Advances in Laser Therapy for Hidradenitis Suppurativa: A Systematic Assessment of Current Evidence
Abstract
1. Introduction
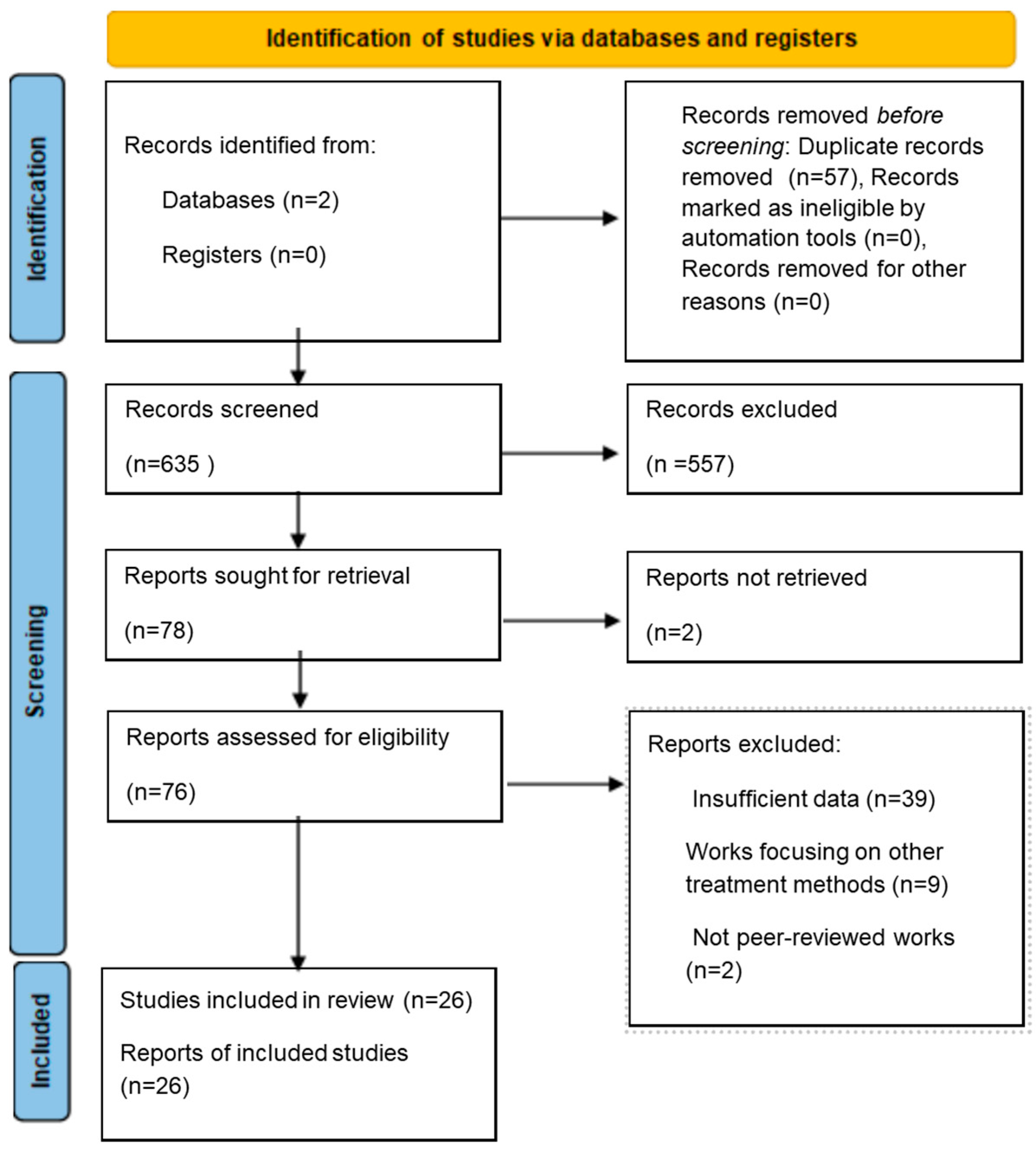
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
- Written in English.
- Original scientific research published in peer-reviewed journals.
- Clearly defined study objectives, methodology, and results.
2.3. Data Extraction
2.4. Outcomes
3. CO2 Laser
4. Neodymium Laser
5. Alternative Laser- and Energy-Based Therapies in HS Management
5.1. Intense Pulsed Light Therapy (IPL) and Radiofrequency (RF)
5.2. Diode Laser
5.3. Alexandrite Laser
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouazzi, D.; Nielsen, S.M.; Hagan, P.G.; Botvid, S.; Hove, L.S.; Prens, E.P.; Boer, J. Prevalence of Hidradenitis Suppurativa: A Meta-Analysis of Global Hidradenitis Suppurativa Atlas Studies. JAMA Dermatol. 2025, 161, 1022–1028. Available online: https://pubmed.ncbi.nlm.nih.gov/40864454/ (accessed on 23 October 2025). [CrossRef]
- Daveluy, S.; Okoye, G.A. Quality of life and the patient journey in hidradenitis suppurativa. J. Am. Acad. Dermatol. 2024, 91, S8–S11. Available online: https://pubmed.ncbi.nlm.nih.gov/39627002/ (accessed on 15 August 2025). [CrossRef]
- Zouboulis, C.C.; Bechara, F.G.; Benhadou, F.; Bettoli, V.; Mokos, Z.B.; Del Marmol, V.; Dolenc-Voljč, M.; Giamarellos-Bourboulis, E.J.; Grimstad, Ø.; Guillem, P.; et al. European S2k guidelines for hidradenitis suppurativa/acne inversa part 2: Treatment. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 899–941. Available online: https://pubmed.ncbi.nlm.nih.gov/39699926/ (accessed on 23 October 2025). [CrossRef] [PubMed]
- Nazzaro, G.; Zerboni, R.; Passoni, E.; Barbareschi, M.; Marzano, A.V.; Muratori, S.; Veraldi, S. High-frequency ultrasound in hidradenitis suppurativa as rationale for permanent hair laser removal. Ski. Res. Technol. 2019, 25, 587–588. Available online: https://pubmed.ncbi.nlm.nih.gov/30609069/ (accessed on 15 April 2025). [CrossRef] [PubMed]
- Lapins, J.; Marcusson, J.; Emtestam, L. Surgical treatment of chronic hidradenitis suppurativa: CO2 laser stripping-secondary intention technique. Br. J. Dermatol. 1994, 131, 551–556. Available online: https://pubmed.ncbi.nlm.nih.gov/7947209/ (accessed on 15 April 2025). [CrossRef] [PubMed]
- Finley, E.M.; Ratz, J.L. Treatment of hidradenitis suppurativa with carbon dioxide laser excision and second-intention healing. J. Am. Acad. Dermatol. 1996, 34, 465–469. Available online: https://pubmed.ncbi.nlm.nih.gov/8609261/ (accessed on 15 April 2025). [CrossRef]
- Lapins, J.; Sartorius, K.; Emtestam, L. Scanner-assisted carbon dioxide laser surgery: A retrospective follow-up study of patients with hidradenitis suppurativa. J. Am. Acad. Dermatol. 2002, 47, 280–285. Available online: https://pubmed.ncbi.nlm.nih.gov/12140476/ (accessed on 15 April 2025). [CrossRef]
- Madan, V.; Hindle, E.; Hussain, W.; August, P. Outcomes of treatment of nine cases of recalcitrant severe hidradenitis suppurativa with carbon dioxide laser. Br. J. Dermatol. 2008, 159, 1309–1314. Available online: https://pubmed.ncbi.nlm.nih.gov/19036028/ (accessed on 15 April 2025). [CrossRef]
- Hazen, P.G.; Hazen, B.P. Hidradenitis suppurativa: Successful treatment using carbon dioxide laser excision and marsupialization. Dermatol. Surg. 2010, 36, 208–213. Available online: https://pubmed.ncbi.nlm.nih.gov/20039918/ (accessed on 15 April 2025). [CrossRef]
- Jain, A.; Jain, V. Use of lasers for the management of refractory cases of hidradenitis suppurativa and pilonidal sinus. J. Cutan. Aesthetic Surg. 2012, 5, 190. Available online: https://pubmed.ncbi.nlm.nih.gov/23112515/ (accessed on 15 April 2025). [CrossRef]
- Krakowski, A.C.; Admani, S.; Uebelhoer, N.S.; Eichenfield, L.F.; Shumaker, P.R. Residual Scarring from hidradenitis suppurativa: Fractionated CO2 laser as a novel and noninvasive approach. Pediatrics 2014, 133, e248–e251. Available online: https://pubmed.ncbi.nlm.nih.gov/24323993/ (accessed on 15 April 2025). [CrossRef] [PubMed]
- Mikkelsen, P.R.; Dufour, D.N.; Zarchi, K.; Jemec, G.B.E. Recurrence rate and patient satisfaction of CO2 laser evapora-tion of lesions in patients with hidradenitis suppurativa: A retrospective study. Dermatol. Surg. 2015, 41, 255–260. Available online: https://pubmed.ncbi.nlm.nih.gov/25654196/ (accessed on 15 April 2025). [CrossRef] [PubMed]
- Crocco, E.I.; Dalapicola, M.C.; Suzuki, N.M.; Alves, R.O. Surgical Treatment of Chronic Hidradenitis Suppurativa: CO2 Laser Stripping-Second Intention Technique. Dermatol. Surg. 2016, 42, 429–431. Available online: https://pubmed.ncbi.nlm.nih.gov/26866288/ (accessed on 15 April 2025). [CrossRef] [PubMed]
- Nicholson, C.L.; Hamzavi, I.; Ozog, D.M. Rapid healing of chronic ulcerations and improvement in range of motion after fractional carbon dioxide (CO2) treatment after CO2 excision of hidradenitis suppurativa axillary lesions: A case report. JAAD Case Rep. 2016, 2, 4–6. Available online: https://pubmed.ncbi.nlm.nih.gov/27051812/ (accessed on 15 April 2025). [CrossRef]
- Abdel Azim, A.A.; Salem, R.T.; Abdelghani, R. Combined fractional carbon dioxide laser and long-pulsed neodymium: Yttrium-aluminium-garnet (1064 nm) laser in treatment of hidradenitis suppurativa; a prospective ran-domized intra-individual controlled study. Int. J. Dermatol. 2018, 57, 1135–1144. Available online: https://pubmed.ncbi.nlm.nih.gov/29907956/ (accessed on 15 April 2025). [CrossRef]
- Lindén, O.; Lönndahl, L.; Erlendsson, A.M.; Sandberg, C.; Killasli, H. Effects of mixed-technology CO2 and Ga-As laser in patients with hidradenitis suppurativa—A case series. JAAD Case Rep. 2022, 30, 124–127. Available online: https://pubmed.ncbi.nlm.nih.gov/36457941/ (accessed on 15 April 2025). [CrossRef]
- Sechi, A.; Caposiena Caro, R.D.; Michelucci, A.; Dini, V.; Piaserico, S.; Zalaudek, I.; Savoia, F.; Tartaglia, J. CO2 Laser Versus Surgical Deroofing for the Treatment of Hidradenitis Suppurativa Tunnels: A Comparative Multicentric, Retrospective Study. Dermatol. Surg. 2025, 51, 389–395. Available online: https://pubmed.ncbi.nlm.nih.gov/39560294/ (accessed on 15 April 2025). [CrossRef]
- Fabbrocini, G.; França, K.; Lotti, T.; Marasca, C.; Annunziata, M.C.; Cacciapuoti, S.; Masarà, A.; Romanelli, M.; Lotti, J.; Wollina, U.; et al. Intralesional Diode Laser 1064 nm for the Treatment of Hidradenitis Suppurativa: A Report of Twenty Patients. Open Access Maced. J. Med. Sci. 2018, 6, 31–34. Available online: https://pubmed.ncbi.nlm.nih.gov/29483975/ (accessed on 15 April 2025). [CrossRef]
- Tierney, E.; Mahmoud, B.H.; Hexsel, C.; Ozog, D.; Hamzavi, I. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol. Surg. 2009, 35, 1188–1198. Available online: https://pubmed.ncbi.nlm.nih.gov/19438670/ (accessed on 15 April 2025). [CrossRef]
- Mahmoud, B.H.; Tierney, E.; Hexsel, C.L.; Pui, J.; Ozog, D.M.; Hamzavi, I.H. Prospective controlled clinical and histo-pathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J. Am. Acad. Dermatol. 2010, 62, 637–645. Available online: https://pubmed.ncbi.nlm.nih.gov/20227579/ (accessed on 15 April 2025). [CrossRef]
- Castrillón Velásquez, M.A.; Kim, M.; Tan, M.H.; Tran, K.; Murrell, D.F. An Atypical Localized Form of Hidradenitis Suppurativa of the Jawline and Neck Mimicking Severe Cystic Acne on Presentation. Ski. Appendage Disord. 2017, 3, 215–218. Available online: https://pubmed.ncbi.nlm.nih.gov/29177152/ (accessed on 15 April 2025). [CrossRef] [PubMed]
- Xu, L.Y.; Wright, D.R.; Mahmoud, B.H.; Ozog, D.M.; Mehregan, D.A.; Hamzavi, I.H. Histopathologic study of hidradenitis suppurativa following long-pulsed 1064-nm Nd:YAG laser treatment. Arch. Dermatol. 2011, 147, 21–28. Available online: https://pubmed.ncbi.nlm.nih.gov/20855672/ (accessed on 15 April 2025). [CrossRef] [PubMed]
- Highton, L.; Chan, W.-Y.; Khwaja, N.; Laitung, J.K.G. Treatment of hidradenitis suppurativa with intense pulsed light: A prospective study. Plast. Reconstr. Surg. 2011, 128, 459–466. Available online: https://pubmed.ncbi.nlm.nih.gov/21788837/ (accessed on 15 April 2025). [CrossRef] [PubMed]
- Wilden, S.; Friis, M.; Tuettenberg, A.; Staubach-Renz, P.; Wegner, J.; Grabbe, S.; von Stebut, E. Combined treatment of hidradenitis suppurativa with intense pulsed light (IPL) and radiofrequency (RF). J. Dermatol. Treat. 2021, 32, 530–537. Available online: https://pubmed.ncbi.nlm.nih.gov/31609667/ (accessed on 15 April 2025). [CrossRef]
- Schultheis, M.; Staubach, P.; Nikolakis, G.; Grabbe, S.; Ruckes, C.; von Stebut, E.; Szepietowski, J.C. LAight® Therapy Significantly Enhances Treatment Efficacy of 16 Weeks of Topical Clindamycin Solution in Hurley I and II Hidradenitis Sup-purativa: Results from Period A of RELIEVE, a Multicenter Randomized, Controlled Trial. Dermatology 2022, 238, 476–486. Available online: https://pubmed.ncbi.nlm.nih.gov/34535610/ (accessed on 15 April 2025). [CrossRef]
- Strobel, A.; Schultheis, M.; Staubach, P.; Grabbe, S.; Mann, C.; Hennig, K.; Szepietowski, J.C.; Matusiak, L.; Krajewski, P.; von Stebut, E.; et al. Real-world effectiveness and safety of the LAight-therapy in patients with hidradenitis suppurativa. J. Dtsch. Dermatol. Ges. 2024, 22, 936–945. Available online: https://pubmed.ncbi.nlm.nih.gov/38807028/ (accessed on 15 April 2025). [CrossRef]
- Özdemir, A.K.; Tamer, E. 808 nm diode laser in the treatment of Hidradenitis Suppurativa: A retrospective study. Arch. Dermatol. Res. 2024, 316, 708. Available online: https://pubmed.ncbi.nlm.nih.gov/39528770/ (accessed on 15 April 2025). [CrossRef]
- Brown, N.K.D.; Kumassah, P.K.; Brown, G.D.; Brookmann, S.; Ambe, P.C.; Agbedinu, K. Minimally invasive management of hidradenitis suppurativa using a 1470 nm diode laser: A step-by-step description of our technique. BMC Surg. 2025, 25, 36. Available online: https://pubmed.ncbi.nlm.nih.gov/39849423/ (accessed on 15 April 2025). [CrossRef]
- Sidhom, S.; Petry, S.U.; Ward, R.; Daveluy, S. Treatment of hidradenitis suppurativa with 755-nm alexandrite laser hair removal: A randomized controlled trial. JAAD Int. 2024, 16, 239–243. Available online: https://pubmed.ncbi.nlm.nih.gov/39072264/ (accessed on 15 April 2025). [CrossRef]
- Molinelli, E.; Sapigni, C.; Simonetti, O.; Brisigotti, V.; Giuliodori, K.; Offidani, A. Alexandrite laser as an adjuvant therapy in the management of mild to moderate hidradenitis suppurativa: A controlled prospective clinical study. J. Am. Acad. Dermatol. 2022, 87, 674–675. Available online: https://pubmed.ncbi.nlm.nih.gov/34774922/ (accessed on 15 April 2025). [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Author (Year) | Population/Disease Duration/Hurley Stage | Laser Settings and Sessions (Parameters and Technique; Sessions) | Healing Time and Time of Follow-Up | Outcome | Adverse Events |
|---|---|---|---|---|---|
| Lapins et al. (1994) [5] | 21 F; 3 M/1–28 years/ Hurley II–III | 30 W, paintbrush technique; 1 session/area | 3–5 weeks; 15–47 months | 33% RF 8% recurrence | Secondary infections in 8% |
| Finley and Ratz (1996) [6] | 7 F/1–10 years/ND | 40 W, continuous wave; 12 procedures (5 bilateral, 2 unilateral) | 4–8 weeks; 10–27 months | 14% recurrence | Temporary paresthesias in 14%; axillary stricture in 14%; inguinal candidiasis in 14% |
| Lapins et al. (2002) [7] | 31 F; 3 M/1–35 years/Hurley II | 20–30 W, continuous wave; 67 operation sites | 3–5 weeks; 7–87 months | 24% RF; 12% recurrence | Temporary axillary paresthesia in 3% |
| Madan et al. (2008) [8] | 8 F; 1 M/4–17 years/severe, multifocal HS | 15–25 W, ultrapulse mode; 27 sites in 19 sessions | 1–4 weeks; 12 months | 67% RF | Axillary contracture scar in 22% (not limiting); dehiscence of the wound in 11% |
| Hazen and Hazen (2010) [9] | 42 F; 19 M/1–25 years/advanced, recurrent HS | 8–30 W, marsupialization; 154 sessions | Average of 8.8 weeks; 1–19 years | 1–17 years RF in treated areas; 3% recurrence | Granulation tissue in 28%; cellulitis in 5%; Sweet’s syndrome in 2%; dehiscence of the wound in 2% |
| Jain and Jain (2012) [10] | 4 F/ND/ND | Nd:YAG laser 1064 nm (30 J, 30 ms, 10 mm spot size) CO2 laser deroofing (30 W) | 15 days; 3 years | 0% recurrence | ND |
| Krakowski et al. (2014) [11] | 1 adolescent F/1 year/post-HS scarring | Fractionated CO2 (15 mJ, 15% density); 2 sessions | 2 days; 6 months | Cosmetic improvement; 0% recurrence | ND |
| Mikkelsen et al. (2015) [12] | 48 F; 10 M/4–39 years/ND | 20–35 W and 4 mm spot size | ND; 1–47 months | 29% recurrence | ND |
| Crocco et al. (2016) [13] | 2 F; 1 M/2–10 years/Hurley III | 30 W, dynamic mode; 1–3 sessions/area | (1) 9 weeks: ND (2) ND; 6 months (3) ND; ND | 100% RF; 0% recurrence | ND |
| Nicholson et al. (2016) [14] | 1 F/ND/Hurley III | 50 J, 5% density, 120 μm spot size; 4 sessions | ND; 16 months | Cosmetic improvement; 0% recurrence | ND |
| Abdel Azim et al. (2018) [15] | 11 F; 9 M/2–10 years/Hurley II–III | Fractional CO2 laser (15 W, 10,600 nm) + Nd:YAG (1064 nm); 80 sessions (4 sessions/patient with 2 weeks intervals) | ND; at 2 weeks and 3 months | (1) Combined treatment: 80% RF (2 weeks), 55% RF (3 months) (2) Nd:YAG only: 35% RF (2 weeks), 20% RF (3 months) | Transient erythema that disappeared within 48 h |
| Lindén et al. (2022) [16] | 5 F; 3 M/1–42 years/Hurley II–III | Skin type-I–III: CO2 16 W, 0.5 ms, 8 mJ and Ga-As 8 W, 6 ms, 48 mJ; Skin type IV–VI: CO2 16 W, 0.25 ms, 4 mJ and Ga-As 8 W, 4 ms, 32 mJ | ND; ND | ND | Minor and transient burning and redness in 100% |
| Sechi et al. (2025) [17] | 3 F; 7 M/ND/Hurley II | 4–10 W; continuous wave mode | 3–7 weeks; 6 months | 10% recurrence | Bleeding in 10% |
| Author (Year) | Population/Disease Duration/Hurley Stage | Laser Settings and Sessions (Parameters and Technique; Sessions) | Healing Time and Time of Follow-Up | Outcome | Adverse Events |
|---|---|---|---|---|---|
| Tierney et al. (2009) [19] | 19 F; 3 M/ND/Hurley II–III | Long-pulsed Nd:YAG; 1064 nm/3 monthly sessions | ND, 4-week follow-up | 65.3% overall HS-LASI reduction; statistically significant only at treated sites (p < 0.02) | ND |
| Mahmoud et al. (2010) [20] | 19 F; 3 M/ND/Hurley II | Long-pulsed Nd:YAG; 1064 nm; 20–35 ms/ 4 monthly sessions; | ND, 8-week follow-up | 72.7% improvement (laser) vs. 22.9% (control) | ND |
| Castrillón Velásquez et al. (2017) [21] | 1 M/3-year duration/Hurley II | Long-pulsed Nd:YAG; 1064 nm/12 monthly sessions | ND, 52-week follow-up | Sartorius score improved from 70 to 14; | ND |
| Fabbrocini et al. (2018) [18] | 14 F; 6 M/ND/Hurley I–II | Long-pulsed Nd:YAG; 1064 nm; 250 J/cm2/4 sessions every 2 weeks | ND, follow-up after last session included | 1 complete, 7 good, 10 partial, 2 no response; no worsening in any case | Mild pain, erythema, swelling; 1 case of fever (self-limited) |
| Xu et al. (2011) [22] | 17 F; 3 M/ND/Hurley II | Long-pulsed Nd:YAG; 1064 nm; stacking pulses; adjusted to skin type | ND, 8.5-week follow-up | HS-LASI score reduced by −31.6% overall | ND |
| Author (Year) | Population/Disease Duration/Hurley Stage | Laser Settings and Sessions (Parameters and Technique; Sessions) | Healing Time and Time of Follow-Up | Outcome | Adverse Events |
|---|---|---|---|---|---|
| Highton et al. (2011) [23] | 15 F, 3 M/ND/Hurley II–III | Intense Pulse Light–Harmony Laser 420 nm; fluence, 7 to 10 J/cm2; pulse width, 30 to 50 msec; 8 sessions (2 per week for 4 weeks) | ND; ND | Significant improvement on the treated side in the mean examination score maintained at 12 months, high patients’ satisfaction | ND |
| Wilden et al. (2021) [24] | 47 MF/at least 6-month duration/at least 3 abscesses or nodules | IPL + RF combination or IPL/RF monotherapy for 12 weeks followed by 12 weeks IPL + RF combination for all | Total treatment time: 24 weeks (2 × 12-week phases); follow-up not extended beyond treatment period | Change in active lesions −3.6 (p = 0.001); DLQI −5.2 (p = 0.003); After 12 weeks active lesions of the IPL + RF group decreased more than in the IPL group | wound healing delay (n = 3), transient mild to moderate pain (n = 2) lasting less than 12 h, a transiently elevated skin sensitivity (n = 2), a transient mild fever (n = 1), and a regional numbness (n = 1) |
| Schultheis et al. (2022) [25] | 88 MF/ND/Hurley I–II | LAight® therapy (IPL + RF); 8 sessions over 16 weeks (bi-weekly) | Total treatment time: 32 weeks First follow-up at week 16 | ΔIHS4: −7.2 (−60%) vs. −1.8 (−17.8%), p < 0.001; combined treatment LAight® + clindamycin superior to monotherapy—significantly higher decrease in disease severity and improvement in quality of life | Erythema (n = 26), edema/swelling (n = 18), blisters/crustification (n = 11), other AE (n = 2) |
| Strobel et al. (2024) [26] | 2 282 F, 1 155 M/ND/Hurley I–III | LAight® therapy (IPL + RF); session frequency not specified | 26-week treatment period; 6 months follow-up | Significant reductions in IHS4, pain-NRS, and DLQI across all Hurley stages; pain response rates at week 26: 80.0% (Hurley I), 70.6% (Hurley II), 42.8% (Hurley III); DLQI response rates: 66.4% (Hurley I), 61.3% (Hurley II), 52.1% (Hurley III) | Mild, transient erythema (5.7%) and edema (2.9%) |
| Author (Year) | Population/Disease Duration/Hurley Stage | Laser Settings and Sessions (Parameters and Technique; Sessions) | Healing Time and Time of Follow-Up | Outcome | Adverse Events |
|---|---|---|---|---|---|
| Brown et al. (2025) [28] | 32 MF/mean duration 8.7 yrs/Hurley I–III | 1470 nm diode laser; 5 W for shallow lesions, 8 W for deep lesions; session frequency not specified | ND; ND | Minimally invasive technique focusing on tissue preservation; emphasized importance of multidisciplinary approach and close follow-up | ND |
| Özdemir and Tamer (2024) [27] | 16 patients (13 men, 3 women), aged 23–61/ND/ND | 808 nm diode laser; 2–4 sessions; no systemic treatment 3 months prior or during therapy | ND; 6-month follow-up | Significant reduction in HS severity (HS-PGA 3.0 → 2.0, p = 0.012); 8/10 patients responded; improved DLQI (4.5 → 1.0, p = 0.002) | Mild pain; no severe adverse effects |
| Author (Year) | Population/Disease Duration/Hurley Stage | Laser Settings and Sessions (Parameters and Technique; Sessions) | Healing Time and Time of Follow-Up | Outcome | Adverse Events |
|---|---|---|---|---|---|
| Sidhom et al. (2024) [29] | 15 adult patients (age 18–60) with bilateral symmetric HS/ND/ND | 755 nm alexandrite laser, 4 monthly sessions on one side; | Total treatment time: 24 weeks (4 monthly sessions); Follow-up: 8 weeks post-treatment | HiSCR response: 75% in treated sites vs. 33.3% in control (p = 0.0046); lesion improvement: axilla 72.7%, inguinal 70%, inframammary 100% | ND |
| Molinelli et al. (2022) [30] | 40 F/ND/Hurley stages I–II | 755 nm Alexandrite laser; 5 sessions at 6-week intervals | Total treatment time: 30 weeks; Follow-up after 15 and 30 weeks | HiSCR achieved in 70% of treated patients vs. 20% in control group. Reduction in acute flares and significantly longer disease-free survival in the treated group | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawroński, M.; Bukowczyk, K.; Chęcińska, J.; Krupiczowicz, J.; Kołomyjec, M.; Łyko, M.; Jankowska-Konsur, A. Advances in Laser Therapy for Hidradenitis Suppurativa: A Systematic Assessment of Current Evidence. J. Clin. Med. 2025, 14, 7683. https://doi.org/10.3390/jcm14217683
Gawroński M, Bukowczyk K, Chęcińska J, Krupiczowicz J, Kołomyjec M, Łyko M, Jankowska-Konsur A. Advances in Laser Therapy for Hidradenitis Suppurativa: A Systematic Assessment of Current Evidence. Journal of Clinical Medicine. 2025; 14(21):7683. https://doi.org/10.3390/jcm14217683
Chicago/Turabian StyleGawroński, Michał, Kinga Bukowczyk, Julia Chęcińska, Julita Krupiczowicz, Michalina Kołomyjec, Magdalena Łyko, and Alina Jankowska-Konsur. 2025. "Advances in Laser Therapy for Hidradenitis Suppurativa: A Systematic Assessment of Current Evidence" Journal of Clinical Medicine 14, no. 21: 7683. https://doi.org/10.3390/jcm14217683
APA StyleGawroński, M., Bukowczyk, K., Chęcińska, J., Krupiczowicz, J., Kołomyjec, M., Łyko, M., & Jankowska-Konsur, A. (2025). Advances in Laser Therapy for Hidradenitis Suppurativa: A Systematic Assessment of Current Evidence. Journal of Clinical Medicine, 14(21), 7683. https://doi.org/10.3390/jcm14217683






