Development and Validation of Prognostic Models for Treatment Response of Patients with B-Cell Lymphoma: Standard Statistical and Machine-Learning Approaches
Abstract
1. Introduction
2. Methods
2.1. Data Source and Study Population
2.2. Study Variables Measurement
2.3. Statistical Analysis
2.4. Model Development
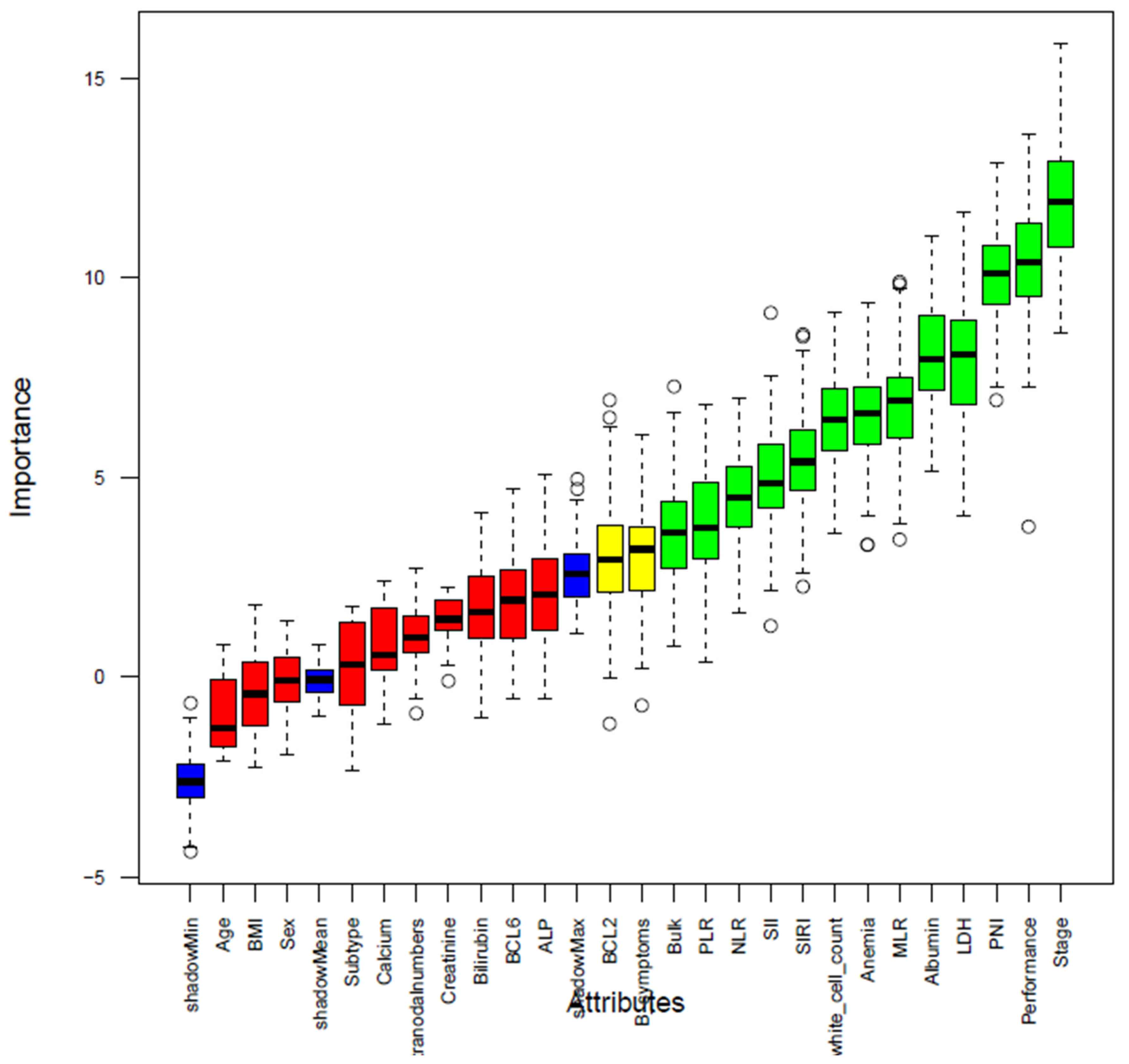
2.5. Feature Selection
2.6. Class Imbalance Management
2.7. Model Performance Evaluation
3. Results
3.1. Determination of Cut-Off Values for Inflammatory Nutritional Indicators
3.2. Background Characteristics
3.3. Features Selection
3.4. Model Development and Performance
3.5. Nomogram Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| CR | Complete response |
| GBM | Gradient Boosting Model |
| IPI | International Prognostic Index |
| LaRDR | Lymphoma and Related Diseases Registry |
| LDH | Lactate Dehydrogenase |
| LR | Logistic Regression |
| ML | Machine Learning |
| MLR | monocyte-to-lymphocyte ratio |
| NCCN-IPI | National Comprehensive Cancer Network International Prognostic Index |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| PFS | Progression Free survival |
| PLR | Platelet-to-Lymphocyte Ratio |
| PNI | Prognostic nutrition |
| RF | Random Forest |
| R-IPI | Revised International Prognostic Index |
| SII | Systemic Immune-Inflammation Index |
| SIRI | Systemic Inflammation Response Index |
| OS | Overall Survival |
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar] [CrossRef]
- Cancer Research Institute. Immunotherapy: For Lymphoma. What Makes Immunotherapy for Lymphoma a Promising Treatment? Available online: https://www.cancerresearch.org/immunotherapy-by-cancer-type/lymphoma (accessed on 23 July 2025).
- Mafra, A.; Laversanne, M.; Gospodarowicz, M.; Klinger, P.; De Paula Silva, N.; Piñeros, M.; Steliarova-Foucher, E.; Bray, F.; Znaor, A. Global patterns of non-Hodgkin lymphoma in 2020. Int. J. Cancer 2022, 151, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Liu, Y.; Fang, X.; Jiang, Y.; Ding, M.; Ge, X.; Yuan, D.; Lu, K.; Li, P.; Li, Y.; et al. The epidemiological patterns of non-Hodgkin lymphoma: Global estimates of disease burden, risk factors, and temporal trends. Front. Oncol. 2023, 13, 1059914. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.P. Treatment challenges in the management of relapsed or refractory non-Hodgkin’s lymphoma—Novel and emerging therapies. Cancer Manag. Res. 2013, 5, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Patrício, A.; Costa, R.S.; Henriques, R. On the challenges of predicting treatment response in Hodgkin’s Lymphoma using transcriptomic data. BMC Med. Genom. 2023, 16 (Suppl. S1), 170. [Google Scholar] [CrossRef]
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar] [CrossRef]
- Sehn, L.H.; Berry, B.; Chhanabhai, M.; Fitzgerald, C.; Gill, K.; Hoskins, P.; Klasa, R.; Savage, K.J.; Shenkier, T.; Sutherland, J. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007, 109, 1857–1861. [Google Scholar] [CrossRef]
- Zhou, Z.; Sehn, L.H.; Rademaker, A.W.; Gordon, L.I.; LaCasce, A.S.; Crosby-Thompson, A.; Vanderplas, A.; Zelenetz, A.D.; Abel, G.A.; Rodriguez, M.A. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood J. Am. Soc. Hematol. 2014, 123, 837–842. [Google Scholar] [CrossRef]
- Solal-Céligny, P.; Roy, P.; Colombat, P.; White, J.; Armitage, J.O.; Arranz-Saez, R.; Au, W.Y.; Bellei, M.; Brice, P.; Caballero, D. Follicular lymphoma international prognostic index. Blood 2004, 104, 1258–1265. [Google Scholar] [CrossRef]
- Hoster, E.; Dreyling, M.; Klapper, W.; Gisselbrecht, C.; Van Hoof, A.; Kluin-Nelemans, H.C.; Pfreundschuh, M.; Reiser, M.; Metzner, B.; Einsele, H. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood J. Am. Soc. Hematol. 2008, 111, 558–565. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, L.; Hua, H.; Zhou, J.; Zhao, Y.; Wang, B. An externally validated nomogram for predicting the overall survival of patients with diffuse large B-cell lymphoma based on clinical characteristics and systemic inflammatory markers. Technol. Cancer Res. Treat. 2023, 22, 15330338231180785. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, H.; Zhang, Q.; Sun, Y.; He, X.; Liao, J.; Liu, Y.; Huang, L. Nomogram based on the systemic immune-inflammation index for predicting the prognosis of diffuse large B-cell lymphoma. Asia Pac. J. Clin. Oncol. 2023, 19, e138–e148. [Google Scholar] [CrossRef]
- Bröckelmann, P.; Müller, H.; Fuchs, M.; Gillessen, S.; Eichenauer, D.; Borchmann, S.; Jacob, A.; Behringer, K.; Momotow, J.; Ferdinandus, J. Correlation between progression-free and overall survival in patients with Hodgkin lymphoma: A comprehensive analysis of individual patient data from randomized GHSG trials. Ann. Oncol. 2024, 36, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Bathe, O.F. Response biomarkers: Re-envisioning the approach to tailoring drug therapy for cancer. BMC Cancer 2016, 16, 850. [Google Scholar] [CrossRef] [PubMed]
- Bommier, C.; Zucca, E.; Chevret, S.; Conconi, A.; Nowakowski, G.; Maurer, M.J.; Cerhan, J.R.; Thieblemont, C.; Lambert, J. Early complete response as a validated surrogate marker in extranodal marginal zone lymphoma systemic therapy. Blood 2024, 143, 422–428. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, L.; Hua, H.; Zhou, J.; Zhao, Y.; Wang, B. A novel and Validated Inflammation-Based Prognosis Score (IBPS) predicts outcomes in patients with diffuse large B-cell lymphoma. Cancer Manag. Res. 2023, 15, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, W.; Hu, Y.-Y.; Li, Z.-M.; Xia, Z.-J.; Lin, X.-P.; Zhang, Y.-R.; Liang, P.-Y.; Li, Y.-H. Qualitative visual trichotomous assessment improves the value of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in predicting the prognosis of diffuse large B-cell lymphoma. Chin. J. Cancer 2015, 34, 264–271. [Google Scholar] [CrossRef]
- Sung, K.H.; Lee, E.H.; Kim, Y.Z. Factors influencing the response to high dose methotrexate-based vincristine and procarbazine combination chemotherapy for primary central nervous system lymphoma. J. Korean Med. Sci. 2011, 26, 551. [Google Scholar] [CrossRef]
- Wang, P.; Chen, K.; Wang, J.; Ni, Z.; Shang, N.; Meng, W. A new nomogram for assessing complete response (CR) in gastric diffuse large B-cell lymphoma (DLBCL) patients after chemotherapy. J. Cancer Res. Clin. Oncol. 2023, 149, 9757–9765. [Google Scholar] [CrossRef]
- Kos, I.A.; Thurner, L.; Bittenbring, J.T.; Christofyllakis, K.; Kaddu-Mulindwa, D. Advances in lymphoma molecular diagnostics. Diagnostics 2021, 11, 2174. [Google Scholar] [CrossRef]
- Huai, Q.; Luo, C.; Song, P.; Bie, F.; Bai, G.; Li, Y.; Liu, Y.; Chen, X.; Zhou, B.; Sun, X. Peripheral blood inflammatory biomarkers dynamics reflect treatment response and predict prognosis in non-small cell lung cancer patients with neoadjuvant immunotherapy. Cancer Sci. 2023, 114, 4484–4498. [Google Scholar] [CrossRef]
- Dong, J.; Sun, Q.; Pan, Y.; Lu, N.; Han, X.; Zhou, Q. Pretreatment systemic inflammation response index is predictive of pathological complete response in patients with breast cancer receiving neoadjuvant chemotherapy. BMC Cancer 2021, 21, 700. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Xiong, Z.; Gu, S.; Xia, X. The Predictive Role of Inflammatory Biomarkers for Treatment Response and Progression-Free Survival in Patients with Hepatocellular Carcinoma Receiving Hepatic Arterial Infusion Chemotherapy with FOLFOX Regimen: A Preliminary Study. J. Hepatocell. Carcinoma 2023, 10, 1037–1049. [Google Scholar] [CrossRef]
- Namikawa, T.; Yokota, K.; Tanioka, N.; Fukudome, I.; Iwabu, J.; Munekage, M.; Uemura, S.; Maeda, H.; Kitagawa, H.; Kobayashi, M. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg. Today 2020, 50, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lei, Y.; Wen, Q.; Zhang, Y.; Kong, X.; Wang, W.; Qian, S.; Hou, H.; Wang, Z.; Wu, S.; et al. The prognostic nutritional index, an independent predictor of overall survival for newly diagnosed follicular lymphoma in China. Front. Nutr. 2022, 9, 981338. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Lei, T.; Zhong, S.; Zhou, J.; Liu, R.; Wu, C.; Li, S. C-reactive protein levels, the prognostic nutritional index, and the lactate dehydrogenase-to-lymphocyte ratio are important prognostic factors in primary central nervous system lymphoma: A single-center study of 223 patients. Neurosurg. Rev. 2023, 47, 17. [Google Scholar] [CrossRef] [PubMed]
- Waley, A.B.; Haggag, R.; Ahmed Barakat, A.A.-e.; Rashied, H.A.; Bayomy, M.F.; Esawy, M.M.; Abdul-Saboor, A.; Bakry, A. Impact of Prognostic Nutritional Index and Systemic Immune-Inflammation Index on the Clinical Outcome of Diffuse Large B Cell Lymphoma Patients Treated with RCHOP. Zagazig Univ. Med. J. 2024, 30, 3907–3917. [Google Scholar] [CrossRef]
- Li, D.; Yuan, X.; Liu, J.; Li, C.; Li, W. Prognostic value of prognostic nutritional index in lung cancer: A meta-analysis. J. Thorac. Dis. 2018, 10, 5298. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Tanaka, M.D.; Geubels, B.M.; Grotenhuis, B.A.; Marijnen, C.A.; Peters, F.P.; Van der Mierden, S.; Maas, M.; Couwenberg, A.M. Validated pretreatment prediction models for response to neoadjuvant therapy in patients with rectal cancer: A systematic review and critical appraisal. Cancers 2023, 15, 3945. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Lymphoma and Related Diseases Registry Investigators. Improving outcomes for patients with lymphoma: Design and development of the Australian and New Zealand Lymphoma and Related Diseases Registry. BMC Med. Res. Methodol. 2022, 22, 266. [Google Scholar]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; WHO Technical Report Series 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Wang, Z.; Zhang, J.; Luo, S.; Zhao, X. Prognostic Significance of Systemic Immune-Inflammation Index in Patients With Diffuse Large B-Cell Lymphoma. Front. Oncol. 2021, 11, 655259. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Liu, Y.; Jiang, Y.; Ge, X.; Yuan, D.; Ding, M.; Qu, H.; Liu, F.; Zhou, X.; Wang, X. Prognosis and complications of patients with primary gastrointestinal diffuse large B-cell lymphoma: Development and validation of the systemic inflammation response index-covered score. Cancer Med. 2023, 12, 9570–9582. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature selection with the Boruta package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Bhalla, D. Select Important Variables Using Boruta Algorithm; TechTarget: Boston, MA, USA, 2017. [Google Scholar]
- Neumann, U.; Riemenschneider, M.; Sowa, J.-P.; Baars, T.; Kälsch, J.; Canbay, A.; Heider, D. Compensation of feature selection biases accompanied with improved predictive performance for binary classification by using a novel ensemble feature selection approach. BioData Min. 2016, 9, 36. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.-L.; Augustin, T. Unbiased split selection for classification trees based on the Gini index. Comput. Stat. Data Anal. 2007, 52, 483–501. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Lunardon, N.; Menardi, G.; Torelli, N. ROSE: A package for binary imbalanced learning. R J. 2014, 6, 79–89. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lam, S.-K.; Zhang, Y.; Yang, R.; Cai, J. Review of methodological workflow, interpretation and limitations of nomogram application in cancer study. Radiat. Med. Prot. 2022, 3, 200–207. [Google Scholar] [CrossRef]
- Wolff, R.F.; Moons, K.G.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S.; Group†, P. PROBAST: A tool to assess the risk of bias and applicability of prediction model studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef]
- Gupta, R.; Day, C.N.; Tobin, W.O.; Crowson, C.S. Understanding the effect of categorization of a continuous predictor with application to neuro-oncology. Neuro-Oncol. Pract. 2022, 9, 87–90. [Google Scholar] [CrossRef]
- Austin, P.C.; Harrell, F.E., Jr.; Steyerberg, E.W. Predictive performance of machine and statistical learning methods: Impact of data-generating processes on external validity in the “large N, small p” setting. Stat. Methods Med. Res. 2021, 30, 1465–1483. [Google Scholar] [CrossRef]
- Shouval, R.; Labopin, M.; Unger, R.; Giebel, S.; Ciceri, F.; Schmid, C.; Esteve, J.; Baron, F.; Gorin, N.C.; Savani, B. Prediction of hematopoietic stem cell transplantation related mortality-lessons learned from the in-silico approach: A European Society for Blood and Marrow Transplantation Acute Leukemia Working Party data mining study. PLoS ONE 2016, 11, e0150637. [Google Scholar] [CrossRef]
- Fan, S.; Zhao, Z.; Zhang, Y.; Yu, H.; Zheng, C.; Huang, X.; Yang, Z.; Xing, M.; Lu, Q.; Luo, Y. Probability calibration-based prediction of recurrence rate in patients with diffuse large B-cell lymphoma. BioData Min. 2021, 14, 38. [Google Scholar] [CrossRef]
- Fan, S.; Zhao, Z.; Yu, H.; Wang, L.; Zheng, C.; Huang, X.; Yang, Z.; Xing, M.; Lu, Q.; Luo, Y. Applying probability calibration to ensemble methods to predict 2-year mortality in patients with DLBCL. BMC Med. Inform. Decis. Mak. 2021, 21, 14. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Luo, Y.; Yu, H.; Wu, S.; Ren, X.; Zheng, C.; Huang, X. Classifying 2-year recurrence in patients with dlbcl using clinical variables with imbalanced data and machine learning methods. Comput. Methods Programs Biomed. 2020, 196, 105567. [Google Scholar] [CrossRef]
- Huang, R.J.; Kwon, N.S.-E.; Tomizawa, Y.; Choi, A.Y.; Hernandez-Boussard, T.; Hwang, J.H. A comparison of logistic regression against machine learning algorithms for gastric cancer risk prediction within real-world clinical data streams. JCO Clin. Cancer Inform. 2022, 6, e2200039. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.I.; Leung, A.A.; Walker, R.L.; Sikdar, K.C.; O’Beirne, M.; Quan, H.; Turin, T.C. A comparison of machine learning algorithms and traditional regression-based statistical modeling for predicting hypertension incidence in a Canadian population. Sci. Rep. 2023, 13, 13. [Google Scholar] [CrossRef]
- Didier, A.J.; Nigro, A.; Noori, Z.; Omballi, M.A.; Pappada, S.M.; Hamouda, D.M. Application of machine learning for lung cancer survival prognostication—A systematic review and meta-analysis. Front. Artif. Intell. 2024, 7, 1365777. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, S.-X.; Liu, P. Machine learning in predicting pathological complete response to neoadjuvant chemoradiotherapy in rectal cancer using MRI: A systematic review and meta-analysis. Br. J. Radiol. 2024, 97, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, L.; Wang, S.; Zhao, X.; Chen, L.; Sun, X.; Liu, Y.; Liu, J.; Sun, S. Utility of contrast-enhanced MRI radiomics features combined with clinical indicators for predicting induction chemotherapy response in primary central nervous system lymphoma. J. Neuro-Oncol. 2024, 166, 451–460. [Google Scholar] [CrossRef]
- Ben Bouallègue, F.; Tabaa, Y.A.; Kafrouni, M.; Cartron, G.; Vauchot, F.; Mariano-Goulart, D. Association between textural and morphological tumor indices on baseline PET-CT and early metabolic response on interim PET-CT in bulky malignant lymphomas. Med. Phys. 2017, 44, 4608–4619. [Google Scholar] [CrossRef]
- Alem, A.Z.; Mohanty, I.; Pati, N.; Niyonsenga, T. Prognostic performance of machine learning in predicting haematological cancer outcomes: Systematic review and meta-analysis. Blood Rev. 2025, 28, 101325. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Vickers, A.J.; van Calster, B.; Steyerberg, E.W. A simple, step-by-step guide to interpreting decision curve analysis. Diagn. Progn. Res. 2019, 3, 18. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Sesques, P.; Johnson, N.A. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood J. Am. Soc. Hematol. 2017, 129, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.-C.; Ye, B.-K.; Yao, D.-J.; Guo, C.-C. A novel lactate dehydrogenase-based risk score model to predict the prognosis of primary central nervous system germ cell tumor treated with chemoradiotherapy. Clin. Neurol. Neurosurg. 2024, 236, 108081. [Google Scholar] [CrossRef]
- Yuan, S.; Xia, Y.; Shen, L.; Ye, L.; Li, L.; Chen, L.; Xie, X.; Lou, H.; Zhang, J. Development of nomograms to predict therapeutic response and prognosis of non-small cell lung cancer patients treated with anti-PD-1 antibody. Cancer Immunol. Immunother. 2021, 70, 533–546. [Google Scholar] [CrossRef]
- Peng, R.-R.; Liang, Z.-G.; Chen, K.-H.; Li, L.; Qu, S.; Zhu, X.-D. Nomogram based on lactate dehydrogenase-to-albumin ratio (LAR) and platelet-to-lymphocyte ratio (PLR) for predicting survival in nasopharyngeal carcinoma. J. Inflamm. Res. 2021, 14, 4019–4033. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Lin, M.; Liu, T.; Li, J. Factors associated with immunotherapy respond and survival in advanced non-small cell lung cancer patients. Transl. Oncol. 2022, 15, 101268. [Google Scholar] [CrossRef]
- Hammarström, K.; Imam, I.; Mezheyeuski, A.; Ekström, J.; Sjöblom, T.; Glimelius, B. A comprehensive evaluation of associations between routinely collected staging information and the response to (chemo) radiotherapy in rectal cancer. Cancers 2020, 13, 16. [Google Scholar] [CrossRef]
- Zhong, J.-H.; Huang, D.-H.; Chen, Z.-Y. Prognostic role of systemic immune-inflammation index in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 75381. [Google Scholar] [CrossRef]
- Kou, J.; Huang, J.; Li, J.; Wu, Z.; Ni, L. Systemic immune-inflammation index predicts prognosis and responsiveness to immunotherapy in cancer patients: A systematic review and meta-analysis. Clin. Exp. Med. 2023, 23, 3895–3905. [Google Scholar] [CrossRef]
- Agyeman, K.B.; Shafi, N.; Contreras, R.; Parackal, V.; Shah, D.N.; Gurram, A.; Keetha, N.R.; Ameen, D. The Association Between Systemic Immune-Inflammation Index and Cardiovascular Diseases: An In-depth Umbrella Review of Meta-Analyses with Grade Assessment. Heliyon 2025, 11, e42736. [Google Scholar] [CrossRef]
- Wang, H.; Nie, H.; Bu, G.; Tong, X.; Bai, X. Systemic immune-inflammation index (SII) and the risk of all-cause, cardiovascular, and cardio-cerebrovascular mortality in the general population. Eur. J. Med. Res. 2023, 28, 575. [Google Scholar] [CrossRef]
- Mir, F.; Mattiello, F.; Grigg, A.; Herold, M.; Hiddemann, W.; Marcus, R.; Seymour, J.F.; Bolen, C.R.; Knapp, A.; Nielsen, T. Follicular Lymphoma Evaluation Index (FLEX): A new clinical prognostic model that is superior to existing risk scores for predicting progression-free survival and early treatment failure after frontline immunochemotherapy. Am. J. Hematol. 2020, 95, 1503–1510. [Google Scholar] [CrossRef]
- Clausen, M.R.; Maurer, M.J.; Ulrichsen, S.P.; Larsen, T.S.; Himmelstrup, B.; Rønnov-Jessen, D.; Link, B.K.; Feldman, A.L.; Slager, S.L.; Nowakowski, G.S. Pretreatment hemoglobin adds prognostic information to the NCCN-IPI in patients with diffuse large B-cell lymphoma treated with anthracycline-containing chemotherapy. Clin. Epidemiol. 2019, 11, 987–996. [Google Scholar] [CrossRef]
- Troppan, K.T.; Melchardt, T.; Deutsch, A.; Schlick, K.; Stojakovic, T.; Bullock, M.D.; Reitz, D.; Beham-Schmid, C.; Weiss, L.; Neureiter, D. The significance of pretreatment anemia in the era of R-IPI and NCCN-IPI prognostic risk assessment tools: A dual-center study in diffuse large B-cell lymphoma patients. Eur. J. Haematol. 2015, 95, 538–544. [Google Scholar] [CrossRef]
- Gaspar, B.L.; Sharma, P.; Das, R. Anemia in malignancies: Pathogenetic and diagnostic considerations. Hematology 2015, 20, 18–25. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, K.; He, Q.; Gu, X.; Jiang, C.; Wu, J. Hypoxia signaling in cancer: Implications for therapeutic interventions. MedComm 2023, 4, e203. [Google Scholar] [CrossRef]
- Bozzini, C.; Busti, F.; Marchi, G.; Vianello, A.; Cerchione, C.; Martinelli, G.; Girelli, D. Anemia in patients receiving anticancer treatments: Focus on novel therapeutic approaches. Front. Oncol. 2024, 14, 1380358. [Google Scholar] [CrossRef]
- Cannavale, K.; Xu, H.; Xu, L.; Sattayapiwat, O.; Rodriguez, R.; Bohac, C.; Page, J.; Chao, C. Epidemiology of chemotherapy-induced anemia in patients with non-hodgkin lymphoma. Perm. J. 2019, 23, 18–252. [Google Scholar] [CrossRef]
- Gong, Y.; Yan, H.; Yang, Y.; Zhai, B.; Huang, Z.; Zhang, Z. Construction and validation of a novel nomogram for predicting the recurrence of diffuse large B cell lymphoma treated with R-CHOP. Pharmacogenomics Pers. Med. 2023, 16, 291–301. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Zhang, T.; Song, Z.; Hu, G.; Li, W.; Li, L.; Qiu, L.; Qian, Z.; Zhou, S. Prognostic Significance of BCL-2 and BCL-6 Expression in MYC-positive DLBCL. Clin. Lymphoma Myeloma Leuk. 2018, 18, e381–e389. [Google Scholar] [CrossRef]
- Elhendawy, H.A.; Ibrahiem, A.T.; Elmahdi, H.S.; Omar, A.M. Prognostic Significance of BCL2 Protein in Diffuse Large Cell Lymphoma of Head and Neck; Relation to Response to Chemotherapy. Open J. Pathol. 2020, 10, 76. [Google Scholar] [CrossRef][Green Version]
- Correia, C.; Maurer, M.J.; McDonough, S.J.; Schneider, P.A.; Ross, P.E.; Novak, A.J.; Feldman, A.L.; Cerhan, J.R.; Slager, S.L.; Witzig, T.E. Relationship between BCL2 mutations and follicular lymphoma outcome in the chemoimmunotherapy era. Blood Cancer J. 2023, 13, 81. [Google Scholar] [CrossRef]
- Liu, Y.; He, P.; Liu, F.; Shi, L.; Zhu, H.; Cheng, X.; Zhao, J.; Wang, Y.; Zhang, M. Prognostic significance of B-cell lymphoma 2 expression in acute leukemia: A systematic review and meta-analysis. Mol. Clin. Oncol. 2014, 2, 411–414. [Google Scholar] [CrossRef]
- Chong, S.J.F.; Lu, J.; Valentin, R.; Lehmberg, T.Z.; Eu, J.Q.; Wang, J.; Zhu, F.; Kong, L.R.; Fernandes, S.M.; Zhang, J. BCL-2 dependence is a favorable predictive marker of response to therapy for chronic lymphocytic leukemia. Mol. Cancer 2025, 24, 62. [Google Scholar] [CrossRef]




| Variables | Treatment Response, N (%) | X2 (p-Value) | |
|---|---|---|---|
| Complete | Incomplete | ||
| Sex | |||
| Male | 1231 (74.9) | 412 (25.1) | 0.93 (0.334) |
| Female | 858 (76.6) | 262 (23.4) | |
| Age | |||
| ≤60 | 675 (77.3) | 198 (22.7) | 1.90 (0.168) |
| >60 | 1414 (74.8) | 476 (25.2) | |
| BMI | |||
| Underweight | 47 (72.1) | 19 (28.8) | 6.91 (0.075) |
| Normal | 668 (73.1) | 246 (26.9) | |
| Overweight | 726 (76.1) | 228 (23.9) | |
| Obese | 648 (78.2) | 181 (21.8) | |
| Stage | |||
| I | 359 (89.5) | 42 (10.5) | 84.89(<0.001) |
| II | 330 (83.8) | 64 (16.2) | |
| III | 402 (75.4) | 131 (24.6) | |
| IV | 998 (69.5) | 437 (30.5) | |
| I or II | 689 (86.7) | 106 (13.3) | 73.19 (<0.001) |
| III or IV | 1400 (71.1) | 568 (28.9) | |
| Subtype | |||
| DLBCL | 1480 (74.1) | 518 (25.9) | 9.63 (0.022) |
| FL | 412 (79.1) | 109 (20.9) | |
| MCL | 144 (80.0) | 36 (20.0) | |
| BL | 53 (82.8) | 11 (17.2) | |
| ECOG performance status | |||
| 0 or 1 | 1863 (78.0) | 525 (22.0) | 54.40 (<0.001) |
| 2–4 | 226 (60.3) | 149 (39.7) | |
| LDH | |||
| Normal | 1109 (83.1) | 225 (16.9) | 78.45 (<0.001) |
| Elevated | 980 (68.6) | 449 (31.4) | |
| B symptoms | |||
| Absent | 1686 (77.1) | 500 (22.9) | 12.74 (<0.001) |
| Present | 403 (69.8) | 174 (30.2) | |
| BCL6 expression | |||
| Negative | 636 (73.8) | 226 (26.2) | 2.12 (0.14) |
| Positive | 1453 (76.4) | 448 (23.6) | |
| BCL2 expression | |||
| Negative | 758 (78.5) | 207 (21.5) | 6.72 (0.009) |
| Positive | 1331 (74.0) | 467 (26.0) | |
| Number of Extranodal sites | |||
| ≤1 | 1419 (77.9) | 402 (22.1) | 15.19 (<0.001) |
| >1 | 670 (71.1) | 272 (28.9) | |
| Bulk disease | |||
| No | 1392 (78.2) | 388 (21.8) | 17.89 (<0.001) |
| Yes | 697 (70.9) | 286 (29.1) | |
| Anemia | |||
| No | 1317 (81.2) | 304 (18.8) | 66.90 (<0.001) |
| Yes | 772 (67.6) | 370 (32.4) | |
| Albumin | |||
| Low | 662 (67.5) | 319 (32.5) | 53.75 (<0.001) |
| High | 1427 (80.1) | 355 (19.9) | |
| Creatinine | |||
| Low (≤95.5) | 1674 (76.4) | 516 (23.6) | 3.75 (0.053) |
| High (>95.5) | 415 (72.4) | 158 (27.6) | |
| Alkaline phosphate | |||
| Low (≤83.5) | 1090 (78.5) | 298 (21.5) | 12.61 (<0.001) |
| High (>83.5) | 999 (72.7) | 376 (27.3) | |
| Bilirubin | |||
| Low (≤40.5) | 2052 (75.9) | 653 (24.1) | 3.85 (0.050) |
| High (>40.5) | 37 (63.8) | 21 (36.2) | |
| PNI | |||
| Low (≤40.93) | 626 (67.8) | 297 (32.2) | 44.90 (<0.001) |
| High (>40.93) | 1463 (79.5) | 377 (20.5) | |
| SII | |||
| Low (≤1686.985) | 1651 (79.1) | 436 (20.9) | 55.97 (<0.001) |
| High (>1686.985) | 438 (64.8) | 238 (35.2) | |
| SIRI | |||
| Low (≤3.529) | 1542 (79.2) | 406 (20.8) | 44.52 (<0.001) |
| High (>3.529) | 547 (67.1) | 268 (32.9) | |
| MLR | |||
| Low (≤0.611) | 1479 (79.0) | 394 (21.0) | 34.99 (<0.001) |
| High (>0.611) | 610 (68.5) | 280 (31.5) | |
| PLR | |||
| Low (≤274.773) | 1561 (78.8) | 419 (21.2) | 38.96 (<0.001) |
| High (>274.773) | 528 (67.4) | 255 (32.6) | |
| NLR | |||
| Low (≤5.123) | 1503 (79.3) | 397 (20.7) | 43.40 (<0.001) |
| High (>5.123) | 586 (67.6) | 281 (32.4) | |
| IPI risk group | |||
| Low | 625 (89.0) | 77 (11.0) | 117.27 (<0.001) |
| Low intermediate | 592 (76.4) | 183 (23.6) | |
| High intermediate | 513 (70.4) | 216 (29.6) | |
| High | 359 (64.5) | 198 (35.5) | |
| Revised IPI risk group | |||
| Low | 159 (91.4) | 15 (8.6) | 87.97 (<0.001) |
| Intermediate | 1058 (81.2) | 245 (18.8) | |
| High | 872 (67.8) | 414 (32.2) | |
| NCCN-IPI risk group | |||
| Low | 175 (88.8) | 22 (11.2) | 106.35 (<0.001) |
| Low intermediate | 919 (82.7) | 192 (17.3) | |
| High intermediate | 819 (70.7) | 340 (29.3) | |
| High | 176 (59.5) | 120 (40.5) | |
| Model | Discrimination and Classification Metrics | Calibration | |||||
|---|---|---|---|---|---|---|---|
| AUC | Accuracy | Sensitivity | Specificity | PPV | NPV | Brier Score | |
| IPI | 0.65 | 0.60 | 0.61 | 0.59 | 0.31 | 0.83 | 0.235 |
| R-IPI | 0.61 | 0.60 | 0.61 | 0.59 | 0.31 | 0.83 | 0.239 |
| NCCN-IPI | 0.63 | 0.55 | 0.68 | 0.51 | 0.30 | 0.84 | 0.238 |
| LR | 0.70 | 0.62 | 0.70 | 0.60 | 0.35 | 0.87 | 0.227 |
| RF | 0.69 | 0.62 | 0.73 | 0.59 | 0.35 | 0.88 | 0.298 |
| XgBoost | 0.70 | 0.61 | 0.73 | 0.58 | 0.34 | 0.88 | 0.231 |
| KNN | 0.69 | 0.61 | 0.76 | 0.57 | 0.35 | 0.89 | 0.233 |
| GBM | 0.69 | 0.61 | 0.68 | 0.60 | 0.34 | 0.86 | 0.232 |
| SVM | 0.69 | 0.62 | 0.70 | 0.59 | 0.34 | 0.87 | 0.229 |
| NB | 0.70 | 0.68 | 0.58 | 0.69 | 0.36 | 0.85 | 0.223 |
| Model | Risk Groups (Score) | ||||
|---|---|---|---|---|---|
| Nomogram | Low (<138) | LI (138–188) | HI (188–225) | High (≥225) | |
| Risk factors and scoring | Stage (I/II = 0; III/IV = 100) ECOG PS (≤1 = 0; >1 = 57) BCL2 Expression (Negative = 0; Positive = 36) SII (Low = 0; High = 70) Anemia (No = 0; Yes = 37) LDH (Normal = 0; Elevated = 52) | ||||
| Frequency (%) | 218 (39.4) | 125 (22.6) | 93 (16.8) | 117 (21.2) | |
| Treatment response | Complete (%) | 90.9 | 74.4 | 67.7 | 60.7 |
| Incomplete (%) | 9.1 | 25.6 | 32.3 | 39.3 | |
| IPI | Low (0–1) | LI (2) | HI (3) | High (4–5) | |
| Risk factors and scoring | Age (≤60 = 0; >60 = 1) Stage (I/II = 0; III/IV = 1) LDH (Normal = 0; Elevated = 1) ECOG PS (≤1 = 0; >1 = 1) Extranodal Sites (≤1 = 0; >1 = 1) | ||||
| Frequency (%) | 136 (25.6) | 167 (30.2) | 140 (25.3) | 110 (19.9) | |
| Treatment response | Complete (%) | 89.7 | 78.4 | 77.9 | 57.3 |
| Incomplete (%) | 10.3 | 21.6 | 22.1 | 42.7 | |
| R-IPI | Very good (0) | Good (1–2) | Poor (3–5) | ||
| Risk factors and scoring | Age (≤60 = 0; >60 = 1) Stage (I/II = 0; III/IV = 1) LDH (Normal = 0; Elevated = 1) ECOG PS (≤1 = 0; >1 = 1) Extranodal Sites (≤1 = 0; >1 = 1) | ||||
| Frequency (%) | 30 (5.4) | 273 (49.4) | 250 (45.2) | ||
| Treatment response | Complete (%) | 93.3 | 82.4 | 68.8 | |
| Incomplete (%) | 6.7 | 17.6 | 31.2 | ||
| NCCN-IPI | Low (0–1) | LI (2–3) | HI (4–5) | High (6–8) | |
| Risk factors and scoring | Age (<40 = 0; 41–60 = 1; 61–75 = 2; >75 = 3) LDH (≤ULN = 0; >ULN–≤3 × ULN = 1; >3 × ULN = 2) Stage (I/II = 0; III/IV = 1) ECOG PS (≤1 = 0; >1 = 1) Major Extranodal Sites (No = 0; Yes = 1) | ||||
| Frequency (%) | 34 (6.1) | 226 (40.9) | 232 (42.0) | 61 (11.0) | |
| Treatment response | Complete (%) | 91.2 | 83.2 | 74.1 | 55.7 |
| Incomplete (%) | 8.8 | 16.8 | 25.9 | 44.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alem, A.Z.; Mohanty, I.; Pati, N.; Wellard, C.; Chung, E.; Hawkes, E.A.; McQuilten, Z.K.; Wood, E.M.; Opat, S.; Niyonsenga, T. Development and Validation of Prognostic Models for Treatment Response of Patients with B-Cell Lymphoma: Standard Statistical and Machine-Learning Approaches. J. Clin. Med. 2025, 14, 7445. https://doi.org/10.3390/jcm14207445
Alem AZ, Mohanty I, Pati N, Wellard C, Chung E, Hawkes EA, McQuilten ZK, Wood EM, Opat S, Niyonsenga T. Development and Validation of Prognostic Models for Treatment Response of Patients with B-Cell Lymphoma: Standard Statistical and Machine-Learning Approaches. Journal of Clinical Medicine. 2025; 14(20):7445. https://doi.org/10.3390/jcm14207445
Chicago/Turabian StyleAlem, Adugnaw Zeleke, Itismita Mohanty, Nalini Pati, Cameron Wellard, Eliza Chung, Eliza A. Hawkes, Zoe K. McQuilten, Erica M. Wood, Stephen Opat, and Theophile Niyonsenga. 2025. "Development and Validation of Prognostic Models for Treatment Response of Patients with B-Cell Lymphoma: Standard Statistical and Machine-Learning Approaches" Journal of Clinical Medicine 14, no. 20: 7445. https://doi.org/10.3390/jcm14207445
APA StyleAlem, A. Z., Mohanty, I., Pati, N., Wellard, C., Chung, E., Hawkes, E. A., McQuilten, Z. K., Wood, E. M., Opat, S., & Niyonsenga, T. (2025). Development and Validation of Prognostic Models for Treatment Response of Patients with B-Cell Lymphoma: Standard Statistical and Machine-Learning Approaches. Journal of Clinical Medicine, 14(20), 7445. https://doi.org/10.3390/jcm14207445







