19.8) | 1127 (27.0) | 5255 (18.7) | 0.198 | <0.001 |
| PaHF (Strictly Defined) | PaHF (Broadly Defined) | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Unadjusted analysis | 3.87 | 3.64–4.12 | <0.001 | 5.90 | 5.59–6.23 | <0.001 |
| Multivariable analysis model 1 * | 3.11 | 2.93–3.32 | <0.001 | 4.85 | 4.59–5.13 | <0.001 |
| Multivariable analysis model 2 * | 2.92 | 2.74–3.11 | <0.001 | 4.39 | 4.16–4.65 | <0.001 |
| Multivariable analysis model 3 * | 2.78 | 2.61–2.96 | <0.001 | 4.25 | 4.02–4.50 | <0.001 |
| Multivariable analysis model 4 * | 2.95 | 2.77–3.14 | <0.001 | 4.66 | 4.41–4.93 | <0.001 |
| Variable | Univariable Analyses | Multivariable Analysis Model 1 | Multivariable Analysis Model 2 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (continuous) | 1.03 (1.02–1.03) | <0.001 | 1.02 (1.02–1.02) | <0.001 | ||
| Age ≥ 65 years | 1.77 (1.64–1.91) | <0.001 | 1.43 (1.32–1.55) | <0.001 | ||
| Male | 1.15 (1.08–1.22) | <0.001 | 1.09 (1.03–1.16) | 0.005 | 1.08 (1.01–1.15) | 0.023 |
| Diabetes mellitus | 1.53 (1.44–1.63) | <0.001 | 1.15 (1.07–1.23) | <0.001 | 1.14 (1.06–1.22) | <0.001 |
| Hypertension | 2.06 (1.90–2.25) | <0.001 | 1.41 (1.29–1.54) | <0.001 | 1.49 (1.36–1.64) | <0.001 |
| CAD | 1.66 (1.56–1.77) | <0.001 | 1.35 (1.26–1.43) | <0.001 | 1.36 (1.28–1.45) | <0.001 |
| PAD | 1.19 (1.11–1.27) | <0.001 | 0.97 (0.90–1.04) | 0.355 | 0.97 (0.90–1.05) | 0.471 |
| CKD/ESRD | 2.13 (1.92–2.37) | <0.001 | 1.48 (1.32–1.65) | <0.001 | 1.49 (1.34–1.67) | <0.001 |
| Valvular heart disease | 1.80 (1.65–1.96) | <0.001 | 1.69 (1.55–1.84) | <0.001 | 1.65 (1.51–1.80) | <0.001 |
| Atrial fibrillation | 1.92 (1.79–2.06) | <0.001 | 1.70 (1.58–1.83) | <0.001 | 1.70 (1.58–1.84) | <0.001 |
| COPD | 1.44 (1.34–1.54) | <0.001 | 1.18 (1.10–1.27) | <0.001 | 1.20 (1.11–1.29) | <0.001 |
| AVB versus SND | 0.90 (0.85–0.96) | 0.001 | 0.99 (0.92–1.05) | 0.679 | 1.00 (0.94–1.07) | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.J.; Lee, S.; Hong, S.; Kim, K.; Kim, J.; Kim, J.Y.; Park, K.-M.; On, Y.K.; Park, S.-J. Temporal Trends and Prognostic Impact of Pacemaker-Associated Heart Failure: Insights from a Nationwide Cohort Study. J. Clin. Med. 2025, 14, 7744. https://doi.org/10.3390/jcm14217744
Park YJ, Lee S, Hong S, Kim K, Kim J, Kim JY, Park K-M, On YK, Park S-J. Temporal Trends and Prognostic Impact of Pacemaker-Associated Heart Failure: Insights from a Nationwide Cohort Study. Journal of Clinical Medicine. 2025; 14(21):7744. https://doi.org/10.3390/jcm14217744
Chicago/Turabian StylePark, Young Jun, Sungjoo Lee, Sungjun Hong, Kyunga Kim, Juwon Kim, Ju Youn Kim, Kyoung-Min Park, Young Keun On, and Seung-Jung Park. 2025. "Temporal Trends and Prognostic Impact of Pacemaker-Associated Heart Failure: Insights from a Nationwide Cohort Study" Journal of Clinical Medicine 14, no. 21: 7744. https://doi.org/10.3390/jcm14217744
APA StylePark, Y. J., Lee, S., Hong, S., Kim, K., Kim, J., Kim, J. Y., Park, K.-M., On, Y. K., & Park, S.-J. (2025). Temporal Trends and Prognostic Impact of Pacemaker-Associated Heart Failure: Insights from a Nationwide Cohort Study. Journal of Clinical Medicine, 14(21), 7744. https://doi.org/10.3390/jcm14217744






