Abstract
Background: Osteoarthritis (OA) is associated with triglyceride–glucose (TyG) index values. However, the evidence supporting this association remains controversial. This study explores the associations of OA with the TyG index and with a combination of the TyG index and obesity in the Korean population. Methods: A total of 15,128 subjects (including 6575 men and 8553 women) were included in this analysis. Complex sample binary logistic regression was used to analyze the associations of OA with TyG, TyG-body mass index (TyG-BMI), TyG-waist circumference (TyG-WC), and the TyG-waist-to-height ratio (TyG-WHtR). We applied the Bonferroni correction to account for multiple comparisons. Results: The prevalence of OA in the Korean population was 7.3% in men and 24.3% in women. The TyG was not associated with OA. In men, the odds ratio (OR) for the TyG was 0.89 (95% CI, 0.79–1.00; p = 0.315) per one standard deviation increase in the crude model, and the adjusted OR was 0.98 (95% CI, 0.86–1.11; adjusted p > 0.999) in the adjusted model. In women, the OR for the TyG was 1.27 (95% CI, 1.19–1.35; p < 0.001), whereas the adjusted OR was 1.08 (95% CI, 1.01–1.16; adjusted p = 0.236). In the adjusted models, TyG-BMI, TyG-WC, and TyG-WHtR were not associated with OA in men but strongly associated with OA in women. Conclusions: The TyG index was not associated with OA in the Korean population. The strength of the associations between OA and the TyG indices combined with obesity was lower than the that of the associations between OA and BMI, WC, and WHtR individually.
1. Introduction
Osteoarthritis (OA) is a major public health issue in middle-aged and older populations and represents the most prevalent form of arthritis [1,2]. According to the reports of GBD 2021 Osteoarthritis [1], 595 million people had OA in 2020, thus accounting or 7.6% of the global population. OA affects quality of life and daily life activities and is a leading cause of chronic pain, disability, morbidity, and mortality [1,2]. OA most commonly occurs in the appendicular joints of the knees, hips, and hands and is the most common pathological disease characterized by subchondral bone perfusion abnormalities [1,3,4]. Modifiable risk factors for OA include joint injury, obesity or high body mass index (BMI), muscle weakness, smoking, and high-level physical occupations [1,5,6,7], whereas nonmodifiable risk factors include age, sex, hypertension, and diabetes [3,7,8,9]. Clinical management or treatments for OA include physical therapy, exercise and weight loss, pain medication, surgical treatment, nonsteroidal anti-inflammatory drugs, and endothelium-targeted strategies [1,3,4].
Recently, several studies have reported an association between triglyceride–glucose (TyG) index values and various diseases. The TyG index is a predictor of insulin resistance (IR), which has been identified as a potential risk factor for OA, metabolic syndrome, stroke, Alzheimer’s disease, depression, diabetes, hypertension, cardiovascular disease, dementia, and nonalcoholic fatty liver disease [7,10,11,12]. In studies suggesting an association between TyG and OA, insulin resistance has been shown to be closely related to metabolic dysfunction, inflammation, bone mass and density, and obesity [7,13]. Adipose tissue is an important source of cytokines and adipokines, which may contribute to the development and progression of arthritis [12,13,14]. Individuals with obesity tend to have higher serum levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) [14]. Elevated levels of TNF-α and IL-6 have also been detected in the subchondral bone and cartilage of OA patients [14]. Furthermore, TNF-α promotes the development of insulin resistance and obesity [14]. Therefore, both adipose tissue and insulin resistance represent important biological risk factors for OA [7,12,13,14]. However, although OA has been associated with TyG index values [7,12,13,15,16], the evidence supporting this association remains controversial. Specifically, several studies have reported that the TyG index is strongly associated with OA [7,12,13,15,16], whereas some studies have argued that the TyG index is only weakly associated or not associated with OA [11,17,18]. For example, the study by Huang et al. [7] demonstrated an association between a higher TyG index and an increased risk of OA (odds ratio [OR] = 7.34 [95% confidence interval (CI) = 2.25–23.93], p = 0.001) after adjusting for various potential covariates in 3921 participants from the U.S. population. In contrast, Lan et al. [11] reported no significant association between TyG index and OA (OR = 1.23 [95% CI = 0.93–1.62], p = 0.151) after adjusting for multiple covariates in 14,715 participants from the adult U.S. population. Furthermore, obesity and IR are major risk factors for OA development [11]. Therefore, several studies have reported that TyG indices combined with obesity are strongly associated with OA [11,17,18,19]. Therefore, this study aimed to examine the association between the TyG index value and OA in the Korean population. Also, we examined the association between OA and TyG indices combined with obesity. Previous studies have mainly focused on the association between OA and the TyG index or TyG indices combined with obesity indices, and the findings remain controversial. In addition, few studies have compared sex differences in the association between OA and TyG index. Therefore, the present study investigated the association of OA with TyG index and TyG indices combined with obesity indices, stratified by sex, through a comprehensive cross-sectional analysis using a large-scale dataset from the Korean population.
2. Materials and Methods
2.1. Study Design and Target Population
In this large-scale cross-sectional study, we used data from the Korea National Health and Nutrition Examination Survey (KNHANES), which focuses on a national representative sample of the Korean population. The KNHANES provides reliable statistics on basic demographic characteristics, nutritional intake status, chronic disease, biochemical profiles, anthropometric measurements and health examinations, and health behavior and status for the Korean population. Additionally, a complex survey sample design that considers weighting, clustering, and stratification was used to address the KNHANES data to ensure that these data were representative of the entire Korean population. Initially, 47,309 subjects participated in the KNHANES from 2014 to 2019. The target population of the present study was adults aged over 45 years because the prevalence of OA in the Korean population aged under 45 years is very low, and high TyG values increase the incidence of arthritis in the population aged over 45 years [12]. Subjects with missing values for major variables, such as biochemical profiles, obesity indices, and important sociodemographic and socioeconomic characteristics, were excluded from the final analysis. Ultimately, a total of 15,128 subjects (6575 men and 8553 women) were included in the study. The specific inclusion and exclusion criteria are presented in Figure 1.
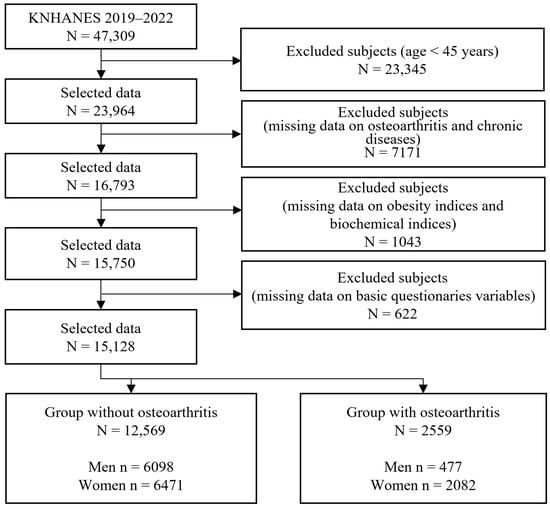
Figure 1.
Sample selection criteria and procedure.
The institutional review board (IRB) of the Korea Disease Control and Prevention Agency (KDCA) approved the KNHANES (IRBs: 2018-01-03-C-A, 2018-01-03-P-A, and 2013-12EXP-03-5C). Furthermore, the IRB of the Korea Institute of Oriental Medicine approved the use of publicly available anonymized KNHANES data (IRB No. I-2501/001-001). All the subjects provided written informed consent before they participated in the KNHANES. Publicly available KNHANES data did not contain any personally identifiable information. The present study was conducted in accordance with the principles of the Declaration of Helsinki.
2.2. Definition of Osteoarthritis
In this study, the OA group was defined on the basis of patients who were diagnosed by a doctor and currently suffering from OA. As in previous studies [12,13,17,19], the medical history of doctor-diagnosed OA was obtained by a self-report questionnaire. Additionally, to mitigate respondent recall bias, face-to-face health interviews were conducted with well-trained staff or experts in accordance with the guidelines. The interviewer asked, “Have you been diagnosed with OA by a doctor?” and “Are you currently suffering from OA?” If the respondents answered “Yes” to both questions, they were included in the OA group. Additionally, subjects who were diagnosed with osteoporosis in the past but had since been cured were included in the non-OA group.
2.3. Covariates
Based on previous studies reporting associations between OA and TyG, we identified potential confounders that may influence the association. The confounders were as follows: age [7,11,12,13,15,16,17,18,19], education level (tercile) [7,11,12,13,15,16,17,18,19], household income (quartile) [7,11,13,17,18,19], alcohol consumption (yes or no) [7,11,12,17,18,19], smoking (yes or no) [7,11,12,16,17,18,19], weekly walking or exercise (yes or no) [7,13,16], marital status (married or single) [12,13,16,19], and menopause (in women only; yes or no) [20,21]. Diabetes or hypertriglyceridemia status was not used as covariates because the TyG index is derived from triglyceride and glucose levels. A detailed description of these confounders is shown in Table 1.

Table 1.
Sociodemographic characteristics of the subjects.
2.4. Measurements and Laboratory Tests
Measurements for obesity indices were performed by specialized staff and experts based on standard protocols. Height and weight measurements of all participants were conducted on the basis of automatic measurement equipment (JENIX DS-102; Dong Sahn Jenix Co., Seoul, Republic of Korea) and measured in units of 0.1 cm and 0.1 kg, respectively. WC was measured with a flexible plastic tape (Seca 200, Hamburg, Germany). The WHtR was calculated by dividing WC by height. BMI was obtained as weight divided by height squared (kg/m2).
For biochemical tests, we obtained blood samples from all participants after a minimum fasting period of 8 h based on standard examination protocols. Fasting plasma glucose and triglyceride concentrations were measured by standard enzymatic methods using an automatic analyzer (Hitachi Automatic Analyzer 7600; Hitachi Co., Ltd., Tokyo, Japan). Diastolic and systolic blood pressures (DBP and SBP, respectively) were measured three times using a mercury sphygmomanometer (Baumanometer Wall Unit 33 (0850); Baum Inc., Copiague, NY, USA). The mean of the second and third measurements was used in the analysis. The TyG index was calculated with formulas based on a previous study [22]: TyG = Ln [TG (mg/dL) × glucose (mg/dL)/2)]. Additionally, TyG indices combined with obesity indices were calculated on the basis of a formula developed in accordance with previous studies [11,15,17,19,23]: TyG-BMI = TyG × BMI, TyG-WC = TyG × WC (cm), and TyG-WHtR = TyG × WHtR.
2.5. Statistical Analysis
All statistical analyses were conducted using the complex sampling design module in SPSS version 28 (IBM SPSS, Inc., Chicago, IL, USA) to account for the survey design of KNHANES. To produce nationally representative estimates, all analyses incorporated the official survey weights, strata, and cluster variables, in accordance with the KNHANES guidelines. This approach was applied to all results, including the descriptive statistics in Table 1 and the odds ratios derived from the complex sample logistic regression models. Further details on the weighting, stratification, and clustering procedures of the KNHANES data are provided in reference [24]. To examine the statistical significance of sex differences between men and women, t-tests with general linear models and Rao–Scott chi-square tests were performed for continuous variables and categorical variables, respectively. The associations of OA with the obesity indices, the TyG index, and the TyG index combined with obesity were examined using complex sample binary logistic regression for both crude and adjusted models after applying standardization. A detailed description and statistical analysis methods of a complex survey sample design are available [24,25]. Before the statistical analysis of the KNHANES data was conducted, we examined the multicollinearity between variables based on the variance inflation factor (VIF). Additionally, we tested the linearity between the logit of the dependent variable and the independent variables using the Box–Tidwell test. The VIF values of all the variables, including BMI, WC, WHtR, TyG, TyG-BMI, TyG-WC, and TyG-WHtR, ranged from 1.00 to 1.33 for men and from 1.10 to 1.47 for women. Additionally, all the continuous independent variables met the linearity assumption because the p values of all the continuous independent variables ranged from 0.09 to 0.81 for men and from 0.40 to 0.99 for women according to the results of the Box–Tidwell test. We applied the Bonferroni correction to account for multiple comparisons arising from testing the seven indices such as BMI, WC, WHtR, TyG, TyG-BMI, TyG-WC, and TyG-WHtR. Accordingly, we set a more stringent threshold for statistical significance at a Bonferroni corrected p value < 0.007 (α = 0.05/7). Odds ratios (ORs) are shown with 95% confidence intervals (CIs), and a Bonferroni corrected p value < 0.05 was considered statistically significant.
3. Results
3.1. Basic Sociodemographic Characteristics of the Subjects
Table 1 presents the basic characteristics of the subjects who participated in this study. The prevalence of OA in the Korean population was 7.3% in men and 24.3% in women. The mean ages of the normal and OA groups were 58.73 ± 0.164 and 66.24 ± 0.53 years, respectively, for men (p < 0.001) and 57.9 ± 0.152 and 66.72 ± 0.253 years, respectively, for women (p < 0.001). In terms of sex differences, all the variables, including anthropometric indices, blood profiles, sociodemographic variables, and chronic diseases, exhibited significant differences between men and women (p < 0.001), except for the weekly working variable (p < 0.01). With respect to the difference between the normal and OA groups, in men, most variables exhibited significant differences except for BMI, glucose level, TyG-BMI, TyG-WC, marital status, weekly working status, and hypercholesterolemia. In women, all variables other than smoking exhibited significant differences between the normal and OA groups.
3.2. Associations of OA with the TyG Index and the TyG Index Combined with Obesity
Table 2 and Table 3 show the association of OA with the TyG index and TyG index combined with obesity among Korean men and women. Based on a Bonferroni corrected p value, the TyG index was not associated with OA in either the crude (OR = 0.89 [0.79–1.00], p = 0.315) or adjusted models (adj. OR = 0.98 [0.86–1.11], adj. p > 0.999) in men. Among the TyG indices combined with obesity, OA was not associated with TyG-BMI and TyG-WC in either the crude or adjusted models. TyG-WHtR was related to OA in the crude model (OR = 1.23 [1.09–1.38], p = 0.007), but this association disappeared in the adjusted model (adj. OR = 1.16 [1.03–1.31], adj. p = 0.103). The magnitudes of these associations were lower than those of the associations of OA with BMI, WC, and WHtR individually in both the crude and adjusted models.

Table 2.
Associations of OA with the TyG index and TyG indices combined with obesity in Korean men.

Table 3.
Associations of OA with the TyG index and TyG indices combined with obesity among Korean women.
In women, the TyG index was associated with OA (OR = 1.27 [1.19–1.35], p < 0.001); however, this association disappeared after adjustment to account for confounders (adj. OR = 1.08 [1.01–1.16], adj. p = 0.236). OA was strongly associated with TyG-BMI, TyG-WC, and TyG-WHtR in both the crude model (OR = 1.60 [1.50–1.70], p < 0.001; OR = 1.75 [1.64–1.87], p < 0.001; OR = 1.93 [1.80–2.07], p < 0.001) and the adjusted model (adj. OR = 1.44 [1.34–1.54], adj. p < 0.001; adj. OR = 1.40 [1.30–1.50], adj. p < 0.001; adj. OR = 1.39 [1.29–1.50], adj. p < 0.001). However, the magnitudes of these associations were slightly lower than those of the associations of OA with BMI, WC, and WHtR individually in both the crude and adjusted models.
In the interaction analysis between sex and TyG indices combined with obesity, the interaction effect was significant for all TyG indices combined with obesity (p for interaction < 0.001), except for the TyG index (p for interaction = 0.06) in the fully adjusted models, indicating that the associations between OA and TyG indices combined with obesity were stratified by sex. Overall, TyG was not associated with OA in both women and men. The magnitudes of the associations between all indices and OA were stronger in women than in men. TyG-BMI, TyG-WC, and TyG-WHtR were associated with OA in women, but not in men. In both men and women, the strengths of these associations were lower than those of the associations of OA with BMI, WC, and WHtR individually.
4. Discussion
In this study, we demonstrated that OA was not associated with the TyG index in both men and women. Additionally, TyG indices combined with obesity, such as the TyG-BMI, TyG-WC, and TyG-WHtR indices, were not associated with OA in men but strongly associated with OA in women. However, the strength of the associations between OA and TyG indices combined with obesity was lower than that of the association between OA and obesity indices individually.
Several studies have reported that the TyG index is associated with OA [7,12,13,15,16], whereas some studies have argued that the TyG index is only weakly associated or not associated with OA [11,17,18]. Cai et al. [19] examined the association between osteoarthritis and two insulin resistance indices (the homeostatic model assessment of insulin resistance (HOMA-IR) index and the TyG index) in a U.S. population. These authors reported that the TyG index was more strongly associated with OA than the HOMA-IR index, and the TyG-WHtR was the best predictor of OA among the TyG, TyG-BMI, TyG-WC, and TyG-WHtR indices. Zhang et al. [17] tested the association of arthritis (rheumatoid arthritis and osteoarthritis) with TyG–BMI, TyG–WC, and TyG–WHtR in Chinese and U.S. populations. These authors argued that the TyG–BMI and TyG–WHtR indices were positively associated with the incidence of arthritis in both populations and that these indices had greater diagnostic power than the TyG index. Additionally, Lan et al. [11] investigated the association of OA with TyG, TyG–BMI, TyG-WC, TyG–WHtR, the visceral adiposity index, and lipid accumulation products and explored the predictive power of these indices in the U.S. population. These authors reported that TyG-BMI, TyG-WHtR, TyG-WC, and LAP were positively related to the incidence of OA in an adjusted model and that the best predictor of OA was TyG-WHtR. However, TyG was not associated with the incidence of OA. Liu et al. [12] examined the association between new-onset arthritis and the TyG index through a prospective cohort study in a Chinese population and reported that the TyG index was an independent risk predictor of the start of new-onset arthritis in individuals aged 45 and above. Yan et al. [13] investigated the association between the TyG index and arthritis in a U.S. population and reported that the TyG index was positively associated with arthritis in older adults with normal weight and no diabetes. Additionally, Huang et al. [7] investigated whether elevated TyG values were associated with OA in a U.S. population and reported that elevated TyG values were linked to a high risk of OA. Kim et al. [18] tested the association of knee OA with HOMA-IR, TyG, TyG-BMI, and TyG-WC in the Korean population. These authors reported that HOMA-IR was not associated with knee OA, the TyG index was weakly associated with knee OA, and the TyG-BMI and TyG-WC indices were strongly associated with knee OA. Furthermore, Que et al. [15] examined the predictive power of estimated glucose disposal rate, TyG, TyG-BMI, TyG-WC, and TyG-WHtR for predicting OA. With respect to the prediction of OA, the estimated glucose disposal rate and the TyG, TyG-BMI, TyG-WC, and TyG-WHtR indices had areas under the receiver operating characteristic curve (AUCs) of 0.68, 0.58, 0.60, 0.62, and 0.64, respectively. Li et al. [16] investigated the potential mediating role of the TyG index in the association between sarcopenic obesity and OA in the U.S. population. These authors reported a significant association between the TyG index and elevated OA risk in subjects with sarcopenic obesity, and in this case, the TyG index had an AUC value of 0.572 for predicting OA risk. Our findings were not consistent with the results of most previous studies on this topic; namely, they indicated that the TyG index is strongly associated with OA [7,12,13,15,16]. However, our results were linked to the findings of previous studies in that they indicated that the TyG index was not associated or only weakly associated with OA [11,17,18]. With respect to TyG indices combined with obesity, both previous studies and this study reported that TyG indices combined with obesity indices such as TyG-BMI were associated with OA in both sexes [15,18,19] or predominantly in women. However, we assume that one of the reasons for these associations is that obesity indices are strongly associated with OA rather than TyG being associated with OA. For example, the results of previous studies [11,17,18,19] and this study revealed that TyG was weakly associated or not associated with OA; however, that TyG indices combined with obesity were strongly associated with OA because obesity indices were strongly associated with OA. Therefore, we speculate that obesity may explain these associations of OA with TyG-BMI, TyG-WC, and TyG-WHtR. In this study, obesity indices were strongly associated with OA, and TyG was weakly associated or not associated with OA. Similarly, Park et al. [21] investigated the association between obesity indices and knee OA using retrospective cohort data in the Korean population and reported that higher BMI and WC were associated with increased knee OA and that decreased obesity reduced the risk of knee OA. However, most previous studies have reported that the TyG value is strongly associated with OA [7,12,13,15,16]. Therefore, further research is needed to examine the interrelationships among obesity, TyG, and OA across various ethnic groups and countries.
This study had several limitations. First, as in other similar studies, the biological mechanism underlying the association between OA and TyG combined with obesity indices is difficult to explain because of the overlaps among various fields of lipids, obesity, and OA. Second, our findings cannot be generalized to other ethnic groups and countries because of the controversy over the association between TyG and OA to date. Third, because of the cross-sectional design, identifying cause–effect relationships between TyG values and OA as well as between TyG indices combined with obesity and OA is difficult. Fourth, the KNHANES dataset used in this study only provides doctor-diagnosed OA and self-reported current OA symptoms based on questionnaires. Therefore, our findings should be interpreted with caution and regarded as preliminary evidence. Fifth, our results were derived from the Korean population, and the criteria for obesity based on BMI differ across ethnic groups. Therefore, further research is needed to examine the associations between TyG index values and OA in other ethnic groups and countries to ensure external validity, as well as to clarify the causal relationships and underlying biological mechanisms. However, this study has several strengths. The statistical findings concerning the relationship of OA with TyG values and TyG values combined with obesity are powerful because of the use in this research of KNHANES data supported by a national representative sample of the Korean population.
5. Conclusions
In this study, we examined the association of OA with the TyG index and TyG indices combined with obesity in the Korean population. Our findings revealed that OA was not associated with the TyG index in both men and women. Additionally, TyG indices combined with obesity were not associated with OA in men but strongly associated with OA in women. Additionally, combining TyG values and obesity indices to identify OA is not appropriate because obesity indices individually were better predictors than combined indices. Our findings were derived from a single cross-sectional study in the Korean population. So, the generalization of our results is limited. Therefore, our findings should be interpreted with caution and considered as preliminary evidence that requires further investigation.
Author Contributions
J.H.C.: Data curation, formal analysis, and writing—original draft; B.J.L.: Conceptualization, investigation, methodology, supervision, validation, writing—original draft, and writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Korea Institute of Oriental Medicine (KIOM; grant Nos. KSN2511012 and KSN2313022), which is funded by the Korean government.
Institutional Review Board Statement
The institutional review board (IRB) of the Korea Disease Control and Prevention Agency (KDCA) approved the Korea National Health and Nutrition Examination Survey (KNHANES) (IRBs: 2018-01-03-C-A, 2018-01-03-P-A, and 2013-12EXP-03-5C, approved on 3 January 2018, 3 January 2018, and 3 December 2013, respectively). Furthermore, the IRB of the Korea Institute of Oriental Medicine approved the use of publicly available anonymized KNHANES data (IRB No. I-2501/001-001, approved on 7 January 2025). All the subjects provided written informed consent before their participation in the KNHANES. Publicly available KNHANES data did not contain any personally identifiable information. The present study was conducted in accordance with the principles of the Declaration of Helsinki.
Informed Consent Statement
All the subjects provided written informed consent before their participation in the Korea National Health and Nutrition Examination Survey (KNHANES).
Data Availability Statement
All the data used in the present study are available from the Korea National Health and Nutrition Examination Survey (KNHANES) conducted by the Korea Centers for Disease Control and Prevention (KCDC). The data can be accessed freely (https://knhanes.kdca.go.kr/knhanes/eng/main.do and https://knhanes.kdca.go.kr/knhanes/main.do) (accessed on 1 September 2025).
Acknowledgments
We thank all the participants who agreed to participate in the Korea National Health and Nutrition Examination Survey.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Yoshimura, N.; Muraki, S.; Oka, H.; Tanaka, S.; Kawaguchi, H.; Nakamura, K.; Akune, T. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: A 3-year follow-up of the ROAD study. Osteoarthr. Cartil. 2012, 20, 1217–1226. [Google Scholar] [CrossRef]
- Ching, K.; Houard, X.; Berenbaum, F.; Wen, C. Hypertension meets osteoarthritis-revisiting the vascular aetiology hypothesis. Nat. Rev. Rheumatol. 2021, 17, 533–549. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Schram, B.; Orr, R.; Pope, R.; Canetti, E.; Knapik, J. Risk factors for development of lower limb osteoarthritis in physically demanding occupations: A narrative umbrella review. J. Occup. Health 2020, 62, e12103. [Google Scholar] [CrossRef]
- Poulsen, E.; Goncalves, G.H.; Bricca, A.; Roos, E.M.; Thorlund, J.B.; Juhl, C.B. Knee osteoarthritis risk is increased 4–6 fold after knee injury-A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 1454–1463. [Google Scholar] [CrossRef]
- Huang, J.; Rozi, R.; Ma, J.; Fu, B.; Lu, Z.; Liu, J.; Ding, Y. Association between higher triglyceride glucose index and increased risk of osteoarthritis: Data from NHANES 2015–2020. BMC Public Health 2024, 24, 758. [Google Scholar] [CrossRef]
- Kendzerska, T.; King, L.K.; Lipscombe, L.; Croxford, R.; Stanaitis, I.; Hawker, G.A. The impact of hip and knee osteoarthritis on the subsequent risk of incident diabetes: A population-based cohort study. Diabetologia 2018, 61, 2290–2299. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, X.; Huang, J.; Meng, B.; Li, F.; Liu, H.; Chen, L.; Zhou, R.; Zou, M.; Wu, X. Association of diabetes mellitus status and hyperglycemia with symptomatic knee osteoarthritis. Arthritis Care Res. 2023, 75, 509–518. [Google Scholar] [CrossRef]
- Yao, K. Association between domain-specific physical activity and triglyceride-glucose (TyG) index among US adults: Evidence from NHANES 2007–2018. BMC Public Health 2025, 25, 159. [Google Scholar] [CrossRef]
- Lan, Y.; Zheng, Q.; Li, M.; Chen, J.; Huang, D.; Lin, L. Associations between surrogate insulin resistance indexes and osteoarthritis: NHANES 2003–2016. Sci. Rep. 2025, 15, 1578. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, J.; Xue, X.; Lv, Y.; Guo, S.; Wei, P. Triglyceride-glucose index in the prediction of new-onset arthritis in the general population aged over 45: The first longitudinal evidence from CHARLS. Lipids Health Dis. 2024, 23, 79. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, L.; La, R.; Jiang, M.; Jiang, D.; Huang, L.; Xu, W.; Wu, Q. The association between triglyceride glucose index and arthritis: A population-based study. Lipids Health Dis. 2023, 22, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.; Chen, D.; Cai, H.; Lan, W.; Huang, Y.; Rui, G. Associations between estimated glucose disposal rate and osteoarthritis risk in US adults: A cross-sectional study. BMC Musculoskelet. Disord. 2025, 26, 302. [Google Scholar] [CrossRef]
- Li, Z.; Yin, S.; Zhao, G.; Cao, X. Association between sarcopenic obesity and osteoarthritis: The potential mediating role of insulin resistance. Exp. Gerontol. 2024, 197, 112611. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, H.; Chen, J.; Chen, J.; Zhou, H.; Qi, T.; Wang, D.; Zeng, H.; Yu, F. Association between different triglyceride-glucose index combinations with obesity indicators and arthritis: Results from two nationally representative population-based study. Eur. J. Med. Res. 2024, 29, 389. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, J.H.; Shin, S.R.; Han, A.L. Association between insulin resistance indices and prevalence of knee osteoarthritis using the Korean National health and examination survey. Sci. Rep. 2025, 15, 18195. [Google Scholar] [CrossRef]
- Cai, H.; Que, Z.; Chen, J.; Chen, D.; Rui, G.; Lan, W. Association between different insulin resistance surrogates and osteoarthritis: A cross-sectional study from NHANES 1999–2018. BMC Musculoskelet. Disord. 2024, 25, 901. [Google Scholar] [CrossRef]
- Zeng, C.M.; He, J.; Wang, D.C.; Xie, H. Association between triglyceride levels and rheumatoid arthritis prevalence in women: A cross-sectional study of NHANES (1999–2018). BMC Womens Health 2025, 25, 129. [Google Scholar] [CrossRef]
- Park, D.; Park, Y.M.; Ko, S.H.; Hyun, K.S.; Choi, Y.H.; Min, D.U.; Han, K.; Koh, H.S. Association of general and central obesity, and their changes with risk of knee osteoarthritis: A nationwide population-based cohort study. Sci. Rep. 2023, 13, 3796. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Zheng, S.; Shi, S.; Ren, X.; Han, T.; Li, Y.; Chen, Y.; Liu, W.; Hou, P.C.; Hu, Y. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: Cross-sectional and prospective cohort study. J. Transl. Med. 2016, 14, 260. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency. The Eighth Korea National Health and Nutrition Examination Survey (KNHANES VIII-1); Korea Disease Control and Prevention Agency: Cheongju, Republic of Korea, 2019. [Google Scholar]
- Lee, B.J.; Chi, J.H. Association between anemia and grip strength indices combined with anthropometry in the Korean population. Sci. Rep. 2023, 13, 18517. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).