Abstract
Background: Cryoglobulinemia (CG) syndrome is a heterogeneous condition characterized by the presence of cryoglobulins in serum, often leading to vasculitis with protean clinical manifestations. Understanding the presentation of cryoglobulinemia-related symptoms based on cryoprecipitate levels, GC type, and severity at diagnosis is essential for effective management. Hence, this study aimed to provide a comprehensive analysis of patients with positive cryoglobulin detection to investigate these aspects. Methods: We conducted a retrospective review of clinical charts from patients with positive cryoglobulin detection at Colmar Hospital between May 2015 and April 2019. Results: Among 166 patients with positive cryoglobulins, the median cryoprecipitate value was 37 mg/L [IQR: 25–70], with 62% of patients below the 50 mg/L threshold. High cryoprecipitate levels were associated with C-virus hepatitis (p = 0.0007), increased fatigue (p = 0.001), fever (p = 0.0013), weight loss (p = 0.028), and musculoskeletal symptoms (p = 0.002). These patients also exhibited decreases in complement fractions (p-values 0.017 to 0.006). At the end of the one-year follow-up, they required frequent renal replacement therapy (p < 0.0001) and had a higher mortality rate (p = 0.02). Based on the CG type, patients with type I GC had splenomegaly (p = 0.039) and hemopathy (p = 0.001). According to severity at initial presentation, the severe patients had more purpura (p < 0.001), Raynaud’s phenomenon (p = 0.039), and leukocytoclastic vasculitis on skin biopsy (p < 0.001), along with higher cryoprecipitate levels (p = 0.011). Multivariate analysis identified purpura (OR: 10.25), hematological malignancy (OR: 7.06), Raynaud’s phenomenon (OR: 6.41), and cryoprecipitate levels (OR: 1.02) as significant markers of disease severity serving for the development of a severity score for clinical practice. Conclusions: This study identifies severity markers in patients with positive cryoprecipitate and proposes a score related to severity at diagnosis.
1. Introduction
Cryoglobulinemia (CG) encompasses a spectrum of heterogeneous conditions characterized by the presence of cryoglobulins in serum [1]. These immunoglobulins (Ig) precipitate below 37 °C in vitro and redissolve upon rewarming [2,3]. CG is classified into types based on the Ig isotype [2], distinguishing type I CG (associated with hematological conditions) from mixed CG (types II and III) [4,5]. Patients experience diverse symptoms, including constitutional, musculoskeletal, and vascular manifestations, which define the spectrum of CG vasculitis [6,7]. While cryoglobulins may sometimes be detected incidentally, some patients present with severe, life-threatening manifestations [8]. Factors influencing disease severity include the type of cryoglobulin (type I or mixed), cryoprecipitate levels, the presence of underlying conditions (e.g., hematological malignancy, autoimmune disorders, chronic infection), and multi-organ involvement [9,10,11]. Despite sharing a common name, type I and mixed CG differ in pathophysiological mechanisms and prognosis CG [4,10].
Cryoprecipitate levels exceeding 50 mg/L are considered clinically significant [12,13,14], but patients with lower levels can also experience severe, life-threatening complications [15,16].
Treatment strategies are tailored according to disease severity, CG type, and the presence of underlying conditions. These approaches include observation, steroid therapy with or without plasmapheresis, and immunosuppressive agents [17,18,19]. Due to delays in identifying cryoglobulins, treatment decisions are often guided by clinical judgment and expert guidelines [1,8,20].
Given these challenges, this study aims to investigate the characteristics of CG patients based on cryoprecipitate levels and CG type and to identify factors associated with disease severity at the time of diagnosis.
2. Methods
2.1. Study Design and Population
We conducted a retrospective review of clinical charts from patients with positive cryoglobulin detection at Colmar Hospital between May 2015 and April 2019. Patients with cryoglobulins present, via laboratory testing [21] in the setting of compatible clinical symptoms, were included. These symptoms included weight loss, arthralgia, vascular manifestations (e.g., skin necrosis or Raynaud’s phenomenon), neuropathy, and glomerulopathy [7].
2.2. Investigations
Cryoprecipitate detection was performed using spectrometry combined with immunofixation to determine Ig-type after 7 days of storage [21]. All patients underwent screening for chronic infectious diseases (human immunodeficiency virus, hepatitis B and C), autoimmune-related disorders (antinuclear antibody and anti-CCP testing), and malignancy (blood immunophenotyping and body computed tomography).
Skin biopsies were performed selectively in patients who presented suspicious cutaneous lesions (e.g., palpable purpura, necrotic ulcers).
Electromyograms (EMG) were only performed in patients with clinical suspicion of neuropathy (e.g., persistent sensory deficits, tingling, numbness, or motor weakness). Compatible neurologic features on EMG referred to findings consistent with peripheral neuropathy (e.g., sensory axonal neuropathy), as interpreted by the neurology team.
2.3. Exclusion Criteria
Patients with vasculitis not clearly attributable to cryoglobulins (e.g., ANCA-associated vasculitis, IgA vasculitis, or middle or large vessel vasculitis) were excluded.
2.4. Data Collection
Collected variables analyzed included demographics, medical history, clinical symptoms, and laboratory results. Treatment regimens varied and included steroids alone, Ig-IV, plasmapheresis, conventional immunosuppressive agents, anti-CD20 therapy, chemotherapy, and combined therapy (defined by the use of at least two agents).
2.5. Outcomes
Outcomes analyzed at one year post-diagnosis included relapse-free survival, progression toward chronic disease (defined by at least 2 relapses), end-stage renal failure requiring replacement therapy, and mortality.
2.6. Study Analysis
1: Cryoprecipitate levels: Patients were stratified by cryoprecipitate levels at initial measurement, with levels above 50 mg/L considered high [14].
2: CG type: Patients were compared based on CG type (type I vs. mixed).
3: Severity: Patients were classified according to disease severity at the initial evaluation. Severity was defined as the initiation of steroids or a >50% increase in the daily dose or initiation of other therapies within three days of diagnosis, based on the physician’s discretion. Further severity analysis included items from the Birmingham vasculitis activity score (BVAS) 2003.
2.7. Statistical Analysis
Quantitative data are presented as medians with interquartile ranges [IQR] and compared using Mann–Whitney tests. Qualitative data, expressed as numbers (percentages), were compared using Chi-squared or Fisher’s exact tests.
To identify factors associated with severe disease, we performed logistic regression analysis with univariate and multivariate models. A predictive score for disease severity was developed using variables with a p-value ≤ 0.20 in the bivariate analysis, followed by stepwise selection. The final score included variables selected through a stepwise procedure and derived from the coefficients in the multivariate analysis. The optimal threshold for severe cryoglobulinemia syndrome was determined by maximizing sensitivity and specificity using the Youden index of receiver operating characteristic (ROC) curve analysis. Statistical significance was set at p-value < 0.05 (two-sided). Statistical analyses were performed using Prism 10 (GraphPad, San Diego, CA, USA), R version 4.0.3, and RStudio version 1.4.1103 (R Foundation for Statistical Computing, Vienna, Austria).
The study was approved by the ethics committee of Colmar Hospital and followed the principles of the Declaration of Helsinki.
3. Results
Among the 166 patients with the presence of cryoglobulins in serum, the median cryoglobulin level was 37 mg/L [IQR: 25–70].
Comparison according to cryoprecipitate levels:
A total of 62% (102/166) of patients exhibited cryoprecipitate levels below 50 mg/L (median: 27 mg/L [IQR: 22–33]), whereas 38% (64/166) had levels above 50 mg/L (median: 90 mg/L [60–157]). The distribution of cryoglobulinemia types (type I and mixed) was similar between the two groups. Patients with high cryoprecipitate levels have more frequently C-virus hepatitis (7/102 (6.8%) vs. 17/64 (26%); p = 0.0007), when other baseline conditions were similarly distributed.
Constitutional symptoms, including fatigue (17/64 (27%) vs. 6/102 (5%); p = 0.001), fever (11/64 (17%) vs. 5/102 (4%); p = 0.013), and weight loss (6/64 (9%) vs. 0/102 (0%); p = 0.028), were significantly more prevalent in patients with high cryoprecipitate levels. Musculoskeletal symptoms were also more frequent in this group (11/64 (17%) vs. 3/102 (2%); p = 0.002), while vascular symptoms (e.g., purpura, Raynaud’s phenomenon, livedo, necrosis, or vasculitis on biopsy) were similarly distributed. Patients with high cryoprecipitate levels exhibited lower complement fractions (CH 50: 43 [20–61] vs. 64 [46–76]; p = 0.017; C3: 0.9 [0.65–1.19] vs. 1.08 [0.82–1.35]; p = 0.028; C4: 0.12 [0.045–0.20] vs. 0.1850 [0.110–0.277]; p = 0.006) compared to those with low cryoprecipitate levels. Treatment strategies were similar according to cryoprecipitate levels.
During the one-year follow-up, patients with low cryoprecipitate levels were more likely to experience chronic disease evolution (>2 relapses) (63/102 (61%) vs. 0/64 (0%); p < 0.0001). In contrast, patients with high cryoprecipitate experienced fewer relapses (32/64 (50%) vs. 28/102 (27%); p = 0.0046) and required renal replacement therapy more frequently (12/64 (18%) vs. 1/102 (0.98%); p < 0.0001) (see Table 1).

Table 1.
Patient characteristics according to cryoglobulins threshold.
Comparison according to cryoglobulinemia type:
When stratified by cryoglobulin type (type I (n = 16) vs. mixed CG patients (n = 150)), type I cryoglobulinemia was associated with a higher prevalence of malignancy (5/16 (31%) vs. 9/150 (6%); p = 0.0068), especially hematological conditions (8/16 (50%) vs. 18/150 (12%); p = 0.001), mainly Waldenström macroglobulinemia (7/16 (43%) vs. 0/150 (0%); p < 0.0001). C-virus hepatitis history tends to be more common in mixed GC (27/150 (18%) vs. 0/16 (0%); p = 0.07). Clinical features were similar except for frequent splenomegaly in type I CG (5/16 (31%) vs. 7/150 (4.6%); p = 0.039) and a trend toward more frequent musculoskeletal symptoms in mixed CG (25/150 (16.6%) vs. 0/16 (0%); p = 0.07). Patients with mixed cryoglobulinemia exhibited lower CH50 levels (median 51 UI/mL [32.25–76] vs. 78 [58–193]; p = 0.0350) (see Table 2). Patients with type I CG required a frequent combined therapeutic approach (6/16 (37%) vs. 22/150 (14%); p = 0.04), resulting in similar outcomes at one year.

Table 2.
Patients characteristics according to cryoglobulinemia type.
Comparison according to severity at initial diagnosis.
Thus, patients were stratified based on severity at diagnosis, with 20% (34/166) categorized as severe.
Severe cryoglobulinemia syndrome was associated with more purpura (14/33 (42%) vs. 15/133 (11%); p < 0.001), Raynaud’s phenomenon (8/33 (26%) vs. 14/133 (10%); p = 0.039), and leukocytoclastic vasculitis on biopsy (10/15 (66%) vs. 4/26 (15%); p < 0.001). Severe patients had frequent type I CG (6/33 (18%) vs. 10/133 (7%); p = 0.048) (and all of them had IgM cryoprecipitate), secondary to hematological malignancies (9/33 (27%) vs. 17/133 (12%); p = 0.02).
Biological differences included a lower white cell count (median [IQR] g/L: 5.8 [4–7.8] vs. 7.0 [5.1–9.4]; p = 0.036) and higher cryoprecipitate level in severe patients (median [IQR] mg/L: 54 [28–191] vs. 34 [25–65]; p = 0.011) (see Figure 1 and Table 3). Proteinuria > 0.5 g/day and hematuria (when available) were more frequent in non-severe patients (4/30 (13.3%) vs. 20/53 (37%) and (16.6%) vs. 23/52 (44%); p = 0.0234 and p = 0.0152, respectively).
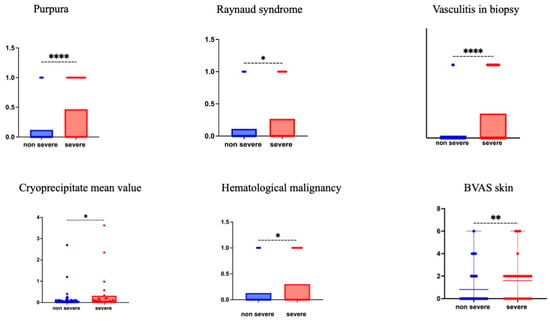
Figure 1.
Patients characteristics according to CG severity at diagnosis. The items purpura, Raynaud’s phenomenon., vasculitis in biopsy, cryoprecipitate value, and hematological malignancy are presented by their mean values for more visibility. The skin items of the 2003 BVAS score are presented as scattered plot means with range. p value is the result of the Mann-Whitney test. * p < 0.05,** p < 0.01, **** p < 0.0001.

Table 3.
Patients characteristics according to CG severity at diagnosis.
These severe patients had a median BVAS [IQR] of 10 [8–12], whereas non-severe patients had a median BVAS [IQR] of 2 [0–4].
Treatment strategies differed slightly, with severe patients more frequently receiving steroids alone within the first three days (15% vs. 4%, p = 0.03). Outcomes at one year were similar across severity groups.
In the overall cohort, 18 patients were dead at one-year follow-up. Mortality was significantly higher in patients with elevated cryoprecipitate levels (11/64 (17.1%) vs. 7/102 (7%)) (p = 0.02). However, no significant associations were found between mortality and CG type (p = 0.47) or initial disease severity (p = 0.7).
Finally, multivariate analysis showed that factors associated with severity at diagnosis included the presence of purpura (odds ratio (OR): 10.25, CI95% [3.6231–59], p = 0.001), hematological conditions (OR: 7.06, CI95% [1.6329–49]; p = 0.007), Raynaud’s phenomenon (OR: 6.41, CI95% [1.9321–99]; p = 0.002), and elevated cryoprecipitate levels (OR: 1.02, CI95% [1.001–4]; p = 0.031). Based on these findings, a severity score was developed incorporating these four factors, each weighted accordingly. The resulting score ranges from ≤2 (with a 5% probability) to ≥40 (with a probability exceeding 99%) for predicting severity (see Table 3). The optimal cut-off value was determined to be 12, demonstrating a sensitivity of 77%, a specificity of 72%, and an overall accuracy of 73%. This scoring system outperformed cryoprecipitate levels alone in predicting severity (see Supplementary Table S1 and Figure S1).
4. Discussion
Our comprehensive cohort analysis of 166 patients with cryoglobulinemia provides critical insights into the clinical manifestations and prognostic factors of this complex condition. By comparing subgroups based on cryoprecipitate thresholds, CG type, and disease severity, we unraveled the heterogeneity of the cryoglobulinemia spectrum.
Recognizing that cryoglobulins can be present in asymptomatic patients, we first analyzed the cohort using a cryoprecipitate threshold of 50 mg/L, based on the literature analysis [12,13,14]. Patients with high cryoprecipitate levels were more likely to exhibit constitutional and musculoskeletal symptoms, suggesting a link between cryoprecipitate levels and symptomatology, consistent with existing studies [1,8,22]. Notably, vascular symptoms, hallmark features of the disease, appeared independent of cryoprecipitate levels and correlated instead with the mere presence of cryoglobulins.
This underscores the need for physicians to consider cryoglobulinemia syndrome in patients with vascular symptoms, even with normal complement fractions, echoing the concept of “idiopathic hypo-cryoglobulinemia” proposed by Roccatello et al. [16].
Moreover, the study sheds light on the clinical presentations associated with CG types. Type I and mixed CG represent two different spectra of the same “entity” with different pathophysiology [1,4,6,8]. In the current study, the difference in clinical presentation (splenomegaly) is more related to the underlying conditions, such as lymphoma, rather than the CG type. Patients with type I CG often experience hyperviscosity syndrome or severe vascular complications related to small vessel thrombosis [10]. The limited numbers of type I GC in the study may contribute to the similarities in clinical presentation. Nevertheless, prior large cohort analyses report a worse prognosis associated with type I CG attributed to thrombotic events and association with hematological malignancies impacting long-term survival [10,23]. Additionally, the low prevalence of C-virus hepatitis reflects the evolution of the cryoglobulinemia spectrum in the modern era.
Regarding severity, patients with severe disease exhibited distinctive clinical features including purpura, Raynaud’s phenomenon, and leukocytoclastic vasculitis on skin biopsy. These findings, often associated with type I CG [10,24], likely result from vascular occlusion.
Although severity was subjectively assessed based on physician judgment, the inclusion of cutaneous items from the BVAS 2003 (a scoring system for ANCA-associated vasculitis) lent partial validation to these assessments. Nevertheless, the patients were mainly managed by rheumatologists or internal medicine practitioners, which may represent a bias explaining the low frequency of renal involvement and absence of pulmonary or gastrointestinal manifestations contributing to lowering the overall BVAS scores overall. Given the real-world nature of this retrospective study, treatment initiation, particularly corticosteroid therapy, was used as a proxy for severity staging, though variations in treatment regimens represent a potential source of bias.
Regarding outcomes, the cryoprecipitate level emerged as a key predictor of disease course, influencing relapse-free survival, progression to chronic disease, the need for renal replacement therapy, and one-year overall survival. Neither CG type nor the initial severity predicted these outcomes. However, the relatively short follow-up period limits the ability to assess long-term effects, as mortality related to cryoglobulinemia or its treatments often unfolds over several years [11,25]. Additionally, the absence of precise documentation on causes of death complicates the interpretation of outcomes, making it challenging to isolate cryoglobulinemia’s role from that of comorbidities.
The study’s most innovative contribution is the development of a clinically applicable severity scoring system. Integrating factors identified through multivariate analysis—purpura, hematological conditions, Raynaud’s phenomenon, and elevated cryoprecipitate levels—demonstrated superior predictive accuracy compared to cryoprecipitate levels alone. These tools recapitulate the spectrum of type I CG by incorporating vascular manifestations (i.e., purpura and severe Raynaud’s phenomenon) and underlying hematological conditions [10,23]. Its reliance on readily available clinical and laboratory parameters within 24 h makes it a practical tool for early diagnosis and treatment decision-making. This scoring system not only supports improved patient outcomes but also reinforces the importance of considering cryoglobulinemia syndrome independently of cryoprecipitate thresholds, aligning with previous concepts such as “idiopathic hypo-cryoglobulinemia.” [16].
However, the current study has several limitations. First, its retrospective nature, with potentially missing data and absence of long-term follow-up, is a major pitfall. Secondly, the dosage of cryoprecipitate and the treatment initiation were at the physician’s appreciation based on clinical experience rather than a standardized protocol. Additionally, the use of cryoprecipitate threshold cutoff based on the literature analysis rather than statistical methods warrants caution. Moreover, an external validation is necessary to confirm the score’s reliability.
Nevertheless, the primary strength of the study lies in its ability to provide an overview of the clinical manifestations of cryoglobulinemia syndrome based on cryoprecipitate levels and, notably, in proposing a severity score applicable for clinical use. The proposed severity score is straightforward, clinically useful, and holds promise for enhancing the management of cryoglobulinemia syndrome. Future studies should aim to validate the score and explore its application in multicentric studies.
In conclusion, cryoglobulinemic vasculitis must be considered independently of the cryoprecipitate threshold and type. Moreover, a novel severity scoring system based on four items (purpura, Raynaud’s phenomenon, hematological conditions, and cryoprecipitate) may predict severity and clinical decision-making and patient care.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14020556/s1, Figure S1: Area under the receiver operating characteristic curve for the severity score and serum cryoglobulin. Table S1: The severity score.
Author Contributions
J.R. and N.A. collected the data and wrote the original draft; J.R. and T.S. statistical analysis. M.B.B., G.B., B.B. provided clinical expertise, participated in data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
According to French Law, retrospective observational database studies do not require approval by an ethics committee or informed signed consent from the patients. This work complies with the protection of personal health data and the protection of privacy within the framework provided by Article 65-2 of the amended Data Protection Act and the general data protection regulations.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no competing financial interests for this study.
References
- Ramos-Casals, M.; Stone, J.H.; Cid, M.C.; Bosch, X. The cryoglobulinaemias. Lancet 2012, 379, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Brouet, J.-C.; Clauvel, J.-P.; Danon, F.; Klein, M.; Seligmann, M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am. J. Med. 1974, 57, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, M.; Franklin, E.C. Cryoglobulinemia—A study of twenty-nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am. J. Med. 1966, 40, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Vieira, M.; Saadoun, D. Cryoglobulinemia-One Name for Two Diseases. N. Engl. J. Med. 2024, 391, 1426–1439. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Trejo, O.; García-Carrasco, M.; Cervera, R.; Font, J. Mixed cryoglobulinemia: New concepts. Lupus 2000, 9, 83–91. [Google Scholar] [CrossRef]
- Desbois, A.C.; Cacoub, P.; Saadoun, D. Cryoglobulinemia: An update in 2019. Jt. Bone Spine 2019, 86, 707–713. [Google Scholar] [CrossRef]
- Quartuccio, L.; Isola, M.; Corazza, L.; Ramos-Casals, M.; Retamozo, S.; Ragab, G.M.; Zoheir, M.N.; El-Menyawi, M.A.-M.; Salem, M.N.; Sansonno, D.; et al. Validation of the classification criteria for cryoglobulinaemic vasculitis. Rheumatology 2014, 53, 2209–2213. [Google Scholar] [CrossRef]
- Roccatello, D.; Saadoun, D.; Ramos-Casals, M.; Tzioufas, A.G.; Fervenza, F.C.; Cacoub, P.; Zignego, A.L.; Ferri, C. Cryoglobulinaemia. Nat. Rev. Dis. Primers 2018, 4, 11. [Google Scholar] [CrossRef]
- Terrier, B.; Karras, A.; Kahn, J.-E.; Le Guenno, G.; Marie, I.; Benarous, L.; Lacraz, A.; Diot, E.; Hermine, O.; de Saint-Martin, L.; et al. The spectrum of type I cryoglobulinemia vasculitis: New insights based on 64 cases. Medicine 2013, 92, 61–68. [Google Scholar] [CrossRef]
- Ghembaza, A.; Boleto, G.; Bommelaer, M.; Karras, A.; Javaugue, V.; Bridoux, F.; Alyanakian, M.; Frenkel, V.M.; Ghillani-Dalbin, P.; Musset, L.; et al. Prognosis and long-term outcomes in type I cryoglobulinemia: A multicenter study of 168 patients. Am. J. Hematol. 2023, 98, 1080–1086. [Google Scholar] [CrossRef]
- Terrier, B.; Carrat, F.; Krastinova, E.; Marie, I.; Launay, D.; Lacraz, A.; Belenotti, P.; Martin, L.d.S.; Quemeneur, T.; Huart, A.; et al. Prognostic factors of survival in patients with non-infectious mixed cryoglobulinaemia vasculitis: Data from 242 cases included in the CryoVas survey. Ann. Rheum Dis. 2013, 72, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Napodano, C.; Gulli, F.; Rapaccini, G.L.; Marino, M.; Basile, U. Cryoglobulins: Identification, classification, and novel biomarkers of mysterious proteins. Adv. Clin. Chem. 2021, 104, 299–340. [Google Scholar] [CrossRef]
- Gorevic, P.D.; Kassab, H.J.; Levo, Y.; Kohn, R.; Meltzer, M.; Prose, P.; Franklin, E.C. Mixed cryoglobulinemia: Clinical aspects and long-term follow-up of 40 patients. Am. J. Med. 1980, 69, 287–308. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, M.; Schifferli, J.A. Cryoglobulins are not essential. Ann. Rheum. Dis. 1998, 57, 3–5. [Google Scholar] [CrossRef]
- Ferri, C.; Zignego, A.L.; Pileri, S.A. Cryoglobulins. J. Clin. Pathol. 2002, 55, 4–13. [Google Scholar] [CrossRef]
- Roccatello, D.; Sciascia, S.; Naretto, C.; Barreca, A.; Solfietti, L.; Battaglia, L.; Viziello, L.; Fenoglio, R.; Rossi, D. Recognizing the new disorder “idiopathic hypocryoglobulinaemia” in patients with previously unidentified clinical conditions. Sci. Rep. 2022, 12, 14904. [Google Scholar] [CrossRef]
- Terrier, B.; Darbon, R.; Durel, C.-A.; Hachulla, E.; Karras, A.; Maillard, H.; Papo, T.; Puechal, X.; Pugnet, G.; Quemeneur, T.; et al. French recommendations for the management of systemic necrotizing vasculitides (polyarteritis nodosa and ANCA-associated vasculitides). Orphanet J. Rare Dis. 2020, 15, 351. [Google Scholar] [CrossRef]
- Fabrizi, F.; Plaisier, E.; Saadoun, D.; Martin, P.; Messa, P.; Cacoub, P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am. J. Kidney Dis. 2013, 61, 623–637. [Google Scholar] [CrossRef]
- Ferri, C.; Cacoub, P.; Mazzaro, C.; Roccatello, D.; Scaini, P.; Sebastiani, M.; Tavoni, A.; Zignego, A.; De Vita, S. Treatment with rituximab in patients with mixed cryoglobulinemia syndrome: Results of multicenter cohort study and review of the literature. Autoimmun. Rev. 2011, 11, 48–55. [Google Scholar] [CrossRef]
- Roccatello, D.; Sciascia, S.; Baldovino, S.; Rossi, D.; Alpa, M.; Naretto, C.; Di Simone, D.; Menegatti, E. Improved (4 Plus 2) Rituximab Protocol for Severe Cases of Mixed Cryoglobulinemia: A 6-Year Observational Study. Am. J. Nephrol. 2016, 43, 251–260. [Google Scholar] [CrossRef]
- Musset, L.; Diemert, M.C.; Taibi, F.; Du, L.T.H.; Cacoub, P.; Leger, J.M.; Boissy, G.; Gaillard, O.; Galli, J. Characterization of cryoglobulins by immunoblotting. Clin. Chem. 1992, 38, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Marie, I.; Launay, D.; Lacraz, A.; Belenotti, P.; de Saint-Martin, L.; Quemeneur, T.; Huart, A.; Bonnet, F.; Le Guenno, G.; et al. Predictors of early relapse in patients with non-infectious mixed cryoglobulinemia vasculitis: Results from the French nationwide CryoVas survey. Autoimmun. Rev. 2014, 13, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Sidana, S.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Hayman, S.R.; Dingli, D.; Kapoor, P.; Gonsalves, W.I.; et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am. J. Hematol. 2017, 92, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Robles, A.; Brito-Zerón, P.; Nardi, N.; Nicolás, J.M.; Forns, X.; Plaza, J.; Yagüe, J.; Sánchez-Tapias, J.M.; Font, J. Life-threatening cryoglobulinemia: Clinical and immunological characterization of 29 cases. Semin. Arthritis Rheum. 2006, 36, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Fayed, A.; Hegazy, M.T.; Biard, L.; Vieira, M.; El Shabony, T.; Saadoun, D.; Casato, M.; Visentini, M.; Ragab, G.; Cacoub, P. Relapse of Hepatitis C Virus Cryoglobulinemic Vasculitis After Sustained Viral Response After Interferon-Free Direct-Acting Antivirals. Am. J. Gastroenterol. 2022, 117, 627–636. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).