Prevention and Treatment of New-Onset Postoperative Atrial Fibrillation in the Acute Care Setting: A Narrative Review
Abstract
1. Introduction
2. Prevention of POAF
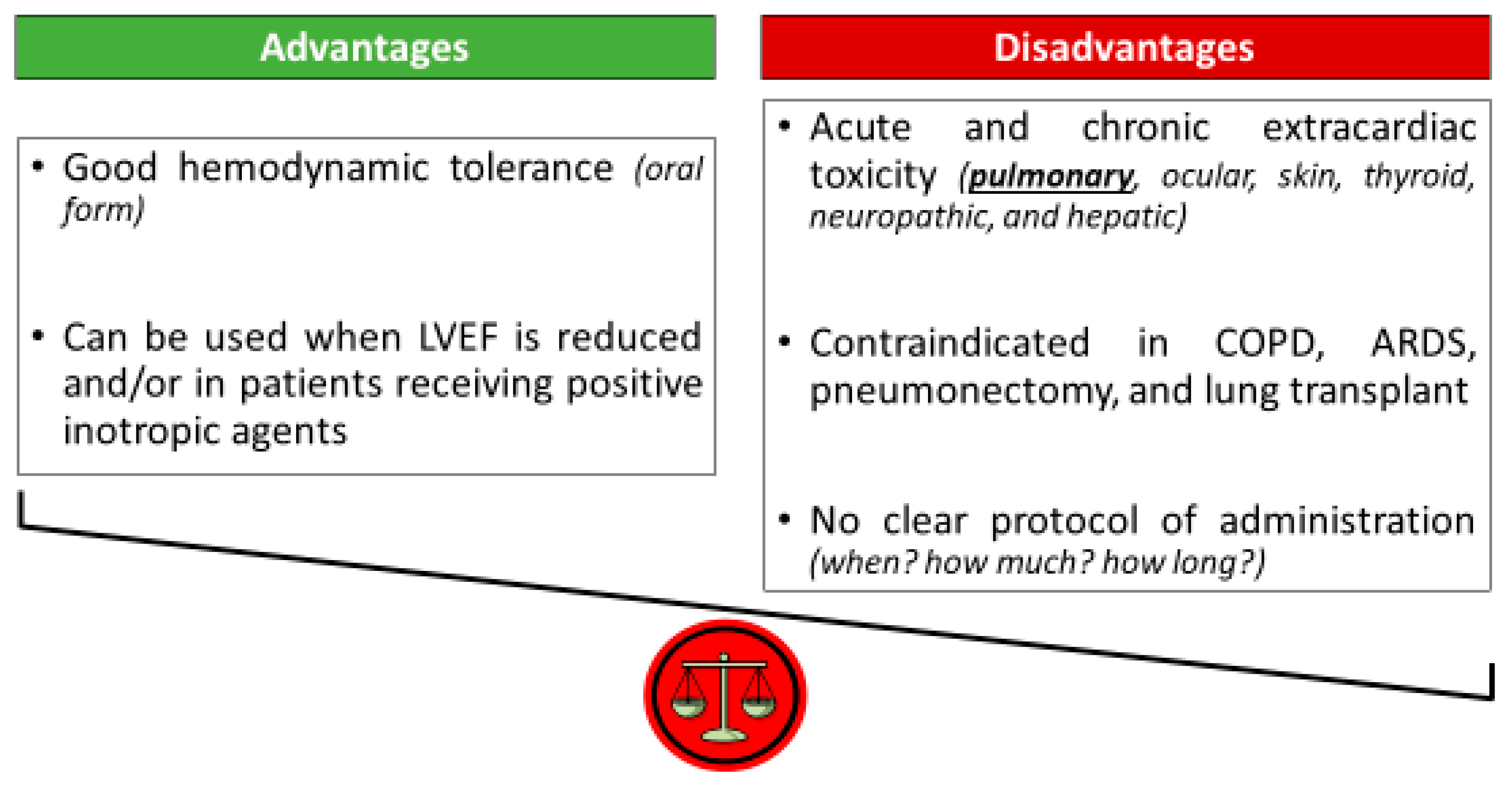
2.1. Amiodarone: A Dead Man Walking [22]
2.2. Perioperative Optimization of Beta-Blockers
2.3. Other Interventions
3. Treatment of POAF
3.1. Treatment of Associated Factors
3.2. Considering a Rate Control Strategy
3.3. Which Place for a Rhythm Control Strategy in the Acute Care Setting?
3.4. Prevention of Thromboembolic Events
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Echahidi, N.; Pibarot, P.; O’Hara, G.; Mathieu, P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J. Am. Coll. Cardiol. 2008, 51, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Gillinov, A.M.; Bagiella, E.; Moskowitz, A.J.; Raiten, J.M.; Groh, M.A.; Bowdish, M.E.; Ailawadi, G.; Kirkwood, K.A.; Perrault, L.P.; Parides, M.K.; et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N. Engl. J. Med. 2016, 374, 1911–1921. [Google Scholar] [CrossRef]
- Gaudino, M.; Di Franco, A.; Rong, L.Q.; Piccini, J.; Mack, M. Postoperative atrial fibrillation: From mechanisms to treatment. Eur. Heart J. 2023, 44, 1020–1039. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Slama, M.; Lakbar, I.; Maizel, J.; Kato, H.; Leone, M.; Okada, M. Landiolol for treatment of new-onset atrial fibrillation in critical care: A systematic review. J. Clin. Med. 2024, 13, 2951. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.P.; Fontes, M.L.; Tudor, I.C.; Ramsay, J.; Duke, P.; Mazer, C.D.; Barash, P.G.; Hsu, P.H.; Mangano, D.T.; Foundation, E. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004, 291, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Villareal, R.P.; Hariharan, R.; Liu, B.C.; Kar, B.; Lee, V.-V.; Elayda, M.; Lopez, J.; Rasekh, A.; Wilson, J.M.; Massumi, A. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J. Am. Coll. Cardiol. 2004, 43, 742–748. [Google Scholar] [CrossRef]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.B.; Nattel, S. Postoperative atrial fibrillation: Mechanisms, manifestations and management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef]
- Conen, D.; Wang, M.K.; Devereaux, P.J.; Whitlock, R.; McIntyre, W.F.; Healey, J.S.; Yuan, F.; Yusuf, S.; Lamy, A. New-onset perioperative atrial fibrillation after coronary artery bypass grafting and long-term risk of adverse events: An analysis from the coronary trial. J. Am. Heart Assoc. 2021, 10, e020426. [Google Scholar] [CrossRef]
- Wang, M.K.; Meyre, P.B.; Heo, R.; Devereaux, P.; Birchenough, L.; Whitlock, R.; McIntyre, W.F.; Chen, Y.C.P.; Ali, M.Z.; Biancari, F.; et al. Short-term and long-term risk of stroke in patients with perioperative atrial fibrillation after cardiac surgery: Systematic review and meta-analysis. CJC Open 2021, 4, 85–96. [Google Scholar] [CrossRef]
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial fibrillation: Epidemiology, pathophysiology, and clinical outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Ascione, R.; Caputo, M.; Calori, G.; Lloyd, C.T.; Underwood, M.J.; Angelini, G.D. Predictors of atrial fibrillation after conventional and beating heart coronary surgery: A prospective, randomized study. Circulation 2000, 102, 1530–1535. [Google Scholar] [CrossRef]
- Ishida, K.; Kimura, F.; Imamaki, M.; Ishida, A.; Shimura, H.; Kohno, H.; Sakurai, M.; Miyazaki, M. Relation of inflammatory cytokines to atrial fibrillation after off-pump coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2006, 29, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.K.; Laurikka, J.; Vikman, S.; Nieminen, R.; Moilanen, E.; Tarkka, M.R. Postoperative interleukin-8 levels related to atrial fibrillation in patients undergoing coronary artery bypass surgery. World J. Surg. 2008, 32, 2643–2649. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Zhao, Y.; Steinberg, B.A.; He, X.; Mathew, J.P.; Fullerton, D.A.; Hegland, D.D.; Hernandez, A.F.; Mills, R.M.; Klaskala, W.; et al. Comparative effectiveness of pharmacotherapies for prevention of atrial fibrillation following coronary artery bypass surgery. Am. J. Cardiol. 2013, 112, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Maesen, B.; Nijs, J.; Maessen, J.; Allessie, M.; Schotten, U. Post-operative atrial fibrillation: A maze of mechanisms. Europace 2012, 14, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, D.R.; Uhm, J.S.; Shim, J.; Sung, J.-H.; Kim, J.-Y.; Pak, H.-N.; Lee, M.-H.; Joung, B. New-onset atrial fibrillation predicts long-term newly developed atrial fibrillation after coronary artery bypass graft. Am. Heart J. 2014, 167, 593–600. [Google Scholar] [CrossRef]
- Konstantino, Y.; Zelnik Yovel, D.; Friger, M.D.; Sahar, G.; Knyazer, B.; Amit, G. Postoperative atrial fibrillation following artery bypass graft surgery predicts long-term atrial fibrillation and stroke. Isr. Med. Assoc. J. 2016, 18, 744–748. [Google Scholar]
- Mitchell, L.B.; Crystal, E.; Heilbron, B.; Page, P. Atrial fibrillation following cardiac surgery. Can. J. Cardiol. 2005, 21 (Suppl. SB), 45B–50B. [Google Scholar]
- European Heart Rhythm Association; Heart Rhythm Society; Fuster, V.; Ryden, L.E.; Cannom, D.S.; Crijns, H.J.; Curtis, A.B.; Ellenbogen, K.A.; Halperin, J.L.; Le Heuzey, J.-Y.; et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for practice guidelines. Circulation 2006, 114, e257–e354. [Google Scholar]
- Nair, S.G. Atrial fibrillation after cardiac surgery. Ann. Card. Anesth. 2010, 13, 196–205. [Google Scholar] [CrossRef]
- Fuster, V.; Ryden, L.E.; Cannom, D.S.; Crijns, H.J.; Curtis, A.B.; Ellenbogen, K.A.; Halperin, J.L.; Kay, G.N.; Le Huezey, J.-Y.; Lowe, J.E.; et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011, 123, e269–e367. [Google Scholar]
- Providencia, R.; Kukendra-Rajah, K.; Barra, S. Amiodarone for atrial fibrillation: A dead man walking? Eur. Heart J. 2024, 45, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Daoud, E.G.; Strickberger, S.A.; Man, K.C.; Goyal, R.; Deeb, G.M.; Bolling, S.F.; Pagani, F.D.; Bitar, C.; Meissner, M.D.; Morady, F. Preoperative amiodarone as prophylaxis against atrial fibrillation after heart surgery. N. Engl. J. Med. 1997, 337, 1785–1791. [Google Scholar] [CrossRef]
- Yagdi, T.; Nalbantgil, S.; Ayik, F.; Apaydin, A.; Islamoglu, F.; Posacioglu, H.; Calkavur, T.; Atay, Y.; Buket, S. Amiodarone reduces the incidence of atrial fibrillation after coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2003, 125, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Auer, J.; Weber, T.; Berent, R.; Puschmann, R.; Hartl, P.; Ng, C.-K.; Schwarz, C.; Lehner, E.; Strasser, U.; Lassnig, E.; et al. A comparison between oral antiarrhythmic drugs in the prevention of atrial fibrillation after cardiac surgery: The pilot study of prevention of postoperative atrial fibrillation (SPPAF), a randomized, placebo-controlled trial. Am. Heart J. 2004, 147, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.S.; Nolan, P.E.; Slack, M.K.; Tisdale, J.E.; Hilleman, D.E.; Copeland, J.G. Amiodarone prophylaxis for atrial fibrillation after cardiac surgery: Meta-analysis of dose response and timing of initiation. Pharmacotherapy 2007, 27, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; E Dilaveris, P.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the diagnosis and management of atrial fibrillation: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Isiadinso, I.; Meshkov, A.B.; Gaughan, J.; Sandhu, P.; Lim, S.; Cordova, F.; Criner, G. Atrial arrhythmias after lung and heart-lung transplant: Effects on short-term mortality and the influence of amiodarone. J. Heart Lung Transplant. 2011, 30, 37–44. [Google Scholar] [CrossRef]
- van Erven, L.; Schalij, M.J. Amiodarone: An effective antiarrhythmic drug with unusual side effects. Heart 2010, 96, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Muehlschlegel, J.D.; Burrage, P.S.; Ngai, J.Y.; Prutkin, J.M.; Huang, C.-C.; Xu, X.; Chae, S.H.; Bollen, B.A.; Piccini, J.P.; Schwann, N.M.; et al. Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anaesthetists practice advisory for the management of perioperative atrial fibrillation in patients undergoing cardiac surgery. Anesth. Analg. 2019, 128, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Couffignal, C.; Amour, J.; Ait-Hamou, N.; Cholley, B.; Fellahi, J.L.; Duval, X.; Costa De Beauregard, Y.; Nataf, P.; Dilly, M.P.; Provenchère, S.; et al. Timing of beta-blocker reintroduction and the occurrence of postoperative atrial fibrillation after cardiac surgery: A prospective cohort study. Anesthesiology 2020, 132, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Blessberger, H.; Lewis, S.R.; Pritchard, M.W.; Fawcett, L.J.; Domanovits, H.; Schlager, O.; Wildner, B.; Kammler, J.; Steinwender, C.; Cochrane Anaesthesia Group. Perioperative beta-blockers for preventing surgery-related mortality and morbidity in adults undergoing non-cardiac surgery. Cochrane Database Syst. Rev. 2019, 9, CD013438. [Google Scholar] [CrossRef] [PubMed]
- Fellahi, J.L.; Heringlake, M.; Knotzer, J.; Fornier, W.; Cazenave, L.; Guarracino, F. Landiolol for managing atrial fibrillation in post-cardiac surgery. Eur. Heart J. Suppl. 2018, 20, A4–A9. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, T.; Allwood, M.; McIntyre, W.F.; Park, L.J.; Daza, J.; Ofori, S.N.; Wang, M.K.; Borges, F.K.; Conen, D.; Marcucci, M.; et al. Landiolol for the prevention of postoperative atrial fibrillation after cardiac surgery: A systematic review and meta-analysis. Can. J. Anesth. 2023, 70, 1828–1838. [Google Scholar] [CrossRef]
- Kaminohara, J.; Hara, M.; Uehara, K.; Suruga, M.; Yunoki, K.; Takatori, M. Intravenous landiolol for the prevention of atrial fibrillation after aortic root, ascending aorta, and aortic arch surgery: A propensity score-matched analysis. JTCVS Open 2022, 11, 49–58. [Google Scholar] [CrossRef]
- Gaudino, M.; Sanna, T.; Ballman, K.V.; Robinson, N.B.; Hameed, I.; Audisio, K.; Rahouma, M.; Di Franco, A.; Soletti, G.J.; Lau, C.; et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: An adaptive, single-centre, single-blind, randomized, controlled trial. Lancet 2021, 398, 2075–2083. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Hafez, A.H.; Elaraby, A.; Roshdy, M.R.; Abdelaziz, M.; Eltobgy, M.A.; Elsayed, H.; El-Samahy, M.; Elbehbeh, N.A.; Philip, K.G.; et al. Posterior pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: A systematic review and meta-analysis. EuroIntervention 2023, 19, e305–e317. [Google Scholar] [CrossRef]
- Piccini, J.P.; Ahlsson, A.; Dorian, P.; Gillinov, M.A.; Kowey, P.R.; Mack, M.J.; Milano, C.A.; Perrault, L.P.; Steinberg, J.S.; Waldron, N.H.; et al. Design and rationale of a phase 2 study of NeurOtoxin (Botulinum toxin type A) for the prevention of postoperative atrial fibrillation—The NOVA study. Am. Heart J. 2022, 245, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; Ross-White, A.; Sibley, S. Magnesium prophylaxis of new-onset atrial fibrillation: A systematic review and meta-analysis. PLoS ONE 2023, 18, e029274. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.; Campbell, N.G.; Allen, E.; Jamal, Z.; Sturgess, J.; Sanders, J.; Opondo, C.; Roberts, N.; Aron, J.; Maccaroni, M.R.; et al. Potassium supplementation and prevention of atrial fibrillation after cardiac surgery. JAMA 2024, 332, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Wang, M.K.; Popova, E.; Chan, M.T.V.; Landoni, G.; Reimer, C.; Reyes, J.C.T.; Grande, A.M.; Tallada, A.G.; I Sessler, D.; et al. Effect of colchicine on perioperative atrial fibrillation and myocardial injury after non-cardiac surgery in patients undergoing major thoracic surgery (COP-AF): An international randomized trial. Lancet 2023, 402, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Fleischmann, K.E.; Smilowitz, N.R.; Fuentes, L.d.L.; Mukherjee, D.; Aggarwal, N.R.; Ahmad, F.S.; Allen, R.B.; Altin, S.E.; Auerbach, A.; et al. 2024 AHA/ACC/ACS/ASNC/HRS/SCA/SCCT/SCMR/SVM Guideline for perioperative cardiovascular management for noncardiac surgery. J. Am. Coll. Cardiol. 2024, 84, 1869–1969. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, J.E.; Padhi, I.; Goldberg, A.; Silverman, N.A.; Webb, C.R.; Higgins, R.S.; Paone, G.; Frank, D.M.; Borzak, S. A randomized, double-blind comparison of intravenous diltiazem and digoxin for atrial fibrillation after coronary artery bypass surgery. Am. Heart J. 1998, 135, 739–747. [Google Scholar] [CrossRef]
- Siu, C.W.; Lau, C.P.; Lee, W.L.; Lam, K.F.; Tse, H.F. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit. Care Med. 2009, 37, 2174–2179. [Google Scholar] [CrossRef]
- Scheuermeyer, F.X.; Grafstein, E.; Stenstrom, R.; Christenson, J.; Heslop, C.; Heilbron, B.; McGrath, L.; Innes, G.; Smith, S.W. Safety and efficiency of calcium channel blockers versus beta-blockers for rate control in patients with atrial fibrillation and no acute underlying medical illness. Acad. Emerg. Med. 2013, 20, 222–230. [Google Scholar] [CrossRef]
- Perrett, M.; Gohil, N.; Tica, O.; Bunting, K.V.; Kotecha, D. Efficacy and safety of intravenous beta-blockers in acute atrial fibrillation and flutter is dependent on beta-1 selectivity: A systematic review and meta-analysis of randomized trials. Clin. Res. Cardiol. 2023, 113, 831–841. [Google Scholar] [CrossRef]
- Darby, A.E.; Dimarco, J.P. Management of atrial fibrillation in patients with structural heart disease. Circulation 2012, 125, 945–957. [Google Scholar] [CrossRef]
- Lin, M.H.; Kamel, H.; Singer, D.E.; Wu, Y.L.; Lee, M. Perioperative/postoperative atrial fibrillation and risk of subsequent strokeand/or mortality. Stroke 2019, 50, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Nielsen, S.J.; Bergfeldt, L.; Ahlsson, A.; Friberg, L.; Björck, S.; Franzén, S.; Jeppsson, A. New-onset atrial fibrillation after coronary artery bypass grafting and long-term outcome: A population-based nationwide from the SWEDEHEART registry. J. Am. Heart Assoc. 2021, 10, e017966. [Google Scholar] [CrossRef] [PubMed]
- Neves, I.A.; Magalhaes, A.; Lima da Silva, G.; Almeida, A.G.; Borges, M.; Costa, J.; Ferreira, J.J.; Pinto, F.J.; Caldeira, D. Anticoagulation therapy in patients with post-operative atrial fibrillation: Systematic review with meta-analysis. Vascul Pharmacol. 2022, 142, 106929. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, E.; Guinot, P.G.; Rozec, B.; Oilleau, J.-F.; Fellahi, J.-L.; Gaudard, P.; Lorne, E.; Mahjoub, Y.; Besnier, E.; Moussa, M.D.; et al. Comparison of landiolol and amiodarone for the treatment of new-onset atrial fibrillation after cardiac surgery (FAAC) trial: Study protocol for a randomized controlled trial. Trials 2023, 24, 353. [Google Scholar] [CrossRef] [PubMed]
- Fornier, W.; Jacquet-Lagrèze, M.; Collenot, T.; Teixeira, P.; Portran, P.; Schweizer, R.; Ovize, M.; Fellahi, J.-L. Microvascular effects of intravenous esmolol in patients with normal cardiac function undergoing postoperative atrial fibrillation: A prospective pilot study in cardiothoracic surgery. Crit. Care 2017, 21, 302. [Google Scholar] [CrossRef]
- Vacheron, C.H.; Allaouchiche, B. Illustration of the loss of haemodynamic coherence during atrial fibrillation using urethral photoplethysmography. BMJ Case Rep. 2019, 12, e230757. [Google Scholar] [CrossRef]
- Pastore, M.C.; Degiovanni, A.; Grisafi, L.; Renda, G.; Sozzani, M.; Giordano, A.; Salvatici, C.; Lorenz, V.; Pierfelice, F.; Cappelli, C.; et al. Left atrial strain to predict postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. Circ. Cardiovasc. Imaging 2024, 17, e015969. [Google Scholar] [CrossRef]
- Schäfer, M.; Contreras, N.; Ramakrishna, S.; Zimmerman, J.M.; Varghese, T.K., Jr.; Mitzman, B. Preoperative atrial deformation indices predict postoperative atrial fibrillation in patients undergoing lung resection surgery. Echocardiography 2025, 42, e70105. [Google Scholar] [CrossRef]
- Fedele, D.; Alvarez, M.C.; Maida, A.; Vasumini, N.; Amicone, S.; Canton, L.; Di Leo, M.; Basile, M.; Manaresi, T.; Angeli, F.; et al. Prevention of atrial fibrillation with SGLT2 inhibitors across the spectrum of cardiovascular disorders: A meta-analysis of randomized controlled trials. Eur. Heart J. Cardiovasc. Pharmacother. 2025, 11, 441–450. [Google Scholar] [CrossRef]

| First Author | Journal | Study Design |
|---|---|---|
| Gillinov AM [2] | N Engl J Med 2016 | Multicenter RCT comparing rate control vs. rhythm control for POAF after cardiac surgery |
| Mathew JP [5] | JAMA 2004 | Large cohort study showing a significant association between perioperative optimization of beta-blockers and POAF |
| Isiadinso I [30] | J Heart Lung Transplant 2011 | Retrospective study showing the association between amiodarone and mortality in POAF after heart–lung transplantation |
| Couffignal C [33] | Anesthesiology 2020 | Prospective multicenter cohort study assessing timing of beta-blockers reintroduction and POAF after cardiac surgery |
| Blessberger H [34] | Cochrane Database Syst rev 2019 | Meta-analysis reporting the role of beta-blockers for preventing morbidity and mortality after noncardiac surgery |
| Cafaro T [36] | Can J Anesth 2023 | Meta-analysis of RCTs showing the efficacy of landiolol in preventing POAF after cardiac surgery |
| Kaminohara J [37] | JTCVS Open 2022 | Propensity score-matched analysis showing the efficacy of landiolol in preventing POAF after cardiac surgery |
| Gaudino M [38] | Lancet 2021 | RCT showing the efficacy of posterior left pericardiotomy for the prevention of POAF after cardiac surgery |
| Curran J [41] | Plos One 2023 | Meta-analysis showing the conflicting results regarding magnesium prophylaxis of new-onset atrial fibrillation |
| O’Brien B [42] | JAMA 2024 | Multicenter RCT showing the lack of effect of potassium supplementation to prevent POAF after cardiac surgery |
| Conen D [43] | Lancet 2023 | International RCT showing the lack of effect of colchicine to prevent POAF after noncardiac surgery |
| Taha A [51] | JAHA 2021 | Population-based nationwide registry studying POAF and long-term outcome after cardiac surgery |
| Neves IA [52] | Vascul Pharmacol 2022 | Meta-analysis on the use of anticoagulation in POAF after cardiac surgery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fellahi, J.-L.; Fischer, M.-O.; Ruste, M.; Jacquet-Lagreze, M. Prevention and Treatment of New-Onset Postoperative Atrial Fibrillation in the Acute Care Setting: A Narrative Review. J. Clin. Med. 2025, 14, 6835. https://doi.org/10.3390/jcm14196835
Fellahi J-L, Fischer M-O, Ruste M, Jacquet-Lagreze M. Prevention and Treatment of New-Onset Postoperative Atrial Fibrillation in the Acute Care Setting: A Narrative Review. Journal of Clinical Medicine. 2025; 14(19):6835. https://doi.org/10.3390/jcm14196835
Chicago/Turabian StyleFellahi, Jean-Luc, Marc-Olivier Fischer, Martin Ruste, and Matthias Jacquet-Lagreze. 2025. "Prevention and Treatment of New-Onset Postoperative Atrial Fibrillation in the Acute Care Setting: A Narrative Review" Journal of Clinical Medicine 14, no. 19: 6835. https://doi.org/10.3390/jcm14196835
APA StyleFellahi, J.-L., Fischer, M.-O., Ruste, M., & Jacquet-Lagreze, M. (2025). Prevention and Treatment of New-Onset Postoperative Atrial Fibrillation in the Acute Care Setting: A Narrative Review. Journal of Clinical Medicine, 14(19), 6835. https://doi.org/10.3390/jcm14196835







