Identification of Key Predictors of Cryoglobulinemia Severity at Diagnosis: Threshold, Type, and Severity Score at Diagnosis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Investigations
2.3. Exclusion Criteria
2.4. Data Collection
2.5. Outcomes
2.6. Study Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos-Casals, M.; Stone, J.H.; Cid, M.C.; Bosch, X. The cryoglobulinaemias. Lancet 2012, 379, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Brouet, J.-C.; Clauvel, J.-P.; Danon, F.; Klein, M.; Seligmann, M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am. J. Med. 1974, 57, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, M.; Franklin, E.C. Cryoglobulinemia—A study of twenty-nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am. J. Med. 1966, 40, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Vieira, M.; Saadoun, D. Cryoglobulinemia-One Name for Two Diseases. N. Engl. J. Med. 2024, 391, 1426–1439. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Trejo, O.; García-Carrasco, M.; Cervera, R.; Font, J. Mixed cryoglobulinemia: New concepts. Lupus 2000, 9, 83–91. [Google Scholar] [CrossRef]
- Desbois, A.C.; Cacoub, P.; Saadoun, D. Cryoglobulinemia: An update in 2019. Jt. Bone Spine 2019, 86, 707–713. [Google Scholar] [CrossRef]
- Quartuccio, L.; Isola, M.; Corazza, L.; Ramos-Casals, M.; Retamozo, S.; Ragab, G.M.; Zoheir, M.N.; El-Menyawi, M.A.-M.; Salem, M.N.; Sansonno, D.; et al. Validation of the classification criteria for cryoglobulinaemic vasculitis. Rheumatology 2014, 53, 2209–2213. [Google Scholar] [CrossRef]
- Roccatello, D.; Saadoun, D.; Ramos-Casals, M.; Tzioufas, A.G.; Fervenza, F.C.; Cacoub, P.; Zignego, A.L.; Ferri, C. Cryoglobulinaemia. Nat. Rev. Dis. Primers 2018, 4, 11. [Google Scholar] [CrossRef]
- Terrier, B.; Karras, A.; Kahn, J.-E.; Le Guenno, G.; Marie, I.; Benarous, L.; Lacraz, A.; Diot, E.; Hermine, O.; de Saint-Martin, L.; et al. The spectrum of type I cryoglobulinemia vasculitis: New insights based on 64 cases. Medicine 2013, 92, 61–68. [Google Scholar] [CrossRef]
- Ghembaza, A.; Boleto, G.; Bommelaer, M.; Karras, A.; Javaugue, V.; Bridoux, F.; Alyanakian, M.; Frenkel, V.M.; Ghillani-Dalbin, P.; Musset, L.; et al. Prognosis and long-term outcomes in type I cryoglobulinemia: A multicenter study of 168 patients. Am. J. Hematol. 2023, 98, 1080–1086. [Google Scholar] [CrossRef]
- Terrier, B.; Carrat, F.; Krastinova, E.; Marie, I.; Launay, D.; Lacraz, A.; Belenotti, P.; Martin, L.d.S.; Quemeneur, T.; Huart, A.; et al. Prognostic factors of survival in patients with non-infectious mixed cryoglobulinaemia vasculitis: Data from 242 cases included in the CryoVas survey. Ann. Rheum Dis. 2013, 72, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Napodano, C.; Gulli, F.; Rapaccini, G.L.; Marino, M.; Basile, U. Cryoglobulins: Identification, classification, and novel biomarkers of mysterious proteins. Adv. Clin. Chem. 2021, 104, 299–340. [Google Scholar] [CrossRef]
- Gorevic, P.D.; Kassab, H.J.; Levo, Y.; Kohn, R.; Meltzer, M.; Prose, P.; Franklin, E.C. Mixed cryoglobulinemia: Clinical aspects and long-term follow-up of 40 patients. Am. J. Med. 1980, 69, 287–308. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, M.; Schifferli, J.A. Cryoglobulins are not essential. Ann. Rheum. Dis. 1998, 57, 3–5. [Google Scholar] [CrossRef]
- Ferri, C.; Zignego, A.L.; Pileri, S.A. Cryoglobulins. J. Clin. Pathol. 2002, 55, 4–13. [Google Scholar] [CrossRef]
- Roccatello, D.; Sciascia, S.; Naretto, C.; Barreca, A.; Solfietti, L.; Battaglia, L.; Viziello, L.; Fenoglio, R.; Rossi, D. Recognizing the new disorder “idiopathic hypocryoglobulinaemia” in patients with previously unidentified clinical conditions. Sci. Rep. 2022, 12, 14904. [Google Scholar] [CrossRef]
- Terrier, B.; Darbon, R.; Durel, C.-A.; Hachulla, E.; Karras, A.; Maillard, H.; Papo, T.; Puechal, X.; Pugnet, G.; Quemeneur, T.; et al. French recommendations for the management of systemic necrotizing vasculitides (polyarteritis nodosa and ANCA-associated vasculitides). Orphanet J. Rare Dis. 2020, 15, 351. [Google Scholar] [CrossRef]
- Fabrizi, F.; Plaisier, E.; Saadoun, D.; Martin, P.; Messa, P.; Cacoub, P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am. J. Kidney Dis. 2013, 61, 623–637. [Google Scholar] [CrossRef]
- Ferri, C.; Cacoub, P.; Mazzaro, C.; Roccatello, D.; Scaini, P.; Sebastiani, M.; Tavoni, A.; Zignego, A.; De Vita, S. Treatment with rituximab in patients with mixed cryoglobulinemia syndrome: Results of multicenter cohort study and review of the literature. Autoimmun. Rev. 2011, 11, 48–55. [Google Scholar] [CrossRef]
- Roccatello, D.; Sciascia, S.; Baldovino, S.; Rossi, D.; Alpa, M.; Naretto, C.; Di Simone, D.; Menegatti, E. Improved (4 Plus 2) Rituximab Protocol for Severe Cases of Mixed Cryoglobulinemia: A 6-Year Observational Study. Am. J. Nephrol. 2016, 43, 251–260. [Google Scholar] [CrossRef]
- Musset, L.; Diemert, M.C.; Taibi, F.; Du, L.T.H.; Cacoub, P.; Leger, J.M.; Boissy, G.; Gaillard, O.; Galli, J. Characterization of cryoglobulins by immunoblotting. Clin. Chem. 1992, 38, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Marie, I.; Launay, D.; Lacraz, A.; Belenotti, P.; de Saint-Martin, L.; Quemeneur, T.; Huart, A.; Bonnet, F.; Le Guenno, G.; et al. Predictors of early relapse in patients with non-infectious mixed cryoglobulinemia vasculitis: Results from the French nationwide CryoVas survey. Autoimmun. Rev. 2014, 13, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Sidana, S.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Hayman, S.R.; Dingli, D.; Kapoor, P.; Gonsalves, W.I.; et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am. J. Hematol. 2017, 92, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Robles, A.; Brito-Zerón, P.; Nardi, N.; Nicolás, J.M.; Forns, X.; Plaza, J.; Yagüe, J.; Sánchez-Tapias, J.M.; Font, J. Life-threatening cryoglobulinemia: Clinical and immunological characterization of 29 cases. Semin. Arthritis Rheum. 2006, 36, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Fayed, A.; Hegazy, M.T.; Biard, L.; Vieira, M.; El Shabony, T.; Saadoun, D.; Casato, M.; Visentini, M.; Ragab, G.; Cacoub, P. Relapse of Hepatitis C Virus Cryoglobulinemic Vasculitis After Sustained Viral Response After Interferon-Free Direct-Acting Antivirals. Am. J. Gastroenterol. 2022, 117, 627–636. [Google Scholar] [CrossRef]
| Patients Characteristics | Total (n = 166) | Cryo ≤ 50 g/L (n = 102) | Cryo > 50 g/L (n = 64) | p Value |
|---|---|---|---|---|
| Age median [IQR] | 66 [53.75–77.0] | 65.0 [51–77.25] | 67.50 [57–77] | 0.160 |
| Sex male n (%) | 76 (45%) | 49 (48%) | 27 (42%) | 0.523 |
| Type of cryoglobulinemia | ||||
| Cryoglobulin type I | 16 (9.6%) | 8 (7.84%) | 8 (12.5%) | 0.4187 |
| Cryoglobulin type II | 77 (46.38%) | 44 (43.13%) | 33 (51.56%) | 0.3383 |
| Cryoglobulin type III | 60 (36%) | 38 (37.25%) | 22 (34.37%) | 0.7423 |
| Underlying conditions | ||||
| Monoclonal gammopathy | 9 (5.42%) | 4 (3.92%) | 5 (7.8%) | 0.2795 |
| Waldenström macroglobulinemia | 7 (4.21%) | 3 (2.94%) | 4 (4.68%) | 0.2416 |
| Diffuse large B-cell lymphoma | 8 (4.18%) | 7(6.98%) | 1 (1.56%) | 0.2930 |
| C-virus hepatitis associated cryoglobulinemia | 24 (14.45%) | 7 (6.8%) | 17 (26%) | 0.0007 |
| Rheumatic arthritis | 12 (7.2%) | 8 (7.84%) | 4 (6.25%) | 0.9211 |
| SLE | 6 (3.61%) | 5 (4.90%) | 1 (1.56%) | 0.4310 |
| Sjogren | 6 (3.61%) | 5 (4.90%) | 1 (1.56%) | 0.4310 |
| Constitutional symptoms | ||||
| Fatigue | 23 (13%) | 6 (5%) | 17 (26%) | 0.001 |
| Fever | 16 (9%) | 5 (4%) | 11 (17%) | 0.013 |
| Weigh loss (>10% of BMI in 6 months or 5% one month) | 6 (3%) | 0 (0%) | 6(9%) | 0.003 |
| Musculoskeletal symptoms | 14 (8%) | 3 (2%) | 11 (17%) | 0.002 |
| Vascular symptoms | ||||
| Purpura | 29 (17%) | 13 (12%) | 16 (25%) | 0.058 |
| Raynaud’s phenomenon/acrocyanosis | 19 (11%) | 10 (9%) | 9 (14%) | 0.457 |
| Livedo reticularis | 7 (4%) | 3 (2%) | 4 (6%) | 0.432 |
| Necrosis | 10 (6%) | 8 (7%) | 2 (3%) | 0.318 |
| Leukocytoclasic vasculitis on skin biopsy | 13/34 (38%) | 5/15 (4%) | 8/19 (42%) | 0.134 |
| Neurological features | ||||
| Sensitive deficiency | 16 (9.6%) | 12 (11.7%) | 4 (6.25%) | 0.02838 |
| Motor deficiency | 11 (6.62%) | 5 (4%) | 6 (9%) | >0.999 |
| Compatible neurologic features at electromyogram | 2 (1%) | 1 (0.9%) | 1 (1%) | >0.999 |
| Enlarged lymph nodes | 9 (5%) | 3 (2%) | 6 (9%) | 0.156 |
| Splenomegaly | 13 (7%) | 7 (6%) | 6 (9%) | 0.768 |
| Proteinuria > 0.5 g/day | 23/67 (34%) | 12/45 (26.6%) | 11/22 (50%) | 0.0988 |
| Hematuria > 10/champ | 28/67 (41.7%) | 17/44 (38.6%) | 11/23 (47%) | 0.6028 |
| Creatinine level (µmol/L) | 76.5 [63–102] | 75 [59–98] | 78.5 [65–117.5] | 0.2926 |
| (CH50) | 52 [39–76] | 64 [46–76] | 43 [20–61] | 0.017 |
| C3 | 1.01 [0.762–1.28] | 1.08 [0.82–1.35] | 0.9 [0.65–1.19] | 0.028 |
| C4 | 0.16 [0.09–0.255] | 0.1850 [0.110–0.277] | 0.12 [0.045–0.20] | 0.006 |
| Outcomes at one year | ||||
| Absence of relapse | 60 (36%) | 28 (27%) | 32 (50%) | 0.0046 |
| Evolution in chronic disease > 2 relapses | 63 (37%) | 63 (61%) | 0 (0%) | <0.0001 |
| Renal replacement therapy | 13 (7.83%) | 1 (0.98%) | 12 (18%) | <0.0001 |
| Patients Characteristics | Total (n = 166) | Type 1 (n = 16) | Mixed Cryo (n = 150) | p Value |
|---|---|---|---|---|
| Age median [IQR] | 66 (53.75–77) | 68.5 (64–77) | 66 (53–68.50) | 0.2952 |
| Sex male n (%) | 76 (45.7%) | 11 (68.7%) | 65 (43%) | 0.1998 |
| Underlying conditions before diagnosis | ||||
| Hematological malignancies | 14 (8.4%) | 5 (31%) | 9 (6%) | 0.0068 |
| C-virus hepatitis | 27 (16.2%) | 0 (0%) | 27 (18%) | 0.0761 |
| SLE | 5 (3%) | 1 (6.25%) | 4 (2.6%) | >0.9999 |
| Sjogren | 1 (6.25%) | 9 (6%) | >0.9999 | |
| Underlying conditions at diagnosis | ||||
| Hematological malignancy | 26 (15.6%) | 8 (50%) | 18 (12%) | 0.0011 |
| Waldenström macroglobulinemia | 7 (4.2%) | 7 (43%) | 0 (0%) | <0.0001 |
| C-virus hepatitis reactivation | 16 (9.6%) | 0 (0%) | 16 (10.6%) | 0.2242 |
| Constitutional symptoms | ||||
| Fatigue | 40 (24%) | 2 (12.5%) | 38 (25.3%) | 0.2362 |
| Fever | 15 (3%) | 0 (0%) | 15 (10%) | 0.2230 |
| Weigh loss (>10% of BMI in 6 months or 5% one month) | 10 (6%) | 2 (12.5%) | 8 (5.3%) | 0.6016 |
| Musculoskeletal symptoms | 25 (15%) | 0 (0%) | 25 (16.6%) | 0.0745 |
| Vascular symptoms | ||||
| Purpura | 25 (15%) | 2 (12.5%) | 23 (15.3%) | 0.7365 |
| Raynaud’s phenomenon/acrocyanosis | 21 (12.6%) | 1 (6.25%) | 20 (13.3%) | 0.4589 |
| Livedo reticularis | 13 (7.8%) | 1 (6.25%) | 12 (8%) | >0.9999 |
| Necrosis | 3 (1.8%) | 0 (0%) | 3 (2%) | >0.9999 |
| Leukocytoclasic vasculitis on skin biopsy | 13/29 44(%) | 1/2 (50%) | 12/27 (8%) | 0.665 |
| Neurological features | ||||
| Sensitive deficiency | 15 (3%) | 1 (6.25%) | 14 (9.3%) | 0.7059 |
| Motor deficiency | 7 (4.2%) | 0 (0%) | 7 (4.6%) | 0.6080 |
| Compatible neurologic features at electromyogram | 2 (1.2%) | 0 (0%) | 2 (1.33%) | >0.9999 |
| Enlarged lymph nodes | 8 (4.8%) | 1 (6.25%) | 7 (4.66%) | >0.9999 |
| Splenomegaly | 12 (7.2%) | 5 (31.25%) | 7 (4.66%) | 0.0039 |
| Proteinuria > 0.5 g/day | 22/65 (33.8%) | 1/10 (10%) | 21/55 (38%) | 0.1449 |
| Hematuria > 10/champ | 26/65 (40%) | 1/10 (10%) | 25/55 (45%) | 0.751 |
| Creatinine level (µmol/L) | 76.5 (63–102) | 76 (65–94) | 78 (64–106) | 0.5333 |
| (CH50) UI/mL | 51 (39–76) | 78 (58–193) | 51 (32.25–76) | 0.0350 |
| C3 g/L | 1.10 (0.76–1.29) | 1.08 (0.94–1.2) | 1.010 (0.75–1.28) | 0.5868 |
| C4 g/L | 0.16 (0.09–0.26) | 0.1450 (0.070) | 0.16 (0.0850) | 0.3997 |
| Patients Characteristics | Total (n = 166) | Non-Severe (n = 133) | Severe (n = 33) | p Value |
|---|---|---|---|---|
| Age median [IQR] | 66 [53.75–77.0] | 66 [54.5–77.5] | 69.5 [53–77.5] | 0.4394 |
| Sex male n (%) | 75 (45.1%) | 61(45.8%) | 14 (42.4%) | >0.9999 |
| Underlying conditions before diagnosis | ||||
| Hematological malignancies | 15 (9%) | 11 (8.2%) | 4 (12.1%) | 0.1433 |
| C-virus hepatitis | 25 (15%) | 20 (15%) | 5 (15.1%) | >0.999 |
| SLE | 6 (3.6%) | 6 (4.5%) | 0 (0%) | 0.3617 |
| Sjogren | 11 (6.6%) | 8 (6%à | 3 (9%) | 0.3537 |
| Underlying conditions at diagnosis | ||||
| Hematological malignancy | 17 (12.7%) | 9 (27%) | 0.0279 | |
| Waldenström macroglobulinemia | 43 (26%) | 36 (27%) | 7 (21%) | 0.673 |
| C-virus hepatitis reactivation | 17 (10%) | 16 (12%) | 1 (3%) | 0.153 |
| Constitutional symptoms | ||||
| Fatigue | 43 (25.9%) | 36 (27%) | 7 (21%) | 0.6573 |
| Fever | 17 (10.2%) | 16 (12%) | 1 (3%) | 0.1526 |
| Weight loss > 10% | 11 (6%) | 8 (6%) | 3 (9%) | 0.691 |
| Musculoskeletal symptoms | 27 (16%) | 22 (16%) | 5 (15%) | 0.893 |
| Vascular symptoms | ||||
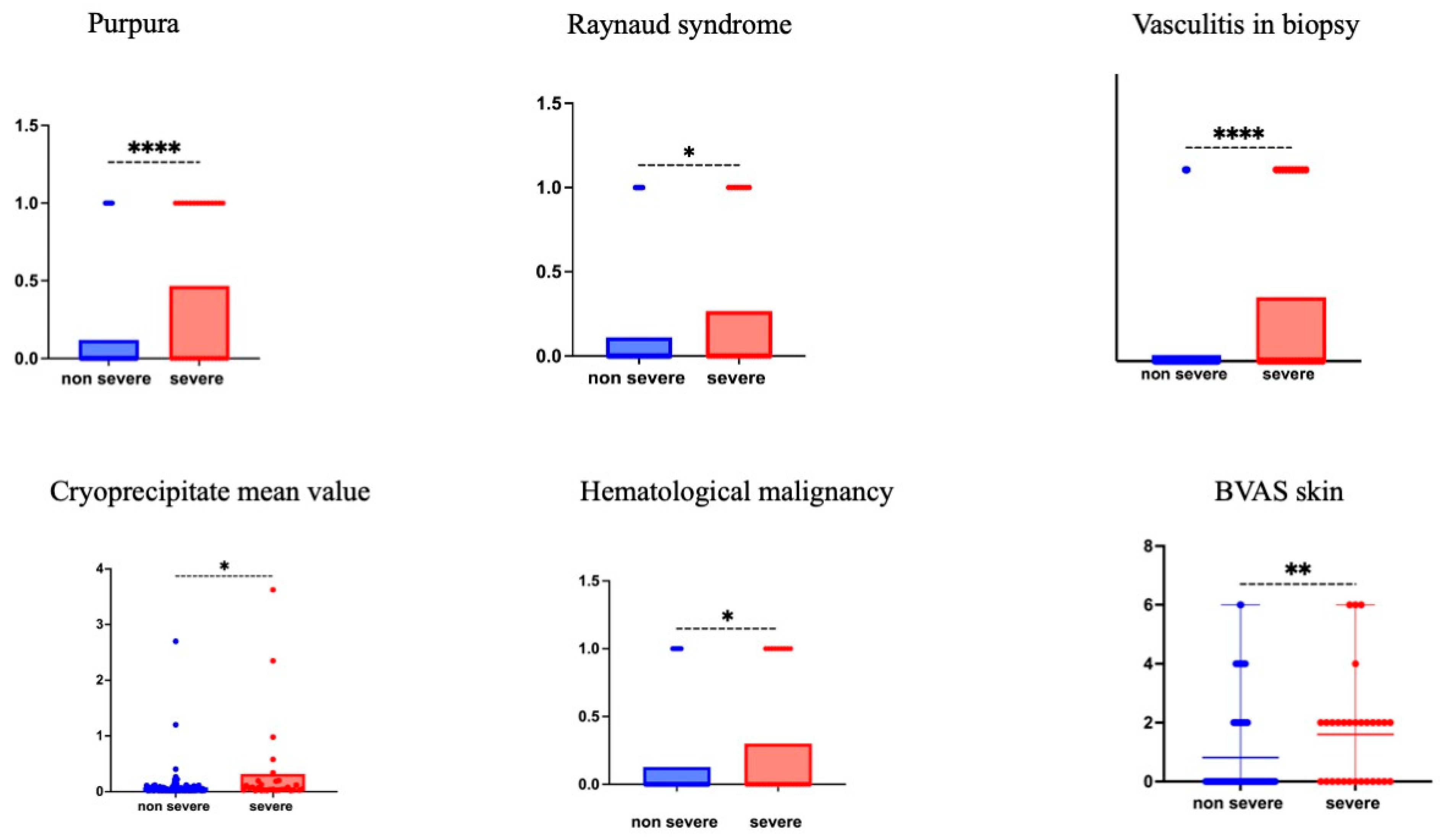
| Purpura | 29 (17%) | 15 (11%) | 14 (42%) | <0.001 |
| Raynaud’s phenomenon/acrocyanosis | 22 (13%) | 14 (10%) | 8 (24%) | 0.039 |
| Livedo reticularis | 14 (8%) | 11 (8%) | 3 (9%) | >0.9999 |
| Necrosis | 3 (1%) | 1 (0.01%) | 2 (6%) | 0.095 |
| Leukocytoclasic vasculitis on skin biopsy | 14 (8%) | 4/26 (15%) | 10/15 (66%) | <0.0001 |
| Neurological features | ||||
| Sensitive deficiency | 16 (9.6%) | 13 (9.7%) | 3 (9%) | >0.999 |
| Motor deficiency | 8 (4.8%) | 7 (5.2%) | 1 (3%) | 0.704 |
| Compatible neurologic features at electromyogram | 6 (3.6%) | 4 (3%) | 2 (6%) | 0.6203 |
| Enlarged lymph nodes | 9 (5.4%) | 8 (6%) | 1 (3%) | 0.6919 |
| splenomegaly | 13 (7%) | 8 (6%) | 5 (15%) | 0.132 |
| Proteinuria > 0.5 g/day | 24/83 (28.8%) | 20/53 (37.7%) | 4/30 (13%) | 0.0234 |
| Hematuria > 10/champ | 28/82 (34%) | 23/52 (44%) | 5/30 (16%) | 0.0152 |
| Creatinine level (µmol/L) | 77 [63–104] | 76 [61.7–104] | 82 (67–102) | 0.5827 |
| Total hemolytic complement (CH50) median [IQR] | 51 [39–76] | 51 [40.5–73.5] | 53 [35.76] | 0.804 |
| C3 median [IQR] | 1.010 [0.7650–1.010] | 1.010 [0.7550–1.280] | 1.015 [0.7825–1.428] | 0.599 |
| C4 median [IQR] | 0.160 [0.090–0.260] | 0.170 [0.100–0.2500] | 0.1250 [0.060–0.3025] | 0.402 |
| Positive rheumatoid factor n(%) | 36 (22%) | 28 (21%) | 8 (26%) | 0.784 |
| Cryoglobulins dosage | 0.0370 [0.0250–0.070] | 0.03360 [0.025–0.0650] | 0.054 [0.0280–0.1915] | 0.010 |
| BVAS SCORE | ||||
| Overall | 3 [0–6] | 3 [0–6] | 2 [1–7.25] | 0.6531 |
| General signs | 0 [0–1] | 0 [0–1] | 0 [0–1] | 0.6914 |
| Skin | 0 [0–2] | 0 [0–2] | 2 [0–6] | 0.0047 |
| Neurological | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0.8957 |
| Kidney | 0 [0–1] | 0 [0–0] | 0 [0–0] | 0.6495 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razanamahery, J.; Aubertin, N.; Bach Bunner, M.; Blaison, G.; Bouldoires, B.; Soumagne, T. Identification of Key Predictors of Cryoglobulinemia Severity at Diagnosis: Threshold, Type, and Severity Score at Diagnosis. J. Clin. Med. 2025, 14, 556. https://doi.org/10.3390/jcm14020556
Razanamahery J, Aubertin N, Bach Bunner M, Blaison G, Bouldoires B, Soumagne T. Identification of Key Predictors of Cryoglobulinemia Severity at Diagnosis: Threshold, Type, and Severity Score at Diagnosis. Journal of Clinical Medicine. 2025; 14(2):556. https://doi.org/10.3390/jcm14020556
Chicago/Turabian StyleRazanamahery, Jerome, Nils Aubertin, Maxime Bach Bunner, Gilles Blaison, Bastien Bouldoires, and Thibaud Soumagne. 2025. "Identification of Key Predictors of Cryoglobulinemia Severity at Diagnosis: Threshold, Type, and Severity Score at Diagnosis" Journal of Clinical Medicine 14, no. 2: 556. https://doi.org/10.3390/jcm14020556
APA StyleRazanamahery, J., Aubertin, N., Bach Bunner, M., Blaison, G., Bouldoires, B., & Soumagne, T. (2025). Identification of Key Predictors of Cryoglobulinemia Severity at Diagnosis: Threshold, Type, and Severity Score at Diagnosis. Journal of Clinical Medicine, 14(2), 556. https://doi.org/10.3390/jcm14020556






