Epistaxis Prevention, Treatment, and Future Perspectives for Hereditary Hemorrhagic Telangiectasia
Abstract
1. Introduction
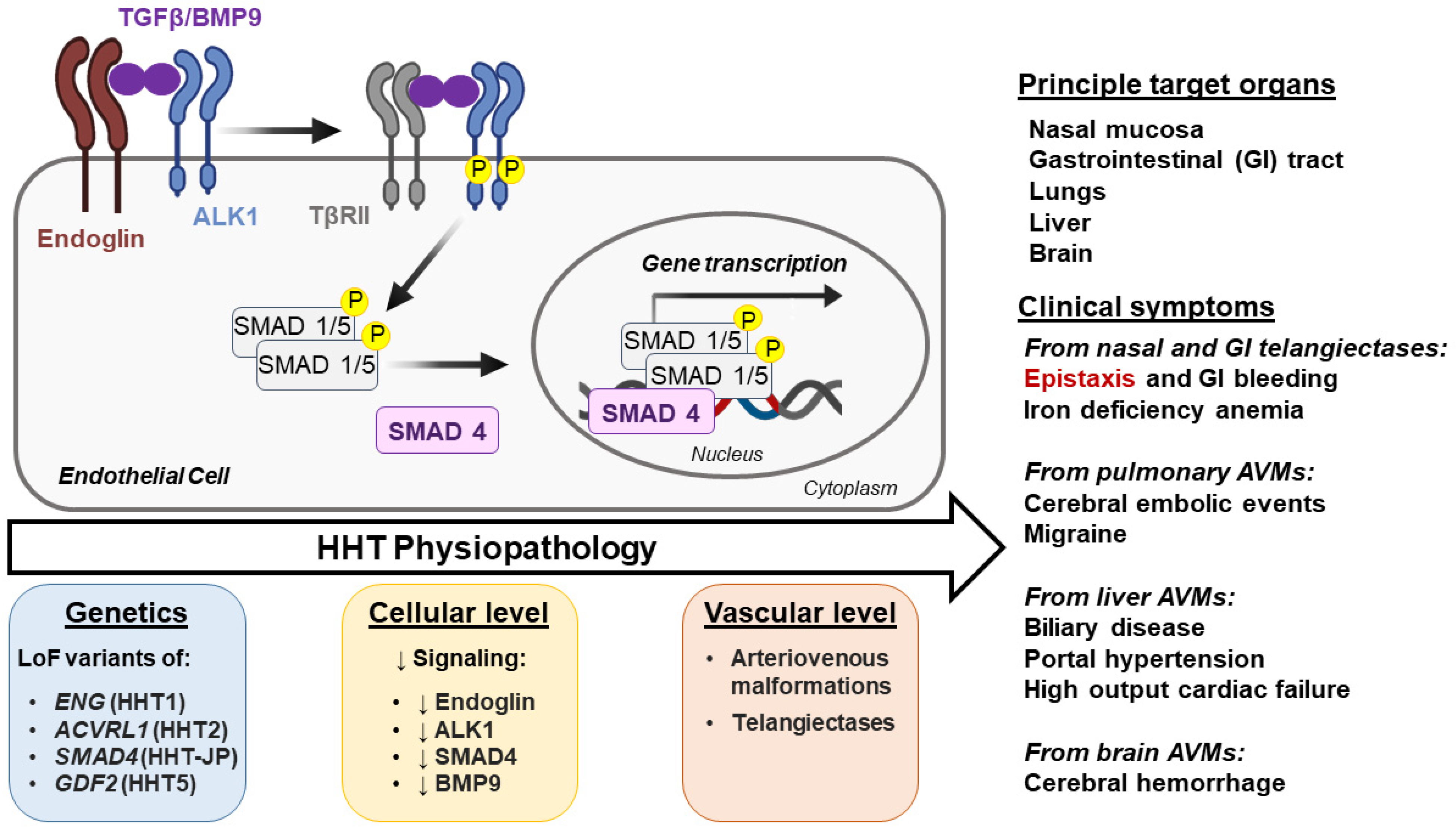
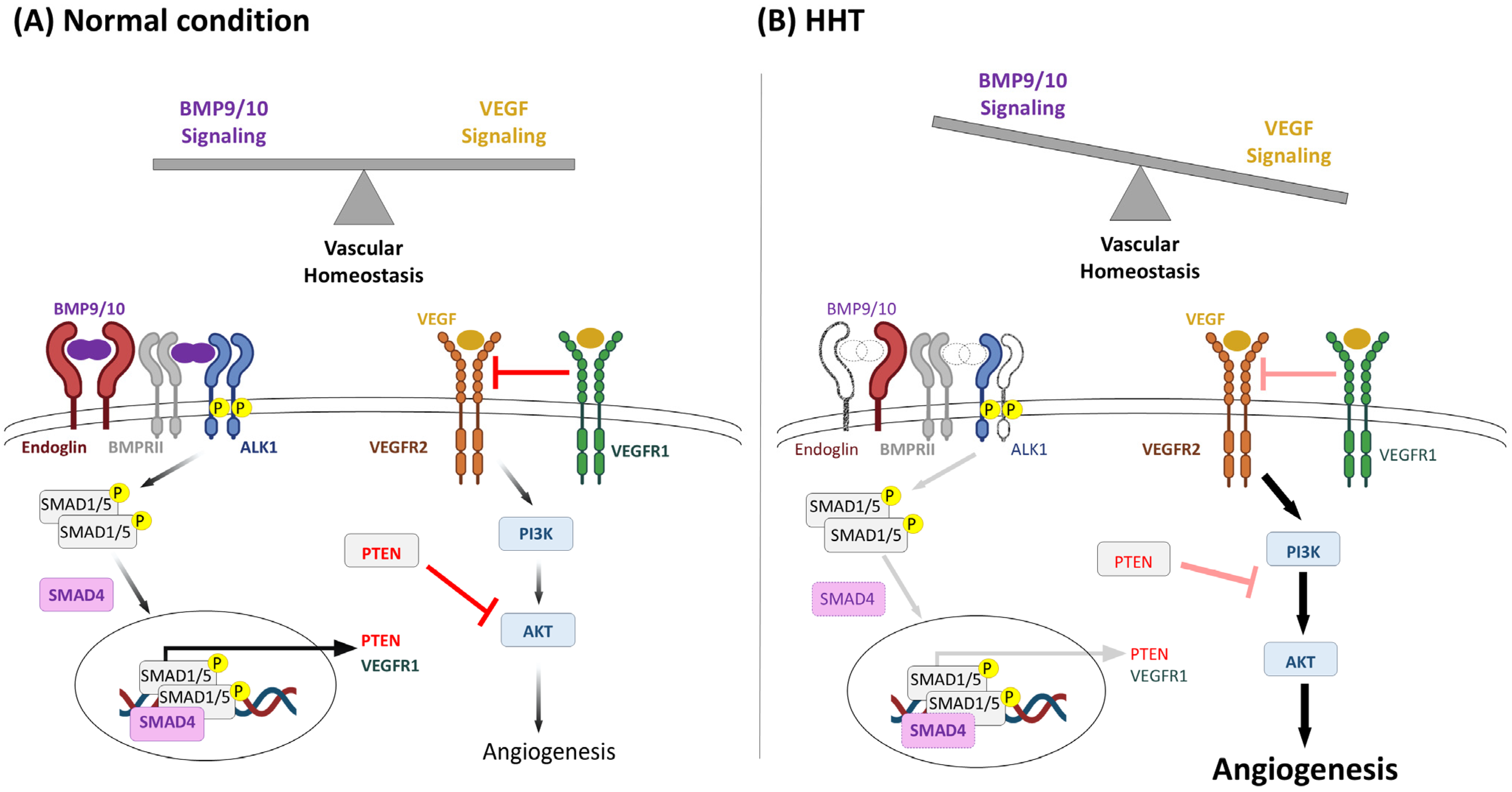
1.1. Genetics and Clinical Manifestations
1.2. Burden of HHT-Induced Epistaxis
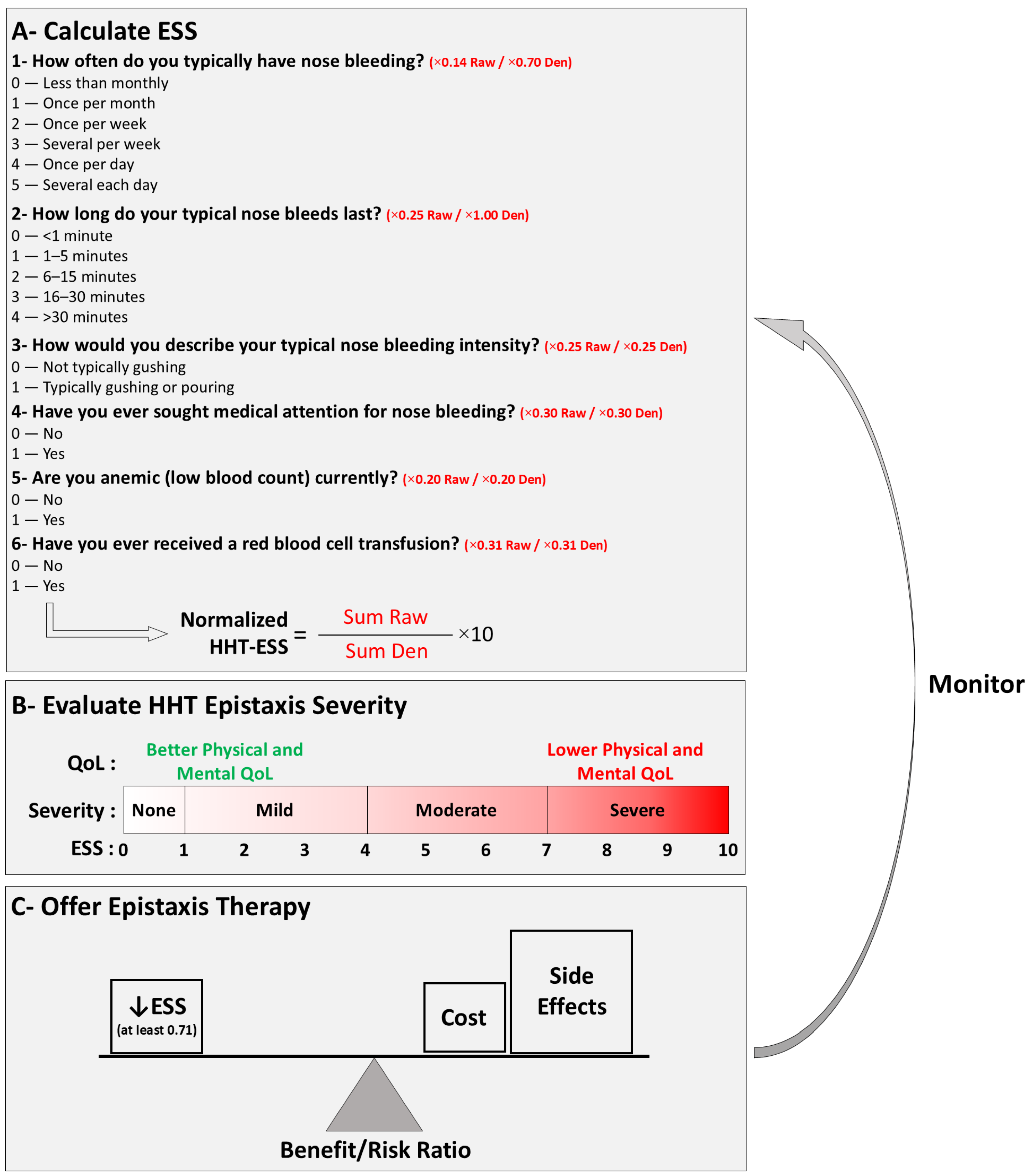
1.3. Prevention of Nosebleeds
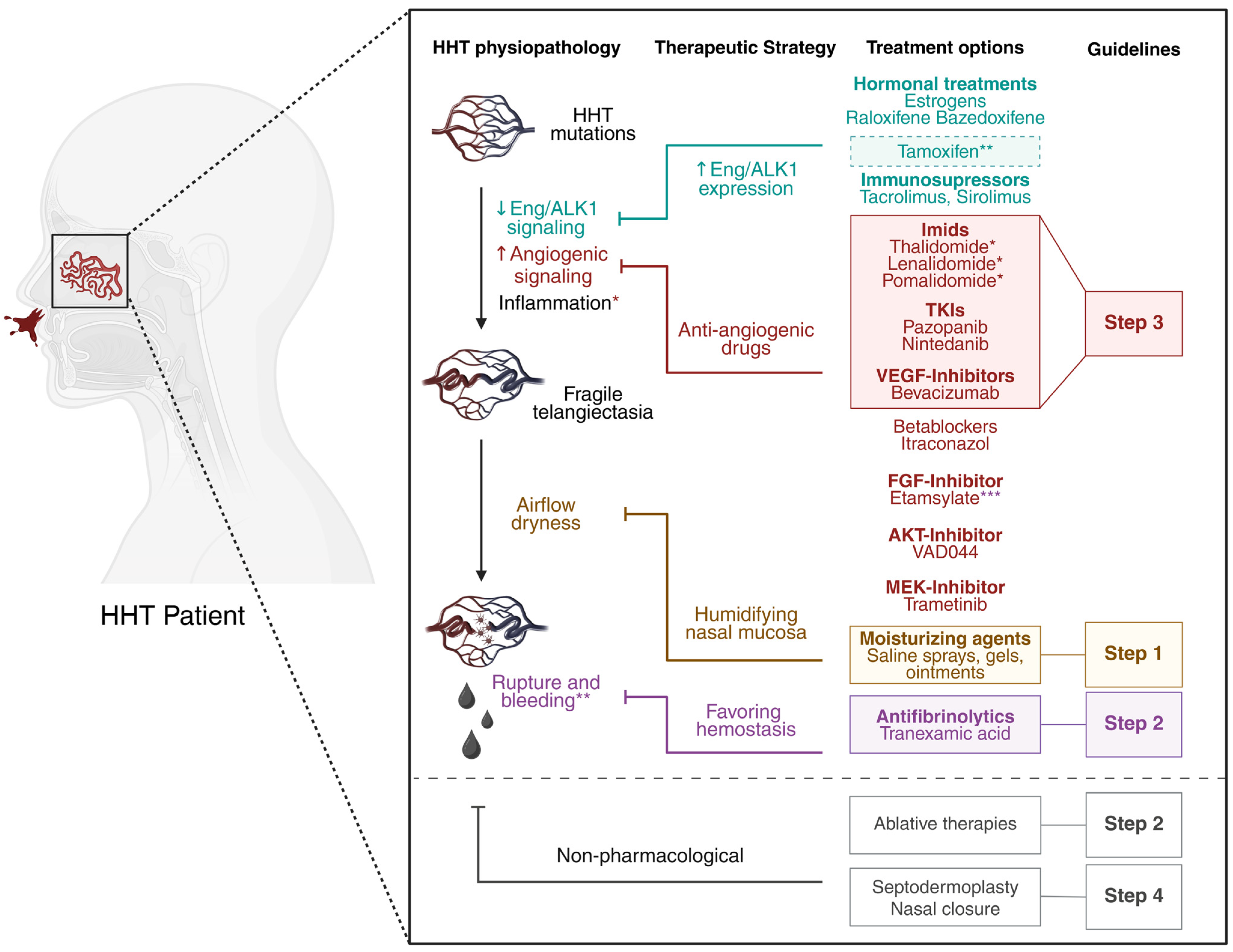
2. Treatment of HHT-Related Epistaxis
2.1. Topical Moisturizing Agents
2.2. Tranexamic Acid
2.3. Nasal Telangiectasia, Ablative Therapies, and Surgical Procedures
2.4. Bevacizumab
3. Treatment Options Under Investigation
3.1. Immunomodulatory Imide Drugs (IMiDs): Thalidomide and Derivatives
3.2. Kinase Inhibitors
3.2.1. Pazopanib and Nintedanib
3.2.2. VAD044
3.2.3. Trametinib
3.2.4. Beta Blockers
3.2.5. Itraconazole
3.2.6. Etamsylate
3.3. Treatments Targeting Endoglin and ALK1 Haploinsufficiency
3.3.1. Hormonal Treatments
3.3.2. Tacrolimus and Sirolimus
3.3.3. Nitric Oxide Targeting for HHT1
4. Discussion and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Glossary
| ALK1 | Activin Receptor-Like Kinase 1 |
| ATF4 | Activating Transcription Factor-4 |
| AVMs | Arteriovenous Malformations |
| BMP | Bone Morphogenetic Protein |
| BOECs | Blood Outgrowth Endothelial Cells |
| EE | Ethinyl Estradiol |
| EMA | European Medicine Agency |
| eNOS | Endothelial NO Synthase |
| ENT ER | Ear Nose Throat Estrogen Receptor |
| EREs | Estrogen-Responsive Elements |
| ERK | Extracellular Signal-Regulated Kinase |
| ESS | Epistaxis Severity Score |
| FDA | Food and Drug Administration |
| FGF | Fibroblast Growth Factor |
| HB-EGF | Heparin Binding EGF Like Growth Factor |
| HHT | Hereditary Hemorrhagic Telangiectasia |
| ICTRP | WHO International Clinical Trials Registry Platform |
| IH | Infantile Hemangiomas |
| IPF | Idiopathic Pulmonary Fibrosis |
| IMiDs | Immunomodulatory Imide Drugs |
| JP-HHT | HHT-Juvenile Polyposis |
| MAPK | Mitogen Activated Protein Kinase |
| MEK | Mitogen-Activated Extracellular Signal-Regulated Kinase 1 |
| MMPs | Metalloproteinases |
| NAC | N-acetylcysteine |
| NMD | Nonsense Mediated Decay |
| PDGF-B | Platelet Derived Growth Factor-B |
| PI3K | Phosphatidyl Inositol 3-Kinase |
| PTC | Premature Termination Codon |
| QoL RCT | Quality of Life Randomized Controlled Trial |
| SERM | Selective Estrogen Receptor Modulator |
| TA | Tranexamic Acid |
| TGF-β | Transforming Growth Factor β |
| TKIs | Tyrosine Kinase Inhibitors |
| TKR | Tyrosine Kinase Receptors |
| TNFα | Tumor Necrosis Factor-α |
| VASCERN | European Reference Network on Rare Multisystemic Vascular Diseases |
| VEGF-A | Vascular Endothelial Growth Factor-A |
References
- Hermann, R.; Shovlin, C.L.; Kasthuri, R.S.; Serra, M.; Eker, O.F.; Bailly, S.; Buscarini, E.; Dupuis-Girod, S. Hereditary haemorrhagic telangiectasia. Nat. Rev. Dis. Primer 2025, 11, 1. [Google Scholar] [CrossRef]
- Faughnan, M.E.; Mager, J.J.; Hetts, S.W.; Palda, V.A.; Lang-Robertson, K.; Buscarini, E.; Deslandres, E.; Kasthuri, R.S.; Lausman, A.; Poetker, D.; et al. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann. Intern. Med. 2020, 173, 989–1001. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Guttmacher, A.E.; Buscarini, E.; Faughnan, M.E.; Hyland, R.H.; Westermann, C.J.; Kjeldsen, A.D.; Plauchu, H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am. J. Med. Genet. 2000, 91, 66–67. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Buscarini, E.; Sabbà, C.; Mager, H.J.; Kjeldsen, A.D.; Pagella, F.; Sure, U.; Ugolini, S.; Torring, P.M.; Suppressa, P.; et al. The European Rare Disease Network for HHT Frameworks for management of hereditary haemorrhagic telangiectasia in general and speciality care. Eur. J. Med. Genet. 2022, 65, 104370. [Google Scholar] [CrossRef]
- Anderson, E.; Sharma, L.; Alsafi, A.; Shovlin, C.L. Pulmonary arteriovenous malformations may be the only clinical criterion present in genetically confirmed hereditary haemorrhagic telangiectasia. Thorax 2022, 77, 628–630. [Google Scholar] [CrossRef]
- Pollak, M.; Gatt, D.; Shaw, M.; Hewko, S.L.; Lamanna, A.; Santos, S.; Ratjen, F. Longitudinal Assessment of Curaçao Criteria in Children with Hereditary Hemorrhagic Telangiectasia. J. Pediatr. 2023, 263, 113665. [Google Scholar] [CrossRef]
- Bideau, A.; Brunet, G.; Heyer, E.; Plauchu, H.; Robert, J.M. An abnormal concentration of cases of Rendu-Osler disease in the Valserine valley of the French Jura: A genealogical and demographic study. Ann. Hum. Biol. 1992, 19, 233–247. [Google Scholar] [CrossRef]
- Westermann, C.J.J.; Rosina, A.F.; De Vries, V.; de Coteau, P.A. The prevalence and manifestations of hereditary hemorrhagic telangiectasia in the Afro-Caribbean population of the Netherlands Antilles: A family screening. Am. J. Med. Genet. A 2003, 116A, 324–328. [Google Scholar] [CrossRef]
- Donaldson, J.W.; McKeever, T.M.; Hall, I.P.; Hubbard, R.B.; Fogarty, A.W. The UK prevalence of hereditary haemorrhagic telangiectasia and its association with sex, socioeconomic status and region of residence: A population-based study. Thorax 2014, 69, 161–167. [Google Scholar] [CrossRef]
- Anzell, A.R.; White, C.M.; Diergaarde, B.; Carlson, J.C.; Roman, B.L. Hereditary Hemorrhagic Telangiectasia Prevalence Estimates Calculated From GnomAD Allele Frequencies of Predicted Pathogenic Variants in ENG and ACVRL1. Circ. Genom. Precis. Med. 2025, 18, e005061. [Google Scholar] [CrossRef] [PubMed]
- Bernabéu-Herrero, M.E.; Patel, D.; Bielowka, A.; Zhu, J.; Jain, K.; Mackay, I.S.; Chaves Guerrero, P.; Emanuelli, G.; Jovine, L.; Noseda, M.; et al. Mutations causing premature termination codons discriminate and generate cellular and clinical variability in HHT. Blood 2024, 143, 2314–2331. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Simeoni, I.; Downes, K.; Frazer, Z.C.; Megy, K.; Bernabeu-Herrero, M.E.; Shurr, A.; Brimley, J.; Patel, D.; Kell, L.; et al. Mutational and phenotypic characterization of hereditary hemorrhagic telangiectasia. Blood 2020, 136, 1907–1918. [Google Scholar] [CrossRef]
- Balachandar, S.; Graves, T.J.; Shimonty, A.; Kerr, K.; Kilner, J.; Xiao, S.; Slade, R.; Sroya, M.; Alikian, M.; Curetean, E.; et al. Identification and validation of a novel pathogenic variant in GDF2 (BMP9) responsible for hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations. Am. J. Med. Genet. A 2022, 188, 959–964. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Almaghlouth, F.I.; Alsafi, A.; Coote, N.; Rennie, C.; Wallace, G.M.; Govani, F.S.; Consortium, G.E.R. Updates on diagnostic criteria for hereditary haemorrhagic telangiectasia in the light of whole genome sequencing of ‘gene-negative’ individuals recruited to the 100 000 Genomes Project. J. Med. Genet. 2024, 61, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Bayrak-Toydemir, P.; McDonald, J.; Akarsu, N.; Toydemir, R.M.; Calderon, F.; Tuncali, T.; Tang, W.; Miller, F.; Mao, R. A fourth locus for hereditary hemorrhagic telangiectasia maps to chromosome 7. Am. J. Med. Genet. A 2006, 140, 2155–2162. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Jackson, J.E.; Bamford, K.B.; Jenkins, I.H.; Benjamin, A.R.; Ramadan, H.; Kulinskaya, E. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 2008, 63, 259–266. [Google Scholar] [CrossRef]
- Vascular Anomalies (VASCA-WG) | VASCERN. Available online: https://vascern.eu/group/vascular-anomalies/ (accessed on 14 May 2025).
- Shovlin, C.L.; Patel, D.; Bielowka, A.; Ledermann, J.A.; Modarresi, A.; Genomics England Research Consortium; Bernabeu-Herrero, M.E.; Aldred, M.A.; Alsafi, A. MEK 1 inhibition and bleeding in hereditary haemorrhagic telangiectasia. Br. J. Haematol. 2024, 204, 361–365. [Google Scholar] [CrossRef]
- Kritharis, A.; Al-Samkari, H.; Kuter, D.J. Hereditary hemorrhagic telangiectasia: Diagnosis and management from the hematologist’s perspective. Haematologica 2018, 103, 1433–1443. [Google Scholar] [CrossRef]
- Jackson, S.B.; Villano, N.P.; Benhammou, J.N.; Lewis, M.; Pisegna, J.R.; Padua, D. Gastrointestinal Manifestations of Hereditary Hemorrhagic Telangiectasia (HHT): A Systematic Review of the Literature. Dig. Dis. Sci. 2017, 62, 2623. [Google Scholar] [CrossRef]
- AAssar, O.S.; Friedman, C.M.; White, R.I. The natural history of epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 1991, 101, 977–980. [Google Scholar] [CrossRef]
- Hoag, J.B.; Terry, P.; Mitchell, S.; Reh, D.; Merlo, C.A. An epistaxis severity score for hereditary hemorrhagic telangiectasia. Laryngoscope 2010, 120, 838–843. [Google Scholar] [CrossRef]
- Merlo, C.A.; Yin, L.X.; Hoag, J.B.; Mitchell, S.E.; Reh, D.D. The effects of epistaxis on health-related quality of life in patients with hereditary hemorrhagic telangiectasia. Int. Forum Allergy Rhinol. 2014, 4, 921–925. [Google Scholar] [CrossRef]
- Devlin, H.L.; Hosman, A.E.; Shovlin, C.L. Antiplatelet and anticoagulant agents in hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 2013, 368, 876–878. [Google Scholar] [CrossRef]
- Livesey, J.A.; Manning, R.A.; Meek, J.H.; Jackson, J.E.; Kulinskaya, E.; Laffan, M.A.; Shovlin, C.L. Low serum iron levels are associated with elevated plasma levels of coagulation factor VIII and pulmonary emboli/deep venous thromboses in replicate cohorts of patients with hereditary haemorrhagic telangiectasia. Thorax 2012, 67, 328–333. [Google Scholar] [CrossRef]
- Buscarini, E.; Leandro, G.; Conte, D.; Danesino, C.; Daina, E.; Manfredi, G.; Lupinacci, G.; Brambilla, G.; Menozzi, F.; De Grazia, F.; et al. Natural History and Outcome of Hepatic Vascular Malformations in a Large Cohort of Patients with Hereditary Hemorrhagic Teleangiectasia. Dig. Dis. Sci. 2011, 56, 2166–2178. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Condliffe, R.; Donaldson, J.W.; Kiely, D.G.; Wort, S.J. British Thoracic Society British Thoracic Society Clinical Statement on Pulmonary Arteriovenous Malformations. Thorax 2017, 72, 1154–1163. [Google Scholar] [CrossRef]
- Al-Sahaf, M.; Anderson, J.; Nandi, J.; Alsafi, A.; Shovlin, C.L. Elective Thoracic Surgical Resections for Pulmonary Arteriovenous Malformations—A 16 Year Single-Center Experience. Pulm. Circ. 2025, 15, e70037. [Google Scholar] [CrossRef] [PubMed]
- Shovlin, C.L.; Chamali, B.; Santhirapala, V.; Livesey, J.A.; Angus, G.; Manning, R.; Laffan, M.A.; Meek, J.; Tighe, H.C.; Jackson, J.E. Ischaemic strokes in patients with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia: Associations with iron deficiency and platelets. PLoS ONE 2014, 9, e88812. [Google Scholar] [CrossRef] [PubMed]
- Topiwala, K.K.; Patel, S.D.; Saver, J.L.; Streib, C.D.; Shovlin, C.L. Ischemic Stroke and Pulmonary Arteriovenous Malformations: A Review. Neurology 2022, 98, 188–198. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Patel, T.; Jackson, J.E. Embolisation of PAVMs reported to improve nosebleeds by a subgroup of patients with hereditary haemorrhagic telangiectasia. ERJ Open Res. 2016, 2, 00035–02016. [Google Scholar] [CrossRef] [PubMed]
- Shovlin, C.L.; Gilson, C.; Busbridge, M.; Patel, D.; Shi, C.; Dina, R.; Abdulla, F.N.; Awan, I. Can Iron Treatments Aggravate Epistaxis in Some Patients With Hereditary Hemorrhagic Telangiectasia? Laryngoscope 2016, 126, 2468–2474. [Google Scholar] [CrossRef]
- Yin, L.X.; Reh, D.D.; Hoag, J.B.; Mitchell, S.E.; Mathai, S.C.; Robinson, G.M.; Merlo, C.A. The minimal important difference of the epistaxis severity score in hereditary hemorrhagic telangiectasia. Laryngoscope 2016, 126, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Kasthuri, R.S.; Mager, H.-J.; Zhou, J.Y.; Serra, M.M.; Samuelson-Bannow, B.T.; Van Doren, L.N.; Piccirillo, J.F.; Clancy, M.S.; McCrae, K.R.; et al. Standardization of Terminology, Definitions, and Outcome Criteria for Bleeding in Hereditary Hemorrhagic Telangiectasia: International Consensus Report. Am. J. Hematol. 2025, 100, 1813–1827. [Google Scholar] [CrossRef]
- Peterson, A.M.; Chakinala, M.M.; Piccirillo, J.F. A Framework for Clinical Trials in Hereditary Hemorrhagic Telangiectasia-Associated Epistaxis-Navigating the PATH. JAMA Otolaryngol.-Head Neck Surg. 2025, 151, 425–426. [Google Scholar] [CrossRef]
- Finnamore, H.; Le Couteur, J.; Hickson, M.; Busbridge, M.; Whelan, K.; Shovlin, C.L. Hemorrhage-Adjusted Iron Requirements, Hematinics and Hepcidin Define Hereditary Hemorrhagic Telangiectasia as a Model of Hemorrhagic Iron Deficiency. PLoS ONE 2013, 8, e76516. [Google Scholar] [CrossRef]
- Chitsuthipakorn, W.; Hoang, M.P.; Kanjanawasee, D.; Seresirikachorn, K.; Snidvongs, K. Treatments of Epistaxis in Hereditary Hemorrhagic Telangiectasia: Systematic Review and Network Meta-Analysis. Curr. Allergy Asthma Rep. 2023, 23, 689–701. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Rivière, S.; Lavigne, C.; Fargeton, A.-E.; Gilbert-Dussardier, B.; Grobost, V.; Leguy-Seguin, V.; Maillard, H.; Mohamed, S.; Decullier, E.; et al. Efficacy and safety of intravenous bevacizumab on severe bleeding associated with hemorrhagic hereditary telangiectasia: A national, randomized multicenter trial. J. Intern. Med. 2023, 294, 761–774. [Google Scholar] [CrossRef]
- Vincent, L.; Robard, L.; Creveuil, C.; Babin, E.; Perreard, M.; Humbert, M. Treatment of Epistaxis in Osler-Weber-Rendu Disease by Bevacizumab Nasal Spray. The EROSB Study: Determining the Effective Dose. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2025, 142, 67–73. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Ambrun, A.; Decullier, E.; Fargeton, A.-E.; Roux, A.; Bréant, V.; Colombet, B.; Rivière, S.; Cartier, C.; Lacombe, P.; et al. Effect of Bevacizumab Nasal Spray on Epistaxis Duration in Hereditary Hemorrhagic Telangectasia: A Randomized Clinical Trial. JAMA 2016, 316, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Dupuis-Girod, S.; Ambrun, A.; Decullier, E.; Samson, G.; Roux, A.; Fargeton, A.-E.; Rioufol, C.; Schwiertz, V.; Disant, F.; Chapuis, F.; et al. ELLIPSE Study: A Phase 1 study evaluating the tolerance of bevacizumab nasal spray in the treatment of epistaxis in hereditary hemorrhagic telangiectasia. mAbs 2014, 6, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Riss, D.; Burian, M.; Wolf, A.; Kranebitter, V.; Kaider, A.; Arnoldner, C. Intranasal submucosal bevacizumab for epistaxis in hereditary hemorrhagic telangiectasia: A double-blind, randomized, placebo-controlled trial. Head Neck 2015, 37, 783–787. [Google Scholar] [CrossRef]
- Whitehead, K.J.; Sautter, N.B.; McWilliams, J.P.; Chakinala, M.M.; Merlo, C.A.; Johnson, M.H.; James, M.; Everett, E.M.; Clancy, M.S.; Faughnan, M.E.; et al. Effect of Topical Intranasal Therapy on Epistaxis Frequency in Patients With Hereditary Hemorrhagic Telangiectasia: A Randomized Clinical Trial. JAMA 2016, 316, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.P.; Sykes, J.; Chandakkar, P.; Marambaud, P.; Vozoris, N.T.; Marchuk, D.A.; Faughnan, M.E. Randomized, double-blind, placebo-controlled, crossover trial of oral doxycycline for epistaxis in hereditary hemorrhagic telangiectasia. Orphanet J. Rare Dis. 2022, 17, 405. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, J.P.; Majumdar, S.; Kim, G.H.; Lee, J.; Seals, K.; Tangchaiburana, S.; Gilbert, S.; Duckwiler, G.R. North American Study for the Treatment of Recurrent Epistaxis with Doxycycline: The NOSTRIL trial. J. Thromb. Haemost. 2022, 20, 1115–1125. [Google Scholar] [CrossRef]
- Lee, J.M.; Wu, V.; Faughnan, M.E.; Lasso, A.; Figol, A.; Kilty, S.J. Prospective Pilot Study of Floseal® for the Treatment of Anterior Epistaxis in Patients with Hereditary Hemorrhagic Telangiectasia (HHT). J. Otolaryngol. Head Neck Surg. 2019, 48, 48. [Google Scholar] [CrossRef]
- Kroon, S.; Snijder, R.J.; Hosman, A.E.; Vorselaars, V.M.M.; Disch, F.J.M.; Post, M.C.; Mager, J.J. Oral itraconazole for epistaxis in hereditary hemorrhagic telangiectasia: A proof of concept study. Angiogenesis 2021, 24, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; Grobost, V.; Le-Guillou, X.; Lavigne, C.; Parrot, A.; Rivière, S.; Séguier, J.; Fargeton, A.-E.; de-Montigny, A.; Huot, M.; et al. Effect of oral nintedanib vs. placebo on epistaxis in hereditary hemorrhagic telangiectasia: The EPICURE multicenter randomized double-blind trial. Angiogenesis 2025, 28, 9. [Google Scholar] [CrossRef]
- Faughnan, M.E.; Gossage, J.R.; Chakinala, M.M.; Oh, S.P.; Kasthuri, R.; Hughes, C.C.W.; McWilliams, J.P.; Parambil, J.G.; Vozoris, N.; Donaldson, J.; et al. Pazopanib may reduce bleeding in hereditary hemorrhagic telangiectasia. Angiogenesis 2019, 22, 145–155. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Kasthuri, R.S.; Iyer, V.N.; Pishko, A.M.; Decker, J.E.; Weiss, C.R.; Whitehead, K.J.; Conrad, M.B.; Zumberg, M.S.; Zhou, J.Y.; et al. Pomalidomide for Epistaxis in Hereditary Hemorrhagic Telangiectasia. N. Engl. J. Med. 2024, 391, 1015–1027. [Google Scholar] [CrossRef]
- Boyer, H.; Fernandes, P.; Le, C.; Yueh, B. Prospective randomized trial of sclerotherapy vs. standard treatment for epistaxis due to hereditary hemorrhagic telangiectasia. Int. Forum Allergy Rhinol. 2015, 5, 435–440. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Fargeton, A.-E.; Grobost, V.; Rivière, S.; Beaudoin, M.; Decullier, E.; Bernard, L.; Bréant, V.; Colombet, B.; Philouze, P.; et al. Efficacy and Safety of a 0.1% Tacrolimus Nasal Ointment as a Treatment for Epistaxis in Hereditary Hemorrhagic Telangiectasia: A Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial. J. Clin. Med. 2020, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, E.; Preis, M.; Hadar, T.; Shvero, J.; Haddad, M. Antiestrogen therapy for hereditary hemorrhagic telangiectasia: A double-blind placebo-controlled clinical trial. Laryngoscope 2009, 119, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, E.; Preis, M.; Shevro, J.; Nageris, B.; Hadar, T. Anti-estrogen therapy for hereditary hemorrhagic telangiectasia—A long-term clinical trial. Rhinology 2011, 49, 214–216. [Google Scholar] [CrossRef]
- Invernizzi, R.; Quaglia, F.; Klersy, C.; Pagella, F.; Ornati, F.; Chu, F.; Matti, E.; Spinozzi, G.; Plumitallo, S.; Grignani, P.; et al. Efficacy and safety of thalidomide for the treatment of severe recurrent epistaxis in hereditary hemorrhagic telangiectasia: Results of a prospective phase II clinical trial. Lancet Haematol. 2015, 2, e465–e473. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Pitiot, V.; Bergerot, C.; Fargeton, A.-E.; Beaudoin, M.; Decullier, E.; Bréant, V.; Colombet, B.; Philouze, P.; Faure, F.; et al. Efficacy of TIMOLOL nasal spray as a treatment for epistaxis in hereditary hemorrhagic telangiectasia. A double-blind, randomized, placebo-controlled trial. Sci. Rep. 2019, 9, 11986. [Google Scholar] [CrossRef]
- Peterson, A.M.; Lee, J.J.; Kallogjeri, D.; Schneider, J.S.; Chakinala, M.M.; Piccirillo, J.F. Efficacy of Timolol in a Novel Intranasal Thermosensitive Gel for Hereditary Hemorrhagic Telangiectasia–Associated Epistaxis: A Randomized Clinical Trial. JAMA Otolaryngol.-Head Neck Surg. 2020, 146, 1006–1014. [Google Scholar] [CrossRef]
- Geisthoff, U.W.; Seyfert, U.T.; Kübler, M.; Bieg, B.; Plinkert, P.K.; König, J. Treatment of epistaxis in hereditary hemorrhagic telangiectasia with tranexamic acid—A double-blind placebo-controlled cross-over phase IIIB study. Thromb. Res. 2014, 134, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Dupuis-Girod, S.; Boutitie, F.; Rivière, S.; Morinière, S.; Hatron, P.-Y.; Manfredi, G.; Kaminsky, P.; Capitaine, A.-L.; Roy, P.; et al. Tranexamic acid for epistaxis in hereditary hemorrhagic telangiectasia patients: A European cross-over controlled trial in a rare disease. J. Thromb. Haemost. 2014, 12, 1494–1502. [Google Scholar] [CrossRef]
- Ng, W.; Jerath, A.; Wąsowicz, M. Tranexamic acid: A clinical review. Anaesthesiol. Intensive Ther. 2015, 47, 339–350. [Google Scholar] [CrossRef]
- Song, Y.; Izumi, N.; Potts, L.B.; Yoshida, A. Tranexamic acid-induced ligneous conjunctivitis with renal failure showed reversible hypoplasminogenaemia. BMJ Case Rep. 2014, 2014, bcr2014204138. [Google Scholar] [CrossRef]
- Kiser, A.S.; Cooper, G.L.; Napier, J.D.; Howington, G.T. Color vision disturbances secondary to oral tranexamic acid. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12456. [Google Scholar] [CrossRef]
- Kaku, Y.; Ito, T.; Kudo, K.; Kido-Nakahara, M.; Nakahara, T.; Moroi, Y.; Furue, M. Generalized fixed drug eruption induced by tranexamic acid. Eur. J. Dermatol. 2014, 24, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Lecker, I.; Wang, D.-S.; Romaschin, A.D.; Peterson, M.; Mazer, C.D.; Orser, B.A. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J. Clin. Investig. 2012, 122, 4654–4666. [Google Scholar] [CrossRef]
- HALT-IT Trial Collaborators. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): An international randomised, double-blind, placebo-controlled trial. Lancet 2020, 395, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Mergoum, A.M.; Mergoum, A.S.; Larson, N.J.; Dries, D.J.; Cook, A.; Blondeau, B.; Rogers, F.B. Tranexamic Acid Use in the Surgical Arena: A Narrative Review. J. Surg. Res. 2024, 302, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Pilbrant, A.; Schannong, M.; Vessman, J. Pharmacokinetics and bioavailability of tranexamic acid. Eur. J. Clin. Pharmacol. 1981, 20, 65–72. [Google Scholar] [CrossRef]
- Ma, T.K.-W.; Chow, K.M.; Kwan, B.C.-H.; Leung, C.B.; Szeto, C.C.; Li, P.K.-T. Manifestation of tranexamic acid toxicity in chronic kidney disease and kidney transplant patients: A report of four cases and review of literature. Nephrology 2017, 22, 316–321. [Google Scholar] [CrossRef]
- Marcos, S.; Botella, L.M.; Albiñana, V.; Arbia, A.; de Rosales, A.M. Sclerotherapy on Demand with Polidocanol to Treat HHT Nosebleeds. J. Clin. Med. 2021, 10, 3845. [Google Scholar] [CrossRef]
- Silva, B.M.; Hosman, A.E.; Devlin, H.L.; Shovlin, C.L. Lifestyle and dietary influences on nosebleed severity in hereditary hemorrhagic telangiectasia. Laryngoscope 2013, 123, 1092–1099. [Google Scholar] [CrossRef]
- Morais, D.; Millás, T.; Zarrabeitia, R.; Botella, L.M.; Almaraz, A. Local sclerotherapy with polydocanol (Aethoxysklerol®) for the treatment of Epistaxis in Rendu-Osler-Weber or Hereditary Hemorrhagic Telangiectasia (HHT): 15 years of experience. Rhinology 2012, 50, 80–86. [Google Scholar] [CrossRef]
- Farneti, P.; Marcos-Salazar, S.; Crocione, C.; Boyer, H.; Puxeddu, R.; Whitehouse, A.B.; Piccirillo, J.F. Consensus on Sclerotherapy for Patients With Hereditary Hemorrhagic Telangiectasia-Related Epistaxis. JAMA Otolaryngol.-Head Neck Surg. 2025, 151, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Lança Gomes, P.; Marino, M.J.; Miglani, A.; Lal, D. Innovations in the management of epistaxis secondary to hereditary hemorrhagic telangiectasia: Our evolution to injection sclerotherapy as the treatment of choice. Front. Allergy 2024, 5, 1456686. [Google Scholar] [CrossRef]
- Woodard, T.D.; Yappel-Sinkko, K.B.; Wang, X.; McCrae, K.R.; Parambil, J.G. Sclerotherapy Versus Cautery/Laser Treatment for Epistaxis in Hereditary Hemorrhagic Telangiectasia. Laryngoscope 2022, 132, 920–925. [Google Scholar] [CrossRef]
- Feller, C.N.; Adams, J.A.; Friedland, D.R.; Poetker, D.M. Duration of effectiveness of coblation for recurrent epistaxis in hereditary hemorrhagic telangiectasia. Am. J. Otolaryngol. 2022, 43, 103409. [Google Scholar] [CrossRef]
- Richer, S.L.; Geisthoff, U.W.; Livada, N.; Ward, P.D.; Johnson, L.; Mainka, A.; Henderson, K.J.; Maune, S.; White, R.I.; Ross, D.A. The Young’s procedure for severe epistaxis from hereditary hemorrhagic telangiectasia. Am. J. Rhinol. Allergy 2012, 26, 401–404. [Google Scholar] [CrossRef]
- Lund, V.J.; Darby, Y.; Rimmer, J.; Amin, M.; Husain, S. Nasal closure for severe hereditary haemorrhagic telangiectasia in 100 patients. The Lund modification of the Young’s procedure: A 22-year experience. Rhinology 2017, 55, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kroetz, D.L. Bevacizumab-Induced Hypertension: Clinical Presentation and Molecular Understanding. Pharmacol. Ther. 2018, 182, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.N.; Apala, D.R.; Pannu, B.S.; Kotecha, A.; Brinjikji, W.; Leise, M.D.; Kamath, P.S.; Misra, S.; Begna, K.H.; Cartin-Ceba, R.; et al. Intravenous Bevacizumab for Refractory Hereditary Hemorrhagic Telangiectasia-Related Epistaxis and Gastrointestinal Bleeding. Mayo Clin. Proc. 2018, 93, 155–166. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Jacobson, B.S. Hereditary hemorrhagic telangiectasia: A model for blood vessel growth and enlargement. Am. J. Pathol. 2000, 156, 737–742. [Google Scholar] [CrossRef]
- Sabbà, C.; Cirulli, A.; Rizzi, R.; Pasculli, G.; Gallitelli, M.; Specchia, G.; Liso, V. Angiogenesis and hereditary hemorrhagic telangiectasia. Rendu-Osler-Weber disease. Acta Haematol. 2001, 106, 214–219. [Google Scholar] [CrossRef]
- Cirulli, A.; Liso, A.; D’Ovidio, F.; Mestice, A.; Pasculli, G.; Gallitelli, M.; Rizzi, R.; Specchia, G.; Sabbà, C. Vascular endothelial growth factor serum levels are elevated in patients with hereditary hemorrhagic telangiectasia. Acta Haematol. 2003, 110, 29–32. [Google Scholar] [CrossRef]
- Sadick, H.; Naim, R.; Sadick, M.; Hörmann, K.; Riedel, F. Plasma level and tissue expression of angiogenic factors in patients with hereditary hemorrhagic telangiectasia. Int. J. Mol. Med. 2005, 15, 591–596. [Google Scholar] [CrossRef]
- Flieger, D.; Hainke, S.; Fischbach, W. Dramatic improvement in hereditary hemorrhagic telangiectasia after treatment with the vascular endothelial growth factor (VEGF) antagonist bevacizumab. Ann. Hematol. 2006, 85, 631–632. [Google Scholar] [CrossRef]
- Mitchell, A.; Adams, L.A.; MacQuillan, G.; Tibballs, J.; vanden Driesen, R.; Delriviere, L. Bevacizumab reverses need for liver transplantation in hereditary hemorrhagic telangiectasia. Liver Transplant. 2008, 14, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Holter, J.L.; Selby, G.B. Bevacizumab in Hereditary Hemorrhagic Telangiectasia. N. Engl. J. Med. 2009, 360, 2143–2144. [Google Scholar] [CrossRef] [PubMed]
- Dupuis-Girod, S.; Ginon, I.; Saurin, J.-C.; Marion, D.; Guillot, E.; Decullier, E.; Roux, A.; Carette, M.-F.; Gilbert-Dussardier, B.; Hatron, P.-Y.; et al. Bevacizumab in Patients With Hereditary Hemorrhagic Telangiectasia and Severe Hepatic Vascular Malformations and High Cardiac Output. JAMA 2012, 307, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.B.; Ross, D.A.; Berard, P.; Figueroa-Bodine, J.; Livada, N.; Richer, S.L. Very low dose bevacizumab for the treatment of epistaxis in patients with hereditary hemorrhagic telangiectasia. Allergy Rhinol. 2014, 5, 91–95. [Google Scholar] [CrossRef]
- Epperla, N.; Kapke, J.T.; Karafin, M.; Friedman, K.D.; Foy, P. Effect of systemic bevacizumab in severe hereditary hemorrhagic telangiectasia associated with bleeding. Am. J. Hematol. 2016, 91, E313–E314. [Google Scholar] [CrossRef]
- Chavan, A.; Schumann-Binarsch, S.; Schmuck, B.; Oltmer, F.; Geisthoff, U.; Hoppe, F.; Wirsching, K.; Klempnauer, J.; Manns, M.; Philip Thomas, R.; et al. Emerging role of bevacizumab in management of patients with symptomatic hepatic involvement in Hereditary Hemorrhagic Telangiectasia. Am. J. Hematol. 2017, 92, E641–E644. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Kasthuri, R.S.; Parambil, J.G.; Albitar, H.A.; Almodallal, Y.A.; Vázquez, C.; Serra, M.M.; Dupuis-Girod, S.; Wilsen, C.B.; McWilliams, J.P.; et al. An international, multicenter study of intravenous bevacizumab for bleeding in hereditary hemorrhagic telangiectasia: The InHIBIT-Bleed study. Haematologica 2021, 106, 2161–2169. [Google Scholar] [CrossRef]
- Buscarini, E.; Botella, L.M.; Geisthoff, U.; Kjeldsen, A.D.; Mager, H.J.; Pagella, F.; Suppressa, P.; Zarrabeitia, R.; Dupuis-Girod, S.; Shovlin, C.L.; et al. Safety of thalidomide and bevacizumab in patients with hereditary hemorrhagic telangiectasia. Orphanet J. Rare Dis. 2019, 14, 28. [Google Scholar] [CrossRef]
- Karnezis, T.T.; Davidson, T.M. Efficacy of intranasal Bevacizumab (Avastin) treatment in patients with hereditary hemorrhagic telangiectasia-associated epistaxis. Laryngoscope 2011, 121, 636–638. [Google Scholar] [CrossRef]
- Khanwalkar, A.R.; Rathor, A.; Read, A.K.; Paknezhad, H.; Ma, Y.; Hwang, P.H. Randomized, controlled, double-blinded clinical trial of effect of bevacizumab injection in management of epistaxis in hereditary hemorrhagic telangiectasia patients undergoing surgical cauterization. Int. Forum Allergy Rhinol. 2022, 12, 1034–1042. [Google Scholar] [CrossRef]
- Wang, D.; Ito, S.; Waldron, C.; Butt, A.; Zhang, E.; Krumholz, H.M.; Al-Samkari, H.; Goshua, G. Cost-effectiveness of bevacizumab therapy in the care of patients with hereditary hemorrhagic telangiectasia. Blood Adv. 2024, 8, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.; Kundra, A.; Jervis, P. The use of thalidomide therapy for refractory epistaxis in hereditary haemorrhagic telangiectasia: Systematic review. J. Laryngol. Otol. 2018, 132, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Lebrin, F.; Srun, S.; Raymond, K.; Martin, S.; van den Brink, S.; Freitas, C.; Bréant, C.; Mathivet, T.; Larrivée, B.; Thomas, J.-L.; et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat. Med. 2010, 16, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Tweedie, D.; Scerba, M.T.; Kim, D.S.; Palmas, M.F.; Pisanu, A.; Carta, A.R.; Greig, N.H. Repurposing Immunomodulatory Imide Drugs (IMiDs) in Neuropsychiatric and Neurodegenerative Disorders. Front. Neurosci. 2021, 15, 656921. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Jung, H.C.; Song, I.S. The effects of thalidomide on the stimulation of NF-kappaB activity and TNF-alpha production by lipopolysaccharide in a human colonic epithelial cell line. Mol. Cells 2004, 17, 210–216. [Google Scholar] [CrossRef]
- Rossi, E.; Bernabeu, C. Novel vascular roles of human endoglin in pathophysiology. J. Thromb. Haemost. 2023, 21, 2327–2338. [Google Scholar] [CrossRef]
- Li, C.; Guo, B.; Ding, S.; Rius, C.; Langa, C.; Kumar, P.; Bernabeu, C.; Kumar, S. TNF alpha down-regulates CD105 expression in vascular endothelial cells: A comparative study with TGF beta 1. Anticancer Res. 2003, 23, 1189–1196. [Google Scholar]
- Hosman, A.; Westermann, C.J.J.; Snijder, R.; Disch, F.; Mummery, C.L.; Mager, J.J. Follow-up of Thalidomide treatment in patients with Hereditary Haemorrhagic Telangiectasia. Rhinology 2015, 53, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Chen, X.; Zhu, B.; Ye, H.; Zhang, W.; Guan, J.; Su, K. Thalidomide for Epistaxis in Patients with Hereditary Hemorrhagic Telangiectasia: A Preliminary Study. Otolaryngol.-Head Neck Surg. 2017, 157, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.C.; Baysal, M.; Umit, E.G. The Role of Thalidomide and Its Analogs in the Treatment of Hereditary Hemorrhagic Telangiectasia: A Systematic Review. J. Clin. Med. 2024, 13, 5404. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Bortolotti, F.; Chiapponi, V.; Buttini, F.; Sonvico, F.; Invernizzi, R.; Quaglia, F.; Danesino, C.; Pagella, F.; Russo, P.; et al. Nasal powders of thalidomide for local treatment of nose bleeding in persons affected by hereditary hemorrhagic telangiectasia. Int. J. Pharm. 2016, 514, 229. [Google Scholar] [CrossRef]
- Kopp, K.O.; Greer, M.E.; Glotfelty, E.J.; Hsueh, S.-C.; Tweedie, D.; Kim, D.S.; Reale, M.; Vargesson, N.; Greig, N.H. A New Generation of IMiDs as Treatments for Neuroinflammatory and Neurodegenerative Disorders. Biomolecules 2023, 13, 747. [Google Scholar] [CrossRef]
- Corral, L.G.; Muller, G.W.; Moreira, A.L.; Chen, Y.; Wu, M.; Stirling, D.; Kaplan, G. Selection of novel analogs of thalidomide with enhanced tumor necrosis factor alpha inhibitory activity. Mol. Med. 1996, 2, 506. [Google Scholar] [CrossRef]
- Dredge, K.; Marriott, J.B.; Macdonald, C.D.; Man, H.-W.; Chen, R.; Muller, G.W.; Stirling, D.; Dalgleish, A.G. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br. J. Cancer 2002, 87, 1166–1172. [Google Scholar] [CrossRef]
- Bowcock, S.J.; Patrick, H.E. Lenalidomide to control gastrointestinal bleeding in hereditary haemorrhagic telangiectasia: Potential implications for angiodysplasias? Br. J. Haematol. 2009, 146, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Swaidani, S.; Kundu, S.; Samour, M.; Silver, B.; Parambil, J.; Thomas, S.; McCrae, K.R. Pomalidomide Reduces Bleeding and Alters Expression of Angiogenesis-Related Proteins in Patients with Hereditary Hemorrhagic Telangiectasia. Blood 2019, 134 (Suppl. S1), 5761. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Kasthuri, R.S.; Iyer, V.; Pishko, A.M.; Decker, J.E.; Whitehead, K.J.; Conrad, M.B.; Weiss, C.; Parambil, J.; Zumberg, M.S.; et al. PATH-HHT, a Double-Blind, Randomized, Placebo-Controlled Trial in Hereditary Hemorrhagic Telangiectasia Demonstrates That Pomalidomide Reduces Epistaxis and Improves Quality of Life. Blood 2023, 142 (Suppl. S2), LBA-3. [Google Scholar] [CrossRef]
- Modarresi, A.; Rennie, C.; Shovlin, C.L. Pomalidomide in Hereditary Hemorrhagic Telangiectasia. N. Engl. J. Med. 2024, 391, 2277–2278. [Google Scholar] [CrossRef]
- Queisser, A.; Seront, E.; Boon, L.M.; Vikkula, M. Genetic Basis and Therapies for Vascular Anomalies. Circ. Res. 2021, 129, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Alsina-Sanchís, E.; García-Ibáñez, Y.; Figueiredo, A.M.; Riera-Domingo, C.; Figueras, A.; Matias-Guiu, X.; Casanovas, O.; Botella, L.M.; Pujana, M.A.; Riera-Mestre, A.; et al. ALK1 Loss Results in Vascular Hyperplasia in Mice and Humans Through PI3K Activation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1216–1229. [Google Scholar] [CrossRef]
- Ola, R.; Dubrac, A.; Han, J.; Zhang, F.; Fang, J.S.; Larrivée, B.; Lee, M.; Urarte, A.A.; Kraehling, J.R.; Genet, G.; et al. PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nat. Commun. 2016, 7, 13650. [Google Scholar] [CrossRef]
- Iriarte, A.; Figueras, A.; Cerdà, P.; Mora, J.M.; Jucglà, A.; Penín, R.; Viñals, F.; Riera-Mestre, A. PI3K (Phosphatidylinositol 3-Kinase) Activation and Endothelial Cell Proliferation in Patients with Hemorrhagic Hereditary Telangiectasia Type 1. Cells 2019, 8, 971. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Haap, M.; Kopp, H.-G.; Lipp, H.-P. Tyrosine kinase inhibitors—A review on pharmacology, metabolism and side effects. Curr. Drug Metab. 2009, 10, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, M.-J.; Choe, S.-W.; Sprecher, D.; Lee, Y.J.; Oh, S.P. Selective effects of oral antiangiogenic tyrosine kinase inhibitors on an animal model of hereditary hemorrhagic telangiectasia. J. Thromb. Haemost. 2017, 15, 1095–1102. [Google Scholar] [CrossRef]
- Parambil, J.G.; Woodard, T.D.; Koc, O.N. Pazopanib effective for bevacizumab-unresponsive epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 2018, 128, 2234–2236. [Google Scholar] [CrossRef] [PubMed]
- Parambil, J.G.; Gossage, J.R.; McCrae, K.R.; Woodard, T.D.; Menon, K.V.N.; Timmerman, K.L.; Pederson, D.P.; Sprecher, D.L.; Al-Samkari, H. Pazopanib for severe bleeding and transfusion-dependent anemia in hereditary hemorrhagic telangiectasia. Angiogenesis 2022, 25, 87–97. [Google Scholar] [CrossRef]
- Kovacs-Sipos, E.; Holzmann, D.; Scherer, T.; Soyka, M.B. Nintedanib as a novel treatment option in hereditary haemorrhagic telangiectasia. BMJ Case Rep. 2017, 2017, bcr2017219393. [Google Scholar] [CrossRef]
- Al-Samkari, H. A Randomized, Placebo-Controlled, Multicenter Proof-of-Concept (POC) Study to Assess the Safety and Efficacy of the Novel Allosteric AKT Inhibitor, VAD044, in Adults with Hereditary Hemorrhagic Telangiectasia (HHT). In ASH. 2024. Available online: https://ash.confex.com/ash/2024/webprogram/Paper206244.html (accessed on 13 December 2024).
- Vaderis Therapeutics AG. Vaderis Receives FDA Fast Track Designation for VAD044 for the Treatment of Hereditary Hemorrhagic Telangiectasia. Vaderis. 2024. Available online: https://vaderis.com/fda-fast-track-designation-for-vad044/ (accessed on 24 April 2025).
- Beuret, L.; Fortier-Beaulieu, S.-P.; Rondeau, V.; Roy, S.; Houde, N.; Balabanian, K.; Espéli, M.; Charron, J. Mek1 and Mek2 Functional Redundancy in Erythropoiesis. Front. Cell Dev. Biol. 2021, 9, 639022. [Google Scholar] [CrossRef]
- Mohammed, K.A.K.; Madeddu, P.; Avolio, E. MEK inhibitors: A promising targeted therapy for cardiovascular disease. Front. Cardiovasc. Med. 2024, 11, 1404253. [Google Scholar] [CrossRef]
- Santibanez, J.F.; Pérez-Gómez, E.; Fernandez-L, A.; Garrido-Martin, E.M.; Carnero, A.; Malumbres, M.; Vary, C.P.H.; Quintanilla, M.; Bernabéu, C. The TGF-beta co-receptor endoglin modulates the expression and transforming potential of H-Ras. Carcinogenesis 2010, 31, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Blobe, G.C. The interaction of endoglin with beta-arrestin2 regulates transforming growth factor-beta-mediated ERK activation and migration in endothelial cells. J. Biol. Chem. 2007, 282, 21507–21517. [Google Scholar] [CrossRef] [PubMed]
- Léauté-Labrèze, C.; Dumas de la Roque, E.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Lachambre, M.-P.; Lord-Dufour, S.; Béliveau, R. Propranolol suppresses angiogenesis in vitro: Inhibition of proliferation, migration, and differentiation of endothelial cells. Vascul. Pharmacol. 2010, 53, 200–208. [Google Scholar] [CrossRef]
- Albiñana, V.; Recio-Poveda, L.; Zarrabeitia, R.; Bernabéu, C.; Botella, L.M. Propranolol as antiangiogenic candidate for the therapy of hereditary haemorrhagic telangiectasia. Thromb. Haemost. 2012, 108, 41–53. [Google Scholar] [CrossRef]
- Mei-Zahav, M.; Blau, H.; Bruckheimer, E.; Zur, E.; Goldschmidt, N. Topical propranolol improves epistaxis in patients with hereditary hemorrhagic telangiectasia—A preliminary report. J. Otolaryngol.-Head Neck Surg. 2017, 46, 58. [Google Scholar] [CrossRef]
- Mei-Zahav, M.; Gendler, Y.; Bruckheimer, E.; Prais, D.; Birk, E.; Watad, M.; Goldschmidt, N.; Soudry, E. Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial. J. Clin. Med. 2020, 9, 3130. [Google Scholar] [CrossRef]
- Li, C.-L.; Fang, Z.-X.; Wu, Z.; Hou, Y.-Y.; Wu, H.-T.; Liu, J. Repurposed itraconazole for use in the treatment of malignancies as a promising therapeutic strategy. Biomed. Pharmacother. 2022, 154, 113616. [Google Scholar] [CrossRef]
- Chong, C.R.; Xu, J.; Lu, J.; Bhat, S.; Sullivan, D.J.; Liu, J.O. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem. Biol. 2007, 2, 263–270. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Han, P.; Yan, S.; You, J.; Guo, C.; Wu, X. Etamsylate-loaded hydrogel composed of carboxymethyl chitosan and oxidized tannic acid for improved wound healing. Int. J. Biol. Macromol. 2024, 279, 135270. [Google Scholar] [CrossRef]
- Albiñana, V.; Giménez-Gallego, G.; García-Mato, A.; Palacios, P.; Recio-Poveda, L.; Cuesta, A.-M.; Patier, J.-L.; Botella, L.-M. Topically Applied Etamsylate: A New Orphan Drug for HHT-Derived Epistaxis (Antiangiogenesis through FGF Pathway Inhibition). TH Open 2019, 3, e230–e243. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.J., Jr.; Escher, G.C.; Lewis, J.S. Hormonal management of hereditary hemorrhagic talangiectasia. J. Am. Med. Assoc. 1952, 149, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- van Cutsem, E.; Rutgeerts, P.; Vantrappen, G. Treatment of bleeding gastrointestinal vascular malformations with oestrogen-progesterone. Lancet 1990, 335, 953–955. [Google Scholar] [CrossRef]
- Vase, P. Estrogen treatment of hereditary hemorrhagic telangiectasia. A double-blind controlled clinical trial. Acta Med. Scand. 1981, 209, 393–396. [Google Scholar] [CrossRef]
- Harrison, D.F. Use of estrogen in treatment of familial hemorrhagic telangiectasia. Laryngoscope 1982, 92, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Bergler, W.; Sadick, H.; Gotte, K.; Riedel, F.; Hörmann, K. Topical estrogens combined with argon plasma coagulation in the management of epistaxis in hereditary hemorrhagic telangiectasia. Ann. Otol. Rhinol. Laryngol. 2002, 111 Pt 1, 222–228. [Google Scholar] [CrossRef]
- Sadick, H.; Naim, R.; Oulmi, J.; Hörmann, K.; Bergler, W. Plasma surgery and topical estriol: Effects on the nasal mucosa and long-term results in patients with Osler’s disease. Otolaryngol.-Head Neck Surg. 2003, 129, 233–238. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Ríus, C.; Smith, J.D.; Almendro, N.; Langa, C.; Botella, L.M.; Marchuk, D.A.; Vary, C.P.; Bernabéu, C. Cloning of the promoter region of human endoglin, the target gene for hereditary hemorrhagic telangiectasia type 1. Blood 1998, 92, 4677–4690. [Google Scholar] [CrossRef]
- Albiñana, V.; Bernabeu-Herrero, M.E.; Zarrabeitia, R.; Bernabéu, C.; Botella, L.M. Estrogen therapy for hereditary haemorrhagic telangiectasia (HHT): Effects of raloxifene, on Endoglin and ALK1 expression in endothelial cells. Thromb. Haemost. 2010, 103, 525–534. [Google Scholar] [CrossRef]
- Danesino, C.; Cantarini, C.; Olivieri, C. Hereditary Hemorrhagic Telangiectasia in Pediatric Age: Focus on Genetics and Diagnosis. Pediatr. Rep. 2023, 15, 129–142. [Google Scholar] [CrossRef]
- Haq, A.U.; Glass, J.; Netchvolodoff, C.V.; Bowen, L.M. Hereditary hemorrhagic telangiectasia and danazol. Ann. Intern. Med. 1988, 109, 171. [Google Scholar] [CrossRef] [PubMed]
- Zacharski, L.R.; Dunbar, S.D.; Newsom, W.A. Hemostatic effects of tamoxifen in hereditary hemorrhagic telangiectasia. Thromb. Haemost. 2001, 85, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.J.; Cave, D.R. Hormonal and antihormonal therapy for epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 2004, 114, 705–709. [Google Scholar] [CrossRef]
- D’Amelio, P.; Isaia, G.C. The use of raloxifene in osteoporosis treatment. Expert Opin. Pharmacother. 2013, 14, 949–956. [Google Scholar] [CrossRef]
- European Medicines Agency; Committee for Orphan Medicinal Products. Raloxifene Hydrochloride for the Treatment of Hereditary Haemorrhagic Telangiectasia. 22 June 2010 EMA/COMP/90982/2010. Available online: https://www.ema.europa.eu/en/documents/orphan-designation/eu310730-public-summary-opinion-orphan-designation-raloxifene-hydrochloride-treatment-hereditary-haemorrhagic-telangiectasia_en.pdf (accessed on 16 May 2025).
- Mosca, L.; Grady, D.; Barrett-Connor, E.; Collins, P.; Wenger, N.; Abramson, B.L.; Paganini-Hill, A.; Geiger, M.J.; Dowsett, S.A.; Amewou-Atisso, M.; et al. Effect of raloxifene on stroke and venous thromboembolism according to subgroups in postmenopausal women at increased risk of coronary heart disease. Stroke 2009, 40, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Zarrabeitia, R.; Ojeda-Fernandez, L.; Recio, L.; Bernabéu, C.; Parra, J.A.; Albiñana, V.; Botella, L.M. Bazedoxifene, a new orphan drug for the treatment of bleeding in hereditary haemorrhagic telangiectasia. Thromb. Haemost. 2016, 115, 1167–1177. [Google Scholar] [CrossRef]
- Albiñana, V.; Cuesta, A.M.; de Rojas-P, I.; Gallardo-Vara, E.; Recio-Poveda, L.; Bernabéu, C.; Botella, L.M. Review of Pharmacological Strategies with Repurposed Drugs for Hereditary Hemorrhagic Telangiectasia Related Bleeding. J. Clin. Med. 2020, 9, 1766. [Google Scholar] [CrossRef]
- Skaro, A.I.; Marotta, P.J.; McAlister, V.C. Regression of cutaneous and gastrointestinal telangiectasia with sirolimus and aspirin in a patient with hereditary hemorrhagic telangiectasia. Ann. Intern. Med. 2006, 144, 226–227. [Google Scholar] [CrossRef]
- Albiñana, V.; Sanz-Rodríguez, F.; Recio-Poveda, L.; Bernabéu, C.; Botella, L.M. Immunosuppressor FK506 increases endoglin and activin receptor-like kinase 1 expression and modulates transforming growth factor-β1 signaling in endothelial cells. Mol. Pharmacol. 2011, 79, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Hessels, J.; Kroon, S.; Boerman, S.; Nelissen, R.C.; Grutters, J.C.; Snijder, R.J.; Lebrin, F.; Post, M.C.; Mummery, C.L.; Mager, J.-J. Efficacy and Safety of Tacrolimus as Treatment for Bleeding Caused by Hereditary Hemorrhagic Telangiectasia: An Open-Label, Pilot Study. J. Clin. Med. 2022, 11, 5280. [Google Scholar] [CrossRef] [PubMed]
- Faughnan, M.E.; Bagheri, N.; Vozoris, N.T.; Sykes, J.; Parlette, A.; Chaparro, C.; Oh, P.; Marchuk, D.A.; Marambaud, P. Low-dose Tacrolimus for Epistaxis in Hereditary hemorrhagic Telangiectasia: Phase II Open Label Trial. Angiogenesis in press.
- Ruiz, S.; Zhao, H.; Chandakkar, P.; Papoin, J.; Choi, H.; Nomura-Kitabayashi, A.; Patel, R.; Gillen, M.; Diao, L.; Chatterjee, P.K.; et al. Correcting Smad1/5/8, mTOR, and VEGFR2 treats pathology in hereditary hemorrhagic telangiectasia models. J. Clin. Investig. 2020, 130, 942–957. [Google Scholar] [CrossRef]
- Toporsian, M.; Gros, R.; Kabir, M.G.; Vera, S.; Govindaraju, K.; Eidelman, D.H.; Husain, M.; Letarte, M. A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ. Res. 2005, 96, 684–692. [Google Scholar] [CrossRef] [PubMed]
- de Gussem, E.M.; Snijder, R.J.; Disch, F.J.; Zanen, P.; Westermann, C.J.J.; Mager, J.J. The effect of N-acetylcysteine on epistaxis and quality of life in patients with HHT: A pilot study. Rhinology 2009, 47, 85–88. [Google Scholar]
- Modarresi, A.; Shovlin, C.L. Integration of genotypic data into clinical trial design and reporting in hereditary hemorrhagic telangiectasia could help personalize treatment. Haematologica 2024, 109, 3826–3827. [Google Scholar] [CrossRef]
- Joyce, K.E.; Onabanjo, E.; Brownlow, S.; Nur, F.; Olupona, K.; Fakayode, K.; Sroya, M.; Thomas, G.A.; Ferguson, T.; Redhead, J.; et al. Whole genome sequences discriminate hereditary hemorrhagic telangiectasia phenotypes by non-HHT deleterious DNA variation. Blood Adv. 2022, 6, 3956–3969. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Aldred, M.A. When “loss-of-function” means proteostasis burden: Thinking again about coding DNA variants. Am. J. Hum. Genet. 2025, 112, 3–10. [Google Scholar] [CrossRef]
- Orlova, V.V.; Nahon, D.M.; Cochrane, A.; Cao, X.; Freund, C.; van den Hil, F.; Westermann, C.J.J.; Snijder, R.J.; Ploos van Amstel, J.K.; Ten Dijke, P.; et al. Vascular defects associated with hereditary hemorrhagic telangiectasia revealed in patient-derived isogenic iPSCs in 3D vessels on chip. Stem Cell Rep. 2022, 17, 1536–1545. [Google Scholar] [CrossRef]
- Al Tabosh, T.; Liu, H.; Koça, D.; Al Tarrass, M.; Tu, L.; Giraud, S.; Delagrange, L.; Beaudoin, M.; Rivière, S.; Grobost, V.; et al. Impact of heterozygous ALK1 mutations on the transcriptomic response to BMP9 and BMP10 in endothelial cells from hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension donors. Angiogenesis 2024, 27, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Ogut, D.; Reel, B.; Gonen Korkmaz, C.; Arun, M.Z.; Cilaker Micili, S.; Ergur, B.U. Doxycycline down-regulates matrix metalloproteinase expression and inhibits NF-κB signaling in LPS-induced PC3 cells. Folia Histochem. Cytobiol. 2016, 54, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Jerkic, M.; Rivas-Elena, J.V.; Prieto, M.; Carrón, R.; Sanz-Rodríguez, F.; Pérez-Barriocanal, F.; Rodríguez-Barbero, A.; Bernabéu, C.; López-Novoa, J.M. Endoglin regulates nitric oxide-dependent vasodilatation. FASEB J. 2004, 18, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Sanz-Rodriguez, F.; Eleno, N.; Düwell, A.; Blanco, F.J.; Langa, C.; Botella, L.M.; Cabañas, C.; Lopez-Novoa, J.M.; Bernabeu, C. Endothelial endoglin is involved in inflammation: Role in leukocyte adhesion and transmigration. Blood 2013, 121, 403–415. [Google Scholar] [CrossRef]
- Rossi, E.; Smadja, D.M.; Boscolo, E.; Langa, C.; Arevalo, M.A.; Pericacho, M.; Gamella-Pozuelo, L.; Kauskot, A.; Botella, L.M.; Gaussem, P.; et al. Endoglin regulates mural cell adhesion in the circulatory system. Cell. Mol. Life Sci. 2016, 73, 1715–1739. [Google Scholar] [CrossRef]
- Rossi, E.; Pericacho, M.; Bachelot-Loza, C.; Pidard, D.; Gaussem, P.; Poirault-Chassac, S.; Blanco, F.J.; Langa, C.; González-Manchón, C.; Novoa, J.M.L.; et al. Human endoglin as a potential new partner involved in platelet-endothelium interactions. Cell. Mol. Life Sci. 2018, 75, 1269–1284. [Google Scholar] [CrossRef]
- Egido-Turrión, C.; Rossi, E.; Ollauri-Ibáñez, C.; Pérez-García, M.L.; Sevilla, M.A.; Bastida, J.M.; González-Porras, J.R.; Rodríguez-Barbero, A.; Bernabeu, C.; Lopez-Novoa, J.M.; et al. Functional Alterations Involved in Increased Bleeding in Hereditary Hemorrhagic Telangiectasia Mouse Models. Front. Med. 2022, 9, 871903. [Google Scholar] [CrossRef]
- Al-Samkari, H. How I treat bleeding in hereditary hemorrhagic telangiectasia. Blood 2024, 144, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Al Tabosh, T.; Al Tarrass, M.; Tourvieilhe, L.; Guilhem, A.; Dupuis-Girod, S.; Bailly, S. Hereditary hemorrhagic telangiectasia: From signaling insights to therapeutic advances. J. Clin. Investig. 2024, 134, e176379. [Google Scholar] [CrossRef]
| ID | Drug/ Intervention | Study Phase | Study Design | Number of Patients | Primary Outcome | Published Results (Peer-Reviewed Journal) |
|---|---|---|---|---|---|---|
| NCT05641142 | ANTIPLATELET | Cohort study | Single Group Assignment, Open Label | 100 | Number of transfusions and/or intravenous iron | N/A |
| ChiCTR2100043253 | BEVACIZUMAB | Phase 4 | Single Arm | 30 | Nose bleeding and SF36 score | N/A |
| NCT04404881 | BEVACIZUMAB | Phase 2 | Single Group Assignment, Open Label | 33 | Change in ESS | N/A |
| NCT03227263 | BEVACIZUMAB | Phase 3 | Double-Blind Multicenter Randomized Phase 2 Trial | 24 | Decrease of at least 50% in the cumulative number of RBC units transfused in a 3-month period | 63.6% of patients (7/11) in the bevacizumab group versus 33.3% of patients (4/12) in the placebo group decreased the number of blood transfusions by at least 50% (p = 0.22) [38] |
| NCT02389959 | BEVACIZUMAB | Phase 4 | Randomized, Parallel, and Quadruple Masking (Participant, Care Provider, Investigator, and Outcomes Assessor) | 40 | Trial improvement in ESS | N/A |
| NCT02157987 | BEVACIZUMAB | Phase 1/Phase 2 | Single Group, Single Masking (Care Provider) | 15 | Decrease of at least 50% in the number of epistaxis at 1 month of treatment compared to the month prior to inclusion for 60% of patients | Not achieved [39]. |
| NCT02106520 | BEVACIZUMAB | Phase 2/Phase 3 | Randomized, Parallel Assignment, and Double Blind | 75 | Mean monthly epistaxis duration for 3 consecutive months immediately after the end of the treatment | No significant difference between treated and placebo group [40] |
| NCT01507480 | BEVACIZUMAB | Phase 1 | Single Group, Double Masking (Participant, Investigator) | 40 | Tolerance of increasing doses of bevacizumab administered as a nasal spray in patients with HHT-related epistaxis | No dose limiting toxicity was observed; no efficacy was observed at any dose in this study [41] |
| NCT01402531 | BEVACIZUMAB | Phase 2 | Single Group Assignment, Open Label | 10 | Epistaxis using the ESS, hematocrit, hemoglobin, and serum ferritin levels | N/A |
| NCT01397695 | BEVACIZUMAB | Phase 2 | Single Group Assignment, Open Label | 20 | Measurement of epistaxis in patients with HHT, as measured by the HHT Foundation ESS, hematocrit, hemoglobin, and serum ferritin levels | N/A |
| EUCTR2009-018049-19-AT; NCT01314274 | BEVACIZUMAB | Phase 2 | Double-Blind, Placebo-Controlled Trial | 15 | Reduction in average visual analog score of epistaxis | No significant change [42] |
| NCT01408030 | BEVACIZUMAB, ESTRIOL, TRANEXAMIC ACID | Phase 2 | Randomized, Parallel, and Quadruple Masking (Participant, Care Provider, Investigator, and Outcomes Assessor) | 106 | Median weekly epistaxis frequency during weeks 5 through 12 | Drug therapy did not significantly reduce epistaxis frequency [43] |
| NCT03397004 | DOXYCYCLINE | Phase 2 | Randomized, Crossover, and Triple Masking (Participant, Care Provider, and Investigator) | 11 | Change in weekly epistaxis duration (WED) | No significant difference between the change in WED [44] |
| NCT04167085 | DOXYCYCLINE | Phase 4 | Randomized, Crossover, and Double Masking | 22 | Frequency of epistaxis; duration of epistaxis; and change in severity of epistaxis | No reduction in the three primary outcome measures [45] |
| EUCTR2016-003982-24-ES | ETHAMSILATE | Phase 2 | Open, Single Arm | 12 | Number of epistaxis per week; bleeding time; amount of bleeding; evolution of anemia; and assessment of QoL | N/A |
| NCT02638012 | FLOSEAL (Hemostatic agent) | Pilot trial | Single Group, Open Label | 7 | Changes in ESS between baseline and one-month follow-up | No statistically significant difference noted in ESS pre-treatment and one-month follow-up [46] |
| EUCTR2017-003272-31-NL | ITRACONAZOL | Phase 2 | Open, Single Arm | 17 | Change in ESS | Median ESS decreased during treatment; no change in hemoglobin levels [47] |
| NCT02963129 | MUPIROCIN | Phase 3 | Randomized Parallel, Double Blind | 40 | Nosebleed by Sadick scale | N/A |
| NCT04976036 | NINTEDANIB | Phase 2 | Randomized, Parallel Assignment, and Quadruple Masking (Participant, Care Provider, Investigator, and Outcomes Assessor) | 48 | Change in epistaxis duration | N/A |
| NCT03954782 | NINTEDANIB | Phase 2 | Randomized, Parallel, and Triple Masking (Participant, Care Provider, and Investigator) | 56 | Reduction of at least 50% in mean monthly epistaxis duration | Primary outcome not achieved, but significant decrease in median epistaxis and increase in median hemoglobin levels [48] |
| NCT04976036 | NINTEDANIB | Phase 2 | Randomized, Parallel Assignment, and Quadruple Masking | 48 | Change in epistaxis duration in minutes under nintedanib treatment, as compared to placebo in HHT patients | N/A |
| EUCTR2018-004179-11-DE | OCTREOTIDE | Phase 3 | Controlled, Randomized, Open Label, Parallel Group, and Not Placebo Controlled | 38 | Decrease of ≥50% in the number of red blood cell transfusions and/or number of IV iron infusions | N/A |
| EUCTR2018-004179-11-NL | OCTREOTIDE | Phase 3 | Randomized, Controlled, and Open | 38 | Decrease of = 50% in the number of units of intravenous iron and/or blood transfusions given | N/A |
| NTR7589 | OCTREOTIDE | Phase 3 | Randomized Controlled Trial, Parallel, and Open | 38 | Decrease of 50% in the number of units of intravenous iron and/or blood transfusions | N/A |
| NCT00004327 | OCTREOTIDE | Phase 2 | Single Group Assignment | 8 | Unknown | N/A |
| NCT03850730 | PAZOPANIB | Phase 1/Phase 2 | Single Group Assignment, Open Label | 30 | Change in epistaxis duration | N/A |
| NCT03850964 | PAZOPANIB | Phase 2/Phase 3 | Randomized, Parallel, and Quadruple Masking (Participant, Care Provider, Investigator, and Outcomes Assessor) | 70 | Change in epistaxis duration in minutes; hemoglobin response rate increase in hemoglobin | N/A |
| NCT02204371 | PAZOPANIB | Phase 2 | Non-Randomized, Single Group, and Open Label | 7 | Change from baseline in hemoglobin at the indicated time points, and change in epistaxis duration, frequency, and intensity | All seven patients realized a benefit, although of variable individual value [49] |
| NCT00588146 | PEGYLATED INTERFERON ALPHA2B | Phase 2 | Randomized, Controlled | 10 | Change in hemoglobin | N/A |
| NCT03910244 | POMALIDOMIDE | Phase 2 | Allocation: Randomized; Intervention Model: Parallel Assignment; Primary Purpose: Treatment; and Masking: Quadruple (Participant, Care Provider, Investigator, and Outcomes Assessor) | 144 | Change in ESS from baseline through week 24 | Pomalidomide significantly decreased epistaxis severity by 0.94 points (p = 0.004); neutropenia, constipation, and rash were reported in pomalidomide group [50] |
| NCT02287558 | POMALIDOMIDE | Phase 1 | Single Group, Open Label | 9 | Transfusion requirement measure | N/A |
| NCT04113187 | PROPANOLOL | Phase 3 | Randomized Parallel, Quadruple Masking (Participant, Care Provider, Investigator, and Outcomes Assessor) | 15 | Cumulative duration of epistaxis (in minutes) | N/A |
| NCT01406639 | RANIBIZUMAB | Phase 1 | Single Group Assignment, Open Label | 10 | Epistaxis as measured by the HHT Epistaxis Severity Score (ESS), hematocrit, and hemoglobin and serum ferritin levels | N/A |
| NCT01408732 | SCLEROTHERAPY WITH SODIUM TETRADECYL SULFATE | Phase 1/Phase 2 | Randomized, Crossover, and Open Label | 17 | Change in ESS | Sclerotherapy with STS (vs standard treatment) significantly reduced ESS [51] |
| NCT05269849 | SIROLIMUS | Phase 2 | Single Group Assignment, Open Label | 10 | Electrolytes; hematology; renal function; liver function; ferritin level; blood glucose level; and lipid assessment | N/A |
| NCT00004654 | SOY PROTEIN | Phase 3 | Randomized, Crossover | 60 | Unknown | N/A |
| NCT04646356 | TACROLIMUS | Phase 2 | Single Group Assignment, Open Label | 30 | Reduction in bleeding minutes per week | N/A |
| EUCTR2019-003585-40-NL | TACROLIMUS | Phase 2 | Open, Single Arm | 20 | Change in hemoglobin level | N/A |
| NCT03152019 | TACROLIMUS | Phase 2 | Randomized, Parallel, and Triple Masking (Participant, Care Provider, and Investigator) | 49 | Percentage of patients experiencing an improvement in their nosebleeds | No significant difference in main objective comparing epistaxis before and after treatment (p = 0.77) [52] |
| NCT00375622 | TAMOXIFEN | Phase 2 | Randomized, Single Group Assignment, and Double Blind | 21 | Frequency of epistaxis; duration of epistaxis; and hemoglobin level | Increase in hemoglobin level in tamoxifen-treated patients [53] |
| NCT00375622 | TAMOXIFEN | Phase 2 | Single Group Assignment | 38 | Self-assessment questionnaire of rhinologic QoL and epistaxis grading scale | Bleeding score and the QoL score improved; hemoglobin concentration also improved [54] |
| JPRN-jRCT2071230066 | THALIDOMIDE | Phase 3 | Randomized Controlled Trial, Double Blind, Placebo Control, Parallel Assignment, and Treatment Purpose | 44 | Change in ESS compared to baseline | N/A |
| JPRN-jRCT2051200141 | THALIDOMIDE | Phase 2 | Single Arm Study, Uncontrolled Control, and Single Assignment | 8 | Improvement rate of ESS | N/A |
| NCT01485224 | THALIDOMIDE | Phase 2 | Single Group Assignment, Open Label | 31 | Percentage of patients showing a decrease in the frequency, intensity, and duration of epistaxis and in the blood transfusion requirement | All 31 (100%, 89–100) patients responded to therapy with a significant decrease in all epistaxis parameters (p < 0·0001 for frequency, intensity, and duration) [55] |
| NCT00389935 | THALIDOMIDE | Phase 2 | Single Group Assignment, Open Label | 14 | Blood transfusion requirements | N/A |
| NCT02484716 | TIMOLOL | Phase 2 | Double-Blind, Placebo-Controlled Study | 58 | Comparison of mean monthly epistaxis duration 3 months before the treatment and 3 months after the end of the treatment | No significant difference between timolol and placebo group [56] |
| NCT01752049 | TIMOLOL | Proof of concept | Randomized, Single Group, and Quadruple Masking (Participant, Care Provider, Investigator, and Outcomes Assessor) | 5 | Mean reduction in lesion area (compared with baseline measurement) of treated telangiectasia | N/A |
| NCT04139018 | TIMOLOL GEL | Phase 2 | Randomized, Parallel, and Quadruple Masking (Participant, Care Provider, Investigator, and Outcomes Assessor) | 23 | Change in ESS | No definitive conclusions on the superiority of timolol can be drawn [57] |
| DRKS00020994 | TIMOLOL SPRAY | Phase 2 | Randomized Controlled Study, Placebo Controlled, and Crossover | 20 | Change in ESS | N/A |
| NCT01031992 | TRANEXAMIC ACID | Phase 3 | Randomized, Crossover, and Double Blind | 23 | Change in hemoglobin level within the phases | No significant change [58] |
| NCT00355108 | TRANEXAMIC ACID | Phase 3 | Randomized, Crossover, and Double Masking | 118 | Average monthly duration of epistaxis | Significant decrease in the duration of epistaxis in HHT patients taking TA [59] |
| NCT05406362 | VAD044 | Phase 1 | Randomized, Quadruple Masking (Participant, Care Provider, Investigator, and Outcomes Assessor) | 80 | Safety and tolerability | N/A |
| EU ID | FDA ID | Product | Indication | Sponsor Europe | Sponsor USA | Designation Date | Designation Date FDA |
|---|---|---|---|---|---|---|---|
| EU/3/23/2766 | N/A | 6-(4-(1-amino-3-hydroxycyclobutyl)phenyl)-1-ethyl-7-phenyl-1H-pyrido [2,3-b][1,4]oxazin-2(3H)-one, L-tartrate salt | Treatment of hereditary hemorrhagic telangiectasia | FGK Representative Service GmbH | Vaderis | 20 March 2023 | 7 December 2022 |
| EU/3/18/2087 | N/A | Etamsylate | Treatment of hereditary hemorrhagic telangiectasia | Dobecure S.L. | 19 November 2018 | ||
| EU/3/17/1845 | N/A | Thalidomide | Treatment of hereditary hemorrhagic telangiectasia | PlumeStars s.r.l. | PlumeStars s.r.l. | 27 February 2017 | 19 July 2017 |
| EU/3/14/1390 | N/A | Bevacizumab | Treatment of hereditary hemorrhagic telangiectasia | Laboratoires Delbert | Terence M. Davidson, MDUCSD Medical Center and Laboratoires Delbert SAS | 16 December 2014 | 20 December 2022 and 10 December 2022 |
| EU/3/14/1367 | N/A | Bazedoxifene acetate | Treatment of hereditary hemorrhagic telangiectasia | Consejo Superior de Investigaciones Científicas (CSIC) | 19 November 2014 | ||
| EU/3/10/730 | N/A | Raloxifene hydrochloride | Treatment of hereditary hemorrhagic telangiectasia | Consejo Superior de Investigaciones Científicas (CSIC) | Consejo Superior de Investigaciones Científicas (CSIC) | 10 June 2010 | 20 August 2010 |
| N/A | Pazopanib | HHT Foundation International (Cure HHT) | 9 October 2019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ficany, A.; Del Alamo, M.; Bernabeu, C.; Shovlin, C.L.; Rossi, E. Epistaxis Prevention, Treatment, and Future Perspectives for Hereditary Hemorrhagic Telangiectasia. J. Clin. Med. 2025, 14, 7724. https://doi.org/10.3390/jcm14217724
Ficany A, Del Alamo M, Bernabeu C, Shovlin CL, Rossi E. Epistaxis Prevention, Treatment, and Future Perspectives for Hereditary Hemorrhagic Telangiectasia. Journal of Clinical Medicine. 2025; 14(21):7724. https://doi.org/10.3390/jcm14217724
Chicago/Turabian StyleFicany, Anthony, Marta Del Alamo, Carmelo Bernabeu, Claire L. Shovlin, and Elisa Rossi. 2025. "Epistaxis Prevention, Treatment, and Future Perspectives for Hereditary Hemorrhagic Telangiectasia" Journal of Clinical Medicine 14, no. 21: 7724. https://doi.org/10.3390/jcm14217724
APA StyleFicany, A., Del Alamo, M., Bernabeu, C., Shovlin, C. L., & Rossi, E. (2025). Epistaxis Prevention, Treatment, and Future Perspectives for Hereditary Hemorrhagic Telangiectasia. Journal of Clinical Medicine, 14(21), 7724. https://doi.org/10.3390/jcm14217724








